Abstract
A hemagglutination inhibition (HAI) assay to assess serum antibody responses following Norwalk virus (NV) infection was developed. HAI activity increased significantly in individuals experimentally infected with NV (n = 18) and correlated with antibody levels measured in a histo-blood group antigen (HBGA) blocking assay. Prechallenge HAI antibody levels also correlated with protection from the development of gastroenteritis (Mann-Whitney test, P = 0.02). The HAI assay is another assay suitable for the detection of antibody that correlates with protection from Norwalk virus-associated disease.
TEXT
Noroviruses (NoVs) are major etiological agents of acute gastroenteritis in humans (15). NoVs are classified into genogroups and further subdivided into genotypes based upon the amino acid sequences of their major capsid protein (4). Norwalk virus, the prototypical and most well-characterized human NoV, is a genogroup I, genotype 1 (GI.1) strain.
Histo-blood group antigens (HBGAs) are glycans that are expressed on the surface of epithelium, found in secretions, and present on the surface of erythrocytes (5). NoVs bind HBGAs in vitro, and HBGAs have been identified as attachment factors necessary to establish infection (7, 8, 11, 13). Human challenge studies demonstrated that only individuals who have a functional fucosyltransferase 2 (FUT2) enzyme, and consequently express certain HBGAs on their mucosae or in secretions, are susceptible to infection with Norwalk virus (8, 11). HBGA binding can vary among different norovirus genotypes (6), but similar dependence on the need for a functional fucosyl transferase gene has been described for other NoV genotypes (16, 17).
Serum antibody that blocks binding of norovirus virus-like particles (VLPs) to HBGAs is the first known correlate of protection from gastroenteritis following experimental infection of persons with Norwalk virus (2, 14). The performance of the HBGA blocking assay is affected by temperature, pH, and the quality, quantity, and availability of the purified HBGAs utilized (12, 14). These technical challenges led us to consider the need for a simpler assay to measure the ability of serum antibody to block the virus-HBGA interaction.
Human erythrocytes are a natural source of HBGA ligands (5). NoVs and VLPs have hemagglutination activity via binding of HBGAs, and hemagglutination inhibition (HAI) activity has been shown to increase significantly following experimental challenge or vaccination of human subjects (3, 7). Therefore, we hypothesized that the HAI assay could be used as an alternative to the HBGA blocking assay to quantitate blocking antibodies in serum.
Serum samples collected during human experimental infection studies with Norwalk virus, the prototypical human NoV, were utilized for this work. All participants provided written informed consent, and the study was performed as described previously (1, 10, 14). Sera were collected prechallenge (day 0 [d0]) and over a 6-month follow-up period. Evidence of infection was defined as a ≥4-fold increase in virus-specific antibody titer between d0 and d28 by enzyme-linked immunosorbent assay (ELISA) or direct detection of viral antigen or RNA in the stool (by either ELISA or reverse transcription-PCR [RT-PCR], respectively). Norwalk virus-infected persons experiencing the following signs and symptoms were considered to have viral gastroenteritis: one episode of vomiting plus one other symptom (abdominal cramps, nausea, bloating, watery stool, headache, or a fever of >37.6°C) or moderate diarrhea (watery feces of at least 200 g) for any continuous 24-hour period (1, 10, 14).
Norwalk virus VLPs were produced using a baculovirus expression system, as described elsewhere (9, 14). Human type O erythrocytes were collected from healthy adult volunteers in Alsever buffer, washed twice with Dulbecco's phosphate-buffered saline (PBS) without Ca2+ and Mg2+ (Invitrogen), and pelleted via centrifugation at 4°C for 10 min at 500 × g. Type O human erythrocytes have previously been demonstrated to hemagglutinate Norwalk virus VLPs (3, 7). Serum samples were pretreated to inactivate nonspecific hemagglutination-inhibiting agents by heating at 56°C for 30 min. Heat-treated sera were then combined in a 1:5 ratio with a 25% (wt/vol) preparation of kaolin (Sigma) and incubated with continuous mixing at room temperature for 30 min to selectively bind and remove any remaining lipid-based nonspecific hemagglutination-inhibiting elements. The treated serum was recovered by centrifugation for 10 min at 10,000 × g and aspiration of the supernatant. The recovered serum was allowed to adsorb to test erythrocytes three times, each for 1 h at 4°C, followed by pelleting of the erythrocytes by centrifugation at 500 × g for 10 min, to eliminate nonspecific hemagglutination activity.
Treated serum was serially 2-fold diluted on 96-well V-bottomed microtiter plates from a starting concentration of 1:10 in PBS with 0.85% saline, pH 5.5. It was incubated for 30 min at room temperature with four hemagglutination units, or ∼20 ng, of Norwalk virus VLPs per reaction, as determined by a hemagglutination assay and confirmed by back-titration on each microtiter plate used for the experiment. Each sample was then combined with an equal volume of 0.5% type O human erythrocytes prepared using 0.85% saline, pH 6.2, and incubated for 2 h at 4°C. The HAI titer was defined as the reciprocal of the highest dilution of serum that completely inhibited hemagglutination by the viral antigen. Geometric mean titers (GMTs) were also calculated for each time point to summarize the overall kinetics of volunteer seroresponses in the study population.
