Abstract
The Polo-like kinases (Plks) are conserved, multifunctional cell cycle regulators that are induced in many forms of cancer and play additional roles in metazoan development. We previously identified plkA in Aspergillus nidulans, the only Plk investigated in filamentous fungi to date, and partially characterized its function through overexpression. Here, we report the plkA null phenotype. Surprisingly, plkA was not essential, unlike Plks in other organisms that contain a single homologue. A subset of cells lacking PLKA contained defects in spindle formation and chromosome organization, supporting some conservation in cell cycle function. However, septa were present, suggesting that PLKA, unlike other Plks, is not a central regulator of septation. Colonies lacking PLKA were compact with multibranched hyphae, implying a role for this factor in aspects of hyphal morphogenesis. These defects were suppressed by high temperature or low concentrations of benomyl, suggesting that PLKA may function during vegetative growth by influencing microtubule dynamics. However, the colonies also showed reduced conidiation and precocious formation of sexual Hülle cells in a benomyl- and temperature-insensitive manner. This result suggests that PLKA may influence reproduction through distinct mechanisms and represents the first example of a link between Plk function and development in fungi. Finally, filamentous fungal Plks have distinct features, and phylogenetic analyses reveal that they may group more closely with metazoan PLK4. In contrast, yeast Plks are more similar to metazoan proteins PLK1 to PLK3. Thus, A. nidulans PLKA shows some conservation in cell cycle function but may also play novel roles during hyphal morphogenesis and development.
INTRODUCTION
The polo-like kinases (Plks) comprise a family of serine/threonine kinases that play multiple roles during cell cycle progression (4, 51). Metazoans contain several Plk homologues, including Polo and PLK4 in Drosophila melanogaster, PLK1 to PLK3 in Caenorhabditis elegans, Plx1 to Plx3 in Xenopus laevis, and PLK1, PLK2/SNK, PLK3/FNK/PRK, PLK4/SAK, and PLK5 in mammals (3, 4, 16). Single Plks exist in fungal species, including Plo1 in Schizosaccharomyces pombe (45), Cdc5p in Saccharomyces cerevisiae (30), Cdc5p in Candida albicans (6), and PLKA in Aspergillus nidulans (5). Trypanosoma brucei also contains a PLK homologue, TbPLK (22). Plks are defined by an N-terminal catalytic domain containing distinct features and a C-terminal polo box domain (PBD), which is important for localization, autoregulation of kinase activity, and interaction with substrates (51). The PBD is typically composed of two polo box motifs, but the divergent and less characterized PLK4 contains a single canonical PBD motif as well as a cryptic polo box sequence (4, 35). Human PLK5, on the other hand, contains a normal PBD but lacks the catalytic domain (16).
Plks from yeast to humans are important for several cell cycle processes (4). During mitotic progression, for example, several Plks function during the G2/M transition (55, 58, 60, 70), centrosome maturation, separation and/or spindle assembly (9, 23, 33, 45, 67), chromosome segregation (2, 66), and mitotic exit (13, 24, 42, 62, 65). Plks are also crucial for cytokinesis or septation (4). For example, Plo1 is an upstream regulator of the septation initiation network (SIN) in S. pombe, and its overexpression can drive septum formation during interphase (45). In addition, overexpression of Cdc5p in S. cerevisiae results in septin deposition, while induction of a truncated form lacking the catalytic domain inhibits septation (64). Similarly, overexpression, depletion, or inactivation of PLK1 in mammals causes defects in cytokinesis (11, 52, 61). Intriguingly, TbPLK is required for cytokinesis but appears to have lost a role in mitosis (32). Although metazoan Plks show some overlap in mitotic function, PLK1 is a primary regulator of mitotic progression, while the proteins PLK2 to PLK4 have additional roles during G1/S or S phase (4, 7, 76). The importance of Plks in cell division is underscored by their modulation in different types of cancers, and Plk1 is a candidate target for anticancer strategies (17). More recent studies revealed additional roles for Plks during development. For example, PLK1 and Polo phosphorylate factors that influence asymmetric cell divisions and cell fate determinants in worms and flies (56, 71), Polo activates meiosis in the fly oocyte (39), and PLK2 is required for neuron differentiation in mice (19). Human PLK5 has no known cell cycle function but is specifically expressed in glial and neuronal cells and is also important for neuronal development (16). Fungal Plks, however, have not demonstrated cell cycle-independent roles in development to date. Although several targets and regulators of Plks have been described (4, 38, 51, 63), the great diversity in Plk function suggests that more may exist and await identification.
The filamentous fungus Aspergillus nidulans is one of the pioneering model organisms for eukaryotic cell cycle research (41). A. nidulans is an attractive system for studying several biological processes due to its sequenced and annotated genome (21), amenability to sophisticated molecular, genetic, and biochemical analyses (37, 46, 47, 68), and ability to form multiple cell types during reproduction (74). We previously identified PLKA in A. nidulans, which is the only Plk characterized in filamentous fungi to date and remains one of the largest members of the Plk family (5). Since initial investigations suggested that plkA was essential, we partially characterized its function through overexpression. Multicopy overexpression resulted in defects in nuclear division, abnormal spindle formation, and chromosome segregation and a delay at the G2/M transition (5). Hyphae could form but were uneven in shape and lacked septa. In this report, we directly address PLKA function through protein depletion and gene deletion, using more recent advances in molecular approaches for A. nidulans. The data support the notion that PLKA has some conservation in cell cycle function. However, the results also reveal several novel features, including the fact that plkA is not essential, not required for septation, and may negatively regulate sexual reproduction. Our findings thus provide the first example of a link between a fungal Plk and development, identify potential new Plk functions, and reveal important insights on the diversity of the Plk family.
MATERIALS AND METHODS
Strains, oligonucleotides, media, and growth conditions.
Strains and oligonucleotides used in the study are listed in Tables 1 and 2. Strains were maintained on YAG medium containing 0.5% yeast extract, 2% glucose, 10 mM MgSO4, 2.0% agar, 0.05 μg/ml pyridoxine, 2 μg/ml nicotinimide, 5.0 μM p-aminobenzoic acid, 0.02 μg/ml biotin, 2.5 μg/ml riboflavin, 10 mM uridine, 10 mM uracil, and 1 ml/liter trace elements (15, 53). Uridine and uracil or riboflavin were omitted in selecting for corresponding prototrophic transformants. Conditional strains carrying plkA under the control of the alcA (alcohol dehydrogenase) promoter [alcA(p)]were grown in minimal medium (MM) containing 0.5 M urea, 0.35 M KCl, 0.1 M MgSO4, 0.5 M monobasic potassium phosphate, 0.5 M dibasic potassium phosphate, 0.8 M sodium thiosulfate, trace elements, and vitamins as described previously (53), as well as 50 mM threonine and 50 mM fructose (minimal medium with threonine and fructose, or mmTF) to induce the alcA promoter. YAG supplemented with uridine and uracil was used as a repressing medium. Cells were grown at 32°C unless otherwise indicated. Standard methods for culture, transformation, and molecular analysis of A. nidulans were employed (28, 48, 53). For experiments that required synchronous germination, 1 × 107 conidia were inoculated into 4 ml of appropriate medium containing 0.8% agar, which was then poured onto standard plates. Growth in the presence of benomyl was determined using YAG or mmTF plates containing 0.2, 0.4, or 0.6 μg/ml of benomyl (Chem Service Inc.). A total of 20,000 conidia were spotted on each plate and incubated for 72 h at 32°C. Benomyl sensitivity assays were also performed by inoculating serial dilutions of conidia on YAG plates containing 0.6 μg/ml of benomyl and incubating the plates for 72 h at 32°C.
Table 1.
A. nidulans strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| TN02A25 | pyrG89 argB2 pabaB22 nkuA::argB riboB2 | 43 |
| KM17 | pyrG89 argB2 pabaB22 nkuA::argB riboB2 alcA(p)::plkA-riboBAf | This study |
| KM5 | pyrG89 argB2 pabaB22 nkuA::argB riboB2 riboBAf+ | This study |
| KM14 | pyrG89 argB2 pabaB22 nkuA::argB riboB2 plkA::pyr4+ | This study |
| KM25 | pyrG89 argB2 pabaB22 nkuA::argB riboB2 pyr4+ | This study |
Table 2.
Oligonucleotides used in this study
| Primer | Sequence |
|---|---|
| KM2F | TAG ATA AAT ACA GAA GCA TAT GTG GTG TAT |
| KM2R | TAA AGT CGC TCG TTA TGT CGT CGG TCG TCA |
| KM3F | ATG GAG AGA CAC CTC CAA CCA ACA ATG GAA |
| KM3R | AGA TCG CGT CTT TCC CAC CGT CAA CAT TCC |
| KM4F | TGA CGA CCG ACG ACA TAA CGA GCG ACT TTA AAG AGG CCG TTC AGG AGT CTG GCT |
| KM4R | TTC CAT TGT TGG TTG GAG GTG TCT CTC CAT TTT TGA GGC GAG GTG ATA GGA TTG |
| KM9F | AGC TCG TGA GAC CAA GTT CT |
| CB38Fa | GAC CTG TCG TAA AAG CC |
| CB38Ra | ATC TCG TCT TGG CCC AGT TC |
| CBPoloF7 | ACTACCGGGATCATCGATTG |
| CBAn80R | CCCGCTAATCGCAGTCGTTC |
| An3F | AGATTTGGCACCACACATTC |
| An3R | GTGACGTGGATACCACCGCT |
Strain construction.
