Abstract
Plasmodium falciparum blood-stage antigens such as merozoite surface protein 1 (MSP-1), apical membrane antigen 1 (AMA-1), and the 175-kDa erythrocyte binding antigen (EBA-175) are considered important targets of naturally acquired immunity to malaria. However, it is not clear whether antibodies to these antigens are effectors in protection against clinical disease or mere markers of exposure. In the context of a randomized, placebo-controlled trial of intermittent preventive treatment in infants conducted between 2002 and 2004, antibody responses to Plasmodium falciparum blood-stage antigens in a cohort of 302 Mozambican children were evaluated by immunofluorescence antibody test and enzyme-linked immunosorbent assay at 5, 9, 12, and 24 months of age. We found that IgG subclass responses to EBA-175 were differentially associated with the incidence of malaria in the follow-up period. A double amount of cytophilic IgG1 or IgG3 was associated with a significant decrease in the incidence of malaria (incidence rate ratio [IRR] = 0.49, 95% confidence interval [CI] = 0.25 to 0.97, and P = 0.026 and IRR = 0.44, CI = 0.19 to 0.98, and P = 0.037, respectively), while a double amount of noncytophilic IgG4 was significantly correlated with an increased incidence of malaria (IRR = 3.07, CI = 1.08 to 8.78, P = 0.020). No significant associations between antibodies to the 19-kDa fragment of MSP-1 (MSP-119) or AMA-1 and incidence of malaria were found. Age, previous episodes of malaria, present infection, and neighborhood of residence were the main factors influencing levels of antibodies to all merozoite antigens. Deeper understanding of the acquisition of antibodies against vaccine target antigens in early infancy is crucial for the rational development and deployment of malaria control tools in this vulnerable population.
INTRODUCTION
In areas where the intensity of transmission of Plasmodium falciparum is high, the greatest burden of malaria occurs in children under age 5 years (21) and that of severe malaria occurs in infants under age 12 months (48). Natural immunity is acquired with age and exposure, protecting quite effectively from disease and high parasitemia (13). The exact immune mediators, mechanisms, and targets underlying such protection are unknown, but immunoglobulin passive transfer studies demonstrated that IgG antibodies are important effectors in protection (6, 45).
Blood-stage antigens such as the 19-kDa fragment of merozoite surface protein 1 (MSP-119) (5, 8, 12, 15, 29, 31, 39), apical membrane antigen 1 (AMA-1) (11, 20, 40), and the 175-kDa erythrocyte binding antigen (EBA-175) (22, 34, 36, 38, 50) are considered important targets of naturally acquired immunity (9), and antibodies against these parasite proteins inhibit invasion of erythrocytes in vitro (7, 31, 37). Recent work has shown an association between IgG to EBA-175 and protection from malaria (26, 42). However, conflicting evidence in immunoepidemiological studies and unsuccessful phase IIb vaccine trials question the extent of the relevance of AMA-1 and MSP-1 in protection against malaria (33). A meta-analysis of antimerozoite antibodies supported the protective effect of total IgG responses to particular antigens against symptomatic falciparum malaria in humans (17) and highlighted the requirement for more prospective cohort studies in different populations examining multiple antigens at multiple time points. In addition, the antibody isotype elicited by P. falciparum antigens is considered to be important, such that the protective effect of IgG has been attributed to the cytophilic (IgG1 and IgG3) rather than the noncytophilic (IgG2 and IgG4) subclasses (16, 28, 32, 44, 53).
Several trials of malaria control strategies (1, 24, 27) are being conducted in Manhiça, an area of southern Mozambique where malaria is endemic. As no previous data on naturally acquired immune responses to blood-stage P. falciparum antigens in the study area were available, a detailed analysis of the development of antibody responses during the first 2 years of life was conducted in the context of a randomized, placebo-controlled trial of intermittent preventive treatment in infants (IPTi) with sulfadoxine-pyrimethamine (SP) (24, 41). This paper sets out to report the evaluation of the age pattern of naturally acquired antibodies to the leading vaccine candidates MSP-119, AMA-1, and EBA-175, the description of the decay of maternal IgG, the pattern of IgG isotype responses, the effect of past and present parasite exposure and neighborhood in the antibody response, and the role of these antibodies in protection against clinical malaria. The majority of prior studies that have attempted to evaluate the role of antimalarial antibody responses with a prospective design have been conducted on older age children rather than infants. The understanding of the acquisition of antibody-mediated natural immunity in early infancy is crucial for the rational development and deployment of malaria control tools, including vaccines, in this most vulnerable population.
MATERIALS AND METHODS
Study area and participants.
The study was conducted at the Centro de Investigação em Saúde da Manhiça (CISM), Manhiça District (Maputo Province), in southern Mozambique. Adjacent to CISM is the Manhiça Health Center, a 110-bed referral health care facility that provides curative and preventive services to the area population. The characteristics of the area have been described in detail elsewhere (2). Malaria transmission is perennial with marked seasonality and an entomological inoculation rate for 2002 of 38 for the whole study area. There is a continuous demographic surveillance system in place covering 36,000 inhabitants of the same ethnicity.
Children included in this analysis participated in an IPTi randomized, placebo-controlled trial (24, 41) with registration number NCT00209795 (http://clinicaltrials.gov). Infants were recruited at age 3 months when attending the Expanded Program on Immunization (EPI) clinic between September 2002 and February 2004. Permanent residents of the CISM study area who did not report allergies to sulfa drugs and did not require admission to the hospital were eligible for inclusion. IPTi-SP or placebo was administered to the study participants at 3, 4, and 9 months of age. Antibody measurements were done at 5, 9, 12, and 24 months of age for the last 302 children (50% under IPTi-SP) recruited out of the total of 1,498 participating in the efficacy trial. At each visit, capillary blood was collected into Microtainer tubes with EDTA to obtain plasma and an erythrocyte pellet. Clinical surveillance for malaria morbidity during the whole study was done through round-the-clock (24 h a day, 7 days a week) passive case detection, documented with standardized questionnaires and databases. Written informed consent for the study was obtained from parents/guardians. Ethical approval was obtained from the ethics review committee of Mozambique (National Health and Bioethics Committee, CNBS) and the ethics review committee of the Hospital Clinic of Barcelona (Ethical Committee of Clinical Investigation, CEIC) in Spain.
Malaria parasitemia.
P. falciparum infections were diagnosed at 12 and 24 months of age and during the passive morbidity surveillance by thick and thin blood films, which were stained and read according to quality-control procedures (3). Parasite density was assessed by counting the number of asexual-stage parasites until 500 leukocytes or parasites had been counted. In addition, submicroscopic P. falciparum infections were assessed by nested PCR targeting the small-subunit rRNA genes, as described previously (52), from erythrocyte pellets collected at the four cross-sectional visits (at 5, 9, 12, and 24 months of age).
Antibody assays.
