SUMMARY
Our understanding of mechanisms that regulate the differentiation of specific classes of synapses is limited. Here, we investigate the formation of synapses between hippocampal dentate gyrus (DG) neurons and their target CA3 neurons and find that DG neurons preferentially form synapses with CA3 rather than DG or CA1 neurons in culture, suggesting that specific interactions between DG and CA3 neurons drive synapse formation. Cadherin-9 is expressed selectively in DG and CA3 neurons, and downregulation of cadherin-9 in CA3 neurons leads to a selective decrease in the number and size of DG synapses onto CA3 neurons. In addition, loss of cadherin-9 from DG or CA3 neurons in vivo leads to striking defects in the formation and differentiation of the DG-CA3 mossy fiber synapse. These observations indicate that cadherin-9 bidirectionally regulates DG-CA3 synapse development and highlight the critical role of differentially expressed molecular cues in establishing specific connections in the mammalian brain.
INTRODUCTION
During the development of neural circuits, axons navigate complex cellular environments to form synapses with specific cell types and at specific subcellular locations. Consequently, a neuron that receives synaptic input from multiple presynaptic sources will often develop distinct types of synapses unique to each input. Although progress has been made in understanding general mechanisms of axon guidance and synaptogenesis, the molecular mechanisms that regulate the formation and differentiation of specific classes of synapses in the mammalian central nervous system are poorly understood.
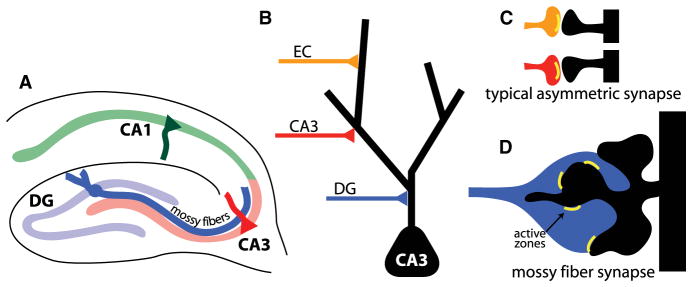
The hippocampus is an excellent model for studying the development of specific classes of synapses because the pattern of connectivity between different cell types is well characterized, and different classes of synapses are structurally distinct (Figures 1A–1D). This is most strikingly exemplified by mossy fiber synapses that connect dentate gyrus (DG) and CA3 neurons. The mossy fiber presynaptic terminal consists of a large and complex presynaptic bouton that grows 50–100 times larger in volume than a typical asymmetric synapse and can contain over 30 separate vesicle release sites (Chicurel and Harris, 1992; Rollenhagen et al., 2007). The postsynaptic structure on the CA3 dendrite consists of an equally elaborate multiheaded spine known as a thorny excrescence (TE) (Figure 1D) (Amaral and Dent, 1981). Because of its enormous size and position near the soma of CA3 neurons, activation of a single mossy fiber synapse can cause the CA3 neuron to fire and, therefore, has been called a “detonator” synapse (McNaughton and Morris, 1987). Farther from the soma, CA3 neurons also receive synaptic input from other CA3 neurons and the entorhinal cortex onto typical asymmetric synapses (Figures 1B and 1C). The molecular mechanisms that drive initial formation and maturation of these unique hippocampal mossy fiber synapses remain unknown and are likely to be distinct from those signals that govern typical asymmetric synapse formation.
Figure 1. Specificity of Synaptic Connections in the Hippocampus.
(A) Diagrammatic representation of mossy fiber pathway of the hippocampus.
(B–D) Types of synapses on the apical dendrite of CA3 pyramidal neurons.
Evidence in support for a role of molecular interactions in regulating the differentiation of specific classes of synapses comes largely from genetic studies in invertebrates (Ackley and Jin, 2004; Rose and Chiba, 2000). For example, in C. elegans the adhesion molecules SYG-1 and SYG-2 are required for the correct localization of a particular synapse, but not for axon growth or synaptogenesis (Shen and Bargmann, 2003; Shen et al., 2004). In vertebrates, molecular signals that regulate topographic mapping (Flanagan, 2006) and laminar restriction (Yamagata and Sanes, 2008) have been identified, but relatively little is known about signals that regulate differentiation of specific subtypes of synapses (Ango et al., 2004; Williams et al., 2010; Zipursky and Sanes, 2010).
Our understanding of the mechanisms that regulate synapse-specific differentiation in mammalian systems has lagged behind other systems partly because it is difficult to assess the complete connectivity of individual neurons in the brain. Cultured neurons are an invaluable tool for investigating general aspects of synapse form and function, but it is often assumed that specificity is lost in dissociated culture. Here, we show that, contrary to expectation, several aspects of synaptic specificity are well preserved in dissociated hippocampal cultures. Using cell- and synapse-specific markers, we developed two in vitro assays and discovered that DG neurons preferentially make synapses with appropriate CA3 target neurons rather than DG or CA1 neurons in culture. We then used the culture system to identify cell adhesion molecules that selectively regulate the formation of one type of synapse over another. Through these experiments we identified cadherin-9 as a critical mediator of DG-CA3 synapse formation.
Cadherins are single-pass transmembrane molecules that mediate cell-cell interactions, and their differential expression in the brain has led to the speculation that they regulate the formation of specific synaptic connections, but this has never been tested. We found that cadherin-9, a classic type II cadherin expressed exclusively by DG and CA3 neurons in the hippocampus (Bekirov et al., 2002), is required specifically for formation of DG but not CA1 or CA3 synapses in culture. In vivo, loss of cadherin-9 from either DG or CA3 neurons severely disrupts mossy fiber bouton and TE spine formation through trans-synaptic interactions. Furthermore, cadherin-9 has a specific role at the mossy fiber synapse because it is not required for typical spine formation on DG and CA1 dendrites. Taken together, these experiments indicate that cadherin-9 regulates the formation and differentiation of DG-CA3 synapses and provide direct evidence that differentially expressed cadherins regulate the formation of specific neural circuits.
RESULTS
DG Neurons Form Synapses with Appropriate Synaptic Targets in Dissociated Culture
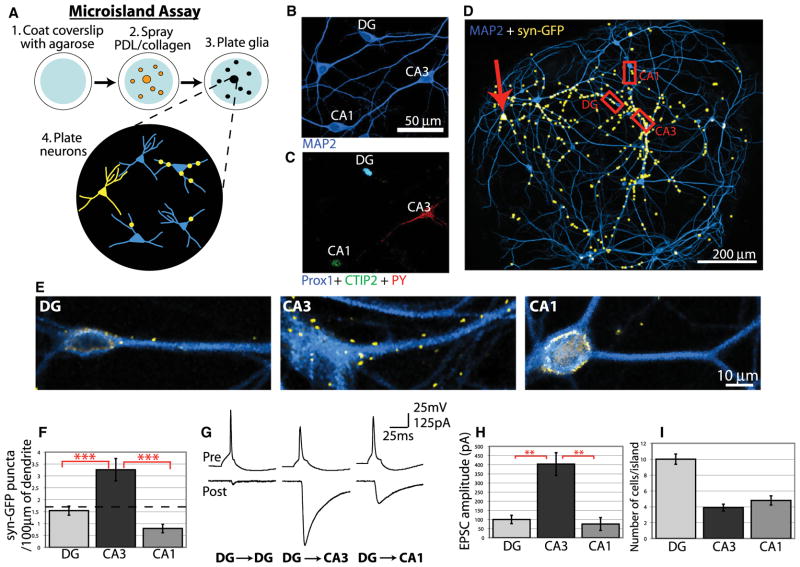
DG neurons synapse onto CA3 pyramidal neurons while avoiding synaptic contact with nearby CA1 pyramidal neurons and other DG neurons. Because primary cultures offer several advantages for molecular perturbation and analysis, we developed two in vitro culture assays to investigate synapse-specific development. We first determined whether the ability of DG neurons to selectively innervate CA3 neurons is preserved in vitro using the “microisland assay.” In this assay, hippocampal neurons from neonatal rats were dissociated and plated to glial microislands on glass coverslips so that each island had approximately 10–30 neurons (Segal and Furshpan, 1990) (Figure 2A). Cultures were transfected with synaptophysin-GFP to visualize synaptic terminals of transfected cells. For analysis only islands containing one transfected DG neuron were selected so that every synaptophysin-GFP punctum could be uniquely associated with the transfected neuron (Figure 2A). To determine whether DG neurons recognize correct targets in microcultures, we used antibodies to identify hippocampal cell types in culture. Anti-Prox1 specifically labels DG neurons (Bagri et al., 2002), anti-PY labels CA3 pyramidal neurons and some interneurons (Woodhams et al., 1989), and anti-CTIP2 labels CA1 pyramidal neurons and most DG neurons (Arlotta et al., 2005). These antibodies, when used in combination, uniquely identify each principal cell type in the hippocampus (Figures 2B and 2C; see Figure S1 available online), and every island analyzed included both correct and incorrect targets so that DG neurons always had a target choice.
