Abstract
Human papillomavirus (HPV) infection can lead to significant disease in males, including anogenital warts, intraepithelial neoplasias, and several types of oral and anogenital cancers. The quadrivalent HPV (type 6/11/16/18) L1 virus-like particle (VLP) vaccine (qHPV vaccine; Gardasil) has recently been demonstrated to prevent persistent infection and associated disease related to vaccine HPV types in males. We report the overall immunogenicity results from a trial of the quadrivalent HPV vaccine in males. Overall, 3,463 heterosexual men and 602 men who had sex with men were enrolled into a randomized, placebo-controlled, double-blind safety, immunogenicity, and efficacy study. Serum samples were collected prior to vaccination at day 1 and at months 7, 24, and 36 postvaccination. Immunogenicity was evaluated with a multiplex, competitive Luminex immunoassay. Almost all subjects (97.4 to 99.2%) seroconverted for vaccine HPV types by month 7. At month 36, 88.9%, 94.0%, 97.9%, and 57.0% of subjects were still seropositive for HPV-6, -11, -16, and -18, respectively. For all vaccine HPV types, black subjects had significantly higher antibody titers at month 7 than did both Caucasian and Asian subjects. An anamnestic antibody response was seen in men seropositive before vaccination. The vaccine was highly immunogenic in males 16 to 23 years of age; responses were comparable to those observed in women. Furthermore, the immune responses were consistent with the established efficacy of the vaccine in the prevention of incident and persistent HPV infection, anogenital warts, and anal intraepithelial neoplasia.
INTRODUCTION
As is the case for women, human papillomavirus (HPV) infection can lead to substantial disease in males, including anogenital warts, intraepithelial neoplasias, and oral and genital cancers. There may be considerable physical and psychological distress associated with these conditions, and treatments are costly (5, 10, 11, 15). The quadrivalent HPV (type 6/11/16/18) L1 virus-like particle (VLP) vaccine (qHPV vaccine; Gardasil) has been available for use in women (in the United States) since June of 2006; the vaccine was approved in November 2009 by the U.S. FDA for use in males 16 to 26 years old (6).
From a public health perspective, the development of a vaccine against common HPV types for use in males is compelling. An effective vaccine that targets HPV-6, -11, -16, and -18 could greatly reduce the burden of anogenital warts and anal cancer in men (6). In addition, as males are the prime vectors for transmission of HPV types to women (1), such a vaccine would also likely reduce the risk of transmission of HPV infection to women. Recent data indicate substantial efficacy in men and a favorable safety profile (6, 7). However, the immunogenicity of the vaccine in men has not yet been described in detail. While the immune responses to the qHPV vaccine should be similar in adult males and females (this has been shown in children ages 9 to 15 years [Reisinger KS, et al., presented at the European Society of Pediatric Infectious Diseases meeting, 5 May 2006]), differences in the immune response between males and females following sexual maturation may result in varied immune responses to vaccines (2, 13).
In this report, we describe the overall immunogenicity results from a trial of the quadrivalent HPV vaccine in men by presenting serum anti-HPV-6, -11, -16, and -18 responses after completion of the 3-dose vaccination regimen. In addition, we present these results stratified by baseline covariates such as age and smoking status, factors that may influence the immune response to vaccination.
MATERIALS AND METHODS
Study population.
Between 3 September 2004 and 29 August 2008, 4,065 healthy men (3,463 heterosexual men [HM; had exclusively female sexual partners] and 602 men who had sex with men [MSM; identified themselves as men who had sex with men and had engaged in either insertive or receptive anal intercourse or oral sex with another male sexual partner within the past year]) were enrolled from 71 sites in 18 countries into a randomized, placebo-controlled, double-blind safety, immunogenicity, and efficacy study (protocol 020; NCT00090285). More-detailed data concerning the design of protocol 020 have been published elsewhere (6).
HM 16 to 23 years old who had 1 to 5 female lifetime sexual partners (LSP) and MSM 16 to 26 years old who had 1 to 5 LSP were eligible. Men with a history of or with current clinically detectable anogenital warts or genital lesions suggesting other sexually transmitted infections were excluded. HPV or anal cytologic prescreening was not performed to determine eligibility for enrollment into the study.