To place plkA (AN1560) under the control of the alcA promoter (1, 72), a PCR fusion construct was utilized (73). Oligonucleotides KM2F and KM2R amplified a 2-kb fragment upstream of the start codon of plkA from cosmid 231 (Fungal Genetics Stock Center), while oligonucleotides KM3F and KM3R amplified a 2-kb fragment immediately downstream and including the start codon. Oligonucleotides KM4F and KM4R amplified the alcA promoter and riboB (Aspergillus fumigatus [riboBAf]) marker from the plasmid pHE13 (a kind gift from B. Oakley). The three products were then combined in a fusion PCR with oligonucleotides KM2F and KM3R. The resulting 7-kb product was gel purified, and 5 μg was transformed into strain TN02A25 (43). Transformants were streaked to single colony three times and screened by PCR with oligonucleotides KM9F and KM3R and by Southern blotting. The positive transformant KM17 was used for subsequent analyses. Negative controls included strain TN02A25 as well as transformant KM5, which did not integrate the construct at the plkA locus but was isogenic to strain KM17 with respect to the riboB marker. In order to delete plkA, a construct containing the pyr4 marker from Neurospora crassa and 2 kb of 5′ and 3′ flanking sequence of plkA was linearized from plasmid pCB150 (5) with XbaI and StuI. After gel purification, 5 μg was transformed into strain TN02A25. Transformants were screened by PCR using oligonucleotides CB38Fa and CB38Ra and by Southern blotting. Strain KM14 was used for subsequent analyses. Strain KM25, which retained plkA but was isogenic to strain KM14 with respect to the pyr4 marker, was used as a negative control in addition to strain TN02A25. All PCRs were performed with Expand Long Template High Fidelity Polymerase (Roche Diagnostics), and Southern blotting utilized a DIG Hybridization System (Roche Diagnostics).
Northern blotting.
RNA was extracted using TRI reagent (Molecular Research Center, Inc.), according to the manufacturer's instructions with minor modifications. Briefly, strains were grown in YAG medium or mmTF for various times, collected, frozen in liquid nitrogen, and ground to powder. Frozen material was added to a volume of 100 μl in Eppendorf tubes, and 1 ml of TRI reagent was added. The samples were vortexed 10 times for 10 s each and incubated at room temperature for 5 min. After the addition of 200 μl of chloroform, the manufacturer's instructions were followed for subsequent isolation and precipitation of total RNA, of which 5 μg was subsequently run on 1.0% agarose gels. RNA was transferred to Zetaprobe membrane (Bio-Rad) and probed with a 1.3-kb DNA fragment amplified from cosmid 231 with oligonucleotides CBPoloF7 and CBAn80R. Fragment labeling with [32P]dCTP and membrane hybridization were performed as previously described (6). In order to compare loading between samples, membranes were stripped and probed with a 32P-labeled fragment homologous to a 580-bp region of the actA open reading frame (ORF). The actA fragment was amplified from genomic DNA (gDNA) with oligonucleotides An3F and An3R. Northern blots were analyzed with a phosphorimager (Typhoon Variable Mode Imager; GE Healthcare). Relative intensities of bands on Northern blots were quantified using ImageJ (http://rsb.info.nih.gov/ij/index.html), according to the method described at http:://lukemiller.org/index.php/2010/11/analyze-gels-and-westernblots-with-image-j/. Briefly, band density for plkA in each lane of a single blot was divided by that of the first lane in order to determine relative densities. The same approach was used for actA. The relative densities of plkA were then divided by the relative densities of actA for the corresponding lane to obtain adjusted relative densities.
Microscopy.
For growth and phenotypic assays of individual cells, 1 × 106 fresh conidia were inoculated into 500 μl of medium on coverslips placed in petri plates and incubated at 32°C for the times indicated in the figure legends. Cells that adhered to coverslips were fixed (6% paraformaldehyde, 50 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], pH 7.0, 25 mM EGTA, pH 7.0, 5.0 mM MgSO4, 5% dimethyl sulfoxide, 10 μg/ml of leupeptin, 3 μg/ml of aprotinin, and 200 μM AEBSF [4-(2-aminoethyl) benzenesulfonyl fluoride]) for 30 min. For visualization of nuclei, coverslips were then washed twice with PE buffer (50 mM PIPES, pH 7.0, 25 mM EGTA, pH 7.0) and incubated in 40 ng/ml of DAPI (4′,6′-diamidino-2-phenylindole; Sigma) for 20 min. To visualize cell walls and septa, fixed cells on coverslips were incubated in 10 μg/ml of calcofluor white (Sigma) for 10 min and rinsed with distilled H2O (dH2O). Coverslips were mounted in SlowFade Gold Antifade Reagent (Invitrogen). To visualize microtubules, immunolocalization of α-tubulin was performed (44). Conidia were inoculated onto coverslips, incubated for the times indicated in the figure legends, fixed for 30 min, rinsed twice with PE buffer at 4°C, and then incubated in digestive solution (50 mM sodium citrate buffer, pH 6.0, 1 mM MgSO4, 2.5 mM EGTA, pH 7.0, 2% bovine serum albumin [BSA], 10 mg/ml Driselase, 1 mg/ml lyticase, 16 mg/ml beta-d-glucanse, 10 μg/ml leupeptin, 3 μg/ml aprotinin, and 200 μM AEBSF) for 30 min. Coverslips were then rinsed with cold PE buffer and incubated in permeabilizer solution (0.1% Nonidet P-40 in PE buffer, 10 μg/ml of leupeptin, 3 μg/ml of aprotinin, and 200 μM AEBSF) for 5 min. After cells were rinsed with PE buffer, they were incubated overnight at room temperature in a 1:200 dilution of monoclonal anti-α tubulin antibody (DM1A; Sigma) in PE buffer containing 2% BSA. Coverslips were then rinsed twice with PE buffer and incubated for 1 h in a 1:200 dilution of anti-mouse IgG F(ab′)2 fragment-fluorescein isothiocyanate ([FITC] Sigma) in PE buffer containing 2% BSA. Coverslips were rinsed twice with PE buffer, once with dH2O, and then incubated with 40 ng/ml of DAPI for 20 min. After being rinsed with water, coverslips were mounted in SlowFade Gold Antifade Reagent. For visualization of conidiophores, the method of Lin and Momany (36) was utilized. Cells were examined on a Leica DM6000B microscope (Leica Microsystems) equipped with a Hamamatsu-ORCA ER camera (Hamamatsu Photonics) using a 63× or 100× objective and DAPI (460 nm) or FITC (520 nm) filter. Images were captured with Openlab software (Improvision, Inc./Perkin Elmer). All growth and phenotypic assays were repeated at least three times and produced similar results.
Protein alignment and phylogenetic analysis.
For protein alignments, the amino acid sequence of PLKA (AN1560) was obtained from the Aspergillus Genome Database (AspGD [http://www.aspgd.org]). A BLASTP search at the Broad Institute (http://www.broadinstitute.org/science/data) using the PBD of PLKA identified single orthologues in select filamentous fungi, including HCEG_00596 from Histoplasma capsulatum, FVEG_01402 from Fusarium verticillioides, NCU09258.4 from Neurospora crassa, MGG_09960 from Magnaporthe oryzae, UMO3234 from Ustilago maydis, fge1_pm_C 6000207 from Aspergillus niger, Afu8g05680 from Aspergillus fumigatus, A0090005000574 from Aspergillus oryzae, and AFL2G_00570 from Aspergillus flavus. Since no hits were obtained with Cryptococcus neoformans, a BLASTP search was performed using the PBD from Cdc5p (YMR001C) from S. cerevisiae (Saccharomyces Genome Database [SGD]; http://www.yeastgenome.org/), which identified CNBG_3036. Additional sequences were obtained from NCBI (http://www.ncbi.nim.nih.gov.protein), the SGD (http://www.yeastgenome.org/), or the Candida Genome Database (CGD [http://www.candidagenome.org/]). Protein names and reference sequence numbers include PLK1 (NP_005021.2), PLK2 (NP_006613.2), PLK3 (NP_004064.2), and PLK4 (NP_055079) from Homo sapiens; Polo (NP_001014592) from D. melanogaster; Plo1 (NP_593647) from S. pombe; Cdc5p (orf19.6010) from C. albicans; and PLK4 (NP_649324) from D. melanogaster. Mek1p (YOR351C) from S. cerevisiae was used as an outgroup. Sequences were aligned and shaded using CLUSTALW and BOXSHADE at the Biology Workbench, version 3.2, website (http://www.workbench.sdsc.edu/). For phylogenetic analysis, full-length sequences were aligned using CLUSTALW. Phylip, version 3.69 (20) (http://www.evolution.genetics.wasgington.edu/phylip/getme.html), was then used to construct a rooted tree. Briefly, SEQBOOT was used to create 100 replicates and calculate bootstrap values. PROTPARS was then used to create maximum-parsimony trees, and CONSENSE was utilized to construct the final consensus trees that included bootstrap values.
RESULTS
plkA and orthologues from several filamentous fungi comprise a divergent group of Plks.
