Abstract
Despite the complexity of tuberculosis (TB) serology, antibodies (Abs) remain attractive biomarkers for TB. Recent evidence of a mycobacterial capsule that consists mainly of the polysaccharides arabinomannan (AM) and glucan provides new options for serologic targets. For this study, Ab responses to AM and glucan for 47 U.S. TB patients (33 HIV negative [HIV−], 14 HIV positive [HIV+]), 42 healthy controls, and 38 asymptomatic HIV+ controls were evaluated by enzyme-linked immunosorbent assays (ELISAs). The results were compared with Ab responses to the mycobacterial glycolipid cell wall antigen lipoarabinomannan (LAM) and to the proteins malate synthase (MS) and MPT51. We found that the main immunoglobulin (Ig) isotype response to polysaccharides was IgG, predominantly of subclass IgG2. IgG responses to AM were significantly higher for HIV− and HIV+ TB cases than for controls (P, <0.0001 and <0.01, respectively); significantly higher for HIV− than for HIV+ TB cases (P, <0.01); and significantly higher in sputum smear-positive than smear-negative patients in both HIV− and HIV+ cases (P, 0.01 and 0.02, respectively). In both TB groups, titers of Ab to glucan were significantly lower than titers of Ab to AM (P, <0.0001). IgG responses to AM and MS or to AM and MPT51 did not correlate with each other in HIV− TB patients, while they correlated significantly in HIV+ TB patients (P, 0.01 and 0.05, respectively). We conclude that Ab responses to AM could contribute to the serodiagnosis of TB, especially for HIV− TB patients. This study also provides new and important insights into the differences in the profiles of Abs to mycobacterial antigens between HIV− and HIV+ TB patients.
INTRODUCTION
New biomarkers for the diagnosis of active tuberculosis (TB) are urgently needed. Despite a history of disappointing results, antibodies (Abs) to Mycobacterium tuberculosis antigens remain attractive biomarkers for TB. Detection of serum Abs to M. tuberculosis antigens (serology) does not require a specimen from the site of disease, and tests could easily be developed into a simple, rapid dipstick format. However, commercially available serodiagnostic tests to date have been limited by a lack of sensitivity and specificity (51; reviewed in references 45 and 46). Therefore, the World Health Organization (WHO) recently cautioned against the use of such tests, while strongly recommending further targeted research in the field of TB serology (26).
Studies show that multiple antigen testing provides higher sensitivities for TB serodiagnostic assays than tests based on single antigens (reviewed in reference 45). Many mycobacterial proteins and a few lipids and glycolipids have been evaluated for their serodiagnostic potential in recent decades, and some promising antigens have been identified (reviewed in reference 44). However, polysaccharide antigens have been insufficiently studied. Recent studies have confirmed the existence of a mycobacterial capsule that consists mainly of the polysaccharides glucan (70 to 80%) and arabinomannan (AM) (10 to 20%) and, to a lesser extent, of proteins and glycolipids (8, 23, 36). Located at the interface between the bacterium and host cells, capsular antigens are involved in mycobacterial pathogenicity (8, 13, 36, 47) and therefore likely elicit host immune responses. Navoa et al. demonstrated that titers of Ab to AM were significantly higher in Indian smear-positive cavitary TB patients (n = 20) than in healthy, tuberculin skin test-negative (TST−) controls (n = 17) (27). Ab responses to glucan have been elicited in M. tuberculosis-infected mice (40), but to our knowledge, the response to this polysaccharide has not been evaluated in humans.
To explore the potential serodiagnostic value of mycobacterial capsular polysaccharide antigens, studies with well-characterized samples from non-HIV-infected (HIV−) and HIV-infected (HIV+) subjects are needed. Comparison of Ab responses in HIV− TB patients to those in HIV+ TB patients is critical for several reasons: (i) HIV− TB patients appear to produce Ab responses to a wider range of M. tuberculosis antigens than do HIV+ TB patients (33, 37); (ii) polyclonal B-cell stimulation in HIV infection affects the spectrum of Ab responses to many antigens (19, 22); and (iii) disease presentations and host responses in TB are strongly influenced by immune competency (1). Therefore, our primary objective was the evaluation of Ab responses to the polysaccharides AM and glucan in TB patients and controls stratified by HIV status. Due to known variations in the capsular composition of bacterial serotypes and to suggested differences between mycobacterial strains (15, 25, 28, 39), our secondary objective was the correlation of Ab responses to capsular antigens isolated from an attenuated Mycobacterium bovis bacillus Calmette-Guérin (BCG) vaccine strain with Ab responses to those isolated from an M. tuberculosis strain. Our third objective was the assessment of an adjunctive serodiagnostic value attributable to capsular Ab responses compared to Ab responses against a selection of other mycobacterial antigens.