Immunofluorescence antibody test (IFAT) with asynchronous cultures of P. falciparum (3D7) and enzyme-linked immunosorbent assays (ELISAs) were done as described previously (41). To prepare IFAT slides, in vitro cultures of P. falciparum (3D7) were used at 3.5% parasitemia and 3 to 5% hematocrit. For the ELISA, plasma samples were assayed for IgG (including isotypes) and IgM to the recombinant proteins MSP-119 (19-kDa fragment, C terminus, 3D7 [25]), AMA-1 (3D7 [23]), and EBA-175 (region F2, CAMP [37]) from the International Centre for Genetic Engineering and Biotechnology (ICGEB; New Delhi, India) expressed in Escherichia coli and purified with a histidine tag. Briefly, high-binding 96-well microplates (Maxisorp; Nunc, Denmark) coated with the proteins were incubated first with plasma (1:200) and later with peroxidase-conjugated goat anti-human IgG or IgM secondary antibodies (Sigma, St. Louis, MO). To develop the plates, phosphate solution with 0.012% H2O2 substrate and o-phenylenediamine chromogen were added, and the colorimetric reaction was stopped with 3 M H2SO4.
IgG isotypes were measured in the samples in which a positive IgG response for the corresponding antigen was detected at a given time point. Identical sets of antigen-coated plates were prepared for the determination of IgG, IgG1, IgG2, IgG3, or IgG4 in assays performed with any one plasma sample (1:200, duplicates) in parallel on the same day. ELISA conditions (including plasma dilutions) had been previously standardized in our laboratory with reference reagents. Wells were incubated for 3 h with peroxidase-conjugated polyclonal sheep anti-human IgG1 (1:6,000), IgG2 (1:3,000), IgG3 (1:6,500), or IgG4 (1:5,000) (Binding Site, Birmingham, United Kingdom) or peroxidase-conjugated rabbit anti-human IgG specific for gamma chains (1:6,000; Dako, Glostrup, Denmark). In parallel, purified human myeloma proteins IgG1, IgG2, IgG3, and IgG4 (Binding Site) were coated on plates at 2-fold dilutions from 2 to 0.001 μg/ml. Peroxidase-conjugated antibodies to each IgG subclass (Binding Site), at the same dilutions used for plasma samples, were reacted with the myeloma proteins and used as positive controls.
In both IFAT and ELISA, positive controls were a pool of plasma from 8 adults with lifelong exposure to malaria and negative controls were 9 plasma samples from nonexposed adults. For the IFATs, positive- and negative-control plasma samples were used in each slide, they were read blind, and the highest dilution giving green positive fluorescence was scored. Data are presented as endpoint IgG titers (reciprocal of last plasma dilution causing positive fluorescence above the negative-control levels). For the ELISA, specific reactivity of plasma samples was obtained as optical density (OD) values at 492 nm (OD492). For total IgG and IgM, OD492 data were normalized against a positive control (1:200) run in the same experiment and used as continuous variables (arbitrary units, percent). IgG isotype data were reported as OD492s. Positive antibody responses were those with an OD value above the cutoff (arithmetic mean of negative controls plus 3 standard deviations).
Definitions and statistical methods.
P. falciparum infection was defined as the presence of asexual P. falciparum parasites of any density in a blood smear or when assessed by PCR. A clinical malaria episode was defined as a positive blood smear plus an axillary temperature of ≥37.5°C. The sensitivity and specificity of these definitions are ∼100% and 84%, respectively, in infants and 100% and 79.4%, respectively, in children 1 to 4 years old (46). Months were defined to be 30.4 days. Incidence was expressed as the number of episodes per person years at risk (PYAR). To estimate the incidence, the time at risk was calculated as the number of PYAR since the beginning of the time at risk until the end of follow-up, migration, death, or withdrawal of consent, whatever was first. Children were not considered to be at risk for 28 days after the start of each episode of clinical malaria.
Antibody variables were logarithmically transformed. Averages within ages are presented as geometric means (GM) plus 95% confidence intervals (CI). Distributions of antibody responses to each antigen at each time point are presented as weighted scatter plots. To identify variables independently associated with antibody levels, multivariate random-effect regression models using a forward stepwise procedure (P-enter = 0.05, from likelihood ratio test) were estimated, including intervention group, age, neighborhood of residence (defined by the established geographical and demographic platforms), previous clinical malaria episodes (detected by the passive morbidity surveillance), and present infection (detected at the four cross-sectional visits by microscopy and/or PCR). In these models, the subject variable was a random effect; thus, we were able to take the sampling variability into account. Because of the strong effect of previous malaria episodes (41), we used a second model including only children with at least one episode before contact to further evaluate the effect of additional variables on antibody values, including intervention group, age, number of previous malaria episodes, time from previous episodes, present infection, and parasite density (number of parasites/μl).
To evaluate the relationship between the level of each of the antibody variables individually at 5 and 12 months and the incidence of clinical malaria episodes to 12 or 24 months, respectively, negative binomial regression models were estimated. Two periods were defined because of the differences between the 2 years (IPTi and maternal antibodies in year 1). IgG responses were treated as continuous values or categorized by tertiles. The incidence rate ratio (IRR) of children with antibody levels in the highest tertile against those in the lowest tertile was estimated, as was the IRR per 2-fold increase in the value of antibodies. Regression analyses were done crude and adjusted by intervention group, sex, neighborhood, present infection, and previous clinical episodes for each antibody variable.
In an advanced analysis to evaluate the relationship between all the antibody variables together (IgG and IgM to all antigens) at 5 and 12 months and the incidence of clinical malaria episodes to 12 or 24 months, respectively, negative binomial regression models using a forward stepwise procedure (P-enter = 0.05, from likelihood ratio test) were estimated and adjusted by intervention group, sex, neighborhood, present infection, and previous clinical episodes. Only children who had at least 70% of the antibody variables measured were included in this advanced analysis; complete ELISA data were obtained from all samples, whereas IFAT data were obtained from 78.35% of samples, due to limited plasma volumes in some children.
Data analysis was performed using Stata (version 11) software (Stata statistical software: release 10; StataCorp LP, College Station, TX, 2007). Statistical significance was defined at a P value of <0.05.
RESULTS
Age pattern of naturally acquired IgG, IgM, and IgG subclass antibodies.