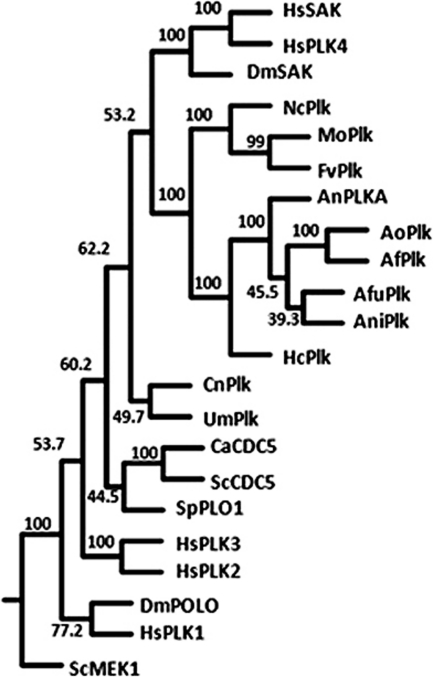
We previously demonstrated that the N-terminal catalytic domain of A. nidulans PLKA was 42% identical to that of PLK1 and that the C terminus contained a region homologous to the PBD (5). However, alignment of PLKA with PBD sequences from more recent reports (51) revealed some distinct features (see Fig. S1 in the supplemental material). While the first 24 residues of the PLKA PBD are 62% identical to the sequence of PLK1, three unique regions of 20 to 50 amino acids each interrupt the remainder of the domain, decreasing the total PBD identity to 21% (see Fig. S1). Despite this divergence, the PLKA PBD contains residues that align with Trp414, Leu490, His538, and Lys540 of PLK1 that are important for phospho-substrate recognition and binding (see Fig. S1) (51). A BLASTP search of select filamentous fungal genomes at the Broad Institute (http://www.broadinstitute.org/science/data#) using the PBD of PLKA recovered single orthologues, each of which contained a conserved N-terminal catalytic domain with Plk-specific features, a large central region, and a PBD with insertions, similar to that of PLKA (see Fig. S2 in the supplemental material). The divergence in PBD sequence appeared to be specific to filamentous fungi; the PBDs from select dimorphic fungi, with the exception of Histoplasma capsulatum, were more similar to those of yeasts and metazoan Plks. The filamentous fungal Plks showed some conservation in the large sequence linking the catalytic domain and PBD and also contained an elongated C terminus compared to metazoan PLK1 (see Fig. S2). Although unrelated in sequence, large central regions of unknown function are also found in Cdc5p, TbPLK, and PLK4. Phylogenetic analysis using full-length sequences suggests that PLKA and filamentous fungal orthologues comprise a distinct group within the Plk family and lie closest to metazoan PLK4 (Fig. 1). In contrast, yeast Plks including Cdc5p and Plo1 from S. cerevisiae and S. pombe, respectively, group closer to the metazoan proteins PLK1 to PLK 3. Thus, our results show that PLKA and orthologues from several filamentous fungi have some distinct features and comprise a divergent group within the Plk family.
Fig 1.
Phylogenetic analysis of select Plk orthologues in filamentous fungi. A consensus tree including bootstrap values (Phylip, version 3.69) (20) based on full-length sequences of select Plks and uncharacterized orthologues in filamentous fungi. Characterized genes include Homo sapiens Sak (HsSak), PLK1 (HsPlk1), PLK2 (HsPlk2), PLK3 (HsPlk3), and PLK4 (HsPlk4); Drosophila melanogaster Sak (DmSak) and Polo (DmPolo); Candida albicans CDC5 (CaCdc5); Saccharomyces cerevisiae CDC5 (ScCdc5); Schizosaccharomyces pombe plo1 (SpPlo1); and Aspergillus nidulans plkA (AnPLKA). Uncharacterized orthologues are indicated by species initials followed by “Plk” for simplicity, including NcPlk (Neurospora crassa), MoPlk (Magnaporthe oryzae), FvPlk (Fusarium verticillioides), AoPlk (Aspergillus oryzae), AfPlk (Aspergillus flavus), AfuPlk (Aspergillus fumigatus), AniPlk (Aspergillus niger), HsPlk (Histoplasma capsulatum), CnPlk (Cryptococcus neoformans), and UmPlk (Ustilago maydis). Saccharomyces cerevisiae Mek1 (ScMek1) represents an outgroup. Numbers at branch points represent bootstrap values from 100 replicates.
plkA is not essential but influences colony growth and hyphal morphogenesis.
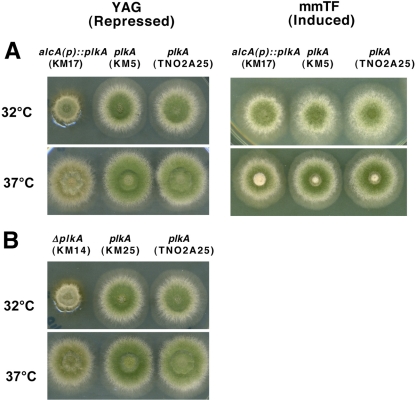
We previously characterized PLKA function through overexpression since attempts to delete or place plkA under the control of the conditional alcA promoter using one-step approaches were not successful, and benomyl-induced haploidization of a plkA heterozygous strain did not uncover ΔplkA spores (5). Based on these results, we concluded that plkA was essential. To further define its roles, we attempted to place plkA under the control of the alcA promoter using different approaches (68). Previously, we transformed a circular plasmid containing the pyr4 marker and a 3′ truncated copy of plkA following the alcA promoter into strain GR5 and plated transformants on inducing medium containing ethanol and fructose (5). However, none of the transformants tested showed correct integration of the plasmid. In this report, a linear promoter-replacement construct was utilized, which consisted of the riboBAf marker and 2-kb sequences homologous to the regions flanking the plkA start codon (see Fig. S3A in the supplemental material). The PCR fusion construct (73) was transformed into the ΔnkuA strain TN02A25, which lacks the orthologue of KU70 and thus reduces nonhomologous end joining of DNA (43). Multiple transformants were obtained on inducing medium containing threonine and fructose and screened using PCR and Southern blotting. Strain KM17 contained a single copy of plkA under the control of the alcA promoter (see Fig. S3A) and was used for subsequent analyses. As negative controls, strain TN02A25 and transformant KM5 were utilized. Strain KM5 retained plkA under the control of its endogenous promoter but was isogenic for the riboB marker. Southern blotting revealed additional bands in KM5, but these likely reflect integration of the construct, which has homology with the probe, at heterologous sites. When conidia were inoculated onto solid inducing medium (mmTF) and incubated at 32°C for 72 h, the strains grew in a similar and normal manner (Fig. 2A). On repressing medium (YAG), however, alcA(p)::plkA colonies were compact, unlike control strains (Fig. 2A). The growth defect was suppressed at a higher temperature of 37°C, suggesting that cells depleted of PLKA are cold sensitive.
Fig 2.
Absence of PLKA results in a temperature-sensitive, compact growth phenotype. (A) Strains KM17 [alcA(p)::plkA riboB+], KM5 (plkA riboB+), and parental strain TN02A25 (plkA) were spot inoculated onto mmTF or YAG medium and incubated for 72 h at 32° or 37°C. (B) Strains KM14 (ΔplkA pyr4+), KM25 (plkA pyr4+), and parental strain TN02A25 (plkA) were spot inoculated onto YAG medium and incubated for 72 h at 32 or 37°C.
The fact that cells depleted of PLKA could still grow, albeit abnormally, suggested either leakiness of the alcA promoter or that plkA was in fact not essential. To clarify this issue, we first investigated transcript levels using Northern blotting. When incubated in repressing medium, control strains KM5 and TN02A25 demonstrated a band of approximately the same intensity and size expected for plkA. However, this band was absent in alcA(p)::plkA cells of strain KM17 (see Fig. S3B in the supplemental material). Alternatively, a much larger band was present but reduced in intensity compared to the smaller band in control strains. In inducing medium, the control strains showed a band similar to that in repressing medium. The alcA(p)::plkA cells contained a slightly smaller band, which is expected, given the site of integration of the alcA promoter. The larger band was also present but reduced in intensity relative to the small band, especially with longer incubation time (see Fig. S3B). Control strain KM5 contained an even larger additional band, but it was most evident during longer incubation. This band may reflect heterologous integration of the transforming construct, as suggested by the Southern blotting, but does not appear to be functionally relevant since strain KM5 was phenotypically indistinguishable from strain TN02A25. Thus, the results show that plkA expression under the control of the alcA promoter is downregulated or upregulated in repressing or inducing medium, respectively. However, the nature and significance of the large band in alcA(p)::plkA cells of strain KM17 are not clear since Southern blotting did not reveal additional, heterologous integration of the transforming construct.