MATERIALS AND METHODS
Study design and subjects.
This was a case-control study with evaluation of Ab responses from 47 patients with culture-confirmed TB, 42 healthy controls, and 38 asymptomatic HIV+ controls. TB patients were recruited from 4 public hospitals in New York City from 2007 to 2010. Inclusion criteria were an age of >21 years and receipt of sputum smears for acid-fast bacilli (AFB) and mycobacterial cultures. Sputum smears were considered positive if one of the initial three smears was positive. Subjects on antituberculous treatment (ATT) for >2 weeks or those with a history of ATT for active TB within the year prior to enrollment were excluded. Controls were (i) healthy volunteers without known risk factors for HIV infection, who were categorized by results of the a tuberculin skin test (TST) and a gamma interferon (IFN-γ) release assay (IGRA) (QuantiFERON-TB Gold blood test [QFT]; Cellestis, Australia), and (ii) asymptomatic HIV-infected (HIV+) persons categorized by TST results. Approval for research on human subjects was obtained from the institutional review boards of the New York University School of Medicine and the Albert Einstein College of Medicine. Written informed consent was obtained from all subjects prior to enrollment.
Mycobacterial antigens.
In addition to the mycobacterial polysaccharide antigens AM and glucan, other antigens were selected based on their different compositions and their serodiagnostic potentials. Lipoarabinomannan (LAM) is a major glycolipid constituent of the mycobacterial cell wall that has serodiagnostic value, albeit with limitations (16, 49). The two mycobacterial proteins malate synthase (MS) (81 kDa; Rv1837c) and MPT51 (27 kDa; Rv3803c) are culture filtrate proteins that elicit Ab responses in most HIV-infected and uninfected patients with TB but not in persons with latent M. tuberculosis infection (LTBI) (2, 35, 50; reviewed in reference 44).
Antigen preparations.
Glucan and AM were isolated and purified from BCG strain Pasteur and the M. tuberculosis strain H37Rv as described previously (39, 42). Briefly, mycobacteria (2 ml from a starter culture) were resuspended in 500 ml of minimal medium in roller bottles that were placed horizontally for stationary growth at 37°C. The cells were collected after 14 days and were centrifuged at 4,000 × g for 15 min at 4°C. Cell pellets were then pooled and treated with glass beads. The bead-treated cells were resuspended and were centrifuged at 8,000 × g for 15 min. The collected supernatant was filtered through 0.22-μm-pore-size filters. The capsular extract antigens were isolated, purified by column chromatography, and then lyophilized.
Purified LAM from H37Rv was obtained from the Biodefense and Emerging Infections Research Resources Repository (BEI; Manassas, VA), and the recombinant proteins MS and MPT51 were expressed in Escherichia coli and were purified as described previously (2).
Antibody detection assays.
Enzyme-linked immunosorbent assays (ELISAs) were performed essentially as described previously (2, 27). Briefly, the wells of 96-well microtiter plates (Immulon 2HB; Fisher Scientific, NY) were coated with either AM, glucan, or LAM at 10 μg/ml or with MS or MPT51 at 4 μg/ml (all antigens were used at 50 μl/well). Serum samples, diluted 1:50, were added in duplicate to the antigen-coated wells, and the bound Abs were detected with either alkaline phosphatase (AP)-conjugated protein A (protein A-AP) (1:1,000; Sigma, St. Louis MO), goat anti-human IgA-AP, or goat anti-human IgM-AP (1:1,000; Sigma), followed by p-nitrophenyl phosphate substrate (60 min at 37°C). Secondary Abs for the detection of IgG subclass Ab responses were mouse anti-human IgG1-AP, IgG2-AP, IgG3-AP, and IgG4-AP (1:1,000; Southern Biotech, Birmingham, AL). Optical densities (OD) were measured at 405 nm. Negative controls were processed in duplicate as described above, except for the addition of serum. Each assay was repeated on two separate days.
Statistical analysis.
Statistical analysis was performed using STATA software, version 9.2 (StataCorp, College Station, TX), and GraphPad Prism software, version 5.02 (GraphPad Inc., San Diego, CA). All Ab responses were compared using nonparametric tests, the Mann-Whitney U test and the Spearman rank correlation test, because Ab responses to some antigens were not normally distributed.
