Abstract
Discriminating among sensory stimuli is critical for animal survival. This discrimination is particularly essential when evaluating whether a stimulus is noxious or innocuous. From insects to humans, TRP channels are key transducers of thermal, chemical and other sensory cues1, 2. Many TRPs are multi-modal receptors that respond to diverse stimuli1–3, but how animals distinguish sensory inputs activating the same TRP is largely unknown. Here we determine how stimuli activating Drosophila TRPA1 are discriminated. While Drosophila TRPA1 responds to both noxious chemicals4 and innocuous warming5, we find that TRPA1-expressing chemosensory neurons respond to chemicals but not warmth, a specificity conferred by a chemosensory-specific TRPA1 isoform with reduced thermosensitivity compared to the previously described isoform. At the molecular level, this reduction results from a unique region that robustly reduces the channel’s thermosensitivity. Cell-type segregation of TRPA1 activity is critical: when the thermosensory isoform is expressed in chemosensors, flies respond to innocuous warming with regurgitation, a nocifensive response. TRPA1 isoform diversity is conserved in malaria mosquitoes, suggesting similar mechanisms may allow discrimination of host-derived warmth, an attractant, from chemical repellents. These findings indicate that reducing thermosensitivity can be critical for TRP channel functional diversification, facilitating their use in contexts where thermal sensitivity can be maladaptive.
Keywords: TRP, polymodal, pain, nociception, thermosensation, chemosensation
Highly temperature-responsive Transient Receptor Potential (TRP) cation channels, thermoTRPs, mediate thermosensation from insects to mammals1, 2 and are important for human pain and inflammation6. Like mammalian thermoTRPs, Drosophila melanogaster TRPA1 is both a thermal and chemical sensor, responding to innocuous warmth (above ~25–27°C)5, 7 and noxious chemicals4. TRPA1 acts in thermosensors within the brain to modulate thermal preference over 18–32°C5, innocuous temperatures compatible with fly survival8, and in gustatory chemosensors to inhibit ingestion of electrophiles4, reactive chemicals like allyl isothiocyanate (AITC, found in wasabi) and N-Methyl Maleimide (NMM) that rapidly incapacitate flies (Supp. Fig. 1). TRPA1’s responsiveness to both innocuous and noxious stimuli raises the question of how these stimuli are distinguished to elicit distinct behavioral responses. Mammals face similar issues; for example, TRPM8 transduces both innocuous and noxious cold1–3.
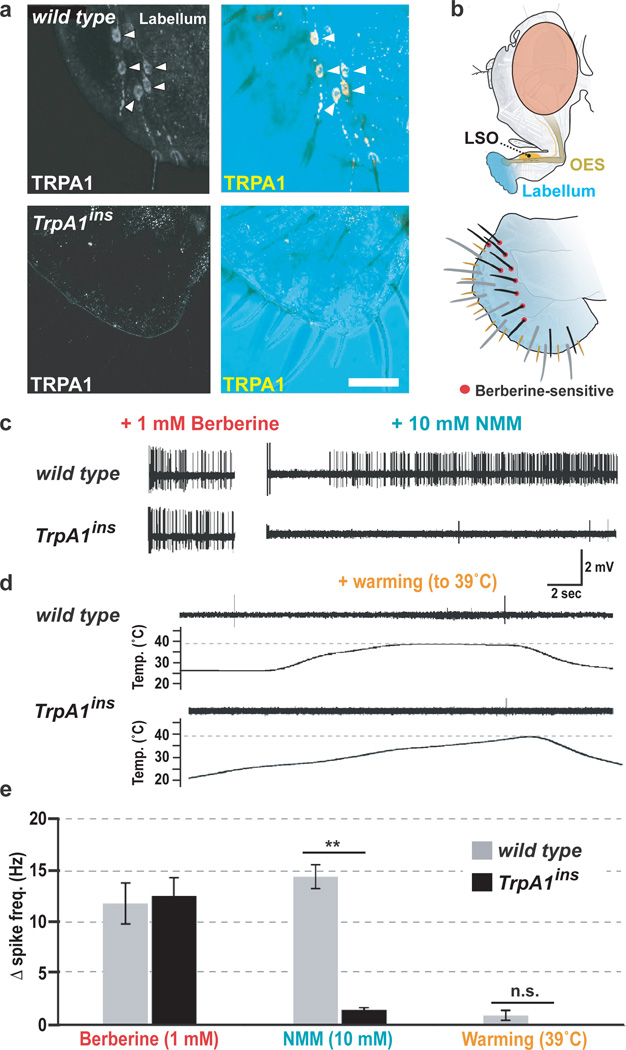
We previously reported TRPA1-expressing chemosensors in the labral sense organ4; using improved immunostaining conditions, we now also detect specific TRPA1 protein expression in labellar chemosensors (Fig. 1a, b). Extracellular tip-recording9 indicated these neurons were TRPA1-dependent chemosensors; they responded to the electrophile NMM with robust spiking in wild type but not TrpA1 mutants (Fig. 1c, Supp. Fig. 2). The mutant defect was electrophile-specific, as TrpA1 mutants responded like wild type to berberine chloride (Fig. 1c), a bitter compound that also activates these neurons10. In contrast, warming to ~39°C, from innocuously warm to the noxious range, elicited no spiking in these cells (Fig. 1d, e). This is striking as TRPA1's effectiveness in conferring warmth sensitivity has led to its use as a thermogenetic tool5, 11. Thus, despite TRPA1’s known sensitivity to both temperature and chemicals, these chemosensors are warmth-insensitive.