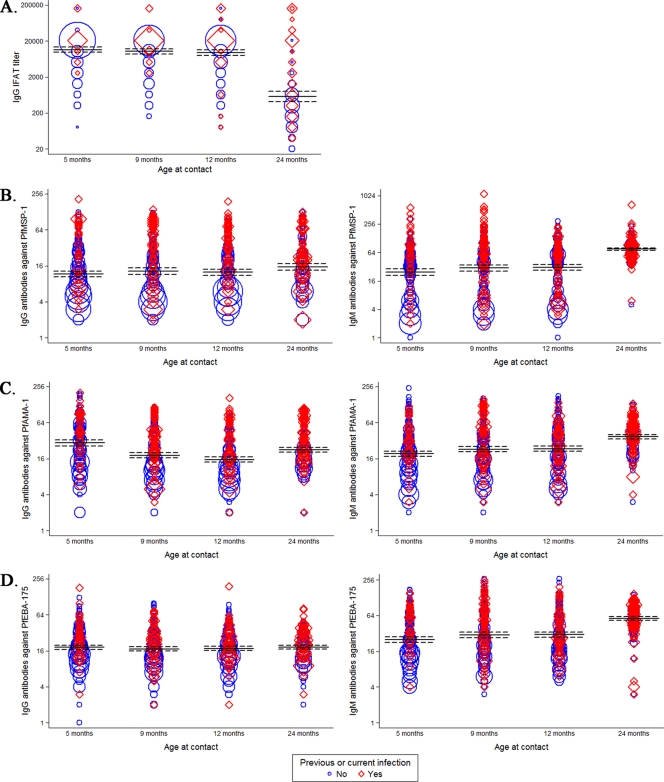
IgG titers to P. falciparum blood stages measured by IFAT were very high during the first year of life (Fig. 1A), decreasing at age 2 years. The table in the supplemental material summarizes the number of samples that were available to be analyzed by ELISA per each time point and the frequency of positive antibody responses. The prevalence of antibody responses to AMA-1 was the highest at all time points (27.4 to 61.4% for IgG), followed by MSP-119 (22.1 to 30.3% for IgG) and, lastly, by EBA-175 (16.9 to 24.23% for IgG). The age patterns of IgG responses against each merozoite antigen were different. IgG values to MSP-119 remained constant during the first year of life and then increased at 2 years, but not significantly (Fig. 1B). IgG responses to AMA-1 were the highest at age 5 months and then gradually decreased up to age 1 year and increased at age 2 years (Fig. 1C); the association between age and levels of IgG to AMA-1 was significant (P < 0.0001). Total IgG responses to EBA-175 remained quite constant over the 2 years but at low levels (Fig. 1D) and were not significantly associated with age. IgM responses to the three merozoite antigens were significantly associated with age (P < 0.0001), increasing from 5 to 9 months and from 12 to 24 months (Fig. 1B to D).
Fig 1.
Antibody responses to asexual blood-stage whole P. falciparum parasites (A), P. falciparum MSP-119 (PfMSP-119) (B), P. falciparum AMA-1 (PfAMA-1) (C), and P. falciparum EBA-175 (PfEBA-175) (D) in children up to 2 years of age. For IFAT data (A), IgG levels (y axes in log scale) are expressed as endpoint titers. For ELISA data (B to D), IgG (left) and IgM (right) levels are expressed as normalized OD values (y axes, percentages). In the weighted scatter plots, the area of the symbol is proportional to the number of observations. Geometric mean IgG titers and 95% confidence intervals are indicated by horizontal continuous and dashed lines, respectively. Red symbols correspond to IgG levels in children with previous or present infection.
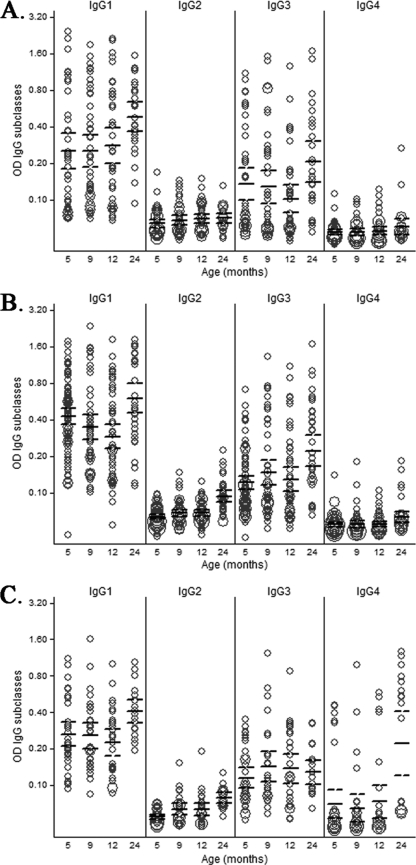
In those plasma samples in which total IgG responses above background levels could be measured, the subclasses of those IgG antibodies were subsequently analyzed. The pattern of IgG isotypes varied depending on the antigen and the age of the child (Fig. 2). MSP-119 and AMA-1 predominantly elicited cytophilic IgG1 and IgG3 subclasses. EBA-175 primarily induced IgG1 and IgG3, followed by induction of IgG4 at 2 years. IgG2 levels were very low for all antigens, and IgG4 levels were low for MSP-119 and AMA-1, consistent with previous reports showing that these P. falciparum proteins predominantly induce IgG1 and IgG3 subclasses (53). Our assay included purified human myeloma protein controls that confirmed the positive reactivity of all the isotype reagents, including IgG2 and IgG4. The kinetics of IgG1, IgG3, and IgG4 were different for each antigen (Fig. 2). IgG1 responses to MSP-119 were quite constant during the first year of life and then increased at age 2 years (P = 0.0207), while IgG3 appeared to decrease over the first year and then increased at 2 years of age (P < 0.0001) (Fig. 2A). In contrast, IgG1 responses to AMA-1 were high at 5 months of age and then decreased up to age 1 year and increased to the highest levels at age 2 years (P < 0.0001), whereas IgG3 remained constant in year 1 and then increased by age 2 years (Fig. 2B). IgG1 responses to EBA-175 remained similar over the first year and then increased in the second year (P = 0.0031) (Fig. 2C). IgG3 to EBA-175 remained moderate at all time points; IgG4 was low in the first year but markedly increased at age 2 years (P < 0.0001).
Fig 2.
IgG subclass responses to MSP-119 (A), AMA-1 (B), and EBA-175 (C) in children up to age 2 years of age. Only data for children receiving IPTi with placebo are depicted here. IgG levels (y axes) are expressed as OD values. In the weighted scatter plots, the area of the symbol is proportional to the number of observations. Geometric mean IgG levels and 95% confidence intervals are indicated by horizontal continuous and dashed lines, respectively.
Effect of exposure to P. falciparum on the magnitude of antibody responses.
There was a strong association between previous clinical malaria episodes or present infection and the magnitude of antibody responses, particularly for MSP-119 and AMA-1 (Table 1). Children with a previous clinical malaria episode had, on average, 1.78 times higher levels of IgG to AMA-1 than children without (CI = 1.54 to 2.05, P < 0.0001). Similarly, children with current parasitemia had 1.26 times higher levels of IgG to AMA-1 than uninfected children (CI = 1.10 to 1.43, P = 0.0007). IgM levels were also strongly associated with present infections but not with past clinical episodes (Table 1). Total IgG levels for EBA-175 were significantly associated with previous episodes and current infection, and IgM levels were significantly associated with current infection. However, in those children with detectable IgG responses to EBA-175, IgG1 isotype levels were significantly associated with previous episodes only, and IgG3 isotype levels were significantly associated with current P. falciparum infection only (Table 1). All these analyses were adjusted by age and neighborhood of residence and also by IPTi treatment group, since for some antigens SP affected antibody levels, as reported previously (41). In all cases, when neighborhood was associated with levels of antibody to an antigen, current infection also appeared to be significantly associated in the same model, suggesting that the effect of neighborhood was linked to the incidence of P. falciparum infection in the area. However, in this study we were unable to explain the spatial associations of antibody levels because none of the geographical, sociological, or entomological characteristics documented (e.g., proximity to swamps, rivers, and housing type) differed significantly among the neighborhoods.