RESULTS
TB cases (n = 47) and controls (n = 80) were categorized by HIV status. No statistically significant differences in sex, age, or the results of sputum smears for AFB were found between HIV− (n = 33) and HIV+ (n = 14) TB patients (Table 1). HIV− controls (n = 42) were younger than HIV+ controls (n = 38) but did not differ in sex (Table 1). Of the HIV− controls, 22/42 (52%) were TST positive (TST+) and had a history of BCG vaccination. Of those, 10/22 (45%) were QFT positive (QFT+), indicating the presence of LTBI. Of the HIV+ controls, 11/38 (29%) were TST+ and were not further categorized by QFT due to the unavailability of this test for this group.
Table 1.
Characteristics of 47 TB cases and 80 controls stratified by HIV status
| Characteristic | TB patients |
Controls |
||||
|---|---|---|---|---|---|---|
| HIV− (n =33) | HIV+ (n = 14) | P | HIV− (n = 42) | HIV+ (n = 38) | P | |
| No. (%) male | 23 (70) | 12 (86) | 0.30a | 17 (40) | 17 (45) | 0.70a |
| Mean age (yr) ± SD | 39 ± 13 | 43 ± 10 | 0.25b | 41 ± 10 | 46 ± 11 | 0.04b |
| No. (%) sputum smear positivec | 17 (52) | 6 (43) | 0.59a | |||
| Median (range) CD4 count | NAd | 122 (17–483) | NA | NA | 547 (11–1,541) | NA |
Determined by the chi-square test.
Determined by the t test.
At least one of initial three sputum smears positive for acid-fast bacilli.
NA, not applicable.
Ab responses to AM.
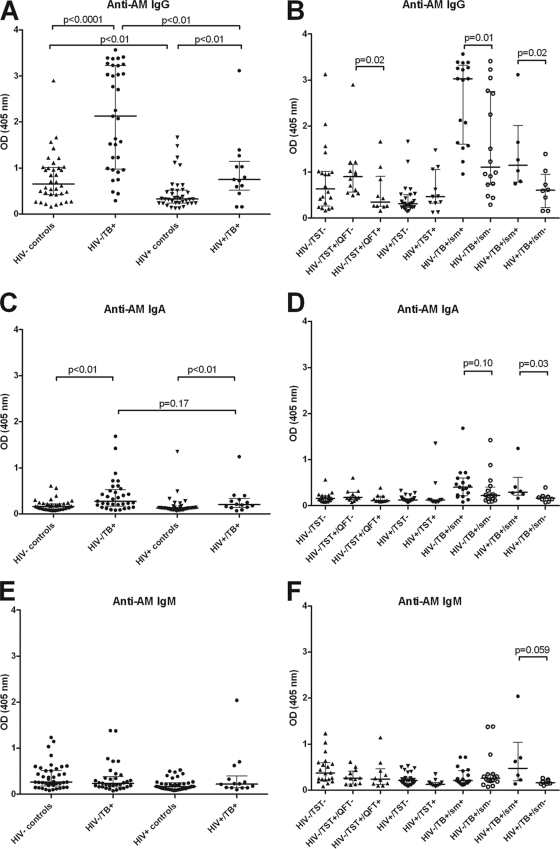
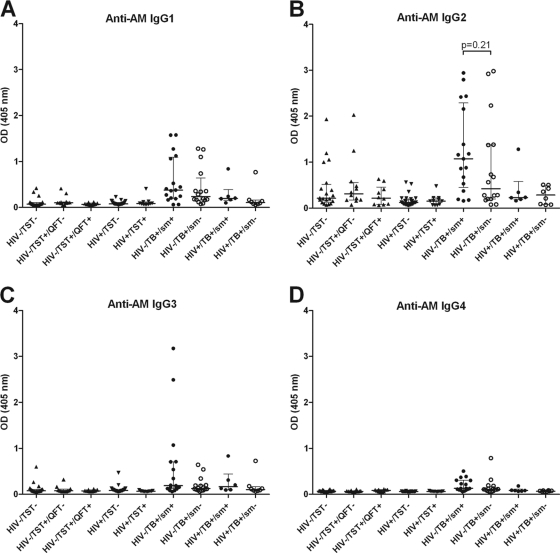
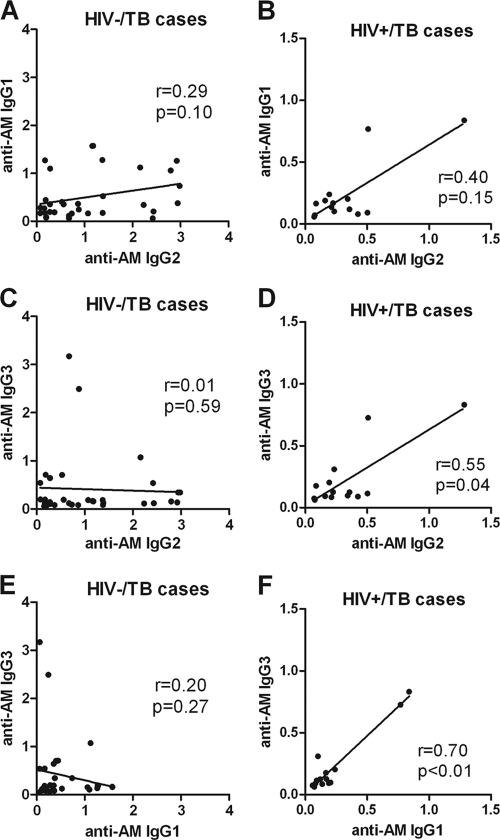
Unless otherwise specified, Ab responses were against AM isolated from the BCG strain Pasteur. Among both HIV− and HIV+ subjects, IgG responses to AM were significantly higher for TB cases than for controls (Fig. 1A). They were also significantly higher in HIV− than in HIV+ TB patients and significantly higher in HIV− than in HIV+ controls. When TB cases were further categorized by AFB smear results, significantly higher IgG responses to AM were seen in smear-positive than in smear-negative TB patients in both the HIV− and HIV+ groups (Fig. 1B). Interestingly, among HIV− controls, there was a wide range of IgG responses to AM that were significantly higher in TST+ QFT− than in TST+ QFT+ individuals. The IgG responses to AM isolated from BCG strain Pasteur versus AM isolated from M. tuberculosis H37Rv correlated strongly and highly significantly with one another in both TB patients (r = 0.90; P < 0.0001) and controls (r = 0.91; P < 0.0001), regardless of HIV status (Fig. 2A and B). In the majority of TB cases, the predominant IgG subclass