Of 34 enrolled volunteers, 5 were randomized to receive placebo and 29 were challenged with one of three different doses of the same challenge pool of Norwalk virus (4,800, 48, or 4.8 RT-PCR units). Of those who received Norwalk virus, 18 became infected, and 12 of these patients experienced gastroenteritis. The majority of the 16 uninfected individuals had a reason to resist infection, including receipt of placebo, a nonfunctional fucosyltransferase 2, or blood group B or AB (14). The serum HAI antibody responses were compared to anti-Norwalk virus antibody responses measured by ELISA and the blocking assay (14). All persons who demonstrated a ≥4-fold rise in anti-Norwalk virus ELISA titer between d0 and d28 also demonstrated a 4-fold rise in HAI titer (Table 1). Conversely, no one who was uninfected demonstrated a 4-fold rise in HAI titer, HBGA blocking titer, or ELISA titer (n = 16).
Table 1.
Seroresponse following challenge with Norwalk virus, detected by HAI ELISA and blocking assays, by study visit (n = 34)a
| Patient parameterb | Day 0 | Day 28 | Day 180 |
|---|---|---|---|
| Infected, gastroenteritis (n = 12) | |||
| HAI | |||
| GMT (95% CI) | 9 (6, 14) | 341 (194, 599) | 78 (52, 118) |
| Seroresponse frequency (%) | NA | 100 | 100 |
| % with titer of ≥40 | 8.3 | 100 | 100 |
| HBGA blocking assay | |||
| GMT BT50 (95% CI) | 34 (23,51) | 449 (260, 777) | 404 (250, 651) |
| Seroresponse frequency (%) | NA | 100 | 100 |
| ELISA | |||
| GMT (95% CI) | 3,800 (1,200, 12,000) | 580,000 (290,000, 1,200,000) | 82,000 (47,000, 140,000) |
| Seroresponse frequency (%) | NA | 100 | 100 |
| Infected, no gastroenteritis (n = 6) | |||
| HAI | |||
| GMT (95% CI) | 32 (15, 68) | 685 (287, 1,633) | 180 (74, 437) |
| Seroresponse frequency (%) | NA | 100 | 100 |
| % with titer of ≥40 | 83.3 | 100 | 100 |
| HBGA blocking assay | |||
| GMT BT50 (95% CI) | 167 (78, 356) | 1,957 (1,051, 3,646) | 903 (494, 1,652) |
| Seroresponse frequency (%) | NA | 100 | 100 |
| ELISA | |||
| GMT (95% CI) | 12,000 (4,400, 30,000) | 1,000,000 (530,000, 2,000,000) | 130,000 (61,000, 280,000) |
| Seroresponse frequency (%) | NA | 100 | 100 |
| Uninfected (n = 16)c | |||
| HAI | |||
| GMT (95% CI) | 9 (6, 14) | 8 (5, 14) | 8 (5, 13) |
| Seroresponse frequency (%) | NA | 0 | 0 |
| % with titer of ≥40 | 6.3 | 6.3 | 6.3 |
| HBGA blocking assay | |||
| GMT BT50 (95% CI) | 40 (25, 64) | 41 (25, 67) | NT |
| Seroresponse frequency (%) | NA | 0 | NT |
| ELISA | |||
| GMT (95% CI) | 1,200 (350, 3,900) | 1,000 (300, 3,400) | 1,000 (300, 3,400) |
| Seroresponse frequency (%) | NA | 0 | 0 |
The seroresponse represents the percentage of persons within each group with a ≥4-fold rise in titer relative to the day 0 (d0) value.
Abbreviations: CI, confidence interval; HAI, hemagglutination inhibition; NA, not applicable; NT, not tested; HBGA, histo-blood group antigen; BT50, the level of antibody that blocks 50% of the signal generated from binding of Norwalk virus VLPs to HBGAs in an HBGA blocking assay.
The majority of the uninfected individuals had a reason to resist infection, including receipt of placebo, a nonfunctional fucosyltransferase 2, or blood group B or AB.
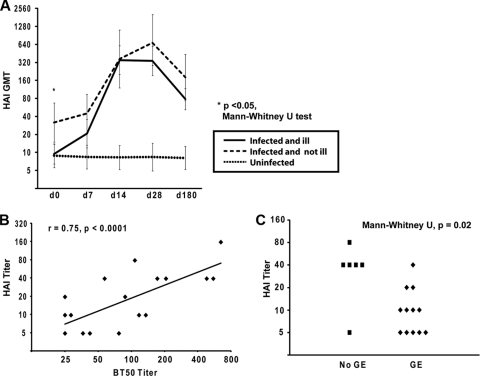
Regardless of clinical outcome, the GMT of serum HAI activity from persons infected with Norwalk virus (n = 18) peaked at 28 days following challenge, following a similar curve to that observed with the HBGA blocking antibody levels (Fig. 1A) (14). By 28 days postchallenge, 100% of infected volunteers had an HAI titer of at least 40. In comparison, volunteers who did not become infected following challenge (n = 16) did not demonstrate any rise in HAI titer at any time point. The HAI titer was significantly correlated (Stata IC10; StataCorp, College Station, TX) with HBGA blocking titer at the baseline (Pearson's r = 0.75 [P < 0.0001]) (Fig. 1B) and at d28 postchallenge (Pearson's r = 0.94 [P < 0.0001]) (data not shown).