Figure 1. TRPA1-dependent gustatory neurons do not respond to heat.
a, TRPA1 immunostaining of wild-type (top) and TrpA1ins (bottom) labella. Right, DIC reveals labellar structures. Arrowheads, chemosensor cell bodies. b, Drosophila gustatory organs (top). LSO, Labral Sense Organ; OES, oesophagus. Labellar bristles (bottom). Brown, s-type; grey, L-type; black, i-type; berberine-sensitive bristles were targeted for electrophysiology. c–d, Bristle responses to: berberine (1 mM) and N-Methyl Maleimide (NMM, 10 mM) (c); warming (d). e, Average spike rate after subtracting electrolyte-only baseline. **P<0.01; ns, not significant (P>0.05), t-test. All data are mean ± s.e.m. Warming reached average maximum temperature of 39.0 ±0.6 SD.
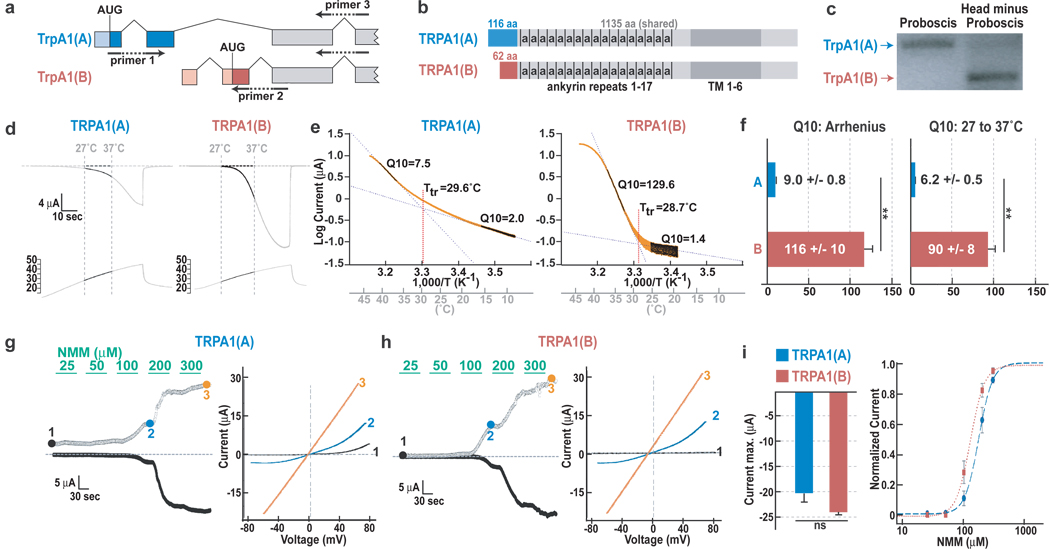
In addition to the previously characterized transcript, TrpA1(B), a transcript with an alternative 5' end, TRPA1(A), has been annotated12 (Fig. 2a). These transcripts encode protein isoforms with distinct amino termini, but the same ankyrin and transmembrane domains (Fig. 2b). RT-PCR demonstrated differential expression: TrpA1(A) was expressed in the proboscis, which houses the TRPA1-expressing chemosensors, while TrpA1(B) predominated elsewhere in the head, where TRPA1-expressing thermosensors are located (Fig. 2c).
Figure 2. TRPA1 isoform diversity yields tissue-specific channels with different thermal sensitivities.
a, TrpA1 gene structure and primer locations. b, Red and blue boxes denote isoform-specific sequences. a, ankyrin repeat. Dark grey, transmembrane region. c, RT-PCR. d–e, TRPA1(A)- and TRPA1(B)-dependent currents (d) and Arrhenius plots (e) in oocytes. f, Q10s from Arrhenius plot (left) or 27–37°C (right). g, h, Left panels, NMM responsiveness of TRPA1(A) (g) and TRPA1(B) (h). Right panels, I–V relationships at points marked at left. i, Mean amplitudes at 300 µM NMM (left) and NMM dose-response (right). All data, mean +/− s.e.m. **P<0.01; n.s., not significant, t-test.