Table 1.
Effect of previous clinical malaria episodes and present infections on antibody levels to whole parasite (IFAT), MSP-119, AMA-1, and EBA-175a
| Antigen | Antibody | Result by exposure to malaria |
|||||
|---|---|---|---|---|---|---|---|
| Previous malaria episodeb |
Present infectionc |
||||||
| Proportional difference | 95% CI | P value | Proportional difference | 95% CI | P value | ||
| Whole P | IgG | 2.85 | 2.10–3.86 | <0.0001 | 1.84 | 1.37–2.47 | <0.0001 |
| MSP-119 | IgG | 2.05 | 1.76–2.39 | <0.0001 | 1.31 | 1.14–1.51 | 0.0001 |
| IgG1 | 2.56 | 2.05–3.19 | <0.0001 | 1.94 | 1.59–2.37 | <0.0001 | |
| IgG2 | 1.14 | 1.06–1.23 | 0.0003 | 1.13 | 1.06–1.21 | 0.0003 | |
| IgG3 | 2.31 | 1.89–2.83 | <0.0001 | 1.74 | 1.42–2.14 | <0.0001 | |
| IgG4 | 1.16 | 1.10–1.23 | <0.0001 | 1.09 | 1.03–1.16 | <0.004 | |
| IgM | NS | NS | NS | 1.28 | 1.11–1.48 | 0.0005 | |
| AMA-1 | IgG | 1.78 | 1.54–2.05 | <0.0001 | 1.26 | 1.10–1.43 | 0.0007 |
| IgG1 | 1.49 | 1.24–1.81 | <0.0001 | 1.44 | 1.22–1.71 | <0.0001 | |
| IgG2 | 1.06 | 1.00–1.12 | 0.0444 | 1.10 | 1.04–1.15 | 0.0006 | |
| IgG3 | 1.86 | 1.57–2.20 | <0.0001 | 1.52 | 1.28–1.82 | <0.0001 | |
| IgG4 | 1.06 | 1.01–1.12 | <0.0111 | 1.09 | 1.04–1.15 | 0.0004 | |
| IgM | NS | NS | NS | 1.25 | 1.12–1.39 | 0.0001 | |
| EBA-175 | IgG | 1.18 | 1.06–1.31 | 0.0025 | 1.12 | 1.01–1.24 | 0.026 |
| IgG1 | 1.22 | 1.00–1.49 | 0.0448 | NS | NS | NS | |
| IgG2 | NS | NS | NS | NS | NS | NS | |
| IgG3 | NS | NS | NS | 1.32 | 1.08–1.62 | 0.007 | |
| IgG4 | NS | NS | NS | NS | NS | NS | |
| IgM | NS | NS | NS | 1.15 | 1.02–1.29 | 0.023 | |
Multivariate random-effect models estimated by stepwise procedure and adjusted by age and neighborhood of residence. NS, not significant in the forward stepwise procedure (P-enter = 0.05, from likelihood ratio test).
Number of children who had previous clinical episodes: 18 at 5 months, 54 at 9 months, 60 at 12 months, and 82 at 24 months.
Number of children with current parasitemia (detected by microscopy or PCR): 36 children at 5 months, 62 at 9 months, 37 at 12 months, and 78 at 24 months.
In a subgroup analysis including only children with previous malaria episodes (103 subjects and 212 antibody measurements), levels of antibodies in children with current parasitemia were significantly higher than in those who were aparasitemic (Table 2).
Table 2.
Levels of antibodies in children with current parasitemia versus those who were aparasitemic in the subgroup of children who had previous episodes of malariaa
| Antigen | Antibody | Proportional difference in IgG levelsb | 95% CI | P value |
|---|---|---|---|---|
| MSP-119 | IgG | 1.61 | 1.24–2.09 | 0.0003 |
| IgG1 | 1.28 | 1.02–1.60 | 0.0332 | |
| IgG3 | 1.73 | 1.32–2.26 | <0.0001 | |
| AMA-1 | IgG1 | 1.44 | 1.12–1.85 | 0.0044 |
| IgG3 | 1.82 | 1.37–2.42 | <0.0001 | |
| IgG4 | 1.11 | 1.02–1.22 | 0.0222 | |
| EBA-175 | IgG3 | 1.46 | 1.15–1.86 | 0.0020 |
Current infection was significantly associated with increased antibody levels for the antigens and IgG isotypes included in the table. The analysis was done by multivariate random-effect regression models using a forward stepwise procedure (P-enter = 0.05, from likelihood ratio test) including the following variables: intervention group, age, number of previous malaria episodes, time from previous episodes, current infection, and parasite density.
Difference in IgG levels in children who were currently parasitemic versus those who were not.
Association between antibody responses and incidence of clinical malaria.
We analyzed the association between individual antibody responses at 5 months and at 12 months and the subsequent incidence of malaria at two different times-at-risk intervals: 5 to 12 and 12 to 24 months. Table 3 summarizes the relationship between magnitude of antibodies and incidence of malaria in the two time periods. Results could be grouped into five different patterns, the most common being no significant association between antibody levels and risk of malaria (e.g., IgM to AMA-1) (pattern i) and high antibody levels being significantly associated with an increased risk of malaria in the crude analysis but with significance disappearing in the adjusted analysis (e.g., IgG to MSP-119) (pattern ii). In general, pattern ii was the trend for all responses, but the trend described by pattern i did not reach statistical significance. There was only one case (pattern iii) in which the association between high antibody levels at age 5 months and increased risk remained significant, after adjusting for previous episodes (IgG1 to MSP-119 at 5 months). We observed a differential pattern for IgG subclasses to EBA-175 in the adjusted analysis, particularly in year 2: 2-fold increments in levels of cytophilic IgG1 and IgG3, each one independently, were significantly correlated with a decreased incidence of malaria (for IgG1, IRR = 0.49, CI = 0.25 to 0.97, and P = 0.026; for IgG3, IRR = 0.44, CI = 0.19 to 0.98, and P = 0.037) (pattern iv), and 2-fold increments in the levels of the noncytophilic IgG4 were correlated with an increased incidence of malaria (IRR = 3.07, CI = 1.08 to 8.78, P = 0.020) (pattern v). Analyses comparing the IRRs of children with antibody levels in the highest tertile against those of children with antibody levels in the lowest tertile were consistent with the results reported in Table 3 (data not shown).