Figure 2. DG Neurons Preferentially Innervate CA3 Neurons in Microcultures.
(A) Diagram of microisland assay.
(B) After 12 DIV, hippocampal neurons were immunostained for the neuronal marker MAP2.
(C) The same field of view in (B) but immunostained for Prox1, CTIP2, and PY to identify DG, CA1, and CA3 neurons, respectively (also see Figure S1).
(D) A 12 DIV microisland with a synaptophysin-GFP transfected DG neuron (red arrow) immunostained for MAP2 and GFP. Multiple fields of view were merged, and the GFP signal was thresholded and enlarged for visibility.
(E) Magnified images of the regions outlined in (D) showing preferential formation of DG synapses on CA3 neurons. For simplicity only MAP2 and synaptophysin-GFP are shown, but all cells were immunostained for Prox1 and CTIP2 to identify cell types. GFP fluorescence around the cell soma is the result of nonspecific background staining, and only synaptophysin-GFP puncta on dendrites were analyzed.
(F) Quantification of synaptophysin-GFP puncta on DG, CA3, and CA1 neurons in hippocampal microislands. The dotted line represents the expected number of synapses per length of dendrite if all cells were innervated equally (n > 50 cells of each type from 21 islands).
(G) Example traces of paired recordings from hippocampal microislands in which single-action potentials were elicited in presynaptic DG neurons (upper traces), and postsynaptic EPSCs were measured by voltage clamp (lower traces).
(H) Average evoked EPSC amplitudes for paired recordings. (I) Average number of DG, CA3, and CA1 neurons on each microisland. Statistics were performed using ANOVA followed by posttests where ***p < 0.001 and ** p < 0.01. Error bars represent SEM.
An island with one transfected DG neuron and several surrounding untransfected neurons is shown in Figure 2D. Synaptophysin-GFP puncta are represented in yellow and were expanded for visibility at this magnification. An example of each cell type is shown at higher magnification with the anti-GFP signal (Figure 2E). Although positioned nearby on the island, many more synaptophysin-GFP puncta are found on the dendrites of the CA3 neuron compared to the DG or CA1 neuron (Figure 2E). To quantify this, the total number of synaptophysin-GFP puncta on every neuron from 21 islands was counted, normalized to dendrite length, and sorted by cell type. Analysis of the data indicates that DG neurons form significantly more synapses with CA3 neurons than with DG or CA1 neurons (Figure 2F). Furthermore, we calculated the expected number of synapses if all cells were innervated equally and represented this average by the dotted line in Figure 2F. Because CA3 neurons are innervated significantly above this value, it suggests that there is a signal that promotes DG synapse formation onto these cells. Conversely, CA1 neurons were innervated significantly below this average value, suggesting the potential presence of a negative cue that inhibits synapse formation by DG neurons. DG neurons formed synapses with other DG neurons near the frequency expected by chance (Figure 2F).
To determine if the synaptic bias of DG neurons for CA3 dendrites measured by synaptophysin-GFP reflects a bias in functional connectivity, we analyzed synaptic responses of neurons in microcultures by whole-cell recordings. For these experiments, pairs of neurons on an island were recorded simultaneously and tested for synaptic connectivity. One neuron was held in current clamp and stimulated to produce action potentials, whereas evoked excitatory postsynaptic currents (EPSCs) were recorded from the second neuron held in voltage clamp. Paired recording was followed by immunofluorescence-based identification of cell type. Recovering cells for immunofluorescence after paired recording has a high failure rate because it requires the integrity of the cell to be maintained when the patch electrodes are withdrawn. Therefore, only those pairs where the identity of both neurons could be unambiguously determined post hoc were used for analysis. Analysis of paired recordings in which a DG neuron was the presynaptic neuron showed that the evoked response varied depending on the postsynaptic cell (Figures 2G and 2H). Whereas DG-DG and DG-CA1 pairs produced weak synaptic responses, DG-CA3 recordings elicited strong evoked responses (Figures 2G and 2H). This suggests that DG neurons make more numerous or stronger synapses onto CA3 neurons than onto other cell types and indicates that DG neurons also develop a functional synaptic bias for CA3 neurons in culture. This selection of correct targets is particularly impressive because, on average, microcultures contain fewer CA3 than DG or CA1 neurons (Figure 2I). These results on synapse function closely correlate with the analysis of synaptophysin-GFP puncta and demonstrate that DG neurons preferentially connect with appropriate targets using only cues present in microcultures.
Formation of DG-CA3 Synapses In Vitro Does Not Require Directed Axon Guidance
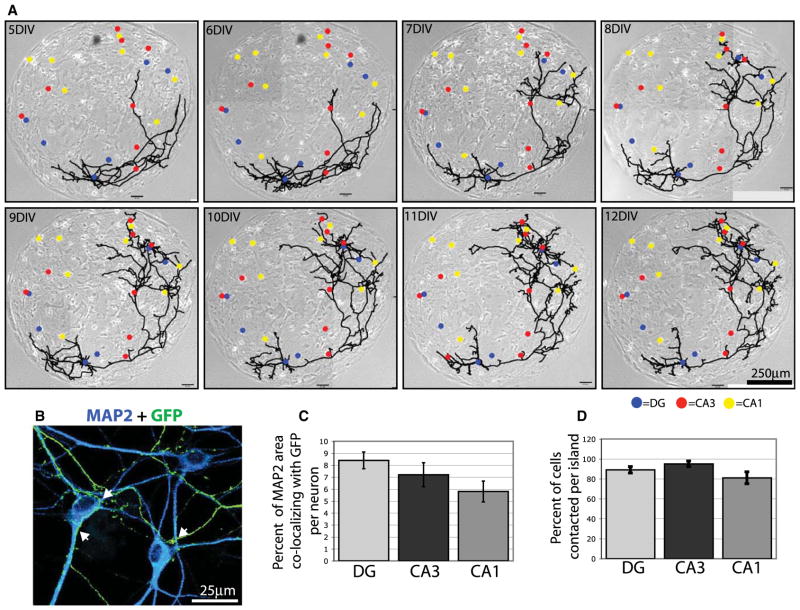
We found that DG neurons synapse primarily with correct targets by 12 days in vitro (DIV), but from our previous experiments we cannot determine whether this specificity arises from a mechanism of biased axon outgrowth or biased synaptogenesis. For example, specificity between DG and CA3 neurons could arise via selective axon growth toward CA3 neurons followed by nonselective synapse formation. Alternatively, DG axons may contact all cell types equally but selectively form synapses with CA3 neurons. To distinguish between these possibilities, we analyzed DG axon growth using time-lapse imaging in the microisland assay.
Islands containing one neuron transfected with GFP were imaged using phase contrast and fluorescence every 24 hr from 5 to 12 DIV and then immunostained to determine the cell type of every neuron on the island (Figure 3A). As shown in the example, cultured DG neurons often develop appropriate morphology with dendrites projecting from one side of the soma and an axon projecting in the opposite direction. There is growth and remodeling of the axon arbor between 5 and 9 DIV, during which several branches are eliminated and others added. From 9 to 12 DIV, the arbor morphology is relatively stable, although there is addition and retraction of minor branches. On this large island the DG axon only grows on half the island, but it contacts dendrites of all neurons on that half of the island regardless of cell type (Figure 3A). For quantification we immunostained similar GFP-transfected islands and analyzed the area of the transfected DG axon that overlapped dendrites of each cell at 12 DIV when synaptic preference is established (Figures 3B and 3C). We also quantified the percentage of each cell type that had contact with the DG axon (Figure 3D). The data indicate that DG axons do not grow preferentially to CA3 neurons but contact all cell types at comparable levels (Figures 3C and 3D). Therefore, DG synapse formation is biased for CA3 neurons in culture, but not through directed axon guidance. Instead, our data suggest that molecular cues on specific cell types actively promote DG synapses with CA3 neurons and prevent DG synapses with CA1 neurons independent of axon guidance mechanisms.
Figure 3. Preferential Synapse Formation Is Independent of Axon Guidance in Microcultures.
(A) Phase-contrast images of a microisland merged with line drawings of a GFP-expressing neuron growing on the island. Cell bodies of all neurons on the island are indicated by color-coded dots. Blue, DG; red, CA3; yellow, CA1.
(B) A magnified region of a microisland with a DG neuron transfected with GFP (transfected cell soma not shown) and immunostained with antibodies against MAP2 to label all neurons and GFP to label the transfected DG axon. Arrowheads indicate points of contact between the axon and target cell dendrites.
(C and D) Quantification of contact between GFP-expressing DG axons and all neurons on microislands. Graph in (C) shows the percentage of MAP2 area that colocalizes with GFP per neuron and, therefore, represents the extent that each potential target neuron comes into contact with the DG axon. Graph in (D) shows the percentage of each cell type that was contacted per island. Error bars represent SEM. No differences among cell types are significant by ANOVA.