Examined in Xenopus oocytes, TRPA1(A) was much less thermosensitive than TRPA1(B), as reflected in its temperature coefficient (Q10), the fold change in current per 10°C change1, 2. Arrhenius plot analysis13 yielded a Q10 of ~9 for TRPA1(A) versus ~116 for TRPA1(B) (Fig. 2d–f). In addition, while TRPA1(B) was essentially inactive at low temperatures, TRPA1(A)-dependent currents were observed ≤ 15°C, further reducing TRPA1(A)’s temperature-dependent activity differential (Supp. Fig. 3). TRPA1(A)’s maximum heat-activated current was also significantly lower (Supp. Fig. 4). Finally, the transition (or threshold) temperature for increased temperature responsiveness was 29.7 +/− 0.3 °C for TRPA1(A) versus 27.8 +/− 0.4 for TRPA1(B) (P<0.01, t-test). As the fly’s innocuous warm temperature range is of particular behavioral relevance, the Q10 from 27°C to 37°C (below the fly’s ~38°C nociceptive threshold14) was also calculated, yielding 6.2 +/− 0.5 for TRPA1(A) and 90 +/− 8 for TRPA1(B) (Fig. 2f). Other properties were largely unaffected; both channels responded robustly to electrophiles and had similar voltage sensitivities (Fig. 2g, h). TRPA1(A) and TRPA1(B) had similar maximum current amplitudes at 300 µM NMM, with EC50s of 176 +/− 12 and 128 +/− 9 µM, respectively (Fig. 2i).
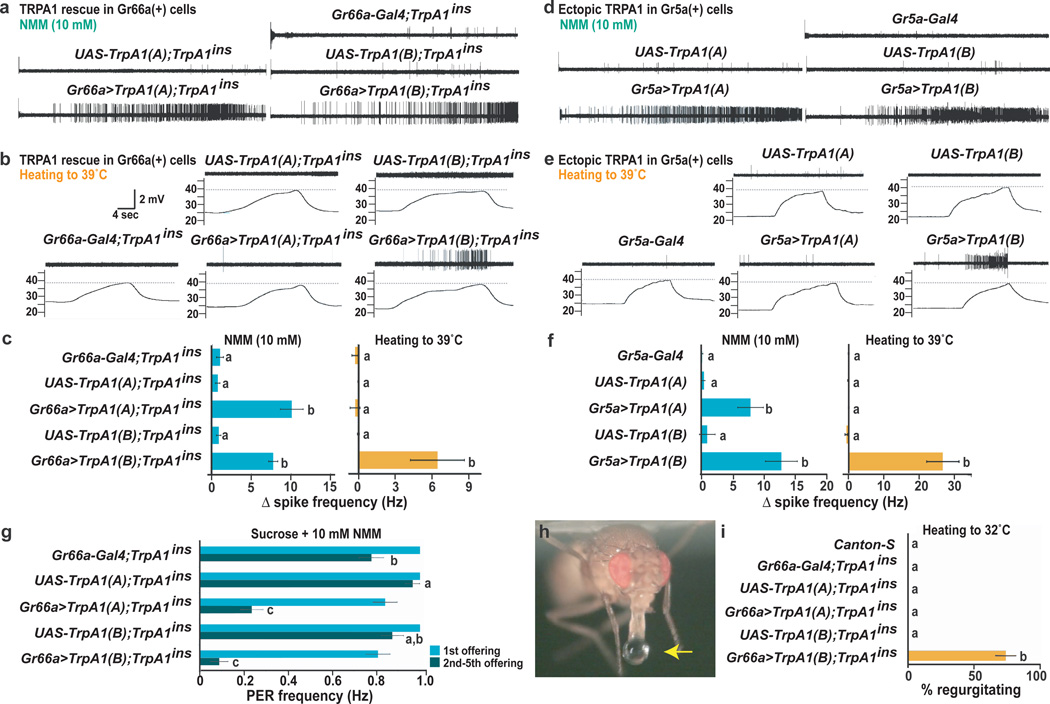
TRPA1(A)’s reduced thermosensitivity could account for the chemosensors’ warmth-insensitivity. But while less temperature-sensitive than TRPA1(B), TRPA1(A)'s Q10 resembles several TRPs suggested to mediate warmth sensitivity15–17. To assess whether TRPA1(A) could confer warmth sensitivity upon fly chemosensors, each isoform was used to rescue a TrpA1 mutant. We previously demonstrated that expressing TRPA1(B) in TRPA1-dependent chemosensors using Gr66a-Gal4 rescues the TrpA1 mutant behavioral defect4. Using electrophysiology, we found both isoforms restored NMM responsiveness (Fig. 3a, Supp. Fig 5a,b), but only TRPA1(B) conferred warmth-sensitivity (Fig. 3b, 3c). These differences did not require properties unique to TRPA1-dependent chemosensors. Each isoform was expressed ectopically in sweet-responsive chemosensors using Gr5a-Gal418. Both isoforms conferred electrophile sensitivity upon these normally electrophile-insensitive neurons, but only TRPA1(B) conferred thermosensitivity (Fig. 3d–f, Supp. Fig. 4c, d). TRPA1(A)’s inability to confer warmth sensitivity on fly chemosensors emphasizes that while a Q10 above 5 makes TRPA1(A) more thermally sensitive than most ion channels, in vivo testing is important in evaluating whether a channel is sufficiently thermosensitive to make a specific neuron warmth-responsive.