The primary immunogenicity analyses were conducted in a population of individuals who were seronegative and PCR negative for the relevant HPV type(s) at day 1, remained HPV PCR negative for the relevant HPV type(s) through 1 month post-dose 3 (month 7), received all 3 vaccinations within prespecified day ranges, and did not deviate from the study protocol in ways that could interfere with the effects of the vaccine. Importantly, subjects who were seropositive and/or PCR positive for a vaccine HPV type through month 7 could still be analyzed for the vaccine HPV types for which they were seronegative and PCR negative through month 7 (excluding HPV-6/11 seropositivity due to antibody cross-reactivity), assuming that all other population criteria were met. Additional analyses were conducted on all randomized participants to evaluate immune responses to vaccine comparing those who entered the study as HPV seropositive/DNA negative with those who entered the study as HPV seronegative/DNA negative for each respective HPV type.
The institutional review board at each participating center approved the protocol, and informed consent was obtained from all subjects. Studies were conducted in conformance with applicable country or local requirements regarding ethical committee review, informed consent, and other statutes or regulations regarding the protection of the rights and welfare of human subjects participating in biomedical research.
Vaccine and randomization.
The quadrivalent HPV (type 6/11/16/18) L1 virus-like particle (VLP) vaccine (qHPV [Gardasil/Silgard; Merck & Co., Inc.]) with amorphous aluminum hydroxyphosphate sulfate (AAHS) adjuvant and a visually indistinguishable AAHS-containing placebo have been described previously (14). Subjects were randomized 1:1 to receive qHPV vaccine or placebo at day 1, month 2 (±3 weeks), and month 6 (±4 weeks). Vaccine or placebo was administered as an 0.5-ml injection in the deltoid muscle.
A computer-generated allocation schedule was produced by the sponsor. Following informed consent and determination that all entry criteria were met, eligible subjects were randomized to a vaccination group. All investigators and site personnel, subjects, monitors, and laboratory personnel remained blinded to treatment allocation throughout the study. Staff of the sponsor were blinded from the study onset through the database lock for this analysis.
Study measurements.
Immunogenicity evaluations focused on an assessment of immunogenicity at the completion of the vaccination regimen (4 weeks post-dose 3) and on characterizing the persistence of vaccine-induced anti-HPV-6, -11, -16, and -18 responses. Serum samples were collected prior to vaccination at day 1 and at months 7, 24, and 36. Immunogenicity was evaluated, including a description of peak and persistent anti-HPV-6, -11, -16, and -18 responses. The response of the immune system to the vaccine was measured with a multiplex, competitive Luminex immunoassay (anti-HPV-6, -11, -16, and -18 cLIA; developed by Merck Research Laboratories, West Point, PA, using technology from the Luminex Corporation, Austin, TX) (9).
Briefly, this assay can simultaneously quantitate neutralizing antibodies to human papillomavirus types 6, 11, 16, and 18 in 50 μl of serum. The HPV-Luminex competitive immunoassay measures titers of polyclonal antibodies in serum capable of displacing phycoerythrin-labeled detection monoclonal antibodies binding to conformationally sensitive, neutralizing epitopes on the respective virus-like particles. Dilution-corrected serostatus cutoffs were 20 milli-Merck units (mMU)/ml for HPV-6, 16 mMU/ml for HPV-11, 20 mMU/ml for HPV-16, and 24 mMU/ml for HPV-18 (epitopes H6.M48, K11.B2, H16.V5, and H18.J4 for HPV types 6, 11, 16, and 18, respectively).
RESULTS
Almost all subjects (>97.4%) seroconverted for vaccine HPV types by month 7, with anti-HPV-6/11/16/18 geometric mean titers (GMTs) reaching peak values 1 month after dose 3 (month 7) (Table 1). After month 7, a gradual decline in HPV-6/11/16/18 GMT was seen. However, by month 36, 88.9%, 94.0%, 97.9%, and 57.0% of subjects remained seropositive for HPV-6, -11, -16, and -18, respectively.
Table 1.