DG-CA3 Synaptic Specificity Is Mediated by Selective Synapse Formation onto Correct Targets
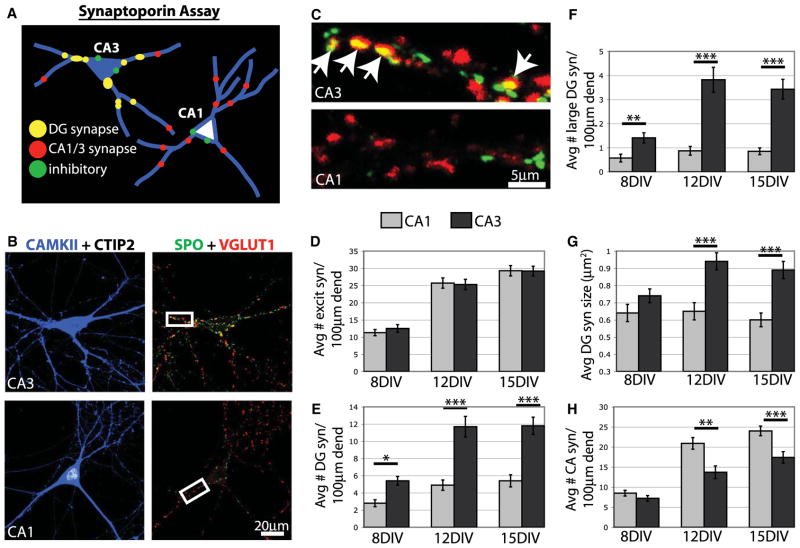
We next investigated two additional questions about the mechanisms regulating synaptic specificity in culture. First, are CA3 neurons simply more synaptogenic than other neurons in culture? Second, does the development of synaptic specificity require elimination of synapses from incorrect targets? To address these questions, we developed a second in vitro assay called the “synaptoporin assay” (Figure 4A). In this assay we use cell and synapse-specific markers to uniquely identify subtypes of synapses made onto an identified postsynaptic target cell grown in standard low-density mass hippocampal cultures.
Figure 4. Specificity Develops at the Onset of Synapse Formation.
(A) Diagram of SPO assay. P0 hippocampal neurons are grown in standard low-density mass cultures and immunostained with cell and synapse markers.
(B) After 15 DIV, hippocampal neurons were immunostained for CAMKII (blue), CTIP2 (white), SPO (green), and VGlut1 (red).
(C) Magnified images of boxed regions in (B). DG synapses express both SPO and VGlut1 and appear yellow. Arrows indicate DG synapses on the CA3 neuron, but none is visible on a comparable region of the CA1 neuron.
(D–H) Graphs showing the total number of excitatory synapses (all VGlut1 puncta), DG synapses (SPO + VGlut1 puncta), large DG synapses (SPO + Vglut1 >1.0 μm2), DG synapse size, and CA synapses (VGlut1 only puncta) on CA1 and CA3 neurons over time. In all cases, n > 30 cells for each cell type from 3 cultures. Statistics were performed using ANOVA followed by posttests where ***p < 0.001, **p < 0.01, and *p < 0.05. Error bars represent SEM. Avg, average; dend, dendrite; excit syn, excitatory synapse.
To establish the synaptoporin (SPO) assay, we determined that DG mossy fiber synapses are identified by coexpression of the presynaptic markers VGlut1 and SPO (synaptophysin II). VGlut1 is expressed at all excitatory, glutamatergic synapses in the hippocampus and, therefore, labels presynaptic sites originating from DG, CA3, and CA1 neurons (Bellocchio et al., 1998; Kaneko et al., 2002). In contrast, SPO has more restricted expression. Although SPO is expressed in a subset of GABAergic synapses, the only excitatory synapses in the hippocampus that express SPO are DG mossy fiber synapses (Figure S2) (Grabs et al., 1994; Grosse et al., 1998; Singec et al., 2002). We confirmed the specificity of these presynaptic markers in sections of postnatal rat brain and in hippocampal cultures by immunostaining individual neurons transfected with synaptophysin-GFP (Figure S2). When a DG neuron expresses synaptophysin-GFP, synapses marked by synaptophysin-GFP express VGlut1 and SPO (Figures S2B and S2C). In contrast when CA3 or CA1 neurons express synaptophysin-GFP, synapses marked by synaptophysin-GFP express VGlut1, but not SPO (Figures S2B and S2C). These results indicate that DG synapses express both VGlut1 and SPO, whereas CA synapses from both CA1 and CA3 neurons express only VGlut1. Therefore, coimmunostaining with antibodies against SPO, VGlut1, and cell type markers allows examination of the development of different kinds of synapses onto different types of hippocampal neurons (Figure 4A).
We carried out the SPO assay on neurons grown for 8, 12, and 15 DIV, by immunostaining cultures with antibodies against SPO and VGlut1 to identify different types of synapses. In addition to these synaptic markers, neurons were immunostained for CAMKII to label the dendrites of excitatory neurons and CTIP2 and Prox1 to identify cell types (Figures 4A–4C). We counted the total number of excitatory synapses, DG synapses, and CA synapses formed onto different neuron types over time. At all time points, dendrites of CA1 and CA3 neurons developed very similar numbers of excitatory synapses. This indicates that neither cell type has any more synaptogenic potential than the other (Figure 4D). However, CA3 neurons developed significantly more DG synapses (up to 2.4 times greater) than CA1 neurons at all time points (Figure 4E). During our analyses we noticed that, like mossy fiber terminals in vivo, SPO-positive synapses were often much larger than typical excitatory presynaptic sites. Therefore, we determined whether these extra-large excitatory presynaptic terminals were also preferentially located on CA3 neurons. Indeed, when we limited our analysis to synapses greater than 1.0 μm2, we discovered that CA3 neurons have up to 4.4 times more extra-large DG synapses than CA1 neurons (Figure 4F), and the average size of a DG synapse is greater on CA3 neurons (Figure 4G). We also observed that CA1 neurons developed significantly more CA synapses than CA3 neurons, which indicates that specificity may not be limited to DG synapses but that other types of synapses also undergo selective formation in culture (Figure 4H).
Together, these experiments support the conclusion that mechanisms driving specific synapse formation in culture function without spatial cues present in the brain. Because we observe a strong synaptic bias as early as 8 DIV, it suggests that this specificity is largely driven by selective synapse formation onto correct targets, and not by elimination of synapses from incorrect targets.
Cadherin-9 Is a Homophilic Type II Cadherin Expressed by DG and CA3 Neurons
To identify molecules that might regulate the formation of DG-CA3 synapses, we analyzed expression patterns of genes that encode transmembrane proteins with extracellular domains that could mediate cell-cell interactions. The initial analysis was based on gene expression data published in the Allen Brain Atlas (http://www.brain-map.org/) and led to identification of the cadherin gene family as potential mediators of connectivity. There are about 20 classic cadherin genes thought to mediate cell-cell interactions, although the specific function of most cadherins is unknown. Several cadherins are expressed in the hippocampus, but only one, cadherin-9, is strongly and specifically expressed in DG and CA3 regions (Figure 5A) (Bekirov et al., 2002). Therefore, we hypothesized that cadherin-9 interactions between DG axons and CA3 dendrites may be important for regulating mossy fiber synapse development but not other types of synapses in the hippocampus.
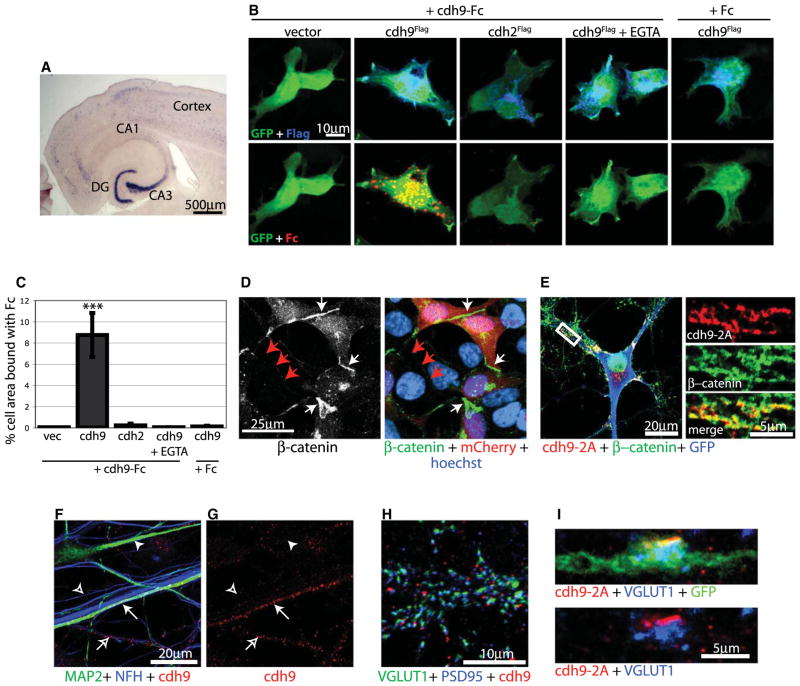
Figure 5. cdh9 Is a Classic Type II Cadherin.