Table 3.
Association between levels of antibodies (2-fold increment) at 5 and 12 months and incidence of malaria at two time periodsa
| Antigen | Antibody | 5-12 mo |
12-24 mo |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crude |
Adjusted |
Crude |
Adjusted |
||||||||||
| IRR | 95% CI | P value | IRR | 95% CI | P value | IRR | 95% CI | P value | IRR | 95% CI | P value | ||
| Whole P | IgG | 1.23 | 0.96–1.58 | 0.100 | 0.99 | 0.79–1.24 | 0.923 | 1.25 | 0.05–1.50 | 0.016 | 1.05 | 0.90–1.24 | 0.528 |
| MSP-119 | IgG | 1.35 | 1.10–1.67 | 0.005 | 1.19 | 0.95–1.50 | 0.129 | 1.46 | 1.21–1.75 | <0.001 | 1.07 | 0.88–1.29 | 0.486 |
| IgG1 | 1.91 | 1.39–2.64 | <0.001 | 1.74 | 1.20–2.52 | 0.003 | 1.69 | 1.20–2.38 | 0.002 | 0.85 | 0.54–1.32 | 0.464 | |
| IgG2 | 3.65 | 1.35–9.84 | 0.013 | 3.41 | 1.36–8.53 | 0.015 | 2.37 | 0.83–6.71 | 0.096 | 0.73 | 0.30–1.82 | 0.504 | |
| IgG3 | 1.62 | 1.12–2.35 | 0.010 | 1.17 | 0.73–1.88 | 0.514 | 1.39 | 0.88–2.18 | 0.135 | 0.74 | 0.48–1.16 | 0.191 | |
| IgG4 | 2.91 | 0.36–23.8 | 0.299 | 0.85 | 0.13–5.42 | 0.863 | 4.58 | 0.89–23.4 | 0.045 | 1.13 | 0.39–3.31 | 0.824 | |
| IgM | 1.17 | 0.99–1.38 | 0.058 | 1.07 | 0.90–1.27 | 0.426 | 1.08 | 0.92–1.27 | 0.362 | 1.02 | 0.89–1.17 | 0.770 | |
| AMA-1 | IgG | 1.20 | 0.94–1.53 | 0.148 | 1.03 | 0.81–1.31 | 0.795 | 1.23 | 1.01–1.51 | 0.042 | 0.93 | 0.76–1.13 | 0.446 |
| IgG1 | 1.31 | 0.91–1.88 | 0.140 | 1.22 | 0.80–1.86 | 0.358 | 1.84 | 1.29–2.63 | 0.001 | 1.10 | 0.67–1.56 | 0.598 | |
| IgG2 | 1.33 | 0.27–6.42 | 0.724 | 0.86 | 0.17–4.24 | 0.853 | 1.62 | 0.43–6.09 | 0.469 | 0.92 | 0.31–2.70 | 0.878 | |
| IgG3 | 1.24 | 0.81–1.89 | 0.326 | 1.15 | 0.71–1.86 | 0.565 | 1.35 | 0.92–1.98 | 0.111 | 0.86 | 0.63–1.18 | 0.356 | |
| IgG4 | 1.42 | 0.33–6.20 | 0.636 | 1.71 | 0.35–8.28 | 0.503 | 1.12 | 0.28–4.53 | 0.871 | 0.71 | 0.24–2.08 | 0.530 | |
| IgM | 1.14 | 0.89–1.47 | 0.308 | 1.07 | 0.85–1.35 | 0.579 | 1.06 | 0.84–1.35 | 0.609 | 1.03 | 0.85–1.26 | 0.750 | |
| EBA-175 | IgG | 1.43 | 1.03–1.99 | 0.029 | 1.34 | 0.99–1.81 | 0.059 | 1.11 | 0.81–1.53 | 0.510 | 0.90 | 0.68–1.18 | 0.430 |
| IgG1 | 1.48 | 0.75–2.94 | 0.257 | 1.77 | 0.98–3.21 | 0.052 | 0.87 | 0.35–2.13 | 0.755 | 0.49 | 0.25–0.97 | 0.026 | |
| IgG2 | 2.50 | 0.07–84.0 | 0.609 | 2.66 | 0.30–23.5 | 0.384 | 0.46 | 0.03–6.10 | 0.561 | 0.37 | 0.06–2.19 | 0.264 | |
| IgG3 | 1.58 | 0.86–2.91 | 0.139 | 1.12 | 0.75–1.67 | 0.581 | 1.50 | 0.73–3.08 | 0.269 | 0.44 | 0.19–0.98 | 0.037 | |
| IgG4 | 0.69 | 0.34–1.40 | 0.300 | 0.85 | 0.51–1.39 | 0.484 | 0.77 | 0.31–1.88 | 0.579 | 3.07 | 1.08–8.78 | 0.020 | |
| IgM | 1.32 | 1.02–1.69 | 0.032 | 1.24 | 0.97–1.59 | 0.086 | 1.09 | 0.87–1.39 | 0.450 | 1.03 | 0.85–1.25 | 0.757 | |
Negative binomial regression model using likelihood ratio test crude and adjusted by gender, IPTi treatment, previous malaria episodes, present infection, and neighborhood. The sample size at the two study periods are those indicated in the table in the supplemental material (antibody prevalences) for each antigen and antibody type and for months 5 and 12. IRR, incidence rate ratio.
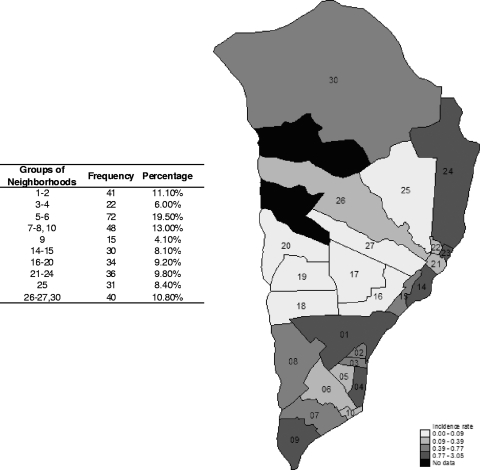
In a multilevel regression analysis including all antibody, clinical, epidemiological, and demographic variables together, the single most significant risk factor for having a malaria episode was having had a previous episode (at 5 months, IRR = 3.52, CI = 1.41 to 8.80, and P = 0.007; at 12 months, IRR = 3.39, CI = 1.58 to 7.29, and P = 0.0018). In addition, neighborhood of residence was the only other variable significantly associated with clinical malaria (P < 0.0001) (Fig. 3).
Fig 3.