Figure 3. TRPA1 isoform diversity determines sensory specificity of gustatory neurons.
a–c TrpA1 mutant, berberine-sensitive i-type bristles expressing different TRPA1 isoforms. Responses to NMM (a) and warming (b). c, Quantitation. d–f, L-type bristles expressing TRPA1 isoforms. Responses to NMM (d) and warming (e). f, Quantitation. g, Rescue of TrpA1 mutant behavioral response to NMM-containing food. PER, proboscis extension response. h, Warmth-induced regurgitation in TrpA1 mutant rescued with TRPA1(B). i, Regurgitation upon warming from room temperature to 32°C. In c, f, g and i, statistically distinct groups marked by different letters (Tukey HSD, α = 0.01). Data are mean ± s.e.m.
These data support a model in which the specificity of TRPA1-expressing gustatory neurons for chemicals is established by their selective expression of TRPA1(A), an isoform unable to confer warmth sensitivity. In contrast, TRPA1(B)’s chemical sensitivity should render TRPA1-dependent thermosensors sensitive to reactive chemicals. However, the location of TRPA1-dependent Anterior Cell (AC) thermosensors inside the head5 should minimize exposure to environmental irritants. Interestingly, multiple TRPV1 and TRPM1 isoforms are present in humans and other mammals2, 17, 19, 20, suggesting the potential generality of isoform diversity in modulating TRP functions.
The behavioral significance of discriminating noxious from innocuous TRPA1 activators was examined by testing gustatory responses of TrpA1 mutants rescued by chemosensor expression of each isoform. TrpA1 mutants exhibit decreased avoidance of reactive electrophile-containing food4. Each isoform rescued this behavior (Fig. 3g). However, TRPA1(B) also triggered a nocifensive response to innocuous warming. When allowed to ingest water to satiation and warmed to ~32°C, neither wild-type nor TRPA1(A) rescue animals showed detectable gustatory responses (Fig. 3h, 3i). However, warming TRPA1(B) rescue flies caused ~75% to regurgitate (Fig. 3h, 3i; Supp. Movie 1). Thus, substituting TRPA1(B) for TRPA1(A) in chemosensors disrupts discrimination of noxious from innocuous stimuli and demonstrates the negative behavioral consequence of misregulated thermosensitivity.
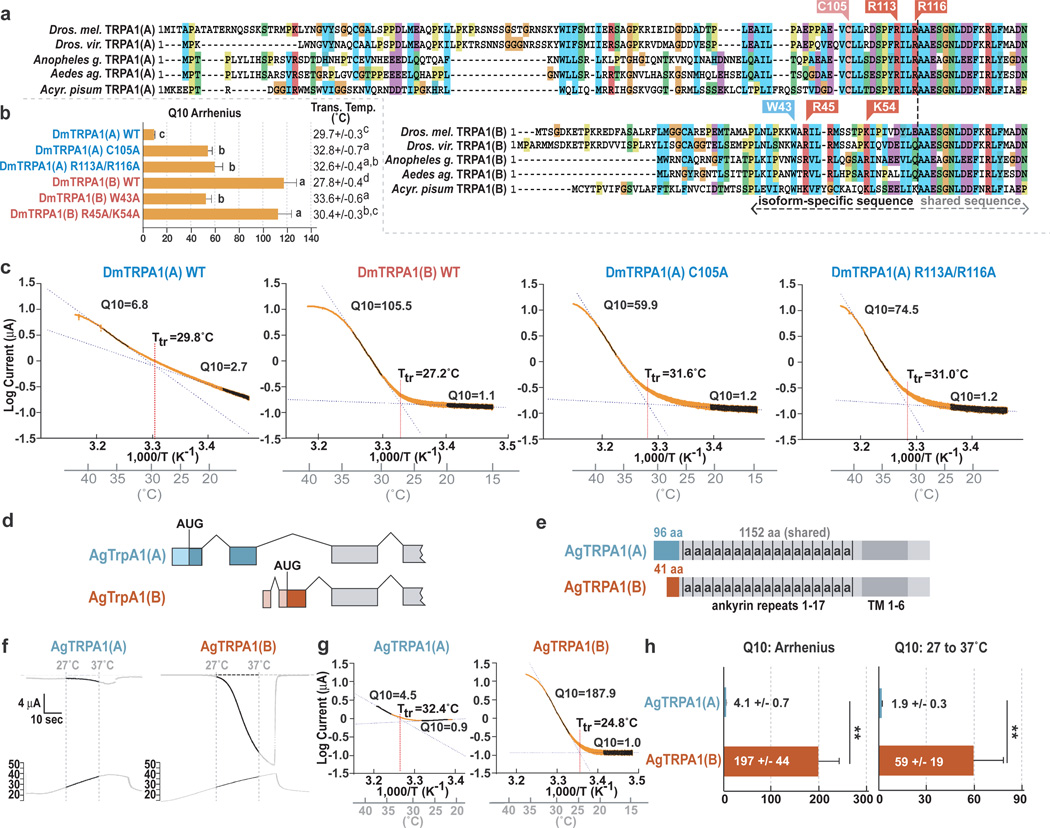
To probe how TRPA1's alternative N-termini confer distinct properties, conserved residues within these regions were mutated (Fig. 4a). Mutating either a cysteine (C105) or two basic residues (R113, R116) in TRPA1(A) dramatically increased temperature-responsiveness (Fig. 4a–c, Supp. Figs. 6 and 7). While wild-type TRPA1(A)'s Q10 was <10, the TRPA1(A) mutants exhibited Q10s >50 (Fig. 4b, c), greater than the reported Q10s of canonical thermoTRPs like TRPM8 (~24)21 and TRPV1 (~40)22. In addition, the TRPA1(A) mutants conducted little current below the threshold, increasing the temperature-dependent activity differential (Fig. 4c, Supp. Fig. 8). The mutants’ enhanced sensitivities appeared temperature-specific, as NMM sensitivity was not increased (Supp. Fig. 8). These data indicate that TRPA1(A) retains all the requirements for robust thermosensation, but contains a modulatory region preventing those elements from exerting their full effect.