In order to further investigate the essentiality of plkA and determine whether the large band in Northern blots of strain KM17 is functionally important, we next attempted to delete plkA in strain TN02A25. Transformation of a deletion construct containing 2-kb sequences homologous to the flanking regions of plkA and the pyr4 marker resulted in several transformants. PCR and Southern blot screening confirmed deletion of plkA in selected strains that grew in a compact manner (see Fig. S3C in the supplemental material) and retention of the gene in select strains that grew normally. Strain KM14 (ΔplkA) was used for subsequent analyses, while strains TN02A25 and KM25, a transformant that retained plkA but was isogenic for the pyr4 marker, were used as negative controls. KM25 was initially mixed, as shown by PCR (see Fig. S3C), but Southern blotting confirmed that it did not contain a wild-type copy of plkA upon subsequent streaking to single colony. Since the Southern probe was homologous to a region of the transforming DNA, the second band on the Southern blot of strain KM25 may represent integration at a heterologous locus. In order to confirm the plkA null phenotype, conidia were incubated on YAG medium for 72 h at 32°C. The ΔplkA colonies grew in a temperature-sensitive, compact manner (Fig. 2B). In contrast, control strains grew normally. Since the ΔplkA phenotype was indistinguishable from that of alcA(p)::plkA cells grown under repressing conditions and since the defects were complemented when the latter were grown in inducing medium (Fig. 2A), the results indicate that the phenotype is due to the absence of PLKA and confirm that plkA is not essential. The results also indicate that the large band observed in Northern blots of alcA(p)::plkA cells is not functionally relevant; it is possible that it represents anti-sense-strand expression. This work represents the first example of a Plk that is not essential for growth in an organism containing a single homologue.
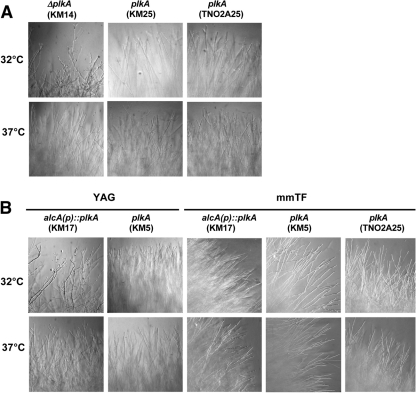
In order to identify additional growth defects, individual hyphal lengths and colony margins were examined. After 7 h in liquid YAG medium, ΔplkA cells were moderately longer than controls (Table 3). However, alcA(p)::plkA cells were more similar in length to control strains in repressing and inducing medium (Table 3). Thus, the absence of PLKA does not have a strong effect on hyphal growth rate. However, ΔplkA colony margins contained hyperbranched hyphae, often with split tips (40), in contrast to control strains (Fig. 3A). The alcA(p)::plkA colonies showed a similar phenotype on repressing but not inducing medium (Fig. 3B), indicating that the effects were due to the absence of PLKA. Incubation at 37°C partially suppressed these hyphal branching defects (Fig. 3). Collectively, the results indicate that PLKA is not essential for hyphal growth but may be important for aspects of hyphal morphogenesis and polar axis formation.
Table 3.
Effects of altering PLKA on hyphal length and number of nucleia
| Strain (genotype) | Medium | Hyphal length (μm ± SEM) | No. of nuclei (μm ± SEM) |
|---|---|---|---|
| KM14 (ΔplkA) | YAG | 32.0 ± 1.9 | 6.0 ± 0.3 |
| KM25 (plkA) | YAG | 26.3 ± 1.7 | 5.3 ± 0.2 |
| TN02A25 (plkA) | YAG | 26.8 ± 1.7 | 5.9 ± 0.3 |
| KM17 [alcA(p)::plkA] | YAG | 30.1 ± 2.7 | 5.7 ± 0.3 |
| KM5 (plkA) | YAG | 29.0 ± 2.0 | 5.4 ± 0.3 |
| KM17 [alcA(p)::plkA] | mmTF | 39.1 ± 1.9 | 3.3 ± 0.2 |
| KM5 (plkA) | mmTF | 34.5 ± 1.7 | 3.0 ± 0.1 |
| TN02A25 (plkA) | mmTF | 38.2 ± 2.1 | 3.7 ± 0.2 |
Cells were incubated in YAG medium at 32°C for 7 h or in mmTF for 12 h, fixed, and then stained with DAPI. Approximately 50 cells were scored for each strain.
Fig 3.
Absence of PLKA results in hyperbranching and split tips. (A) Strains KM14 (ΔplkA pyr4+), KM25 (plkA pyr4+), and TN02A25 (plkA) were spot inoculated onto YAG medium and incubated for 72 h at 32°C. (B) Strains KM17 [alcA(p)::plkA riboB+], KM5 (plkA riboB+), and TN02A25 (plkA) were spot inoculated onto YAG or mmTF plates and incubated for 72 h at 32°C.
PLKA influences nuclear distribution and several aspects of mitotic progression.
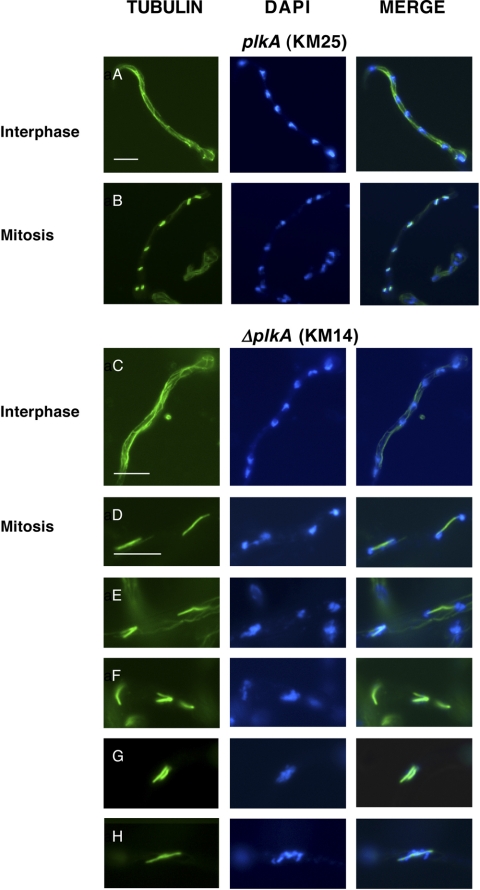
Plks regulate multiple stages of mitosis, including mitotic entry, spindle organization, chromosome segregation, and mitotic exit, for example (4). Consistent with this, overexpression of PLKA impaired nuclear division, spindle formation, and chromosome segregation (5). To determine whether the absence of PLKA influenced these processes, conidia were incubated in YAG medium for 7 h, fixed, processed for immunolocalization of α-tubulin, and/or stained with DAPI. The numbers of nuclei were similar in ΔplkA (KM14) and control cells (KM25 and TN0A25) (Table 3), despite the fact that the former were moderately longer. However, under repressing conditions, alcA(p)::plkA (KM17) and control (KM5 and TN02A25) cells also demonstrated similar numbers of nuclei (Table 3) and did not dramatically differ in length, indicating that absence of PLKA does not strongly influence nuclear division. The mitotic defects resulting from overexpression of PLKA may reflect a strong dominant negative effect of multicopy overexpression (5). However, we noted some effect on mitotic progression because the spindle mitotic index was 10.7% in ΔplkA cells, or double that observed in control strains (Table 4). While interphase cells contained normal cytoplasmic arrays (Fig. 4A and C), 16.4% of the mitotic ΔplkA cells showed abnormal spindles, including bent, monopolar, or frayed arrangements (Fig. 4E and F), compared to 0 to 2.0% in control cells (Table 4). Consistently, similar spindle defects were reported in cells overexpressing PLKA (5), although at a higher frequency. Moreover, bent and discontinuous spindles were reported in 25.0% of S. cerevisiae cells carrying a temperature-sensitive CDC5 mutation (50), and monopolar spindles result from loss of Plks in several other systems (33, 67). A higher proportion of mitotic ΔplkA cells were also in telophase (Table 4), suggesting that PLKA may be important for mitotic exit, and showed additional defects in chromosome organization and separation (Table 4), including uncondensed or fragmented chromatin that was unevenly distributed on and/or dissociated from long spindles (Fig. 4D, F, and H). An increase in the spindle mitotic index and in the proportion of cells containing spindle abnormalities was also observed in alcA(p)::plkA cells in repressing versus inducing medium (Table 4), but only moderate increases in telophase spindles or chromosome defects were present. Intriguingly, DAPI staining demonstrated that 15.1% (n = 258) of ΔplkA cells showed abnormal clustering of three or more nuclei in the germ tube, compared to 3.5% (n = 200) and 3.0% (n = 200) in control strains KM25 and TN02A25, respectively (Fig. 5). This effect was due to the absence of PLKA because 9.4% (n = 180) of alcA(p)::plkA cells showed abnormal clustering of nuclei within the hypha under repressing conditions compared to 4.5% (n = 200) in control strain KM5, and only 1.0% (n = 200), 1.5% (n = 200), or 1.0% (n = 200) of cells from strains KM17, KM5, and TN02A25, respectively, showed these defects in inducing medium. Thus, PLKA influences nuclear distribution and several aspects of mitotic progression but is not essential for these processes.
Table 4.
Effects of altering PLKA on spindle mitotic index and spindle and chromosome organization
| Strain (genotype) | Mediuma | SMI (%)b | Spindle pattern (%)c |
Abnormal chromosome pattern (%)d | |
|---|---|---|---|---|---|
| Telophase | Abnormal | ||||
| TN02A25 (plkA) | YAG | 4.2 | 17.6 | 2.0 | 2.0 |
| KM25 (plkA) | YAG | 5.2 | 17.0 | 0 | 3.4 |
| KM14 (ΔplkA) | YAG | 10.7 | 27.0 | 16.4 | 12.7 |
| KM17 [alcA(p)::plkA] | YAG | 9.0 | 19.5 | 17.0 | 9.8 |
| KM5 (plkA) | YAG | 4.5 | 15.5 | 5.1 | 6.9 |
| TN02A25 (plkA) | mmTF | 5.2 | 17.3 | 5.8 | 3.8 |
| KM5 (plkA) | mmTF | 5.6 | 13.8 | 5.2 | 0 |
| KM17 [alcA(p)::plkA] | mmTF | 3.6 | 12.5 | 3.1 | 3.1 |
Strains were incubated in YAG for 7 h or in mmTF for 7 h, processed for immunolocalization of α-tubulin, and stained with DAPI.