Map of the Manhiça area with the neighborhoods colored as a function of malaria incidence (number of episodes per PYAR) for children selected for the study during the study period. The map illustrates the geographical microheterogeneity and the significant effect of neighborhood on malaria episodes and antibodies in the multilevel regression models. The study area expands a distance of less than 20 km from north to south and approximately 10 km from east to west and has two study clinics (Manhiça and Maragra). The number of children by groups of neighborhoods is indicated in the table.
DISCUSSION
This was a prospective study measuring antibody responses to P. falciparum merozoite antigens at multiple time points performed in an area where malaria is endemic with established demographic and morbidity surveillance systems, which allowed a rigorous and complete documentation of clinical malaria cases over 2 years. This type of design provides useful insights into the acquisition and duration of specific antibody responses, how they are boosted by P. falciparum exposure, and the correlation between these responses and protection from symptomatic malaria. The most important findings are that previous episodes and neighborhood of residence were the main factors influencing levels of antibodies to merozoite antigens, and having taken those into account, only IgG1 and IgG3 antibodies to EBA-175 at 12 months appeared to be significantly associated with reduced malaria incidence between 12 and 24 months of age.
Overall, there was an age-related acquisition of antigen-specific IgM and IgG antibodies to the merozoite antigens examined, supporting the notion that these may be important targets of immunity. In addition, a contribution from maternally transmitted antibodies was suggested in early infancy, particularly for IgG and IgG1 antibodies to AMA-1, which gradually decreased up to 12 months of age, and for IgG to whole P. falciparum blood stages (IFAT), which remained high and appeared to last for up to a year. The kinetics of decay of maternal IgG antibodies and the kinetics of acquisition of infant IgG antibodies, but not of IgM, varied for each antigen studied. The isotype patterns resembled what has been described previously, but the significant induction of noncytophilic IgG4 by EBA-175 at age 2 years represents a novel finding (34, 54).
Past and present exposure to P. falciparum is widely known to affect antibody responses. The occurrence of previous episodes was the strongest factor affecting IgG antibody values and the single most significant risk factor for having a malaria episode, consistent with data of IPTi studies in Tanzania (47) and Manhiça (24). Different neighborhoods of residence had diverse malaria incidences, and this was reflected in antibody levels; ongoing studies in the area are investigating what factors might explain the spatial relationship between geography and malaria risk. Age-adjusted P. falciparum antibody levels have been used as markers of microgeographical variations in exposure to malaria parasite infection (55). Therefore, analyses of the relationship between antibody levels and risk of malaria must be adjusted for the effect of previous malaria episodes and neighborhood. Indeed, in the crude analysis, a common finding was that high antibody levels were associated with an increased risk of malaria. In another study in Manhiça, high maternal and fetal IgG levels were associated with a higher risk of malaria in the infant (49), and this was consistent with the findings of other similar studies (30, 43). Nevertheless, when the analyses were adjusted for prior malaria episodes, the association disappeared, indicating that the main risk factor was the occurrence of previous episodes and that the antibodies were mere markers of the prior exposure. In only one case (MSP-119 IgG1 at 5 months), the positive association between IgG1 and risk of malaria up to 12 months remained significant after adjustment. This might be explained by exposure to malaria occurring during the prenatal and/or perinatal period, before enrollment, and not captured in this study. It was hypothesized that if the placental/maternal infections had been registered and adjusted for, this significance would have disappeared.
Studies restricted to infants are considered less adequate to analyze correlations between antibodies and malaria risk because of the confounding effect of maternal in utero exposure and of maternal transferred immunity. Therefore, more weight is given to the analyses in the 12- to 24-month period. In most cases, there was no association between antibody levels and incidence of malaria in the adjusted analysis. Inconsistencies in the associations between antibodies to MSP-119 and AMA-1 and protection among different studies could partially be explained because of the different recombinant antigen reagents used. It was only for EBA-175 antibody responses that a significant pattern emerged. IgG1 and IgG3 at 12 months had 51% and 56% protective effects, respectively, up to 24 months, whereas IgG4 was associated with an increased risk of malaria in this age period. In fact, IgG1 and IgG3 to MSP-119 and IgG3 to AMA-1 also showed a protective IRR of <1, but this was not statistically significant. The same conclusions were reached when we analyzed data by 2-fold increments or by tertiles. Another study also reported that high IgG4 levels specific for blood-stage antigens were associated with an enhanced risk of infection and malaria attacks (4).
Findings are consistent with those from recently published studies in which high levels of IgG (particularly IgG1 and IgG3) to EBA antigens were associated with protection from malaria (26, 42). Other studies failed to find an association between responses to EBA-175 and the incidence of P. falciparum malaria (22, 34, 36), although Okenu et al. (34) reported a protective trend for high levels of IgG to region II (which includes F2, used in our study). However, these studies examined total IgG, whereas subclass-specific responses to merozoite antigens provide further insights into protective targets and mechanisms of acquired immunity in children. Other studies have found an association between IgG1 and IgG3 to various merozoite proteins and protection (44, 53). Indeed, in this study, an association between total IgG and incidence of malaria was not found; the combination of the predominant IgG cytophilic isotypes with low-level noncytophilic isotypes having opposite effects could partially compensate for any small effect with total IgG.
IgG antibodies function in vivo by inhibiting merozoite invasion of erythrocytes, opsonizing merozoites for phagocytosis, and antibody-dependent cellular inhibition (ADCI). Antibodies against region II of EBA-175 may mediate protection against P. falciparum by blocking the binding of native EBA-175 to erythrocytes and subsequent invasion (9). In addition, cytophilic IgG1 and IgG3 antibodies may be involved in opsonizing merozoites for phagocytosis, activating complement, and/or operate through ADCI mechanisms, as proposed for glutamate-rich protein (GLURP), serine repeat antigen (SERA), or MSP-3 (32). However, not all parasites use EBA-175 for invasion, and there is substantial variability in the levels of the expression of this ligand compared to other ligands in populations in which malaria is endemic (19). Therefore, it is possible that antibodies are partially protective depending on the expression of EBA-175 and use of this ligand in invasion, a variable that may contribute to differences among populations.
In this study, there are a number of puzzling findings about EBA-175 (F2) antibodies, particularly with regard to IgG4. There is a significant rise in IgG4 at 24 months, and two distinct groups can be identified in the weighted scattered plots (very high and very low responders), but their significance remains unknown. Intriguingly, levels of noncytophilic IgG4 to EBA-175 were not associated with either previous malaria episodes or present infection, IgG1 and IgG3 were associated only with previous or current infections, and there was a general trend that IgG4 levels were higher in placebo than SP recipients (41); all these results contrast to the patterns found for MSP-119 and AMA-1. Therefore, it appears overall that IgG subclass responses to EBA-175 may have intrinsic differences compared to IgG subclass responses to MSP-119 or AMA-1, and this might have functional implications toward the mechanisms of protection and development of natural immunity.