Figure 4. Regulation of insect TRPA1 thermosensitivity by alternative N-termini.
a, TRPA1 sequence alignments. b, Q10 and transition temperatures of wild type and mutant TRPA1s. Letters denote statistically distinct groups (Tukey HSD, α = 0.02). c, Arrhenius plots of indicated channels. d, AgTrpA1 gene structure. e, AgTRPA1 isoforms. 'a’: an ankyrin repeat. Light blue and maroon: isoform-specific amino acids. Dark grey: transmembrane region. f–h, Temperature sensitivity of AgTRPA1(A) and AgTRPA1(B). Traces (f) and Arrhenius plots (g) of temperature dependent current recordings at −60 mV in Xenopus oocytes. h, Q10s from Arrhenius plot (left) or 27–37°C (right) (**P<0.01, t-test).
For TRPA1(B), mutating either a conserved tryptophan or two basic residues in the N-terminus yielded channels retaining robust thermosensitivity (Q10 >50; Fig. 4b, Supp. Fig. 6). The thresholds of the TRPA1(A) and TRPA1(B) mutants were all ~30–34°C, within the innocuous warm range but above wild-type TRPA1(B)’s ~28°C (Fig. 4b, c). Thus, while TRPA1(B)-specific sequences are unnecessary for robust responsiveness to innocuous warming, they may tune channel threshold within this range.
In insect disease vectors, TRPA1 orthologs have been implicated in detecting both warmth and chemical repellents4, 23, 24, cues with opposing effects on host-seeking. We found the malaria mosquito Anopheles gambiae also contains TRPA1(A) and TRPA1(B) isoforms of differing thermosensitivity (Fig. 4d–h). In oocytes, AgTRPA1(A) had a Q10 of ~4 versus AgTRPA1(B)’s ~200; from 27 to 37°C, AgTRPA1(A)’s Q10 was ~2 versus AgTRPA1(B)’s ~60 (Fig. 4h). AgTRPA1(A) yielded lower maximum heat-induced current than AgTRPA1(B) (Supp. Fig. 4) and had a higher threshold (34.2 +/− 1.8°C vs. 25.2 +/− 0.9°C, P<0.01). AgTRPA1(A) also exhibited significant conductance below threshold (Fig. 4f, g). Both channels responded to electrophiles (Supp. Fig. 9). TRPA1(A) and TRPA1(B) are conserved in other hematophagous insects including Aedes aegypti and Culex quinquefasciatus mosquitoes and Pediculus humanus corporis lice (Fig. 4a, Supp. Fig. 10), which transmit dengue, West Nile fever and typhus, respectively. TRPA1’s functional diversity provides a potential explanation for how insect vectors discriminate noxious chemicals from host-derived warmth, suggesting TRPA1 presents two distinct molecular targets for disrupting pest behavior.
TRPA1-based electrophile detection likely emerged ≥500 million years ago in a common vertebrate/invertebrate ancestor4. However, the larger TRPA family extends to choannoflagellates, separated from animals ≥600 million years4. As divergent TRPA clades contain highly temperature-sensitive channels1, thermosensitivity may be ancestral. In this scenario, TRPA1’s specialization for noxious chemical detection would necessitate reducing thermosensitivity, consistent with the N-terminus’ effect in TRPA1(A). The ability of N-terminal variation to sculpt channel properties is intriguing as the N-terminus is the most divergent region of TRPA1 within insects and from flies to humans5.