Fig 1.
(A) Kinetics of Norwalk virus-specific antibody by hemagglutination inhibition (HAI) assay. Infected, asymptomatic individuals (n = 6) had a higher baseline geometric mean titer (GMT) than infected individuals who developed gastroenteritis (n = 12). Uninfected individuals did not demonstrate a rise in HAI GMT (n = 16). Data are presented with 95% confidence intervals of the GMT. (B) Among infected persons (n = 18), the HAI titer correlated with the blocking antibody titer at the baseline (Pearson's correlation). BT50, the level of antibody that blocks 50% of the signal generated from binding of Norwalk virus VLPs to HBGAs in vitro. (C) Among infected persons, baseline HAI titer is associated with clinical outcome. GE, gastroenteritis.
Among infected volunteers, those who did not develop viral gastroenteritis postchallenge had a significantly higher HAI titer at baseline than those who did (Mann-Whitney U test, P = 0.02). These data suggest that an HAI titer of 40 may represent a threshold for protective immune response with regard to clinical outcome in susceptible, infected individuals (Fig. 1C). HAI antibody decreased to ≤50% of the peak HAI titer by d180. However, the d180 HAI titer remained above 40 in the majority (17 of 18) of infected individuals. Larger trials will be needed to confirm the reliability of this cutoff.
Vaccination has been proposed as an approach for preventing norovirus infection, and vaccine candidates are undergoing clinical trials (2, 3, 4). Tools for evaluating and defining protective immune responses are critical for the development and assessment of vaccines and diagnostics. The serum HAI assay can measure immune responses after vaccination (3) and is an alternative serological correlate of protection that is easier to perform than the HBGA blocking assay.
ACKNOWLEDGMENTS
We acknowledge Fred Neill for technical support.
This work was conducted with support from the National Institutes of Health (grants P01 AI 57788, N01 AI 25465, P30 DK56336, M01 RR-000188, and T32 GM88129).
Footnotes
Published ahead of print 21 December 2011
REFERENCES
- 1. Atmar RL, et al. 2008. Norwalk virus shedding after experimental human infection. Emerg. Infect. Dis. 14:1553–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atmar RL, et al. 2011. Norovirus vaccine protects against experimental human Norwalk virus illness. N. Engl. J. Med. 365:2178–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. El-Kamary SS, et al. 2010. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J. Infect. Dis. 202:1649–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glass RI, Parashar UD, Estes MK. 2009. Norovirus gastroenteritis. N. Engl. J. Med. 361:1776–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hakomori S. 1999. Antigen structure and genetic basis of histo-blood groups A, B and O: their changes associated with human cancer. Biochim. Biophys. Acta 1473:247–266 [DOI] [PubMed] [Google Scholar]
- 6. Huang P, et al. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 79:6714–6722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hutson AM, Atmar RL, Marcus DM, Estes MK. 2003. Norwalk virus-like particle hemagglutination by binding to H histo-blood group antigens. J. Virol. 77:405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hutson AM, Airaud F, LePendu J, Estes MK, Atmar RL. 2005. Norwalk virus infection associates with secretor status genotyped from sera. J. Med. Virol. 77:116–120 [DOI] [PubMed] [Google Scholar]
- 9. Jiang X, Wang M, Graham DY, Estes MK. 1992. Expression self-assembly, and antigenicity of the Norwalk virus capsid protein. J. Virol. 66:6527–6532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kavanagh O, et al. 2011. Serological responses to experimental Norwalk virus infection measured using a quantitative duplex time-resolved fluorescence immunoassay. Clin. Vaccine Immunol. 18:1187–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lindesmith LC, et al. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548–553 [DOI] [PubMed] [Google Scholar]
- 12. Lindesmith LC, et al. 2010. Heterotypic humoral and cellular immune responses following Norwalk virus infection. J. Virol. 84:1800–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marionneau S, et al. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reeck A, et al. 2010. Serological correlate of protection against norovirus-induced gastroenteritis. J. Infect. Dis. 202:1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scallan E, et al. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg. Infect. Dis. 17:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan M, et al. 2008. Outbreak studies of a GII-3 and a GII-4 norovirus revealed an association between HBGA phenotypes and viral infection. J. Med. Virol. 80:1296–1301 [DOI] [PubMed] [Google Scholar]
- 17. Thorven M, et al. 2005. A homozygous nonsense mutation (428G→A) in the human secretor (FUT2) gene provides resistance to symptomatic norovirus (GGII) infections. J. Virol. 79:15351–15355 [DOI] [PMC free article] [PubMed] [Google Scholar]