Future studies need to investigate in more depth how different antibody isotypes induced by P. falciparum are transferred from the mother to the infant and the kinetics of production in early life (14). Different IgG isotypes appear to inherently vary in their capacity to transfer to the fetus (18), with IgG1 being the most efficiently transported subclass and IgG2 the least (51), and these differences may partly explain the susceptibility of newborns to various pathogens. P. falciparum-specific IgG1 and IgG3 isotypes appear to transfer to the offspring more often and more efficiently than IgG2 and IgG4 (10). Transplacental IgG subclass transfer is also influenced by malaria parasite infection and maternal hypergammaglobulinemia (35). During placental malaria, there is production of more IgG in the mother (49) and it appears that less IgG1 is transferred to the fetus, while IgG3 seems unaffected (35).
Considering the few studies examining the association of IgG responses to EBA-175 with the incidence of P. falciparum malaria, the findings reported here are remarkable. However, among the study limitations, the relatively small number of children having high IgG1, IgG3, and IgG4 responses to EBA-175, the fact that the subgroups of children in which IgG isotypes were measured were not exactly the same for each antigen, and the multiple comparisons performed lead to a cautious interpretation of the protection data, which will require further confirmation in different and larger studies. Advanced investigations at the cellular and molecular levels are required to better understand what the implications of the data might be in relation to the mechanisms by which EBA-175 antibodies mediate protection. This will help in the rational design and development of a vaccine against this leading target candidate.
Supplementary Material
ACKNOWLEDGMENTS
We thank the volunteer children and their mothers and families for their participation in the study. We are grateful to Lázaro Quimice, Nelito José, Ana Rosa Manhiça, Alfons Jiménez, and Pau Cisteró for technical support.
This work was funded by the Bill and Melinda Gates Foundation (grant IPTi03-0) and the Banco de Bilbao-Vizcaya-Argentaria Foundation (grant BBVA 02-0). C.D. is supported by the Ministerio de Ciencia e Innovación (RYC-2008-02631), D.Q. was supported by WHO/TDR (grant OD/TS-07-00017), and A.M. was supported by the Instituto de Salud Carlos III (CP-04/00220). The Centro de Investigaçao em Saúde da Manhiça receives core support from the Spanish Agency for International Cooperation and Development (AECID).
Footnotes
Published ahead of print 14 December 2011
Supplemental material for this article may be found at http://cvi.asmusa.org/.
REFERENCES
- 1. Alonso PL, et al. 2004. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 364:1411–1420 [DOI] [PubMed] [Google Scholar]
- 2. Alonso PL, et al. 2002. Manhiça DSS, Mozambique, p 189–195 In INDEPTH (ed), Population and health in developing countries. Population, health, and survival at INDEPTH sites, 1st ed, vol 1 International Development Research Centre (IDRC), Ottawa, Ontario, Canada [Google Scholar]
- 3. Alonso PL, et al. 1994. Randomised trial of efficacy of SPf66 vaccine against Plasmodium falciparum malaria in children in southern Tanzania. Lancet 344:1175–1181 [DOI] [PubMed] [Google Scholar]
- 4. Aucan C, et al. 2000. High immunoglobulin G2 (IgG2) and low IgG4 levels are associated with human resistance to Plasmodium falciparum malaria. Infect. Immun. 68:1252–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cavanagh DR, et al. 2004. Antibodies to the N-terminal block 2 of Plasmodium falciparum merozoite surface protein 1 are associated with protection against clinical malaria. Infect. Immun. 72:6492–6502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cohen S, McGregor IA, Carrington S. 1961. Gamma-globulin and acquired immunity to human malaria. Nature 192:733–737 [DOI] [PubMed] [Google Scholar]
- 7. Coley AM, et al. 2007. Structure of the malaria antigen AMA1 in complex with a growth-inhibitory antibody. PLoS Pathog. 3:1308–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Conway DJ, et al. 2000. A principal target of human immunity to malaria identified by molecular population genetic and immunological analyses. Nat. Med. 6:689–692 [DOI] [PubMed] [Google Scholar]
- 9. Chitnis CE. 2001. Molecular insights into receptors used by malaria parasites for erythrocyte invasion. Curr. Opin. Hematol. 8:85–91 [DOI] [PubMed] [Google Scholar]
- 10. Deloron P, et al. 1997. Isotypic analysis of maternally transmitted Plasmodium falciparum-specific antibodies in Cameroon, and relationship with risk of P. falciparum infection. Clin. Exp. Immunol. 110:212–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dobaño C, et al. 2008. Differential antibody responses to Plasmodium falciparum merozoite proteins in Malawian children with severe malaria. J. Infect. Dis. 197:766–774 [DOI] [PubMed] [Google Scholar]
- 12. Dodoo D, et al. 2008. Cohort study of the association of antibody levels to AMA1, MSP119, MSP3 and GLURP with protection from clinical malaria in Ghanaian children. Malar. J. 7:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Doolan DL, Dobaño C, Baird JK. 2009. Acquired immunity to malaria. Clin. Microbiol. Rev. 22:13–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duah NO, Miles DJ, Whittle HC, Conway DJ. 2010. Acquisition of antibody isotypes against Plasmodium falciparum blood stage antigens in a birth cohort. Parasite Immunol. 32:125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Egan AF, et al. 1996. Clinical immunity to Plasmodium falciparum malaria is associated with serum antibodies to the 19-kDa C-terminal fragment of the merozoite surface antigen, PfMSP-1. J. Infect. Dis. 173:765–769 [DOI] [PubMed] [Google Scholar]
- 16. Eisenhut M. 2007. Immunity to blood stages of Plasmodium falciparum is dependent on a specific pattern of immunoglobulin subclass responses to multiple blood stage antigens. Med. Hypotheses 69:804–808 [DOI] [PubMed] [Google Scholar]
- 17. Fowkes FJ, Richards JS, Simpson JA, Beeson JG. 2010. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. PLoS Med. 7:e1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. 1994. Placental transfer of immunoglobulin G subclasses. Clin. Diagn. Lab. Immunol. 1:667–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gomez-Escobar N, et al. 2010. Erythrocyte invasion and merozoite ligand gene expression in severe and mild Plasmodium falciparum malaria. J. Infect. Dis. 201:444–452 [DOI] [PubMed] [Google Scholar]
- 20. Gray JC, et al. 2007. Profiling the antibody immune response against blood stage malaria vaccine candidates. Clin. Chem. 53:1244–1253 [DOI] [PubMed] [Google Scholar]
- 21. Greenwood B, Marsh K, Snow R. 1991. Why do some African children develop severe malaria? Parasitol. Today 7:277–281 [DOI] [PubMed] [Google Scholar]
- 22. John CC, et al. 2005. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am. J. Trop. Med. Hyg. 73:222–228 [PubMed] [Google Scholar]