TRPs are a large family of channels, with 27 human and 13 fly members, that vary greatly in thermosensitivity and function2. Significant diversity is evident even among closely related TRPs. In mammals, for example, TRPM8 (Q10 ~2421) mediates thermosensation3, while the less thermosensitive TRPM4 and TRPM5 (Q10 ~8.5 to 1015) mediate insulin secretion25 and TRPM7 (with no reported thermal sensitivity) is implicated in ion homeostasis2. The mechanisms underlying such diversification are unclear. While studies of thermal sensing by TRPs have focused on identifying regions promoting thermosensitivity1, 26–29, our work indicates that regions reducing thermosensitivity are also critical. Here we find that selectively reducing insect TRPA1’s thermosensitivity facilitates its use in a context where thermosensitivity is undesirable. Similar mechanisms could mediate functional diversification not only among isoforms of a single TRP, but also contribute to the remarkable functional diversification observed between different TRP family channels.
METHODS SUMMARY
Fly strains and immunohistochemistry
UAS-TrpA1(B) and Gr66a-Gal4 transgenic strains and the TrpA1ins mutant have been described4. The UAS-TrpA1(A) transgene was amplified from fly cDNA with an isoform specific primer (5’-TATAAAGCTTAAGCCACCATGATTACAGCTCCGGCCACGGCCA-3’) and a reverse primer (5’-GAGACTCGAGCTACATGCTCTTATTGAAGCTCAGGGCG-3’). As detailed in methods, the UAS-TrpA1(A) transgene was inserted in the same genomic location used for the UAS-TrpA1(B) transgene to control for transgene position effects. Anti-TRPA1 immunohistochemistry was as described 4, except secondary antibody was incubated three days.
Behavior
Proboscis extension assay was as described 4, with seven flies per experiment, three experiments per genotype. For heat-sensitive regurgitation, >20 flies per genotype (2 to 3 days old) were starved overnight with water, then glued to glass slides. After 2–3 hour recovery, flies were satiated with water. Only flies drinking longer than 5 sec were tested. Drinking times did not significantly differ between wild type and rescue flies (E.C. and P.G., unpub. data), consistent with similar ingestion behaviors. Flies were heated with a radiant heater at 800 W (H-4438, Optimus, USA) and temperature monitored by adjacent thermocouple microprobe (IT-23, Physitemp Instruments Inc., USA) wrapped in fly cuticle.
Physiology
Oocyte physiology was performed as described 4, with additional details provided in Methods. Extracellular recordings of gustatory neurons were obtained by tip-recording9, as detailed in Methods.
Molecular Biology
Complementary DNA was prepared from dissected Drosophila melanogaster tissue (RETROscript, Ambion, Austin, TX, USA) and subjected to PCR with three primers, two forward isoform-specific primers and a reverse common primer detailed in Methods. Each primer was designed to straddle a splice junction, minimizing amplification of contaminating genomic DNA. In all cases, similar results were obtained from four independent tissue preparations.
METHODS
Fly strains and immunohistochemistry
The UAS-TrpA1(A) transgene was inserted into the genome by site-specific transgenesis 30 at same landing site as UAS-TrpA1(B), attp16 31. TRPA1(A) Genbank accession number is JQ015263.
Behavioral analysis
Chemicals used in incapacitation assays were Sucrose (Calbiochem LC8510 Gibbstown NJ), Sorbitol (Sigma S-1876, St. Louis, MO), Ficoll (Sigma F-4375), Agarose (Invitrogen 15510-027, Carlsbad, CA), Caffeine (Sigma C0750), NMM (Sigma 389412), Isopropanol (100%, J.T.Baker 9083-03 Phillipsburg NJ), Ethanol (100%, Decon Lab 2716,†King of Prussia, PA) and Allyl isothiocyanate (95%, Sigma 377430).
Characterization of TRPA1 isoforms in Xenopus oocytes
TRPA1 currents were recorded as described4, 5. To evaluate temperature sensitivities, oocytes were perfused in the recording buffer (96 mM NaCl, 1 mM MgCl2, 4 mM KCl, and 5 mM HEPES, pH 7.6), the temperature of which was increased ~0.5°C/sec from 10 to 45°C by SC-20 in-line heater/cooler (Warner Instruments, Hamden, CT, USA) with a CL100 bipolar temperature controller (Warner Instruments, Hamden, CT, USA). Temperature-evoked current was recorded at −60 mV. From the recorded current, Q10 was calculated as described13, 16. Arrhenius Q10=exp[10×(−Sarrhe)/(T1×T2)], where Sarrhe is the slope of linear phase of an Arrhenius plot between absolute temperatures, T1 and T2. Transition temperature was assessed as the temperature at which the least-squares fit lines from the two linear phases intersect13, 16. Q10 from 27–37°C was calculated from currents at temperatures of interest using the equation, Q10=(I2/I1)10/(T2-T1), where I1and I2 are currents observed at temperatures of T1 and T2, respectively. Q10 determinations were validated by using Crotalus atrox TRPA116 as a control with known Q10 (K.K. and P.G. unpub.). To assess sensitivity to NMM, voltage across the membrane was initially held at −80 mV, and a 300-ms voltage ramp (−80 mV to 80 mV) per second was applied. The oocytes were perfused for 1 min with the recording buffer containing indicated concentrations of NMM with 30-second washes between NMM applications. Current amplitudes at −80 mV after application of each NMM concentration were fitted to the Hill equation through Sigmaplot 10. The first coding exon of AgTrpA1(B) was chemically synthesized (Genscript, Piscataway, NJ, USA).