SMI, spindle mitotic index. Data represent the proportion of total cells that contained a spindle. Approximately 300 to 500 cells were scored for each strain.
Proportion of spindles in telophase or abnormal in structure. Approximately 50 spindles were scored for each strain.
Proportion of mitotic cells that demonstrated abnormal chromosome segregation.
Fig 4.
Absence of PLKA results in abnormal spindle assembly, chromosome organization, and chromosome segregation in a proportion of cells. Strains KM14 (ΔplkA pyr4+) and KM25 (plkA pyr4+) were incubated in YAG medium for 8 h at 32°C. The cells were then fixed, processed for immunolocalization of α-tubulin, and stained with DAPI. (A and C) Normal cytoplasmic microtubules in interphase cells of strains KM25 and KM14, respectively. (B) Normal metaphase mitotic spindles with associated condensed chromosomes in strain KM25. (D to H) Spindle and chromosome organization defects in mitotic cells of strain KM14. Scale bar, 10 μm.
Fig 5.
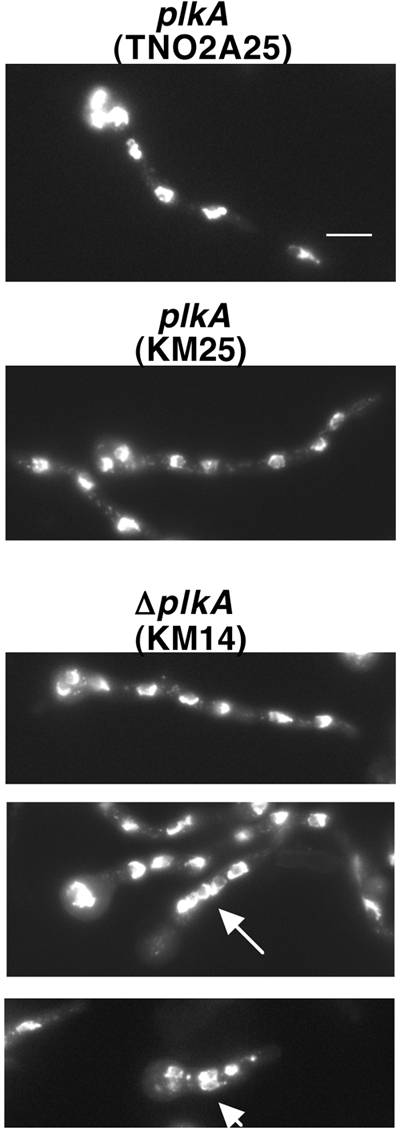
Absence of PLKA results in pleiotropic effects on nuclear distribution. Strains KM14 (ΔplkA pyr4+), KM25 (plkA pyr4+), and TN02A25 (plkA) were incubated in YAG medium for 7 h at 32°C, fixed, then stained with DAPI. Arrows indicate clustered nuclei. Scale bar, 10 μm.
PLKA is not required for septation.
Plks are critical for septation in fungi and cytokinesis in higher organisms (4). In A. nidulans, the first septum is deposited at the germ tube base, after approximately three rounds of mitosis, and along the length of growing hyphae (25). The fact that septa did not form when PLKA was overexpressed suggested that either PLKA was an important regulator of septation or that primary defects in nuclear division inhibited the process (5). To distinguish between the possibilities, strains were incubated in YAG medium for 9 h, fixed, and stained with calcofluor. A septum was located close to the germ tube base in 42.5% (n = 188) or 47.0% (n = 137) of control strain KM25 or TN02A25, respectively, and in 52.3% (n = 151) of ΔplkA cells (KM14) (Fig. 6). Similar proportions of cells containing septa were also observed in alcA(p)::plkA (KM17) and its control strain (KM5) (52.3%, n = 167, versus 51.5%, n = 163, respectively) in repressing medium, confirming that the absence of PLKA does not prevent septum formation. The mean interseptal distances of subapical compartments of hyphae incubated for 12 h were also similar in the absence of PLKA (30.2 ± 1.7 [n = 50], 33.1 ± 2.1 [n = 47], and 30.0 ± 2.0 [n = 46] for strains KM25, TN02A25 and KM14, respectively). Although we cannot conclude whether septa in ΔplkA cells are completely normal, the similarity in their appearance and position and the fact that conidia can form suggest that a large part of the septation process can occur independently of PLKA, in contrast to the situation in yeast (45).
Fig 6.
Cells lacking PLKA can form septa. Strains KM14 (ΔplkA pyr4+), KM25 (plkA pyr4+), and TN02A25 (plkA) were incubated in YAG medium at 32°C for 9 h (top row) or 12 h (bottom row). Cells were fixed and then stained with calcofluor (top row) or calcofluor and DAPI (bottom row). Scale bar, 10 μm.
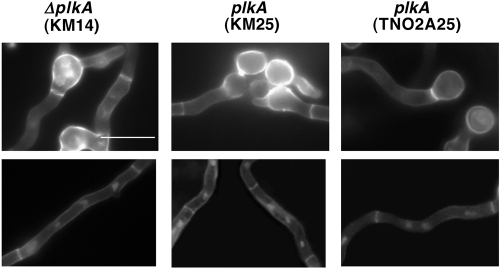
Absence of PLKA results in reduced conidiation and precocious formation of sexual Hülle cells.
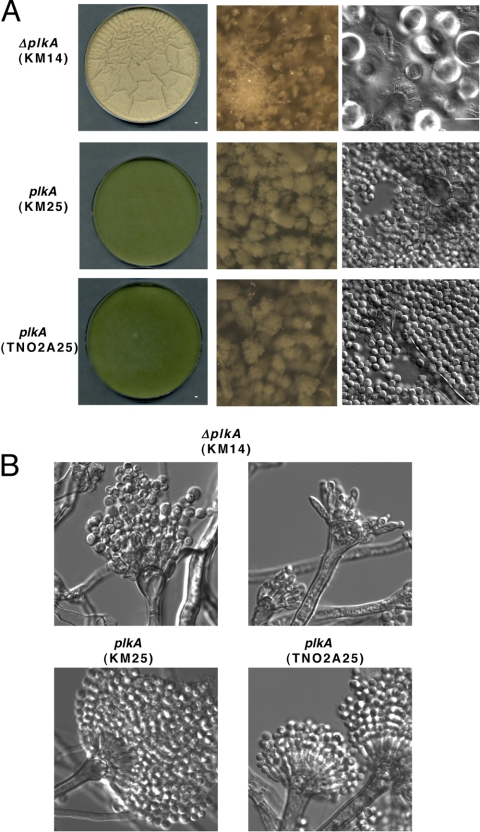
Asexual development in A. nidulans initiates approximately 20 h after vegetative growth and is characterized by production of conidiophores that give rise to chains of pigment-containing conidia (1, 69). Sexual development occurs when carbon sources are depleted and is characterized by the formation of structures including Hülle cells that surround developing fruiting bodies called cleistothecia (14). Since ΔplkA colonies showed reduced pigmentation (Fig. 2) and did not yield high concentrations of isolated conidia, PLKA may influence development. In order to explore this possibility further, top agar cultures of conidia in YAG medium were prepared, which permits more synchronous germination and growth. After 72 h at 32°C, control strains (KM25 and TN02A25) showed abundant conidiophores and pigmented conidia (Fig. 7A). In contrast, pigmentation and conidiation were reduced in ΔplkA (KM14) colonies, but Hülle cells and aerial hyphae were abundant. Structures resembling young cleistothecia were also present (Fig. 7A). Thus, PLKA may play a role in repressing sexual development. When conidiophore structures were analyzed more closely (36), some abnormal metulae and phialides were observed in the ΔplkA strain, whereas others appeared normal, albeit with less dense conidia (Fig. 7B). Top agar-inoculated alcA(p)::plkA (KM17) cultures grown in repressing medium similarly showed reduced pigmentation and abundant aerial hyphae and Hülle cells (see Fig. S4 in the supplemental material). In contrast, abundant conidia were present in control strains (KM5 and TN02A25) under repressing conditions and in all strains in inducing medium (see Fig. S4). A moderate reduction in pigmentation was noted on inducing medium when top agar was used (see Fig. S4) in comparison to the point inoculation method (Fig. 2A), but this affected all strains equally. Thus, the results suggest that PLKA may positively influence asexual development and/or negatively regulate sexual reproduction and provide the first example of a regulatory link between Plks and development in fungi.
Fig 7.
Absence of PLKA impairs asexual development and derepresses aspects of sexual development. (A) Strains KM14 (ΔplkA pyr4+), KM25 (plkA pyr4+), and TN02A25 (plkA) were inoculated into YAG top agar that was poured over standard YAG plates and incubated for 72 h at 32°C. First column, low magnification of plates; second column, high magnification of plate surface; third column, surface cells collected from the plates. (B) Conidiophores collected (36) from strains KM14, KM25, and TN02A25.