(A) In situ hybridization for cdh9 mRNA in a horizontal section of a P14 mouse brain.
(B) 293T cells cotransfected with GFP and Flag-tagged cadherin constructs were treated with Fc or cdh9-Fc as indicated. Top row shows GFP (green) and Flag (blue) expression. Bottom row shows the same cells as above stained for GFP (green) and bound Fc (red).
(C) Quantification of Fc binding. The percentage of GFP cell area (green) that colocalized with Fc (red) was determined. The only condition to show significant binding was cdh9-Fc treatment on cdh9 cells. Statistics determined by ANOVA and error bars represent SEM. ***p > 0.001 compared to all other conditions.
(D) 293T cells cotransfected with mCherry and cdh9 were immunostained for β-catenin (green), mCherry (red), and Hoechst (blue). Note that β-catenin clusters at junctions between transfected cells expressing cdh9 and mCherry (white arrows), but not between untransfected cells (red arrows).
(E) Cultured hippocampal neuron transfected with cdh9-2A-GFP and immunostained for cdh9-2A (red), GFP (blue), and β-catenin (green). The white box indicates a region of higher magnification at right showing that cdh9-2A colocalizes with β-catenin in dendrites, and colocalization appears yellow.
(F and G) Hippocampal neurons immunostained for MAP2 (green) to label dendrites, NFH (blue) to label axons, and endogenous cdh9 (red). cdh9 localizes to dendrites (closed arrow) and axons (open arrow) of a subset of neurons in mixed hippocampal cultures. Dendrites (closed arrowhead) and axons (open arrowhead) not expressing cdh9 are also shown.
(H) Hippocampal neurons immunostained for VGlut1 (green) to label presynaptic sites, PSD95 (blue) to label postsynaptic sites, and endogenous cdh9 (red). cdh9 is adjacent but not overlapping synaptic release sites. (I) DG axon infected in vivo with a lentivirus expressing cdh9-2A-GFP. In DG axons, cdh9-2A (red) was found adjacent to clusters of synaptic vesicles identified by VGlut1 (blue).
Cadherin-9 is a relatively uncharacterized gene predicted to encode a classic type II cadherin, and therefore, cadherin-9 may signal via homophilic binding. To test this, we cloned the predicted full-length cadherin-9 cDNA from postnatal day 7 (P7) mouse hippocampal mRNA and generated an Fc fusion of the extracellular domain of cadherin-9. We found that cadherin-9-Fc but not control Fc binds the surface of 293T cells expressing cadherin-9 in a calcium-dependent manner indicating that, like other classic cadherins, cadherin-9 undergoes homophilic binding (Figures 5B and 5C) (Shimoyama et al., 2000). One consequence of cadherin binding is recruitment and activation of β-catenin (Arikkath and Reichardt, 2008; Stepniak et al., 2009). To determine if cadherin-9-mediated adhesion recruits β-catenin, we expressed cadherin-9 in 293T cells and found that β-catenin is recruited to the interface between cadherin-9 cells (Figure 5D). Additionally, epitope-tagged cadherin-9 colocalizes with β-catenin in neurons (Figure 5E). These results suggest that cadherin-9 is capable of intracellular signaling via catenin proteins.
We next analyzed the location of cadherin-9 protein in neurons. Consistent with in situ hybridization results showing that cadherin-9 is expressed selectively by DG and CA3 neurons, we found that endogenous cadherin-9 protein is found in puncta along axons and dendrites in a subset of cultured hippocampal neurons (Figures 5F and 5G). Cadherin-9 puncta do not directly overlap with synaptic vesicles or the postsynaptic density but are frequently found adjacent to synaptic active zones (Figure 5H), similar to what has been observed for other cadherins (Fannon and Colman, 1996; Uchida et al., 1996). Antibody incompatibility prevented us from determining precisely which cell types are cadherin-9 positive in culture and from analyzing endogenous cadherin-9 protein in vivo. However, we were able to examine the location of epitope-tagged cadherin-9 in DG neurons in the brain. DG neurons of P5 rats were infected with a lentivirus expressing cadherin-9 and GFP using a 2A peptide to drive expression of both genes from a single promoter (Figure S3A). Because the 2A peptide remains attached to cadherin-9, it was used as an epitope tag to visualize cadherin-9 with anti-2A antibodies. We found that at P14 cadherin-9-2A localizes to dendrites and axons of DG neurons (Figures S3B–S3E). Although cadherin-9-2A was found diffusely throughout the dendrites, it localized to discrete bands at mossy fiber terminals adjacent to but not overlapping presynaptic vesicles marked by VGlut1 (Figures 5I and S3B–S3E), consistent with a role as a synaptic organizer.
Loss of Cadherin-9 Selectively Disrupts DG-CA3 Synapse Formation
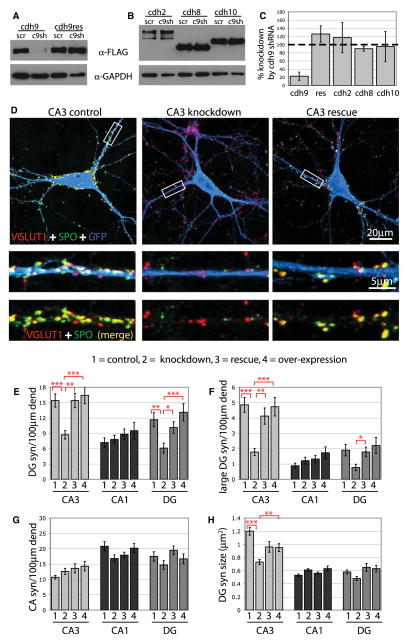
To examine the role of cadherin-9 in synapse formation, we generated a cadherin-9 shRNA that markedly reduces expression of a tagged cadherin-9 construct in 293T cells (Figure 6A). The construct does not significantly affect expression of several other cadherins expressed in the hippocampus, including cadherin-2 (N-cadherin), cadherin-8, and cadherin-10, based on western blot and quantitative PCR analysis (Figures 6B and 6C, and S4). We also generated a codon-substituted cadherin-9 rescue construct insensitive to the cadherin-9 shRNA (Figures 6A and 6C). To determine whether knockdown of cadherin-9 selectively affects DG-CA3 synapse formation, we transfected hippocampal neurons with scrambled control or cadherin-9 shRNA, and examined the formation of DG and CA synapses onto different types of neurons using the SPO assay. Knockdown of cadherin-9 led to significant reductions in the total number of DG synapses and the number of extra-large DG synapses onto CA3 neurons but did not affect the number of CA synapses onto the same cells (Figures 6D–6G), indicating that cadherin-9 regulates a specific class of synapses. In addition, DG synapses that remained on knockdown CA3 neurons were significantly reduced in size compared to controls (Figure 6H), suggesting that cadherin-9 might also control the growth of DG-CA3 synapses. These defects were rescued by coexpression of shRNA-insensitive cadherin-9, which indicates that the observed effects are due to specific loss of cadherin-9 (Figures 6D–6H). As a further test of specificity, we examined the effects of cadherin-9 shRNA on CA1 neurons, which do not express cadherin-9 (Figure 5A). We found no significant effect of cadherin-9 shRNA expression on the formation of synapses onto CA1 neurons, suggesting that the cadherin-9 shRNA does not cause general synaptic defects (Figures 6E–6H). These results indicate that cadherin-9 plays a specific role in regulating DG synapses onto CA3 neurons, and does not regulate non-DG synapses.
Figure 6. cdh9 Specifically Regulates DG Synapse Formation In Vitro.
(A and B) 293T cells were cotransfected with C-terminal FLAG-tagged cadherin constructs and scrambled shRNA (scr) or cdh9 shRNA (c9sh) as indicated, and cell lysates were analyzed by immunoblotting. cdh9 expression is significantly reduced by cdh9 shRNA but not a cdh9 rescue construct containing silent point mutations, or the cadherins cdh2 (N-cadherin), cdh8, or cdh10.
(C) Quantification of cdh9 shRNA western blots (n = 3).
(D) CA3 neurons expressing GFP and scrambled shRNA (control), cdh9 shRNA (knockdown), or cdh9 shRNA plus cdh9res (rescue) were immunostained for GFP (blue), SPO (green), and VGlut1 (red). White boxes indicate regions of higher magnification shown below each neuron. DG synapses appear yellow and are reduced on CA3 knockdown neurons.
(E–H) Graphs show the average number of DG synapses (syn), CA synapses, and DG synapse size on neurons expressing scrambled shRNA (1), cdh9 shRNA3 (2), cdh9 shRNA plus cdh9res (3), or cdh9 overexpression (4) (n > 30 cells for each cell type from at least 3 cultures). Error bars represent SEM. Statistics were performed using ANOVA followed by posttests (***p < 0.001, **p < 0.01, and *p < 0.05). dend, dendrite.