Gustatory Neuron Electrophysiology
Extracellular recordings of gustatory neurons were obtained using the tip-recording method9. Adult female flies, aged 1–4 days, were prepared by inserting a glass reference electrode containing Drosophila Ringer’s solution into the thorax and advancing the electrode through the head to the labellum. A glass recording electrode with an ~15 µm opening was used to apply tastants to individual sensilla. Raw signals were amplified using a TasteProbe preamplifier (Syntech, The Netherlands) and were digitized and analyzed using a PowerLab data-acquisition system with LabChart software (ADInstruments Inc., Australia). Amplified signals were digitized at a rate of 20 kb/s and filtered using a 100 Hz-3000 Hz band-pass filter prior to analysis. Individual action potentials were sorted using a visually-adjusted threshold and average spike rate was calculated beginning 200 ms after electrode contact. Recording times varied by experiment: berberine chloride and sucrose positive controls, 5 s; electrolyte-only, 20 s; NMM on i-type bristles, 60 s; NMM on L-type bristles, 120 s; heat-ramps, >60 s. For heat-ramp experiments, recordings were performed using electrolyte-only as tastant. After ~30 s of recording to determine baseline activity, heat was applied manually to the fly using a radiant heater (PRESTO HeatDish, National Presto Industries, Inc., USA). Application of heat was maintained for ~10–30 s and the distance between the heat source and the preparation was reduced to obtain a temperature of ≥39°C. Bristle temperature was estimated using thermocouple microprobe (IT-23, Physitemp Instruments Inc., USA) wrapped in fly cuticle. All tastants were dissolved in 30 mM tricholine citrate as the electrolyte to inhibit the activity of the water cell in L-type bristles32. Tastants were stored at −20°C and aliquots maintained at 4°C for up to one week. For all experiments, a positive control was used to confirm the viability of the target bristle. For i-type bristles, 1 mM berberine chloride was used as control. For L-type bristles, 30 mM sucrose was used. Individual tastant presentations were separated by a minimum delay of 60 s. At least two animals and six bristles were examined for each condition.
Molecular biology
Primers for RT-PCR reactions:
Drosophila melanogaster TrpA1(A)-F: 5'GCC GGA ACA GCA AGT ATT3'
Drosophila melanogaster TrpA1(B)-F: 5'GTG GAC TAT CTG GAG GCG3'
Drosophila melanogaster TrpA1 common-R: 5'TAT CCT TCG CAT TAA AGT CGC3'
Mutagenesis of Drosophila TRPA1 was performed as described 4. Briefly, for a desired mutation, each of two mutually complementary mutant primers was paired for PCR with a primer (outer primer) that anneals outside of either SalI or HpaI restriction recognition site. The two resulting PCR fragments that overlap only in the region of the two mutant primers were combined and served as template for the next PCR reaction that contained only outer primers. The second PCR product was digested by SalI and HpaI, and subsequently replaced the corresponding wild type region of TRPA1 cDNA. The fragment between the two restriction sites was sequenced. Sequences were aligned using MUSCLE 3.733.
Supplementary Material
Acknowledgments
We thank the Garrity lab, H. Garrity, L. Griffith, L. Huang and M. Rosbash for helpful comments, and F. Marion-Poll and A. Dahanukar for guidance with tip-recording. This work was supported by grants from National Science Foundation (IOS-1025307), National Institute of Mental Health (EUREKA R01 MH094721) and National Institute of Neurological Disorders and Stroke (NINDS) (PO1 NS044232) to P.A.G., a National Research Service Award from NINDS to V.C.P. (F31 NS071897-02) and the Boston College DeLuca Professorship to M.A.T.M.
Footnotes
Author contributions: K.K., V.C.P., and P.A.G. designed experiments. K.K performed molecular biology, genetics, and oocyte physiology. V.C.P. performed genetics and sensory neuron electrophysiology. E.C.C. performed genetics and behavior. A.M.D. performed behavior. L.N. performed immunohistochemistry. A.M.J., K.R. and M.A.T.M. grew and harvested mosquitoes. P.A.G. performed bioinformatics. K.K., V.C.P., and P.A.G. wrote the paper.