response to AM was IgG2 (Fig. 3B), levels of which were significantly higher in HIV− than in HIV+ TB cases (P < 0.01). However, some patients had an IgG1 or IgG3 response, while there was little IgG4 response overall (Fig. 3A, C, and D). In HIV− TB patients, specific IgG subclass responses to AM did not correlate significantly with one another (Fig. 4A, C, and E), indicating a heterogeneous subclass response. In contrast, for HIV+ TB patients, certain IgG subclass responses correlated more and significantly with one another despite the lower number of cases in this group (Fig. 4B, D, and F). In TB patients, IgA responses to AM were statistically significantly lower than IgG responses (P < 0.0001), but these responses correlated strongly with one another (r = 0.71; P < 0.001). For both HIV− and HIV+ subjects, IgA Ab responses to AM were statistically significantly higher in TB patients than in controls (Fig. 1C). Like IgG responses, IgA responses to AM were higher in smear-positive than in smear-negative patients, although this difference was significant only for HIV+ TB patients (Fig. 1D). Overall, IgM responses to AM were much lower than IgG responses, and there was no statistically significant difference in IgM responses between TB cases and controls (Fig. 1E). No significant differences among TB cases according to AFB smear results were seen, although there was a trend toward higher IgM responses for smear-positive than for smear-negative HIV+ TB patients (Fig. 1F).
Fig 1.
Antibody responses to arabinomannan (AM) isolated from the BCG strain Pasteur in tuberculosis (TB) patients and controls stratified by HIV status. (A) IgG responses to AM in TB patients and controls stratified by HIV status. (B) IgG responses to AM in HIV− and HIV+ controls further categorized by tuberculin skin test (TST) and QuantiFERON test (QFT) results and in HIV− and HIV+ TB patients further categorized by sputum smear (sm) results for AFB. (C) IgA responses to AM in TB patients and controls stratified by HIV status. (D) IgA responses to AM in HIV− and HIV+ controls further categorized by TST and QFT results and in HIV− and HIV+ TB patients further categorized by sm results for AFB. (E) IgM responses to AM in TB patients and controls stratified by HIV status. (F) IgM responses to AM in HIV− and HIV+ controls further categorized by TST and QFT results and in HIV− and HIV+ TB patients further categorized by sm results for AFB. The Mann-Whitney U test was used to test for statistical significance in two-group comparisons.
Fig 2.