Because DG neurons express cadherin-9, and there are some ectopic DG-DG synapses in culture, we determined if the number of DG-DG synapses would be affected by downregulation of cadherin-9. Expression of cadherin-9 shRNA led to a decrease in the number of DG-DG synapses, suggesting that ectopic synapse formation between DG neurons in culture is driven, at least in part, by cadherin-9-mediated interactions (Figures 6E–6G). We also overexpressed cadherin-9 in cultured hippocampal neurons to determine whether it is sufficient to induce synapses and found that overexpression does not increase DG synapses on any cell type in culture (Figures 6E–6H). This is consistent with previous studies on N-cadherin, which was also shown to be insufficient to induce synapses or spines (Mendez et al., 2010; Scheiffele et al., 2000; Togashi et al., 2002). Together, these observations indicate that cadherin-9 regulates the formation of synapses when it is expressed both in the pre- and postsynaptic neuron, supporting a mode of action via homophilic binding.
Reduction of Cadherin-9 in DG Neurons Disrupts Mossy Fiber Bouton Formation In Vivo
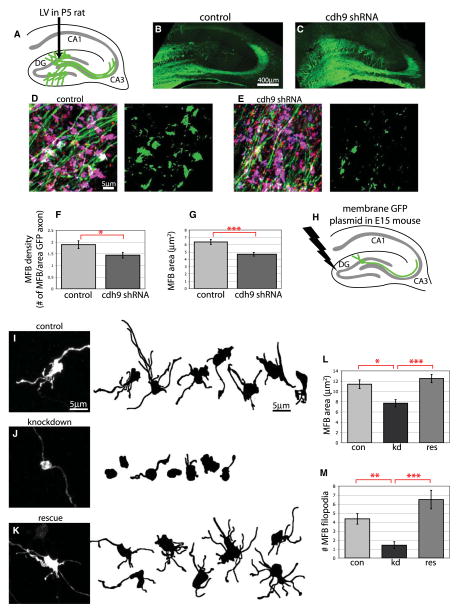
To determine if endogenous cadherin-9 is required for the differentiation of DG-CA3 mossy fiber synapses in vivo, we generated lentiviruses that express cadherin-9 shRNA under control of the human H1 promoter and GFP under control of the rat synapsin promoter. Rat DG neurons were infected with control or cadherin-9 shRNA lentivirus at P5 and assessed by immunofluorescence at P16 (Figure 7A). In these experiments antibodies against GFP were used to label the infected axons, and SPO and VGlut1 were used to label mossy fiber terminals. As shown in Figures 7B and 7C, lentivirus injection into the DG leads to clear labeling of the mossy fiber pathway, and DG axons expressing the shRNA appear to grow normally. Analysis of DG mossy fiber boutons following control virus injection revealed large, complex boutons characteristic of mossy fiber terminals (Figure 7D). In marked contrast, expression of cadherin-9 shRNA revealed significant defects in mossy fiber morphology and density (Figure 7E). Quantification of these experiments revealed that cadherin-9 knockdown neurons had 24% fewer mossy fiber presynaptic boutons compared to controls (Figure 7F), and the average size of the boutons that remained was 26% smaller (Figure 7G). Together, these defects in synapse size and number reduce the total synaptic area in knockdown neurons by 50%. Thus, in DG neurons, cadherin-9 is not required for axon growth but, instead, is specifically involved in mossy fiber bouton formation in vivo.
Figure 7. Reduction of cdh9 Expression in DG Neurons In Vivo Disrupts Mossy Fiber Bouton Formation.
(A) Diagram of DG lentivirus injection strategy used for (B)–(G).
(B and C) Lentivirus infections of the DG with GFP control or GFP plus cdh9 shRNA. Overall development and axon growth appear normal in both control and cdh9 shRNA-expressing cells.
(D and E) DG mossy fibers in the stratum lucidum following lentiviral infection. Sections were immunostained with antibodies against GFP (green) to label infected axons and SPO (red) and VGlut1 (blue) to label mossy fiber synapses, which appear purple in the merged images. To the right, the same images have been thresholded to reveal the areas of triple colocalization. Axons infected with cdh9 shRNA have fewer mossy fiber boutons.
(F and G) Graphs showing that both the number (F) and size (G) of mossy fiber boutons (MFB) are significantly reduced in axons expressing cdh9 shRNA compared to control (n > 20 fields of view from at least 3 different injections per condition).
(H) Diagram of in utero electroporation strategy used for (I)–(M).
(I–K) Examples of individual mossy fiber boutons coexpressing membrane GFP with scrambled shRNA (control), cdh9 shRNA (knockdown), or cdh9 shRNA plus cdh9res (rescue). Confocal z projection of a mossy fiber bouton immunostained for GFP is shown at left, and line drawings of several other boutons from each condition are shown at right.
(L and M) Graphs showing that mossy fiber bouton size and complexity are reduced upon cdh9 knockdown and are fully rescued by coexpression of cdh9res (n = 14–22 boutons from at least 3 different animals for each condition). For all graphs, error bars represent SEM; ***p < 0.001, **p < 0.01, *p < 0.05 by two-tailed t test (F and G) or ANOVA (L and M).
Although lentiviral infection of DG neurons allowed us to visualize the mossy fiber pathways, the fine morphology of individual mossy fiber boutons is difficult to analyze due to the large number of nearby axons that are labeled. It is also difficult to carry out in vivo rescue experiments because of DNA packaging limits of viral tools. To overcome these limitations, we sought to characterize the presynaptic phenotype of cadherin-9 knockdown more precisely by sparsely transfecting DG neurons in vivo using in utero electroporation (Figure 7H). In these experiments hippocampal neurons were electroporated with a plasmid expressing membrane GFP together with either scrambled shRNA control or cadherin-9 shRNA at E15, and then individual mossy fiber boutons were analyzed at P14. At this age, mossy fiber boutons developed their characteristic shape consisting of a large main bouton and several presynaptic filopodia (Figure 7I). Consistent with the lentiviral experiments, expression of the cadherin-9 shRNA caused a significant 33% reduction in the size of the main bouton area and a 67% reduction in the number of presynaptic filopodia, which were completely rescued by coelectroporation of an shRNA resistant cadherin-9 cDNA (Figures 7I–7M). These results indicate that cadherin-9 regulates the density, size, and complexity of mossy fiber boutons.
Reduction of Cadherin-9 in CA3 Neurons Disrupts Pre- and Postsynaptic Development In Vivo
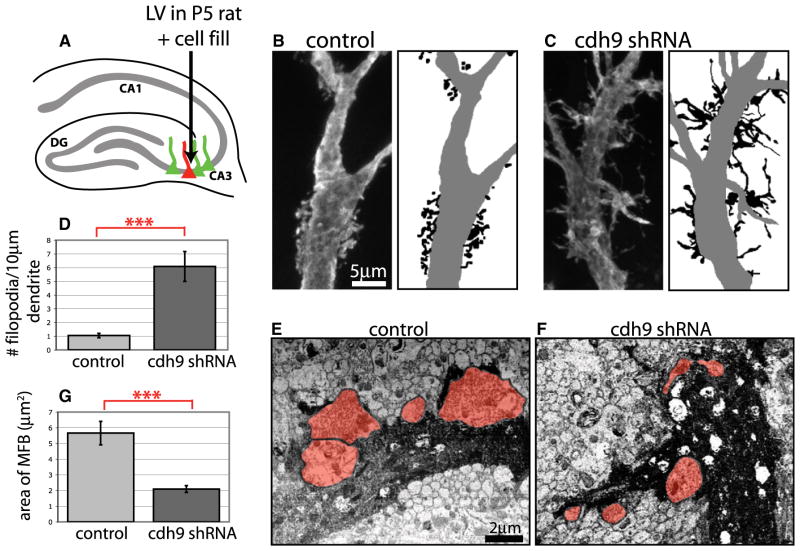
Because cadherin-9 is expressed by both DG and CA3 neurons, and undergoes homophilic interactions, we hypothesized that cadherin-9 is also required in CA3 neurons for the formation of postsynaptic structures apposed to mossy fiber terminals. To examine this possibility, CA3 neurons were infected with control or cadherin-9 shRNA lentivirus at P5 and analyzed at P16 (Figure 8A). To visualize the specialized spines known as TEs, infected CA3 neurons identified by expression of GFP were filled with lucifer yellow (LY) using current-driven microinjection in fixed tissue (Figures 8A–8C and S5). Analysis of filled neurons showed that loss of cadherin-9 expression from CA3 neurons severely disrupted formation of TEs. Under control conditions, TEs appear as discrete clusters of spine heads on the proximal dendrites of CA3 neurons (Figure 8B). In contrast, CA3 neurons expressing cadherin-9 shRNA have few compact TEs and, instead, develop dysmorphic filopodia-like extensions emerging from the main dendritic shaft (Figure 8C). Quantification of the average number of filopodia per length of dendrite revealed that cadherin-9 knockdown neurons have 5.8 times more filopodia than control neurons (Figure 8D). To determine if cadherin-9 knockdown affects spine formation in general, we also examined spine formation at typical spines of DG and CA1 neurons. We found no significant alterations in either spine density or spine length between neurons expressing the scramble or cadherin-9 shRNA for either cell type (Figure S6). We are certain that the shRNA was expressed because the same DG neurons used for spine analysis showed presynaptic defects at their mossy fiber boutons that could be rescued by expression of cadherin-9 (Figures 7I–7M). These results indicate that cadherin-9 is required specifically for stabilization and maturation of TE spines.