Summary of anti-HPV geometric mean titers and seroconversion percentage over timea
| Response | Study time | n | GMT (mMU/ml) | 95% CI | m | Seroconversion (%) | 95% CI |
|---|---|---|---|---|---|---|---|
| Anti-HPV-6 | Day 1 | 1,092 | <7 | <7, <7 | 0 | 0.0 | 0.0, 0.3 |
| Mo 7 | 1,092 | 447.6 | 422.6, 474.1 | 1,080 | 98.9 | 98.1, 99.4 | |
| Mo 24 | 941 | 79.8 | 75.8, 84.1 | 855 | 90.9 | 88.8, 92.6 | |
| Mo 36 | 847 | 71.5 | 67.5, 75.8 | 753 | 88.9 | 86.6, 90.9 | |
| Anti-HPV-11 | Day 1 | 1,092 | <8 | <8, <8 | 0 | 0.0 | 0.0, 0.3 |
| Mo 7 | 1,092 | 624.0 | 594.1, 655.4 | 1,083 | 99.2 | 98.4, 99.6 | |
| Mo 24 | 941 | 94.6 | 90.0, 99.5 | 900 | 95.6 | 94.1, 96.9 | |
| Mo 36 | 847 | 82.6 | 78.3, 87.1 | 796 | 94.0 | 92.2, 95.5 | |
| Anti-HPV-16 | Day 1 | 1,135 | <11 | <11, <11 | 0 | 0.0 | 0.0, 0.3 |
| Mo 7 | 1,135 | 2,404.3 | 2,272.2, 2,544.0 | 1,121 | 98.8 | 97.9, 99.3 | |
| Mo 24 | 979 | 342.7 | 324.7, 361.7 | 970 | 99.1 | 98.3, 99.6 | |
| Mo 36 | 877 | 293.3 | 276.5, 311.2 | 859 | 97.9 | 96.8, 98.8 | |
| Anti-HPV-18 | Day 1 | 1,174 | <10 | <10, <10 | 0 | 0.0 | 0.0, 0.3 |
| Mo 7 | 1,174 | 402.3 | 380.2, 425.7 | 1,143 | 97.4 | 96.3, 98.2 | |
| Mo 24 | 1,011 | 38.4 | 36.0, 41.0 | 630 | 62.3 | 59.2, 65.3 | |
| Mo 36 | 905 | 33.1 | 30.9, 35.4 | 516 | 57.0 | 53.7, 60.3 |
The estimated GMTs and associated CIs are calculated using an analysis of variance model with a term for vaccination group. Seroconversion percent is calculated as 100 × (m/n). The seroconversion CIs are computed based on exact methods. n, number of subjects contributing to the analyses; m, number of subjects with the indicated response.
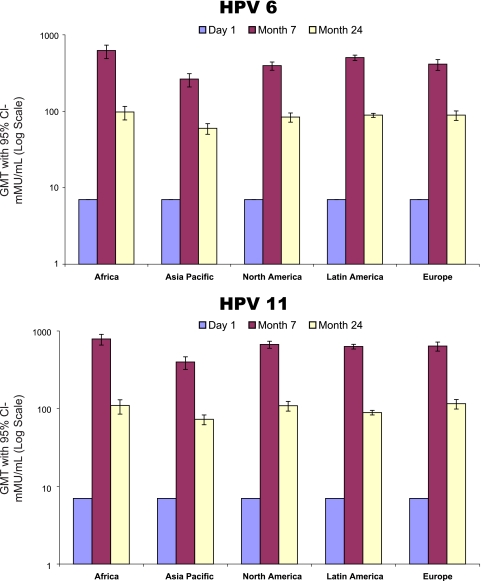
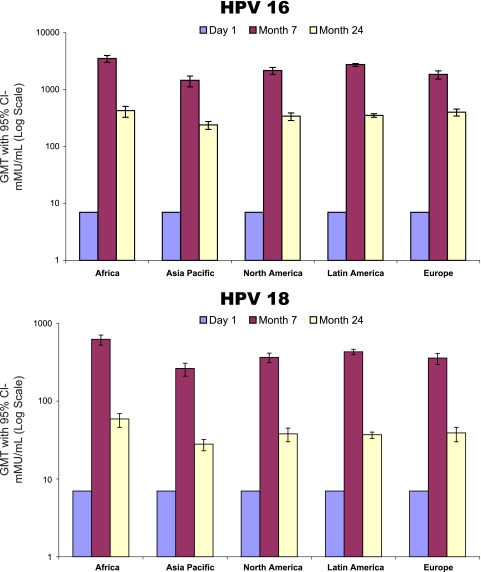
Vaccine immunogenicity at month 7 stratified by baseline subject characteristics can be seen in Table 2. For all vaccine HPV types, black subjects had significantly higher GMTs at month 7 than did both Caucasian and Asian subjects. HPV-18 GMTs for black subjects were also significantly higher than those of Hispanic American subjects. Only HPV-18 GMTs were significantly different between younger (age 15 to 20 years) and older (age 21 to 27 years) subjects, with the GMTs of younger subjects being higher (473.5 mMU/ml [95% confidence interval (CI), 427.5 to 524.6] versus 339.1 mMU/ml [95% CI, 304.7 to 377.3], respectively). Tobacco use and lifetime number of sexual partners were not associated with month 7 GMT for vaccine HPV types. Overall, males from Africa had the highest month 7 GMT for vaccine HPV types, and males from the Asia-Pacific region had the lowest. GMTs at day 1, month 7, and month 24 stratified by HPV type and region can be seen in Fig. 1. The GMTs among men residing in Africa remained higher than those of men residing in other world regions at 24 months as well.