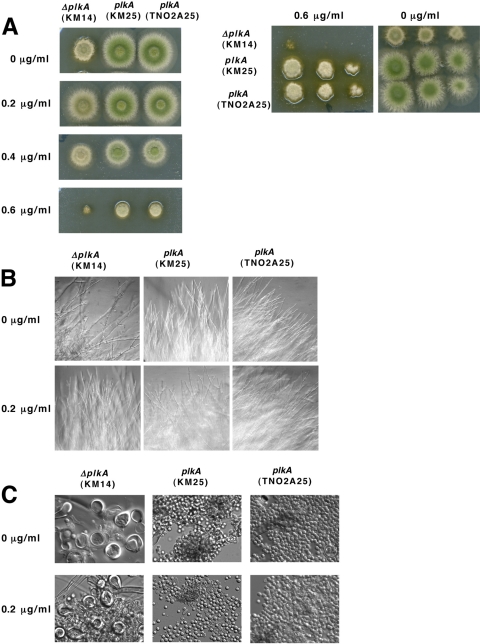
Low concentrations of benomyl suppress some defects in growth, but not development, in cells lacking PLKA.
Plks play important roles in microtubule function and organization in many systems (4). Since the absence of PLKA resulted in cold-sensitive growth, as well as abnormalities in hyphal branching, nuclear distribution, and spindle formation, PLKA may be important for microtubule function and/or dynamics. In order to test this possibility, sensitivity to benomyl was investigated. After 72 h at 32°C in the presence of 0.2 to 0.4 μg/ml of benomyl, ΔplkA (KM14) colonies no longer appeared compact, and diameters approached those of the control strains (KM25 and TN0A25) (Fig. 8A). In addition, hyphal branching defects were less prevalent (Fig. 8B). However, at 0.6 μg/ml of benomyl, ΔplkA colonies were significantly smaller than those of the controls (Fig. 8A). The alcA(p)::plkA colonies (KM17) grown on repressing medium responded to benomyl in a similar manner (see Fig. S5 in the supplemental material). Therefore, low concentrations of benomyl suppress the compact growth and branching defects of colonies lacking PLKA, but the cells are more sensitive to higher concentrations of the drug. A related response to benomyl was reported for a CDC5 mutant in S. cerevisiae (50) and in specific γ-tubulin mutants of A. nidulans (27). These results suggest that PLKA may be important for microtubule dynamics in a complex fashion, and this, in turn, could influence growth patterns. However, the presence of 0.2 μg/ml benomyl did not suppress other phenotypes resulting from absence of PLKA. The mitotic index (12.6%; n = 332) and proportion of abnormal spindles (20.6%; n = 69) in ΔplkA cells (KM14) were similar to values obtained in the absence of benomyl (Table 4). Benomyl also did not affect the mitotic index (5.5%, n = 326; 5.4%, n = 497) or proportion of cells containing abnormal spindles (3.8%, n = 52; 1.8%, n = 56) in control strains KM25 and TN02A25, respectively (Table 4). While investigating nuclear distribution, we noted that all strains demonstrated an increase in the number of cells containing enlarged conidia with 3 or more nuclei in the presence of 0.2 μg/ml benomyl (data not shown). However, when nuclear distribution was scored within the hyphal tube, the frequency of ΔplkA cells containing defects in the presence of the drug (15.1%, n = 258) did not differ from that in its absence (14.4%, n = 221). When the effect of 0.2 μg/ml benomyl on development was explored, ΔplkA colonies demonstrated reduced pigmentation and abundant Hülle cells (Fig. 8A and C), similar to results obtained in the absence of the drug (Fig. 7). These developmental phenotypes also remained in alcA(p)::plkA colonies grown on repressing medium in the presence of benomyl (see Fig. S5). Together with the fact that the developmental defects were not suppressed by higher temperature (Fig. 2), the results suggest that PLKA influences reproduction and other processes in a microtubule-independent manner and thus may utilize different mechanisms during growth and development.
Fig 8.
Low concentrations of benomyl suppress the compact growth and branching defects of cells lacking PLKA but do not prevent Hülle cell formation or a reduction in pigmentation. (A) A total of 2 × 104 conidia of strains KM14 (ΔplkA pyr4+), KM25 (plkA pyr4+), and TN02A25 (plkA) were inoculated onto YAG medium containing different concentrations of benomyl and incubated for 72 h at 32°C. Serial dilutions of strains plated on YAG medium with or without 0.6 μg/ml benomyl are shown on the right. (B) Colony edges of strains grown on YAG medium for 72 h at 32°C in the presence or absence of 0.2 μg/ml benomyl. (C) Surface cells collected from plates shown in panel B.
DISCUSSION
plkA is not essential in A. nidulans.
Plks are critical regulators of cell cycle progression in diverse organisms. PLKA of A. nidulans is the only Plk characterized in filamentous fungi to date, and its functions were previously inferred from overexpression analyses (5). Here, we report that the plkA null phenotype consists of conserved and also novel features, including the fact that PLKA is not required for cell viability. This represents the first example of a nonessential Plk in any organism containing a single homologue. In agreement with our results, plkA (AN1560) was recently identified as being nonessential in a large-scale deletion screen of A. nidulans kinases (S. Osmani and C. De Souza, personal communication). In a previous study, we reached an alternate conclusion based on our inability to delete plkA or place it under the control of a conditional promoter (5). However, this was likely due to a combination of technical issues and the recent incorporation of different strategies. For example, our current work utilized a ΔnkuA strain in order to enhance the yield of homologous integration (43). In addition, linear constructs as opposed to disrupting plasmids (5) were used to place plkA under the control of the alcA promoter, and potential alcA(p)::plkA transformants were plated on threonine and fructose (43, 57), in contrast to ethanol and fructose (5). Furthermore, our recent finding that ΔplkA cells are more sensitive to high concentrations of benomyl may explain why we were previously unable to recover ΔplkA conidia upon benomyl-induced haploidization of a ΔplkA/plkA strain (5). Our inability to identify ΔplkA strains during a previous attempt with the heterokaryon rescue technique may be due in part to some limitations with the method (47) and insufficient screening of weak-growing, primary transformants as transformation itself often results in some poor-growing colonies. Regardless, the phenotype of ΔplkA cells strongly resembled that of alcA(p)::plkA cells grown under repressing conditions, and the defects were suppressed in the latter in inducing medium. Thus, the null phenotype is clearly due to the absence of PLKA. Since the null phenotype could be generated or suppressed in the same strain, it should not be influenced by the ΔnkuA background (47, 68). Consistent with this, we recently succeeded in deleting plkA in a nkuA+ strain, which produced a similar null phenotype (data not shown). We previously reported that overexpression of PLKA resulted in strong growth defects, but this was due to multicopy integration of an alcA(p)::plkA plasmid; transformants with a single copy grew normally (5), similar to our current alcA(p)::plkA strain on inducing medium. Furthermore, dominant negative effects arising from multicopy gene overexpression, for example, can produce stronger phenotypes than deletion of a gene. The fact that plkA was not essential suggested that A. nidulans may contain another homologue. However, blasting the genome (http://www.aspergillusgenome.org) with the PLK1 PBD or PLK4 cryptic PBD did not reveal additional factors. Thus, proteins unrelated in sequence may play redundant cell cycle roles with PLKA. It is not clear why PLKA and many filamentous fungal orthologues have features distinct from yeast Plks, but the data suggest the occurrence of multiple events during Plk evolution. It will be informative to discover whether other filamentous fungal Plks are essential and to determine the functional significance of sequence variations, particularly within the PBD.
PLKA influences aspects of mitotic progression and microtubule dynamics.
Our results suggest that PLKA has some conservation in function, in that it influences processes that are regulated by Plks in other systems. First, the higher spindle mitotic index in cells lacking PLKA suggests some influence on mitotic progression, despite the fact that nuclei could still divide. Specifically, more cells contained defects in spindle and chromosome organization, suggesting that PLKA may be important for spindle formation and chromosome dynamics, like other Plks (4). The higher proportion of ΔplkA cells with telophase spindles implies an additional role in mitotic exit (4). This specific effect was not as strong in alcA(p)::plkA cells under repressing conditions and may be due to undetectable leakiness from the alcA promoter. Overexpression of PLKA generated similar defects in spindle and chromosome organization but also severely impaired mitosis in a manner that suggested a role at the G2/M transition (5). Although a similar number of nuclei were present in ΔplkA and control cells, we cannot exclude the possibility of subtle differences in the timing of the G2/M transition; the more dramatic effects observed upon overexpression could be due to titration of multiple factors required for mitotic entry. Second, the suppression of some ΔplkA phenotypes by high temperature or low concentrations of benomyl suggests that PLKA may be important for microtubule dynamics. Consistent with this, Plks can influence microtubule functions through interactions with microtubule-associated proteins (MAPS), including γ-tubulin, for example (4, 50). In addition, specific A. nidulans γ-tubulin mutants contained abnormal spindles and responded to benomyl in a manner similar to that of ΔplkA cells (27). Thus, PLKA is important but not essential for several aspects of mitosis and may in part influence microtubule dynamics.
PLKA influences nuclear distribution and hyphal morphogenesis but is dispensable for septation.