Figure 8. Reduction of cdh9 in CA3 Neurons In Vivo Causes Pre- and Postsynaptic Defects.
(A) Diagram of CA3 lentivirus injection strategy.
(B and C) CA3 neurons infected with lentiviruses and filled with LY dye to reveal spine morphology. Line drawings of images illustrating disruption of mossy fiber TEs in cdh9 shRNA-expressing neurons are shown at right. Dendrites are gray, and spines and spines/filopodia are black.
(D) Graph showing that the number of filopodia per 10 μm of proximal dendrite is significantly greater in CA3 neurons infected with cdh9 shRNA compared to control (n = 7 neurons for each).
(E and F) Scanning electron microscopy images of photoconverted, LY-filled CA3 neurons infected with lentiviruses. Dendrites of filled neurons are dark, and mossy fiber boutons are shaded red.
(G) Graph showing that the average size of mossy fiber boutons contacting cdh9 shRNA dendrites is significantly smaller compared to control as determined by electron microscopy (n = 16 boutons from 1 control neuron and n = 35 boutons from 2 cdh9 shRNA neurons). In all cases, error bars represent SEM; ***p < 0.001, two-tailed t test.
Finally, we examined whether reduction of cadherin-9 in the postsynaptic CA3 neuron has cell nonautonomous affects on presynaptic bouton formation. Because synaptic contact between pre- and postsynaptic elements cannot be definitively resolved using light microscopy, we performed electron microscopy on photoconverted LY fills of CA3 neurons infected with cadherin-9 shRNA or control lentivirus. In this experiment postsynaptic structures are filled by the photoconverted dye, and uninfected presynaptic mossy fiber boutons in contact with the filled dendrites can be clearly identified (Figures 8E and 8F). Wild-type presynaptic boutons contacting dendrites of cadherin-9 knockdown neurons were 63% smaller than those contacting control neurons (Figure 8G). This suggests that loss of cadherin-9 on the postsynaptic dendrite leads to a trans-synaptic defect in the presynaptic axon terminal and supports the model that cadherin-9 homophilic interactions specifically regulate mossy fiber synapse formation in the developing hippocampus.
DISCUSSION
The formation of synapses between specific cell types with unique synaptic properties is essential for the function of the nervous system, yet the mechanisms that mediate such specificity are largely unknown. In this study, we investigated the mechanisms that regulate the formation of the DG-CA3 synapse in the hippocampus using a combination of in vitro and in vivo approaches. Using two novel in vitro assays for synaptic specificity, we found that DG neurons show a strong preference to form synapses with their target CA3 neurons rather than other DG and CA1 neurons. We then investigated the regulation of DG-CA3 synapse formation and found that cadherin-9, which is selectively expressed in DG and CA3 neurons, plays a critical role in the formation and differentiation of this synapse. Our results indicate that many aspects of DG-CA3 mossy fiber synapse development, including synapse density, presynaptic bouton complexity, and postsynaptic morphology, are regulated by trans-synaptic, homophilic cadherin-9-mediated interactions.
Development of In Vitro Models for Studying Synaptic Specificity
Cultured hippocampal neurons have long been recognized as a valuable system for investigating synapse formation and function. It is often assumed that synaptic specificity is lost in dissociated neurons, but this assumption is largely unsubstantiated by experimental evidence. In fact previous studies suggested that synapse formation in culture is not random. For instance mechanosensory neurons cultured from the mollusk Aplysia californica form specific synaptic connections (Camardo et al., 1983), and in the mammalian CNS, cultured cortical neurons form nonrandom synaptic connections independent of axon guidance (Vogt et al., 2005). It was also shown that cultured CA3 hippocampal neurons develop large, zinc-filled synapses resembling mossy fiber synapses (Kavalali et al., 1999). In these studies precise cell and synapse-specific markers were not used to unambiguously identify cell types, and preferential synapse formation in mixed hippocampal cultures containing all cell types has never been examined.
Here, we developed two approaches, the microisland assay and the SPO assay, to investigate the formation of specific classes of synapses in vitro. The two assays are not simply two methods to examine similar processes but are complementary to one another. The microisland assay allows examination of target selection by an identified presynaptic neuron, whereas the SPO assay allows examination of specific types of inputs onto an identified postsynaptic neuron. Remarkably, both assays reveal that DG neurons preferentially synapse with their correct targets, CA3 neurons, in culture. Although, DG axons are guided to the CA3 region by positional cues in the brain, our results indicate that DG-CA3 synaptic specificity does not depend exclusively on directed axon guidance but that distinct mechanisms promote synapse formation specifically between these cell types.
Our observation that preferential synapse formation occurs early in development suggests that specificity is primarily achieved by selective synapse formation with correct target neurons, and not by elimination from incorrect targets. Synapse elimination is an essential process for the refinement of many circuits. However, synapse elimination typically involves late, activity-dependent processes whereby excess synapses are removed from a target cell population as a means to refine synapse number and strength rather than as a mechanism to remove synapses from incorrect target cells (Kano and Hashimoto, 2009; Katz and Shatz, 1996). We find that initial cell type selection occurs early in synapse formation and in the absence of neural activity (M.E.W. and A.G., unpublished data), and therefore, we hypothesize that genetically determined molecular cues mediate target selection prior to synapse formation. These may include positive cues promoting synapse formation on CA3 neurons and negative cues preventing synapse formation on CA1 neurons. We do not rule out the possibility that the number of correct synapses is refined over time through other mechanisms. In addition it is important to note that although we always observe a highly significant bias toward correct target innervation, we also detect incorrect synapses in culture that are not normally found in the brain. This likely reflects the fact that the brain uses several mechanisms (i.e., axon guidance, specific target recognition, synapse elimination) to ensure that neural circuits form with high fidelity.
Role of Cadherin-9 in Synapse Formation In Vitro
The formation of specific classes of synapses requires communication between two neurons. For this reason transmembrane cell adhesion molecules that interact with the extracellular environment and transmit information inside the cell are attractive candidates for mediating specific synapse formation. The classic cadherin gene family consists of approximately 20 members, and their differential expression in the brain has raised interest in the possibility that cadherin-mediated interactions play an important role in synaptic specificity (Arikkath and Reichardt, 2008; Bekirov et al., 2002). However, much of our understanding of the role of cadherins at synapses is based on N-cadherin, which is broadly expressed and appears to have a general role in modulating synaptogenesis, spine formation, and plasticity in response to activity (Arikkath and Reichardt, 2008; Bozdagi et al., 2004, 2010; Mendez et al., 2010; Saglietti et al., 2007; Togashi et al., 2002). N-cadherin is also involved in earlier events including axon guidance and laminar targeting (Inoue and Sanes, 1997; Kadowaki et al., 2007; Poskanzer et al., 2003), and DG axons respond differentially to N-cadherin versus cadherin-8 (Bekirov et al., 2008). Despite extensive analysis of N-cadherin function, the role of most other cadherins in synapse formation remains unknown.
Cadherin-9 is unique because it is the only cadherin with highly specific expression in DG and CA3 neurons. We found that cadherin-9 is homophilic, localizes to mossy fiber synapses, and is specifically required for formation of a subset of synapses (DG synapses) in culture and in vivo. To our knowledge, this is the first direct evidence that a cadherin regulates the differentiation of a specific class of synapses. Hippocampal neurons express multiple cadherins and, therefore, it is possible that different kinds of hippocampal synapses are specified by a unique cadherin or combination of cadherins. Cadherins participate in both homophilic and heterophilic interactions, and this feature increases the diversity of synapses that may be regulated by individual cadherins (Patel et al., 2006; Shimoyama et al., 2000; Volk et al., 1987). The role of molecules in regulating laminar targeting and subcellular specificity, both as positive (Ango et al., 2004; Shen and Bargmann, 2003; Shen et al., 2004; Yamagata and Sanes, 2008; Yamagata et al., 2002) and negative (Inaki et al., 2007; Klassen and Shen, 2007; Pecho-Vrieseling et al., 2009; Tran et al., 2009) cues, is now fairly well established, and it appears that cadherin-mediated interactions may play a similar role in the generation of synaptic diversity.