Table 2.
Vaccine immunogenicity at month 7 stratified by baseline subject characteristicsa
| Baseline characteristic | HPV-6 |
HPV-11 |
HPV-16 |
HPV-18 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | GMT (mMU/ml) | 95% CI | n | GMT (mMU/ml) | 95% CI | n | GMT (mMU/ml) | 95% CI | n | GMT (mMU/ml) | 95% CI | |
| Race | ||||||||||||
| Caucasian | 349 | 378.0 | 331.7, 430.8 | 349 | 595.0 | 537.1, 659.0 | 361 | 1,902.3 | 1,675.8, 2,159.5 | 378 | 328.3 | 288.0, 374.1 |
| Black | 196 | 620.1 | 519.8, 739.8 | 196 | 793.4 | 686.8, 916.6 | 204 | 3,342.0 | 2,908.0, 3,840.9 | 215 | 596.8 | 503.1, 708.0 |
| Asian | 134 | 297.1 | 236.4, 373.4 | 134 | 441.1 | 365.2, 532.7 | 136 | 1,674.5 | 1,341.6, 2,090.0 | 136 | 297.8 | 239.7, 370.1 |
| Hispanic American | 234 | 488.5 | 413.2, 577.5 | 234 | 650.6 | 565.3, 748.8 | 241 | 2,741.5 | 2,307.7, 3,257.0 | 248 | 407.1 | 340.4, 486.8 |
| Other | 179 | 528.1 | 462.7, 602.7 | 179 | 646.3 | 583.9, 715.4 | 193 | 2,881.2 | 2,584.0, 3,212.6 | 197 | 468.5 | 404.7, 542.3 |
| Age (yr) | ||||||||||||
| 15–20 | 571 | 484.1 | 435.9, 537.6 | 571 | 669.1 | 615.5, 727.5 | 584 | 2,615.4 | 2,380.3, 2,873.7 | 601 | 473.5 | 427.5, 524.6 |
| 21–27 | 521 | 410.8 | 370.7, 455.3 | 521 | 578.0 | 531.7, 628.4 | 551 | 2,199.1 | 1,986.6, 2,434.3 | 573 | 339.1 | 304.7, 377.3 |
| Region | ||||||||||||
| Africa | 125 | 623.1 | 492.6, 788.2 | 125 | 789.6 | 652.5, 955.6 | 131 | 3,529.3 | 2,954.2, 4,216.4 | 132 | 622.4 | 506.0, 765.6 |
| Asia-Pacific | 122 | 264.3 | 212.0, 329.6 | 122 | 396.9 | 328.6, 479.4 | 126 | 1,450.7 | 1,164.6, 1,807.2 | 126 | 262.7 | 211.7, 326.0 |
| Europe | 146 | 414.2 | 332.9, 515.3 | 146 | 641.5 | 545.2, 754.9 | 148 | 1,856.5 | 1,483.9, 2,322.6 | 155 | 358.2 | 288.7, 444.5 |
| Latin America | 489 | 503.8 | 455.8, 557.0 | 489 | 630.9 | 580.2, 685.9 | 515 | 2,762.5 | 2,516.9, 3,032.1 | 533 | 430.1 | 387.3, 477.8 |
| North America | 210 | 400.1 | 336.6, 475.5 | 210 | 674.7 | 591.1, 770.1 | 215 | 2,191.9 | 1,857.9, 2,585.9 | 228 | 365.9 | 304.1, 440.2 |
| Tobacco use status | ||||||||||||
| Current | 387 | 443.7 | 394.9, 498.6 | 387 | 621.1 | 566.3, 681.2 | 410 | 2,374.9 | 2,131.0, 2,646.7 | 416 | 393.5 | 348.6, 444.2 |
| Ex-user | 79 | 363.7 | 272.3, 485.7 | 79 | 531.8 | 415.5, 680.6 | 80 | 2,020.5 | 1,521.3, 2,683.7 | 86 | 349.7 | 257.3, 475.4 |
| Nonuser | 626 | 462.0 | 418.0, 510.7 | 626 | 638.6 | 589.1, 692.2 | 645 | 2,476.0 | 2,252.8, 2,721.3 | 672 | 415.2 | 376.1, 458.4 |