REFERENCES
- 1.Dhaka A, Viswanath V, Patapoutian A. TRP Ion Channels and Temperature Sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- 2.Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacological reviews. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Daniels RL, McKemy DD. Mice left out in the cold: commentary on the phenotype of TRPM8-nulls. Molecular pain. 2007;3:23. doi: 10.1186/1744-8069-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang K, et al. Analysis of Drosophila TRPA1 reveals an ancient origin for human chemical nociception. Nature. 2010;464:597–600. doi: 10.1038/nature08848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nature Reviews Drug Discovery. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswanath V, et al. Opposite thermosensor in fruitfly and mouse. Nature. 2003;423:822–823. doi: 10.1038/423822a. [DOI] [PubMed] [Google Scholar]
- 8.Cohet Y. Epigenetic influences on the lifespan of the Drosophila: Existence of an optimal growth temperature for adult longevity. Experimental Gerontology. 1975;10:181–184. doi: 10.1016/0531-5565(75)90029-7. [DOI] [PubMed] [Google Scholar]
- 9.Hodgson ES, Lettvin JY, Roeder KD. Physiology of a primary chemoreceptor unit. Science. 1955;122:417–418. doi: 10.1126/science.122.3166.417-a. [DOI] [PubMed] [Google Scholar]
- 10.Weiss LA, Dahanukar A, Kwon JY, Banerjee D, Carlson JR. The Molecular and Cellular Basis of Bitter Taste in Drosophila. Neuron. 69:258–272. doi: 10.1016/j.neuron.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Philipsborn AC, et al. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Graveley BR, et al. The developmental transcriptome of Drosophila melanogaster. Nature. 2011;471:473–479. doi: 10.1038/nature09715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vyklicky L, et al. Temperature coefficient of membrane currents induced by noxious heat in sensory neurones in the rat. J Physiol. 1999;517(Pt 1):181–192. doi: 10.1111/j.1469-7793.1999.0181z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tracey WD, Jr, Wilson RI, Laurent G, Benzer S. painless, a Drosophila gene essential for nociception. Cell. 2003;113:261–273. doi: 10.1016/s0092-8674(03)00272-1. [DOI] [PubMed] [Google Scholar]
- 15.Talavera K, et al. Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature. 2005;438:1022–1025. doi: 10.1038/nature04248. [DOI] [PubMed] [Google Scholar]
- 16.Gracheva EO, et al. Molecular basis of infrared detection by snakes. Nature. 2010;464:1006–1011. doi: 10.1038/nature08943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gracheva EO, et al. Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature. 2011;476:88–91. doi: 10.1038/nature10245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marella S, et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 19.Lu G, Henderson D, Liu L, Reinhart PH, Simon SA. TRPV1b, a functional human vanilloid receptor splice variant. Mol Pharmacol. 2005;67:1119–1127. doi: 10.1124/mol.104.009852. [DOI] [PubMed] [Google Scholar]
- 20.Vos MH, et al. TRPV1b overexpression negatively regulates TRPV1 responsiveness to capsaicin, heat and low pH in HEK293 cells. Journal of neurochemistry. 2006;99:1088–1102. doi: 10.1111/j.1471-4159.2006.04145.x. [DOI] [PubMed] [Google Scholar]
- 21.Brauchi S, Orio P, Latorre R. Clues to understanding cold sensation: thermodynamics and electrophysiological analysis of the cold receptor TRPM8. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15494–15499. doi: 10.1073/pnas.0406773101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu B, Hui K, Qin F. Thermodynamics of heat activation of single capsaicin ion channels VR1. Biophysical journal. 2003;85:2988–3006. doi: 10.1016/S0006-3495(03)74719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang G, et al. Anopheles gambiae TRPA1 is a heat-activated channel expressed in thermosensitive sensilla of female antennae. Eur J Neurosci. 2009;30:967–974. doi: 10.1111/j.1460-9568.2009.06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maekawa E, et al. The role of proboscis of the malaria vector mosquito Anopheles stephensi in host-seeking behavior. Parasites & vectors. 2011;4:10. doi: 10.1186/1756-3305-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uchida K, Tominaga M. The role of thermosensitive TRP (transient receptor potential) channels in insulin secretion. Endocrine journal. 2011 doi: 10.1507/endocrj.ej11-0130. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Grandl J, et al. Pore region of TRPV3 ion channel is specifically required for heat activation. Nature neuroscience. 2008;11:1007–1013. doi: 10.1038/nn.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grandl J, et al. Temperature-induced opening of TRPV1 ion channel is stabilized by the pore domain. Nature neuroscience. 2010;13:708–714. doi: 10.1038/nn.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang F, Cui Y, Wang K, Zheng J. Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proceedings of the National Academy of Sciences of the United States of America. 2011;107:7083–7088. doi: 10.1073/pnas.1000357107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yao J, Liu B, Qin F. Modular thermal sensors in temperature-gated transient receptor potential (TRP) channels. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11109–11114. doi: 10.1073/pnas.1105196108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods references
- 30.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markstein M, Pitsouli C, Villalta C, Celniker SE, Perrimon N. Exploiting position effects and the gypsy retrovirus insulator to engineer precisely expressed transgenes. Nat Genet. 2008;40:476–483. doi: 10.1038/ng.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wieczorek H, Wolff G. The labellar sugar receptor of Drosophila. Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 1989;164:825–834. [Google Scholar]
- 33.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.