The SPO specificity assay reveals that decreasing cadherin-9 expression in postsynaptic neurons in culture has two main effects. First, it reduces the number of DG synapses onto CA3 neurons, without affecting non-DG synapses, indicating that it is specifically required for DG-CA3 synapse formation. Second, the DG-CA3 synapses that do form onto neurons with reduced cadherin-9 expression are much smaller than controls, indicating that cadhein-9 signaling also regulates the growth of this unusually large synapse. Furthermore, it is intriguing that cadherin-9 also appears to play a role in the formation of the few DG-DG synapses that form in culture. This is most likely due to an interaction between axonal and dendritic cadherin-9 in DG neurons. In vivo, DG axons normally do not have access to DG dendrites due to the trajectory of axon growth in the mossy fiber pathway; however, DG-DG synapses do sometimes develop in response to seizure activity through a process known as mossy fiber sprouting (Dudek and Sutula, 2007). It will be interesting to determine if cadherin-9 plays a role in this process and whether inhibiting cadherin-9 function can ameliorate the effects of generating a seizure-induced back-projecting DG circuit.
Role of Cadherin-9 in DG-CA3 Mossy Fiber Synapse Formation In Vivo
Our results provide strong evidence in support of a role for cadherin-9 in regulating mossy fiber synapse formation in vivo. Cadherin-9 knockdown results in multiple-related phenotypes, suggesting that cadherin-9 may have a multifaceted role in target recognition, synapse formation, and synapse maturation at the mossy fiber synapse.
Because cadherin-9 is expressed specifically in DG and CA3 neurons, cadherin-9-mediated homophilic adhesion may provide a target recognition cue between DG and CA3 neurons. Support for this comes from the fact that reduction of cadherin-9 in CA3 neurons generates extremely long thin filopodia-like spines. Filopodia are thought to be precursors to mature spines. Their length and flexibility are thought to make them more motile so that they may sample the environment in search of potential synaptic partners. It is possible that without cadherin-9, CA3 dendrites are largely unable to recognize DG axons and, therefore, do not initiate maturation of TE spines. Despite the severe effects of cadherin-9 knockdown on mossy fiber synapse morphology in vivo, some DG-CA3 synapses are still made. If cadherin-9 is important for specificity, why do mossy fiber synapses develop at all? This could reflect an inherent drive in neurons to make synaptic connections. Neurons that fail to generate functional synapses often undergo axon retraction and cell death (Conforti et al., 2007; Verhage et al., 2000). Therefore, with such dire consequences it may not be surprising that in the absence of normal synaptic cues, other compensatory cellular mechanisms drive synapse formation. Because cadherin-9 knockdown does not alter axon guidance, the only available postsynaptic targets are the defective CA3 neurons. In addition it is highly unlikely that a single molecule functions alone to govern specificity, and there may be other molecules that work together with cadherin-9 to ensure that synapses form with high fidelity and precision.
Our in vivo experiments indicate that cadherin-9 plays a critical role in regulating the differentiation of the mossy fiber synapse. Presynaptic boutons are consistently reduced in size, and complexity upon cadherin-9 knockdown and postsynaptic spine formation is severely disrupted. It remains unknown precisely how cadherin-9 mediates pre- and postsynaptic development, but our experiments show that, like other classic cadherins, cadherin-9 recruits β-catenin. β-Catenin is a multifunctional protein that binds PDZ proteins linked to both pre- and postsynaptic differentiation (Arikkath and Reichardt, 2008). Presynaptically, β-catenin recruits synaptic vesicles, and postsynaptically, it regulates spine formation via other catenin molecules and the actin cytoskeleton (Arikkath, 2009; Arikkath and Reichardt, 2008; Bamji et al., 2003). Such mechanisms may be important in conferring the structural features that define the mossy fiber synapse.
In summary we describe a novel approach to identify molecular signals that regulate the differentiation of specific classes of CNS synapses. Our approach allowed us to gain new insight into the function of cadherins in this process, which have long been proposed to mediate the formation of specific connections based on their differential expression patterns but direct evidence for a role in specificity has been lacking. Using the DG-CA3 mossy fiber synapse as a model, we provide several lines of evidence that cadherin-9 plays a critical role in the differentiation of this synapse in vitro and in vivo. Finally, because the DG mossy fiber synapse has been suggested to play a crucial role in pattern separation, selective disruption of cadherin-9 in vivo may provide a useful tool to dissect the contribution of this synapse to hippocampus-dependent behavior.
EXPERIMENTAL PROCEDURES
Hippocampal Neuron Culture
For the microisland assay, P0 cortical glia were cultured on agarose-coated coverslips sprayed with a mixture of poly-D-lysine and collagen to generate glial islands (Segal and Furshpan, 1990). Then, dissociated P0 hippocampal neurons were plated at 4 × 104 cells/ml. For the SPO assay, neurons were plated at the same density onto coverslips preplated with a confluent monolayer of glia. See Supplemental Experimental Procedures for details on all culture procedures, antibodies, and analysis.
Electrophysiology
After 10–14 DIV, microisland cultures were superfused with artificial cerebrospinal fluid. The presynaptic cell was held in current clamp at −55 mV, and the postsynaptic cell was held in voltage clamp at −65 mV. The presynaptic cell was stimulated at the minimum threshold to produce an action potential, and EPSCs were recorded at a sampling rate of 100 kHz. After recording, coverslips were immunostained to identify cell types. See Supplemental Experimental Procedures for details.
Cadherin-9 Cloning, shRNA, and Lentivirus Production
cDNA encoding the predicted full-length cadherin-9 gene described in GenBank NM_009869.1 was amplified from a P7 mouse hippocampal cDNA library. This clone was used to generate an in situ probe, and PCR subcloning was used to generate all other constructs. Cadherin-9 shRNA was made by annealing oligos into the pSRretro.neo system (OligoEngine). The cadherin-9 target sequence is GATGTCAACAACAACCCTC. For lentiviruses a cassette encoding the H1promoter and shRNA was ligated into pFsy1.1GW (Dittgen et al., 2004). For details see Supplemental Experimental Procedures.
In Vivo Spine/Mossy Fiber Bouton Analysis
Timed pregnant E15 mice were in utero electroporated with plasmid DNA at 1–4 μg/μl using standard methods. Confocal stacks of spines or individual mossy fiber boutons were collected on an Olympus FluoView 300, and stacks were analyzed using ImageJ, Excel, and Instat. For details see Supplemental Experimental Procedures.
Intracellular Injection of LY and Electron Microscopy
Mice were perfused with 4% PFA, and 100 μm thick coronal sections were cut. Penetrating microelectrodes were pulled from standard borosilicate capillary glass with filament (1.0 mm outer/0.58 mm inner diameter) and back filled with 5% LY dye. Virally infected CA3 neurons were filled via iontophoresis under visual guidance. For each filled CA3 neuron, viral infection was confirmed based on GFP expression at the cell body by immunostaining after filling with anti-LY (555) and anti-GFP (647). See Supplemental Experimental Procedures for more details and complete electron microscopy methods.
Supplementary Material
Acknowledgments
We thank M. Webb for the PY antibody, H. Cline for the synaptophysin-GFP plasmid, P. Caroni for the membrane GFP plasmid, Y. Zou for confocal use, K. Tiglio, J. Fakhoury, and E. Kang for technical assistance, and A. Kolodkin, Y. Zou, Y. Jin, N. Spitzer, D. Berg, D. Tränkner, and members of the Ghosh lab for comments and discussion. This work was supported by Autism Speaks (to M.E.W.) and NIH Grants R01 NS052772 (to A.G.) and R01 NS067216 (to A.G.).
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and six figures and can be found with this article online at doi:10.1016/j.neuron.2011.06.019.