| Circumcision status | ||||||||||||
| Circumcised | 352 | 421.3 | 366.2, 484.6 | 352 | 623.9 | 560.5, 694.5 | 358 | 2,222.0 | 1,967.0, 2,510.0 | 375 | 369.2 | 321.5, 424.1 |
| Not circumcised | 740 | 460.7 | 422.8, 502.1 | 740 | 624.1 | 581.2, 670.1 | 777 | 2,493.2 | 2,292.0, 2,712.2 | 799 | 418.8 | 383.5, 457.3 |
| No. of sexual partners | ||||||||||||
| 0 | 7 | 202.3 | 28.7, 1,428.3 | 7 | 211.4 | 35.5, 1,259.2 | 7 | 655.8 | 73.0, 5,892.1 | 7 | 179.7 | 23.9, 1,349.4 |
| 1 | 260 | 521.3 | 443.9, 612.3 | 260 | 703.5 | 621.3, 796.6 | 260 | 2,851.5 | 2,461.6, 3,303.2 | 260 | 495.2 | 423.0, 579.8 |
| 2 | 198 | 416.6 | 355.0, 488.9 | 198 | 576.6 | 499.1, 666.1 | 206 | 2,281.6 | 1,941.0, 2,681.9 | 211 | 379.0 | 321.2, 447.4 |
| 3 | 225 | 455.3 | 387.2, 535.4 | 225 | 619.7 | 543.3, 706.8 | 238 | 2,489.0 | 2,176.7, 2,846.2 | 242 | 400.8 | 339.6, 473.1 |
| 4 | 205 | 440.1 | 369.9, 523.6 | 205 | 597.4 | 524.5, 680.5 | 220 | 2,254.0 | 1,916.5, 2,650.9 | 228 | 364.6 | 305.1, 435.7 |
| 5 or more | 197 | 404.0 | 343.2, 475.7 | 197 | 632.1 | 555.4, 719.3 | 204 | 2,195.7 | 1,857.1, 2,596.0 | 226 | 380.6 | 323.1, 448.4 |
| Presence of sexually transmitted disease | ||||||||||||
| Chlamydia | 14 | 215.5 | 83.8, 554.2 | 14 | 372.7 | 146.3, 949.4 | 19 | 1,073.6 | 391.4, 2,945.4 | 19 | 224.5 | 97.6, 516.3 |
| Gonorrhea | 1 | 192.0 | NA | 1 | 256.0 | NA | 1 | 356.0 | NA | 1 | 36.0 | NA |
The per-protocol immunogenicity population includes all subjects who were not general protocol violators, received all 3 vaccinations within acceptable day ranges, were seronegative at day 1 and PCR negative at day 1 through month 7 for the relevant HPV type(s), and had a month 7 serum sample collected within an acceptable day range. n, number of subjects contributing to the analysis; NA, not available.
Fig 1.
Quadrivalent HPV vaccine immunogenicity by world region.
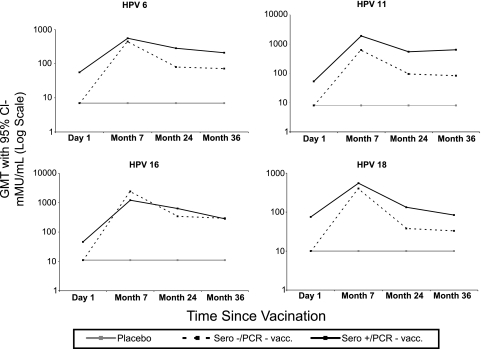
As seen in Fig. 2, subjects who were seropositive for a vaccine HPV type at baseline had a greater antibody response to that HPV type after vaccination than did men who were seronegative for that HPV type, indicating an anamnestic response. This trend is less evident for males who were seropositive for HPV-16 at baseline; however, low numbers of subjects in this category limit this analysis.
Fig 2.
Immunogenicity by baseline serology status.