Our results suggest that PLKA may also have several novel functions. For example, the coenocytic nature of A. nidulans hyphae highlighted defects in nuclear distribution in a proportion of cells lacking PLKA. This phenotype was not suppressed by low concentrations of benomyl and was reminiscent of the A. nidulans nud (nuclear distribution) mutants (41). NUDC, which associates with the cytoplasmic dynein/dynactin complex and is important for nuclear movement in both fungi and higher organisms, interacts with PLK1 in mammals (75). However, PLKA-interacting factors have yet to be identified. We also showed that PLKA influenced colony branching, implying a link with aspects of hyphal morphogenesis. Since these effects were suppressed by low concentrations of benomyl, they may be indirect and microtubule dependent. However, Plks also influence the dynamics of the Golgi apparatus (18, 54, 59), which is important for polarized growth of hyphae (26, 49). Future investigations of PLKA may provide novel insights on the mechanisms by which Plks can influence hyphal morphogenesis.
Another unexpected feature of ΔplkA cells was the presence of septa since all Plks to date are required for septation or cytokinesis (4). Although we cannot rule out some minor role for PLKA in septation, this is the first example of a Plk that does not play a central role in the process. In S. pombe, Plo1 is an upstream regulator of the septation initiation network (SIN) (31). A similar network governs septation in A. nidulans (25, 29) but with some differences, and PLKA is not the functional equivalent of Plo1 in this context. It is not clear why PLKA would play such a different role in septation, but this may relate to the fact that the process is more complex in coenocytic hyphae than in yeast and is not coupled to every round of mitosis (25). Indeed, the dimorphic pathogen C. albicans forms hyphae with a single nucleus per subapical compartment, and CaCdc5p, which is more closely related to yeast Plk orthologues, is required for septation (6). Whether the loss of a primary role in septation correlates with the divergent PBD remains to be determined; it will be informative to investigate the extent to which filamentous fungal Plks with similar PBDs influence the septation process.
PLKA is important for development.
One of the most striking results of our investigation was the strong influence of PLKA on reproduction. The reduced pigment and abundance of aerial hyphae in ΔplkA colonies suggest that PLKA may play a positive role in asexual development. However, the concomitant derepression of sexual structures implies that PLKA may alternatively negatively regulate aspects of the sexual development program. The fact that a high temperature and low concentration of benomyl did not suppress the reproductive defects, unlike the compact growth phenotype, implies that PLKA influences development through independent mechanisms. Activation of sexual structures in the absence of PLKA is novel since Plks have been linked to developmental pathways only in metazoans. Cdc5p of S. cerevisiae is important for meiosis (34), but its absence does not correlate with a switch in development. In addition, while Plks of worms, flies, and mice function during development (19, 39, 56, 71), none were shown to negatively regulate a sexual reproduction program. The mechanisms by which PLKA influences reproduction are not yet clear, but future work will determine how it may impinge on the known circuitry governing developmental decisions in A. nidulans (8, 10, 12).
Overall, our results suggest that PLKA may play separate roles during growth and development. Given that A. nidulans is an established model organism for studying diverse areas of cell biology (21), future investigations of PLKA will provide important insights on the variations in Plk structure and function, the diversity in mechanisms controlling the cell cycle, and the evolution of an important group of cell cycle and developmental regulators.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Osmani (Ohio State University) and S. Kaminskyj (University of Saskatchewan) for comments on the manuscript and B. Oakley and L. Oakley (University of Kansas) for plasmids and discussions.
This work was supported by Fonds Quebecois de la Recherché sur la Nature et les Technologies (FQRNT) Establissement de Nouveaux chercheurs, grant number 107532, and Natural Sciences and Engineering Research Council of Canada Discovery Grant number 312035-2005, both to C.B.
Footnotes
Published ahead of print 2 December 2011
Supplemental material for this article may be found at http://ec.asm.org/.
REFERENCES
- 1. Adams TH, Boylan MT, Timberlake WE. 1988. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell 54:353–362 [DOI] [PubMed] [Google Scholar]
- 2. Alexandru G, Uhlmann F, Mechtler K, Poupart MA, Nasmyth K. 2001. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105:459–472 [DOI] [PubMed] [Google Scholar]
- 3. Andrysik Z, et al. 2010. The novel mouse Polo-like kinase 5 responds to DNA damage and localizes in the nucleolus. Nucleic Acids Res. 38:2931–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Archambault V, Glover DM. 2009. Polo-like kinases: conservation and divergence in their functions and regulation. Nat. Rev. Mol. Cell Biol. 10:265–275 [DOI] [PubMed] [Google Scholar]
- 5. Bachewich C, Masker K, Osmani S. 2005. The polo-like kinase PLKA is required for initiation and progression through mitosis in the filamentous fungus Aspergillus nidulans. Mol. Microbiol. 55:572–587 [DOI] [PubMed] [Google Scholar]
- 6. Bachewich C, Thomas DY, Whiteway M. 2003. Depletion of a polo-like kinase in Candida albicans activates cyclase-dependent hyphal-like growth. Mol. Biol. Cell 14:2163–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bahassi el M, et al. 2002. Mammalian Polo-like kinase 3 (Plk3) is a multifunctional protein involved in stress response pathways. Oncogene 21:6633–6640 [DOI] [PubMed] [Google Scholar]
- 8. Bayram O, Braus GH, Fischer R, Rodriguez-Romero J. 2010. Spotlight on Aspergillus nidulans photosensory systems. Fungal Genet. Biol. 47:900–908 [DOI] [PubMed] [Google Scholar]
- 9. Bettencourt-Dias M, et al. 2005. SAK/PLK4 is required for centriole duplication and flagella development. Curr. Biol. 15:2199–2207 [DOI] [PubMed] [Google Scholar]
- 10. Braus GH, Irniger S, Bayram O. 2010. Fungal development and the COP9 signalosome. Curr. Opin. Microbiol. 13:672–676 [DOI] [PubMed] [Google Scholar]
- 11. Burkard ME, et al. 2007. Chemical genetics reveals the requirement for Polo-like kinase 1 activity in positioning RhoA and triggering cytokinesis in human cells. Proc. Natl. Acad. Sci. U. S. A. 104:4383–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Calvo AM. 2008. The VeA regulatory system and its role in morphological and chemical development in fungi. Fungal Genet. Biol. 45:1053–1061 [DOI] [PubMed] [Google Scholar]
- 13. Charles JF, et al. 1998. The Polo-related kinase Cdc5 activates and is destroyed by the mitotic cyclin destruction machinery in S. cerevisiae. Curr. Biol. 8:497–507 [DOI] [PubMed] [Google Scholar]
- 14. Clutterbuck AJ. 1992. Sexual and parasexual genetics of Aspergillus species. Biotechnology 23:3–18 [PubMed] [Google Scholar]
- 15. Cove DJ. 1966. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim. Biophys. Acta 113:51–56 [DOI] [PubMed] [Google Scholar]
- 16. de Carcer G, et al. 2011. Plk5, a polo box domain-only protein with specific roles in neuron differentiation and glioblastoma suppression. Mol. Cell. Biol. 31:1225–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Degenhardt Y, Lampkin T. 2010. Targeting Polo-like kinase in cancer therapy. Clin. Cancer Res. 16:384–389 [DOI] [PubMed] [Google Scholar]
- 18. de Graffenried CL, Ho HH, Warren G. 2008. Polo-like kinase is required for Golgi and bilobe biogenesis in Trypanosoma brucei. J. Cell Biol. 181:431–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Draghetti C, et al. 2009. Functional whole-genome analysis identifies Polo-like kinase 2 and poliovirus receptor as essential for neuronal differentiation upstream of the negative regulator alphaB-crystallin. J. Biol. Chem. 284:32053–32065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Felsenstein J. 1997. An alternating least squares approach to inferring phylogenies from pairwise distances. Syst. Biol. 46:101–111 [DOI] [PubMed] [Google Scholar]
- 21. Galagan JE, et al. 2005. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438:1105–1115 [DOI] [PubMed] [Google Scholar]
- 22. Graham TM, Tait A, Hide G. 1998. Characterisation of a polo-like protein kinase gene homologue from an evolutionary divergent eukaryote, Trypanosoma brucei. Gene 207:71–77 [DOI] [PubMed] [Google Scholar]
- 23. Habedanck R, Stierhof YD, Wilkinson CJ, Nigg EA. 2005. The Polo kinase Plk4 functions in centriole duplication. Nat. Cell Biol. 7:1140–1146 [DOI] [PubMed] [Google Scholar]
- 24. Hansen DV, Loktev AV, Ban KH, Jackson PK. 2004. Plk1 regulates activation of the anaphase promoting complex by phosphorylating and triggering SCFbetaTrCP-dependent destruction of the APC inhibitor Emi1. Mol. Biol. Cell 15:5623–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Harris SD. 2001. Septum formation in Aspergillus nidulans. Curr. Opin. Microbiol. 4:736–739 [DOI] [PubMed] [Google Scholar]
- 26. Hubbard MA, Kaminskyj SG. 2008. Rapid tip-directed movement of Golgi equivalents in growing Aspergillus nidulans hyphae suggests a mechanism for delivery of growth-related materials. Microbiology 154:1544–1553 [DOI] [PubMed] [Google Scholar]