References
- Ackley BD, Jin Y. Genetic analysis of synaptic target recognition and assembly. Trends Neurosci. 2004;27:540–547. doi: 10.1016/j.tins.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Dent JA. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981;195:51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Ango F, di Cristo G, Higashiyama H, Bennett V, Wu P, Huang ZJ. Ankyrin-based subcellular gradient of neurofascin, an immunoglobulin family protein, directs GABAergic innervation at purkinje axon initial segment. Cell. 2004;119:257–272. doi: 10.1016/j.cell.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Arikkath J. Regulation of dendrite and spine morphogenesis and plasticity by catenins. Mol Neurobiol. 2009;40:46–54. doi: 10.1007/s12035-009-8068-x. [DOI] [PubMed] [Google Scholar]
- Arikkath J, Reichardt LF. Cadherins and catenins at synapses: roles in synaptogenesis and synaptic plasticity. Trends Neurosci. 2008;31:487–494. doi: 10.1016/j.tins.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlotta P, Molyneaux BJ, Chen J, Inoue J, Kominami R, Macklis JD. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ. The chemokine SDF1 regulates migration of dentate granule cells. Development. 2002;129:4249–4260. doi: 10.1242/dev.129.18.4249. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekirov IH, Needleman LA, Zhang W, Benson DL. Identification and localization of multiple classic cadherins in developing rat limbic system. Neuroscience. 2002;115:213–227. doi: 10.1016/s0306-4522(02)00375-5. [DOI] [PubMed] [Google Scholar]
- Bekirov IH, Nagy V, Svoronos A, Huntley GW, Benson DL. Cadherin-8 and N-cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus. 2008;18:349–363. doi: 10.1002/hipo.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Valcin M, Poskanzer K, Tanaka H, Benson DL. Temporally distinct demands for classic cadherins in synapse formation and maturation. Mol Cell Neurosci. 2004;27:509–521. doi: 10.1016/j.mcn.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozdagi O, Wang XB, Nikitczuk JS, Anderson TR, Bloss EB, Radice GL, Zhou Q, Benson DL, Huntley GW. Persistence of coordinated long-term potentiation and dendritic spine enlargement at mature hippocampal CA1 synapses requires N-cadherin. J Neurosci. 2010;30:9984–9989. doi: 10.1523/JNEUROSCI.1223-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camardo J, Proshansky E, Schacher S. Identified Aplysia neurons form specific chemical synapses in culture. J Neurosci. 1983;3:2614–2620. doi: 10.1523/JNEUROSCI.03-12-02614.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel ME, Harris KM. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol. 1992;325:169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Conforti L, Adalbert R, Coleman MP. Neuronal death: where does the end begin? Trends Neurosci. 2007;30:159–166. doi: 10.1016/j.tins.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Dittgen T, Nimmerjahn A, Komai S, Licznerski P, Waters J, Margrie TW, Helmchen F, Denk W, Brecht M, Osten P. Lentivirus-based genetic manipulations of cortical neurons and their optical and electro-physiological monitoring in vivo. Proc Natl Acad Sci USA. 2004;101:18206–18211. doi: 10.1073/pnas.0407976101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- Fannon AM, Colman DR. A model for central synaptic junctional complex formation based on the differential adhesive specificities of the cadherins. Neuron. 1996;17:423–434. doi: 10.1016/s0896-6273(00)80175-0. [DOI] [PubMed] [Google Scholar]
- Flanagan JG. Neural map specification by gradients. Curr Opin Neurobiol. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Grabs D, Bergmann M, Schuster T, Fox PA, Brich M, Gratz M. Differential expression of synaptophysin and synaptoporin during pre- and postnatal development of the rat hippocampal network. Eur J Neurosci. 1994;6:1765–1771. doi: 10.1111/j.1460-9568.1994.tb00569.x. [DOI] [PubMed] [Google Scholar]
- Grosse G, Tapp R, Wartenberg M, Sauer H, Fox PA, Grosse J, Gratzl M, Bergmann M. Prenatal hippocampal granule cells in primary cell culture form mossy fiber boutons at pyramidal cell dendrites. J Neurosci Res. 1998;51:602–611. doi: 10.1002/(SICI)1097-4547(19980301)51:5<602::AID-JNR7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Inaki M, Yoshikawa S, Thomas JB, Aburatani H, Nose A. Wnt4 is a local repulsive cue that determines synaptic target specificity. Curr Biol. 2007;17:1574–1579. doi: 10.1016/j.cub.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Inoue A, Sanes JR. Lamina-specific connectivity in the brain: regulation by N-cadherin, neurotrophins, and glycoconjugates. Science. 1997;276:1428–1431. doi: 10.1126/science.276.5317.1428. [DOI] [PubMed] [Google Scholar]
- Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K. Synapse elimination in the central nervous system. Curr Opin Neurobiol. 2009;19:154–161. doi: 10.1016/j.conb.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kavalali ET, Klingauf J, Tsien RW. Activity-dependent regulation of synaptic clustering in a hippocampal culture system. Proc Natl Acad Sci USA. 1999;96:12893–12900. doi: 10.1073/pnas.96.22.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen MP, Shen K. Wnt signaling positions neuromuscular connectivity by inhibiting synapse formation in C. elegans. Cell. 2007;130:704–716. doi: 10.1016/j.cell.2007.06.046. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- Mendez P, De Roo M, Poglia L, Klauser P, Muller D. N-cadherin mediates plasticity-induced long-term spine stabilization. J Cell Biol. 2010;189:589–600. doi: 10.1083/jcb.201003007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, Shapiro L. Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell. 2006;124:1255–1268. doi: 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Pecho-Vrieseling E, Sigrist M, Yoshida Y, Jessell TM, Arber S. Specificity of sensory-motor connections encoded by Sema3e-Plxnd1 recognition. Nature. 2009;459:842–846. doi: 10.1038/nature08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer K, Needleman LA, Bozdagi O, Huntley GW. N-cadherin regulates ingrowth and laminar targeting of thalamocortical axons. J Neurosci. 2003;23:2294–2305. doi: 10.1523/JNEUROSCI.23-06-02294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen A, Sätzler K, Rodríguez EP, Jonas P, Frotscher M, Lübke JH. Structural determinants of transmission at large hippocampal mossy fiber synapses. J Neurosci. 2007;27:10434–10444. doi: 10.1523/JNEUROSCI.1946-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose D, Chiba A. Synaptic target recognition at Drosophila neuromuscular junctions. Microsc Res Tech. 2000;49:3–13. doi: 10.1002/(SICI)1097-0029(20000401)49:1<3::AID-JEMT2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Saglietti L, Dequidt C, Kamieniarz K, Rousset MC, Valnegri P, Thoumine O, Beretta F, Fagni L, Choquet D, Sala C, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Segal MM, Furshpan EJ. Epileptiform activity in microcultures containing small numbers of hippocampal neurons. J Neurophysiol. 1990;64:1390–1399. doi: 10.1152/jn.1990.64.5.1390. [DOI] [PubMed] [Google Scholar]
- Shen K, Bargmann CI. The immunoglobulin superfamily protein SYG-1 determines the location of specific synapses in C. elegans. Cell. 2003;112:619–630. doi: 10.1016/s0092-8674(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Shen K, Fetter RD, Bargmann CI. Synaptic specificity is generated by the synaptic guidepost protein SYG-2 and its receptor, SYG-1. Cell. 2004;116:869–881. doi: 10.1016/s0092-8674(04)00251-x. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Tsujimoto G, Kitajima M, Natori M. Identification of three human type-II classic cadherins and frequent heterophilic interactions between different subclasses of type-II classic cadherins. Biochem J. 2000;349:159–167. doi: 10.1042/0264-6021:3490159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singec I, Knoth R, Ditter M, Hagemeyer CE, Rosenbrock H, Frotscher M, Volk B. Synaptic vesicle protein synaptoporin is differently expressed by subpopulations of mouse hippocampal neurons. J Comp Neurol. 2002;452:139–153. doi: 10.1002/cne.10371. [DOI] [PubMed] [Google Scholar]
- Stepniak E, Radice GL, Vasioukhin V. Adhesive and signaling functions of cadherins and catenins in vertebrate development. Cold Spring Harb Perspect Biol. 2009;1:a002949. doi: 10.1101/cshperspect.a002949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Abe K, Mizoguchi A, Takaoka K, Chisaka O, Takeichi M. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- Tran TS, Rubio ME, Clem RL, Johnson D, Case L, Tessier-Lavigne M, Huganir RL, Ginty DD, Kolodkin AL. Secreted semaphorins control spine distribution and morphogenesis in the postnatal CNS. Nature. 2009;462:1065–1069. doi: 10.1038/nature08628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Honjo Y, Johnson KR, Wheelock MJ, Takeichi M. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. J Cell Biol. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, et al. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Vogt AK, Brewer GJ, Decker T, Böcker-Meffert S, Jacobsen V, Kreiter M, Knoll W, Offenhäusser A. Independence of synaptic specificity from neuritic guidance. Neuroscience. 2005;134:783–790. doi: 10.1016/j.neuroscience.2005.04.039. [DOI] [PubMed] [Google Scholar]
- Volk T, Cohen O, Geiger B. Formation of heterotypic adherens-type junctions between L-CAM-containing liver cells and A-CAM-containing lens cells. Cell. 1987;50:987–994. doi: 10.1016/0092-8674(87)90525-3. [DOI] [PubMed] [Google Scholar]
- Williams ME, de Wit J, Ghosh A. Molecular mechanisms of synaptic specificity in developing neural circuits. Neuron. 2010;68:9–18. doi: 10.1016/j.neuron.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams PL, Webb M, Atkinson DJ, Seeley PJ. A monoclonal antibody, Py, distinguishes different classes of hippocampal neurons. J Neurosci. 1989;9:2170–2181. doi: 10.1523/JNEUROSCI.09-06-02170.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR. Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature. 2008;451:465–469. doi: 10.1038/nature06469. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Weiner JA, Sanes JR. Sidekicks: synaptic adhesion molecules that promote lamina-specific connectivity in the retina. Cell. 2002;110:649–660. doi: 10.1016/s0092-8674(02)00910-8. [DOI] [PubMed] [Google Scholar]
- Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, proto-cadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.