In general, HM subjects had higher GMT levels than did MSM for all vaccine HPV types at their peak (month 7) and at the end of the study (month 36) (Table 3). The differences observed are statistically significant for all of these time points except the HPV-11 and HPV-18 month 36 GMTs. Though not statistically significant, a higher proportion of HM subjects seroconverted for vaccine HPV types than did MSM subjects (Table 4).
Table 3.
Summary of anti-HPV geometric mean titers over time among HM and MSM subjectsa
| Response | Study time | Vaccinated HM (N = 1,726) |
Vaccinated MSM (N = 299) |
||||
|---|---|---|---|---|---|---|---|
| n | GMT (mMU/ml) | 95% CI | n | GMT (mMU/ml) | 95% CI | ||
| Anti-HPV-6 | Day 1 | 978 | <7 | <7, <7 | 114 | <7 | <7, <7 |
| Mo 7 | 978 | 473.9 | 446.8, 502.7 | 114 | 274.3 | 222.5, 338.3 | |
| Mo 24 | 851 | 81.6 | 77.4, 86.1 | 90 | 64.6 | 53.7, 77.8 | |
| Mo 36 | 792 | 73.4 | 69.2, 77.8 | 55 | 49.2 | 37.3, 64.8 | |
| Anti-HPV-11 | Day 1 | 978 | <8 | <8, <8 | 114 | <8 | <8, <8 |
| Mo 7 | 978 | 651.5 | 620.7, 683.7 | 114 | 431.3 | 348.2, 534.2 | |
| Mo 24 | 851 | 94.9 | 90.1, 100.0 | 90 | 91.6 | 76.7, 109.4 | |
| Mo 36 | 792 | 83.8 | 79.4, 88.5 | 55 | 66.2 | 51.8, 84.6 | |
| Anti-HPV-16 | Day 1 | 999 | <11 | <11, <11 | 136 | <11 | <11, <11 |
| Mo 7 | 999 | 2,622.1 | 2,484.9, 2,766.9 | 136 | 1,271.6 | 996.0, 1,623.4 | |
| Mo 24 | 869 | 355.7 | 335.8, 376.7 | 110 | 255.5 | 219.5, 297.4 | |
| Mo 36 | 811 | 309.3 | 291.5, 328.1 | 66 | 153.0 | 116.1, 201.5 | |
| Anti-HPV-18 | Day 1 | 1,032 | <10 | <10, <10 | 142 | <10 | <10, <10 |
| Mo 7 | 1,032 | 439.3 | 415.7, 464.3 | 142 | 212.1 | 170.0, 264.6 | |
| Mo 24 | 897 | 39.4 | 36.8, 42.2 | 114 | 31.4 | 25.9, 38.0 | |
| Mo 36 | 836 | 33.9 | 31.6, 36.4 | 69 | 24.7 | 19.0, 32.1 | |
The estimated GMTs and associated CIs are calculated using an analysis of variance model with a term for vaccination group. N, number of subjects randomized to the respective vaccination group who received at least 1 injection; n, number of subjects contributing to the analysis.
Table 4.
Summary of anti-HPV seroconversion over time among HM and MSM subjectsa
| cLIA (mMU/ml) | Study time | Vaccinated HM (N = 1,726) |
Vaccinated MSM (N = 299) |
||||
|---|---|---|---|---|---|---|---|
| n | Seroconversion (%) | 95% CI | n | Seroconversion (%) | 95% CI | ||
| HPV-6 (≥20) | Day 1 | 978 | 0.0 | 0.0, 0.4 | 114 | 0.0 | 0.0, 3.2 |
| Mo 7 | 978 | 99.2 | 98.4, 99.6 | 114 | 96.5 | 91.3, 99.0 | |
| Mo 24 | 851 | 91.3 | 89.2, 93.1 | 90 | 86.7 | 77.9, 92.9 | |
| Mo 36 | 792 | 89.5 | 87.2, 91.6 | 55 | 80.0 | 67.0, 89.6 | |
| HPV-11 (≥16) | Day 1 | 978 | 0.0 | 0.0, 0.4 | 114 | 0.0 | 0.0, 3.2 |
| Mo 7 | 978 | 99.4 | 98.7, 98.4 | 114 | 97.4 | 92.5, 99.5 | |
| Mo 24 | 851 | 95.5 | 93.9, 96.8 | 90 | 96.7 | 90.6, 99.3 | |
| Mo 36 | 792 | 94.3 | 92.5, 95.8 | 55 | 89.1 | 77.8, 95.9 | |
| HPV-16 (≥20) | Day 1 | 999 | 0.0 | 0.0, 0.4 | 136 | 0.0 | 0.0, 2.7 |
| Mo 7 | 999 | 99.4 | 98.7, 99.8 | 136 | 94.1 | 88.7, 97.4 | |
| Mo 24 | 869 | 99.2 | 98.3, 99.7 | 110 | 98.2 | 93.6, 99.8 | |
| Mo 36 | 811 | 98.3 | 97.1, 99.1 | 66 | 93.9 | 85.2, 98.3 | |
| HPV-18 (≥24) | Day 1 | 1,032 | 0.0 | 0.0, 0.4 | 142 | 0.0 | 0.0, 2.6 |
| Mo 7 | 1,032 | 98.4 | 97.5, 99.1 | 142 | 89.4 | 83.2, 94.0 | |
| Mo 24 | 897 | 62.9 | 59.6, 66.0 | 114 | 57.9 | 48.3, 67.1 | |
| Mo 6 | 836 | 57.3 | 53.9, 60.7 | 69 | 53.6 | 41.2, 65.7 | |
Abbreviations: N, number of subjects randomized to the respective vaccination group who received at least 1 injection; n, number of subjects contributing to the analysis.