- 23. Kocken CH, et al. 2002. High-level expression of the malaria blood-stage vaccine candidate Plasmodium falciparum apical membrane antigen 1 and induction of antibodies that inhibit erythrocyte invasion. Infect. Immun. 70:4471–4476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Macete E, et al. 2006. Intermittent preventive treatment for malaria control administered at the time of routine vaccinations in Mozambican infants: a randomized, placebo-controlled trial. J. Infect. Dis. 194:276–285 [DOI] [PubMed] [Google Scholar]
- 25. Mazumdar S, Sachdeva S, Chauhan VS, Yazdani SS. 2010. Identification of cultivation condition to produce correctly folded form of a malaria vaccine based on Plasmodium falciparum merozoite surface protein-1 in Escherichia coli. Bioprocess Biosyst. Eng. 33:719–730 [DOI] [PubMed] [Google Scholar]
- 26. McCarra MB, et al. 2011. Antibodies to Plasmodium falciparum erythrocyte-binding antigen-175 are associated with protection from clinical malaria. Pediatr. Infect. Dis. J. 30:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Menendez C, et al. 2008. A randomized placebo-controlled trial of intermittent preventive treatment in pregnant women in the context of insecticide treated nets delivered through the antenatal clinic. PLoS One 3:e1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mewono L, et al. 2008. Interleukin-21 is associated with IgG1 and IgG3 antibodies to erythrocyte-binding antigen-175 peptide 4 of Plasmodium falciparum in Gabonese children with acute falciparum malaria. Eur. Cytokine Netw. 19:30–36 [DOI] [PubMed] [Google Scholar]
- 29. Nebie I, et al. 2008. Humoral responses to Plasmodium falciparum blood-stage antigens and association with incidence of clinical malaria in children living in an area of seasonal malaria transmission in Burkina Faso, West Africa. Infect. Immun. 76:759–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ned RM, et al. 2008. Effect of placental malaria and HIV infection on the antibody responses to Plasmodium falciparum in infants. J. Infect. Dis. 198:1609–1619 [DOI] [PubMed] [Google Scholar]
- 31. O'Donnell RA, et al. 2001. Antibodies against merozoite surface protein (MSP)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 193:1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oeuvray C, et al. 1994. Merozoite surface protein-3: a malaria protein inducing antibodies that promote Plasmodium falciparum killing by cooperation with blood monocytes. Blood 84:1594–1602 [PubMed] [Google Scholar]
- 33. Ogutu BR, et al. 2009. Blood stage malaria vaccine eliciting high antigen-specific antibody concentrations confers no protection to young children in Western Kenya. PLoS One 4:e4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Okenu DM, et al. 2000. Analysis of human antibodies to erythrocyte binding antigen 175 of Plasmodium falciparum. Infect. Immun. 68:5559–5566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Okoko BJ, et al. 2001. The influence of placental malaria infection and maternal hypergammaglobulinemia on transplacental transfer of antibodies and IgG subclasses in a rural West African population. J. Infect. Dis. 184:627–632 [DOI] [PubMed] [Google Scholar]
- 36. Osier FH, et al. 2008. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect. Immun. 76:2240–2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pandey KC, et al. 2002. Bacterially expressed and refolded receptor binding domain of Plasmodium falciparum EBA-175 elicits invasion inhibitory antibodies. Mol. Biochem. Parasitol. 123:23–33 [DOI] [PubMed] [Google Scholar]
- 38. Pattnaik P, et al. 2007. Immunogenicity of a recombinant malaria vaccine based on receptor binding domain of Plasmodium falciparum EBA-175. Vaccine 25:806–813 [DOI] [PubMed] [Google Scholar]
- 39. Perraut R, et al. 2005. Antibodies to the conserved C-terminal domain of the Plasmodium falciparum merozoite surface protein 1 and to the merozoite extract and their relationship with in vitro inhibitory antibodies and protection against clinical malaria in a Senegalese village. J. Infect. Dis. 191:264–271 [DOI] [PubMed] [Google Scholar]
- 40. Polley SD, et al. 2004. Human antibodies to recombinant protein constructs of Plasmodium falciparum apical membrane antigen 1 (AMA1) and their associations with protection from malaria. Vaccine 23:718–728 [DOI] [PubMed] [Google Scholar]
- 41. Quelhas D, et al. 2008. Impact of intermittent preventive treatment with sulfadoxine-pyrimethamine on antibody responses to erythrocytic-stage Plasmodium falciparum antigens in infants in Mozambique. Clin. Vaccine Immunol. 15:1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richards JS, et al. 2010. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin. Infect. Dis. 51:e50–e60 [DOI] [PubMed] [Google Scholar]
- 43. Riley EM, et al. 2000. Lack of association between maternal antibody and protection of African infants from malaria infection. Infect. Immun. 68:5856–5863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Roussilhon C, et al. 2007. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. PLoS Med. 4:e320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sabchareon A, et al. 1991. Parasitologic and clinical human response to immunoglobulin administration in falciparum malaria. Am. J. Trop. Med. Hyg. 45:297–308 [DOI] [PubMed] [Google Scholar]
- 46. Saute F, et al. 2003. Malaria in southern Mozambique: malariometric indicators and malaria case definition in Manhica district. Trans. R. Soc. Trop. Med. Hyg. 97:661–666 [DOI] [PubMed] [Google Scholar]
- 47. Schellenberg D, et al. 2005. Intermittent preventive antimalarial treatment for Tanzanian infants: follow-up to age 2 years of a randomised, placebo-controlled trial. Lancet 365:1481–1483 [DOI] [PubMed] [Google Scholar]
- 48. Schellenberg D, et al. 1999. African children with malaria in an area of intense Plasmodium falciparum transmission: features on admission to the hospital and risk factors for death. Am. J. Trop. Med. Hyg. 61:431–438 [DOI] [PubMed] [Google Scholar]
- 49. Serra-Casas E, et al. 2010. The effect of intermittent preventive treatment during pregnancy on malarial antibodies depends on HIV status and is not associated with poor delivery outcomes. J. Infect. Dis. 201:123–131 [DOI] [PubMed] [Google Scholar]
- 50. Sim BK, et al. 1990. Primary structure of the 175K Plasmodium falciparum erythrocyte binding antigen and identification of a peptide which elicits antibodies that inhibit malaria merozoite invasion. J. Cell Biol. 111:1877–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Simister NE. 2003. Placental transport of immunoglobulin G. Vaccine 21:3365–3369 [DOI] [PubMed] [Google Scholar]
- 52. Snounou G, et al. 1993. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol. Biochem. Parasitol. 61:315–320 [DOI] [PubMed] [Google Scholar]
- 53. Stanisic DI, et al. 2009. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect. Immun. 77:1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Toure FS, Deloron P, Migot-Nabias F. 2006. Analysis of human antibodies to erythrocyte binding antigen 175 peptide 4 of Plasmodium falciparum. Clin. Med. Res. 4:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wilson S, et al. 2007. Age-adjusted Plasmodium falciparum antibody levels in school-aged children are a stable marker of microgeographical variations in exposure to Plasmodium infection. BMC Infect. Dis. 7:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.