- 27. Jung MK, Prigozhina N, Oakley CE, Nogales E, Oakley BR. 2001. Alanine-scanning mutagenesis of Aspergillus gamma-tubulin yields diverse and novel phenotypes. Mol. Biol. Cell 12:2119–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kafer E. 1977. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv. Genet. 19:33–131 [DOI] [PubMed] [Google Scholar]
- 29. Kim JM, Lu L, Shao R, Chin J, Liu B. 2006. Isolation of mutations that bypass the requirement of the septation initiation network for septum formation and conidiation in Aspergillus nidulans. Genetics 173:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kitada K, Johnson AL, Johnston LH, Sugino A. 1993. A multicopy suppressor gene of the Saccharomyces cerevisiae G1 cell cycle mutant gene dbf4 encodes a protein kinase and is identified as CDC5. Mol. Cell. Biol. 13:4445–4457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krapp A, et al. 2006. The Schizosaccharomyces pombe septation initiation network (SIN) is required for spore formation in meiosis. J. Cell Sci. 119:2882–2891 [DOI] [PubMed] [Google Scholar]
- 32. Kumar P, Wang CC. 2006. Dissociation of cytokinesis initiation from mitotic control in a eukaryote. Eukaryot. Cell 5:92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lane HA, Nigg EA. 1996. Antibody microinjection reveals an essential role for human polo-like kinase 1 (Plk1) in the functional maturation of mitotic centrosomes. J. Cell Biol. 135:1701–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee BH, Amon A. 2003. Role of Polo-like kinase CDC5 in programming meiosis I chromosome segregation. Science 300:482–486 [DOI] [PubMed] [Google Scholar]
- 35. Leung GC, et al. 2002. The Sak polo-box comprises a structural domain sufficient for mitotic subcellular localization. Nat. Struct. Biol. 9:719–724 [DOI] [PubMed] [Google Scholar]
- 36. Lin X, Momany M. 2003. The Aspergillus nidulans swoC1 mutant shows defects in growth and development. Genetics 165:543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu HL, et al. 2010. Single-step affinity purification for fungal proteomics. Eukaryot. Cell 9:831–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lowery DM, et al. 2007. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 26:2262–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mirouse V, Formstecher E, Couderc JL. 2006. Interaction between Polo and BicD proteins links oocyte determination and meiosis control in Drosophila. Development 133:4005–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Momany M. 2002. Polarity in filamentous fungi: establishment, maintenance and new axes. Curr. Opin. Microbiol. 5:580–585 [DOI] [PubMed] [Google Scholar]
- 41. Morris NR, Enos AP. 1992. Mitotic gold in a mold: Aspergillus genetics and the biology of mitosis. Trends Genet. 8:32–37 [DOI] [PubMed] [Google Scholar]
- 42. Moshe Y, Boulaire J, Pagano M, Hershko A. 2004. Role of Polo-like kinase in the degradation of early mitotic inhibitor 1, a regulator of the anaphase promoting complex/cyclosome. Proc. Natl. Acad. Sci. U. S. A. 101:7937–7942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nayak T, et al. 2006. A versatile and efficient gene-targeting system for Aspergillus nidulans. Genetics 172:1557–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Oakley BR, Osmani SA. 1993. Cell-cycle analysis using the filamentous fungus Aspergillus nidulans, p 127–142 In Brooks B. (ed), The cell cycle: a practical approach. Oxford University Press, New York, NY [Google Scholar]
- 45. Ohkura H, Hagan IM, Glover DM. 1995. The conserved Schizosaccharomyces pombe kinase Plo1, required to form a bipolar spindle, the actin ring, and septum, can drive septum formation in G1 and G2 cells. Genes Dev. 9:1059–1073 [DOI] [PubMed] [Google Scholar]
- 46. Osmani AH, Davies J, Liu HL, Nile A, Osmani SA. 2006. Systematic deletion and mitotic localization of the nuclear pore complex proteins of Aspergillus nidulans. Mol. Biol. Cell 17:4946–4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Osmani AH, Oakley BR, Osmani SA. 2006. Identification and analysis of essential Aspergillus nidulans genes using the heterokaryon rescue technique. Nat. Protoc. 1:2517–2526 [DOI] [PubMed] [Google Scholar]
- 48. Osmani SA, May GS, Morris NR. 1987. Regulation of the mRNA levels of nimA, a gene required for the G2-M transition in Aspergillus nidulans. J. Cell Biol. 104:1495–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pantazopoulou A, Penalva MA. 2009. Organization and dynamics of the Aspergillus nidulans Golgi during apical extension and mitosis. Mol. Biol. Cell 20:4335–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Park CJ, et al. 2008. Requirement for the budding yeast polo kinase Cdc5 in proper microtubule growth and dynamics. Eukaryot. Cell 7:444–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Park JE, et al. 2010. Polo-box domain: a versatile mediator of polo-like kinase function. Cell. Mol. Life Sci. 67:1957–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Petronczki M, Glotzer M, Kraut N, Peters JM. 2007. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Dev. Cell 12:713–725 [DOI] [PubMed] [Google Scholar]
- 53. Pontecorvo G, Roper JA, Hemmons LM, Macdonald KD, Bufton AW. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141–238 [DOI] [PubMed] [Google Scholar]
- 54. Preisinger C, Barr FA. 2005. Kinases regulating Golgi apparatus structure and function. Biochem. Soc. Symp. 72:15–30 [DOI] [PubMed] [Google Scholar]
- 55. Qian YW, Erikson E, Taieb FE, Maller JL. 2001. The polo-like kinase Plx1 is required for activation of the phosphatase Cdc25C and cyclin B-Cdc2 in Xenopus oocytes. Mol. Biol. Cell 12:1791–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rivers DM, Moreno S, Abraham M, Ahringer J. 2008. PAR proteins direct asymmetry of the cell cycle regulators Polo-like kinase and Cdc25. J. Cell Biol. 180:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Romero B, Turner G, Olivas I, Laborda F, De Lucas JR. 2003. The Aspergillus nidulans alcA promoter drives tightly regulated conditional gene expression in Aspergillus fumigatus permitting validation of essential genes in this human pathogen. Fungal Genet. Biol. 40:103–114 [DOI] [PubMed] [Google Scholar]
- 58. Roshak AK, et al. 2000. The human polo-like kinase, PLK, regulates cdc2/cyclin B through phosphorylation and activation of the cdc25C phosphatase. Cell Signal. 12:405–411 [DOI] [PubMed] [Google Scholar]
- 59. Ruan Q, et al. 2004. Polo-like kinase 3 is Golgi localized and involved in regulating Golgi fragmentation during the cell cycle. Exp. Cell Res. 294:51–59 [DOI] [PubMed] [Google Scholar]
- 60. Sakchaisri K, et al. 2004. Coupling morphogenesis to mitotic entry. Proc. Natl. Acad. Sci. U. S. A. 101:4124–4129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Seong YS, et al. 2002. A spindle checkpoint arrest and a cytokinesis failure by the dominant-negative polo-box domain of Plk1 in U-2 OS cells. J. Biol. Chem. 277:32282–32293 [DOI] [PubMed] [Google Scholar]
- 62. Shirayama M, Zachariae W, Ciosk R, Nasmyth K. 1998. The Polo-like kinase Cdc5p and the WD-repeat protein Cdc20p/fizzy are regulators and substrates of the anaphase promoting complex in Saccharomyces cerevisiae. EMBO J. 17:1336–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Snead JL, et al. 2007. A coupled chemical-genetic and bioinformatic approach to Polo-like kinase pathway exploration. Chem. Biol. 14:1261–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Song S, Lee KS. 2001. A novel function of Saccharomyces cerevisiae CDC5 in cytokinesis. J. Cell Biol. 152:451–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stegmeier F, Visintin R, Amon A. 2002. Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108:207–220 [DOI] [PubMed] [Google Scholar]
- 66. Sumara I, et al. 2002. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol. Cell 9:515–525 [DOI] [PubMed] [Google Scholar]
- 67. Sunkel CE, Glover DM. 1988. polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J. Cell Sci. 89:25–38 [DOI] [PubMed] [Google Scholar]
- 68. Szewczyk E, et al. 2006. Fusion PCR and gene targeting in Aspergillus nidulans. Nat. Protoc. 1:3111–3120 [DOI] [PubMed] [Google Scholar]
- 69. Timberlake WE. 1980. Developmental gene regulation in Aspergillus nidulans. Dev. Biol. 78:497–510 [DOI] [PubMed] [Google Scholar]
- 70. Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. 2001. Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase. Nature 410:215–220 [DOI] [PubMed] [Google Scholar]
- 71. Wang H, Ouyang Y, Somers WG, Chia W, Lu B. 2007. Polo inhibits progenitor self-renewal and regulates Numb asymmetry by phosphorylating Pon. Nature 449:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Waring RB, May GS, Morris NR. 1989. Characterization of an inducible expression system in Aspergillus nidulans using alcA and tubulin-coding genes. Gene 79:119–130 [DOI] [PubMed] [Google Scholar]
- 73. Yang L, et al. 2004. Rapid production of gene replacement constructs and generation of a green fluorescent protein-tagged centromeric marker in Aspergillus nidulans. Eukaryot. Cell 3:1359–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yu JH, Mah JH, Seo JA. 2006. Growth and developmental control in the model and pathogenic aspergilli. Eukaryot. Cell 5:1577–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou T, Aumais JP, Liu X, Yu-Lee LY, Erikson RL. 2003. A role for Plk1 phosphorylation of NudC in cytokinesis. Dev. Cell 5:127–138 [DOI] [PubMed] [Google Scholar]
- 76. Zimmerman WC, Erikson RL. 2007. Polo-like kinase 3 is required for entry into S phase. Proc. Natl. Acad. Sci. U. S. A. 104:1847–1852 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.