DISCUSSION
We have demonstrated that the qHPV vaccine was highly immunogenic for all vaccine types in HM (aged 16 to 23 years) and MSM (aged 16 to 26 years). Almost all subjects seroconverted for vaccine HPV types by month 7. Some interesting differences in immune responses were noted. For example, HM subjects had higher GMT levels for all vaccine HPV types at their peak than did MSM. Likewise, black subjects had significantly higher GMTs at month 7 than did both Caucasian and Asian subjects. Consistent with this observation, seroconversion for vaccine HPV types was higher for men residing in Africa than for those in Asia. There was also a suggestion of an age-dependent response, with the vaccine being more immunogenic in younger men than in older men. Given that high efficacy for all 4 vaccine HPV types was demonstrated across a wide range of antibody levels, the results suggest that any demonstrated differences in immune responses are not relevant to protective efficacy. Overall, the vaccine was highly immunogenic in all groups; titers achieved after vaccination were substantially higher than those seen during natural HPV infection.
In general, the GMTs to vaccine types were lower in men than were those seen in earlier studies of women. The GMTs to vaccine HPV types in males at month 7 were 448 mMU/ml for HPV-6, 624 mMU/ml for HPV-11, 2,404 mMU/ml for HPV-16, and 402 mMU/ml for HPV-18. In comparison, month 7 GMTs in females 16 to 23 years of age for HPV-6, -11, -16, and -18 were 549 mMU/ml, 635 mMU/ml, 3,870 mMU/ml, and 741 mMU/ml, respectively (8). It is not possible to make direct statistical comparisons across the populations as the trials were different from each other with respect to countries included, populations enrolled, number of sexual partners, and other factors.
Of note, other potential factors that might have affected immune responses to the vaccine such as tobacco use and lifetime number of sexual partners did not adversely influence month 7 mean GMTs for vaccine HPV types. As observed previously (8), subjects seropositive for a vaccine HPV type at baseline had a greater antibody response to that HPV type after vaccination, indicating an anamnestic response. Considering that the vaccine was shown to offer an excellent level of protection in this trial, there is no evidence that these differences in antibody levels are clinically relevant.
Our data show that immune responses to the qHPV vaccine are broadly comparable in men and women (3, 4). Furthermore, the observed responses were substantially higher than those seen during natural HPV infection and consistent with the established efficacy of the vaccine in the prevention of incident and persistent HPV infection, anogenital warts, and anal intraepithelial neoplasia.
ACKNOWLEDGMENTS
We thank all study participants as well as all investigators and their staff who enrolled subjects (Australia: Jonathan Anderson; Brazil: Esper Kallas, Edison Fedrizzi, Bernadete Nonnenmacher, and Joao Mendonca; Canada: Charles Lynde, Stephen Schafran, Danielle Rouleau, and Irving Salit; Costa Rica: Javier Moya Rodriguez, Ana Guzman, Javier Bejarano, and Jose Saenz; Croatia: Mihael Skerlev; Finland: Dan Apter, Liisa Lahti, Timo Hakala, and Robert Zilliacus; Germany: Stefan Esser and Carl Knud Schewe; Mexico: Eduardo Lazcano; Netherlands: Hans Rumke and A. G. J. van der Zee; Peru: Robinson Cabello; Philippines: Ricardo Manalastas; Portugal: Jorge Cardoso, Daniel Silva, and Joao Dias; South Africa: Ezio Baraldi, Mohammed Haffejee, and Arthi Ramikisson; Spain: Bonaventura Clotet Sala; Sweden: Arne Wikstrom and Annika Johnsson; Taiwan: Wayne Chang, Jun Chen, Guang-Huan Sun, Ming Li Hseih, and Chi-Rei Yang; United States: Brian Allen, Karl Beutner, Darron Brown, Archana Chatterjee, Daniel Cohen, Eluterio Delfin, Ian Fox, Evan Goldfischer, Dan Henry, Peter Leone, Robert Lipetz, Anita Mercado, Myron Murdock, Natalie Neu, Keith Reisinger, Leigh Roberts, Stephen Richardson, David Whitaker, Michael Wiatrak, Robert Winn, and Philippe Chiliade).
Some of the subjects were studied at the UCSF General Clinical Research Center, supported by grants NIH/NCRR M01-RR-00079 and UL1 RR024131.
The trial was designed by the sponsor (Merck Sharp & Dohme Corp.) in collaboration with external investigators (A.R.G., J.M.P., and S.G.) and an external data and safety monitoring board. The sponsor collated data (J.B.M.), monitored the conduct of the trial (D.G., E.I.O.G., and R.M.H.), performed statistical analyses (J.B.M.), and coordinated manuscript writing with all authors (S.V.). Authors were actively involved in the collection, analysis, and interpretation of the data; creation and revision of the manuscript for intellectual content; and approval of the final manuscript. The first draft was written by R.J.H., A.R.G., and S.V., with contributions from S.G., D.G., E.I.O.G., R.M.H., and J.M.P. All authors met the ICMJE guidelines for authorship, had access to data (with confidentiality agreements), and took part in the decision on where to submit the manuscript for publication.
Footnotes
Published ahead of print 7 December 2011
REFERENCES
- 1. Castellsague X, Bosch FX, Munoz N. 2003. The male role in cervical cancer. Salud. Publica Mex. 45(Suppl. 3):S345–S353 [DOI] [PubMed] [Google Scholar]
- 2. Cook IF. 2008. Sexual dimorphism of humoral immunity with human vaccines. Vaccine 26:3551–3555 [DOI] [PubMed] [Google Scholar]
- 3. FUTURE II Study Group 2007. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 356:1915–1927 [DOI] [PubMed] [Google Scholar]
- 4. Garland SM, et al. 2007. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N. Engl. J. Med. 356:1928–1943 [DOI] [PubMed] [Google Scholar]
- 5. Giuliano AR, et al. 2008. The human papillomavirus infection in men study: human papillomavirus prevalence and type distribution among men residing in Brazil, Mexico, and the United States. Cancer Epidemiol. Biomarkers Prev. 17:2036–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Giuliano AR, et al. 2011. Efficacy of quadrivalent HPV vaccine against HPV infection and disease in males. N. Engl. J. Med. 364:401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moreira ED, Jr, et al. 2011. Safety and reactogenicity of a quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 viral-like particle vaccine in older adolescents and young adults. Hum. Vaccin. 7:768–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olsson S-E, et al. 2007. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like-particle vaccine. Vaccine 25:4931–4939 [DOI] [PubMed] [Google Scholar]
- 9. Opalka D, et al. 2003. Simultaneous quantitation of antibodies to neutralizing epitopes on virus-like particles for human papillomavirus types 6, 11, 16 and 18 by a multiplexed luminex assay. Clin. Diagn. Lab. Immunol. 10:108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Palefsky JM. 2007. HPV infection in men. Dis. Markers 23:261–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palefsky JM, et al. 2005. Anal intraepithelial neoplasia in the highly active antiretroviral therapy era among HIV-positive men who have sex with men. AIDS 19:1407–1414 [DOI] [PubMed] [Google Scholar]
- 12. Reference deleted.
- 13. Stanberry LR, et al. 2002. Glycoprotein-d-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 347:1652–1661 [DOI] [PubMed] [Google Scholar]
- 14. Villa LL, et al. 2005. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16 and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 6:271–278 [DOI] [PubMed] [Google Scholar]
- 15. Woodhall S, et al. 2008. Estimation of the impact of genital warts on health-related quality of life. Sex. Transm. Infect. 84:161–166 [DOI] [PubMed] [Google Scholar]