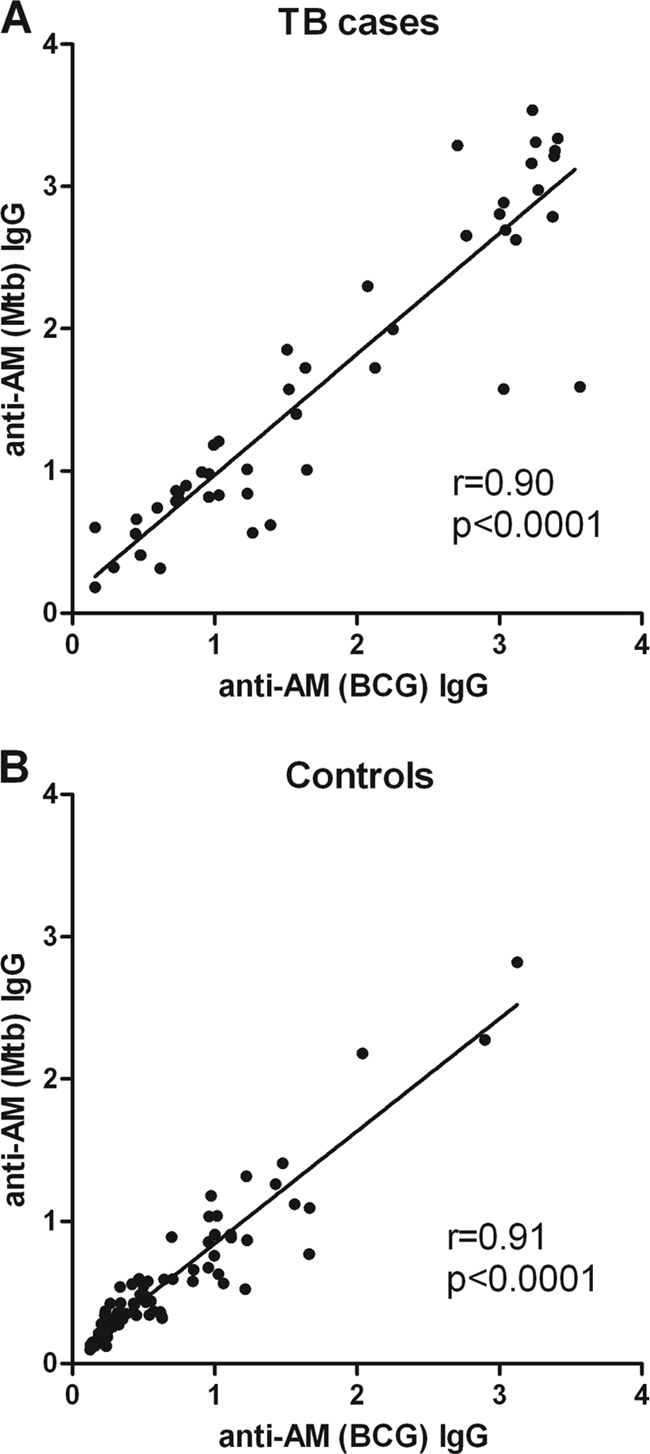
Correlation of IgG responses to arabinomannan (AM) isolated from the BCG strain Pasteur with IgG responses of AM isolated from Mycobacterium tuberculosis H37Rv. (A) Correlation for all tuberculosis (TB) patients combined; (B) correlation for all controls combined. The Spearman rank test was used to test for statistical significance.
Fig 3.
IgG subclass responses to arabinomannan (AM) in tuberculosis (TB) patients and controls stratified by HIV status. HIV− and HIV+ controls are further categorized by tuberculin skin test (TST) and QuantiFERON test (QFT) results, and HIV− and HIV+ TB patients are further categorized by sputum smear (sm) results for acid-fast bacilli. (A) IgG1; (B) IgG2; (C) IgG3; (D) IgG4. The Mann-Whitney U test was used to test for statistical significance in two-group comparisons.
Fig 4.
Correlation of IgG subclass responses to arabinomannan (AM) in tuberculosis (TB) patients stratified by HIV status. (A and B) Correlation between IgG2 and IgG1 responses to AM in HIV− (A) and HIV+ (B) TB patients. (C and D) Correlation between IgG2 and IgG3 responses to AM in HIV− (C) and HIV+ (D) TB patients. (E and F) Correlation between IgG1 and IgG3 responses to AM in HIV− (E) and HIV+ (F) TB patients. The Spearman rank correlation test was used to test for statistical significance.
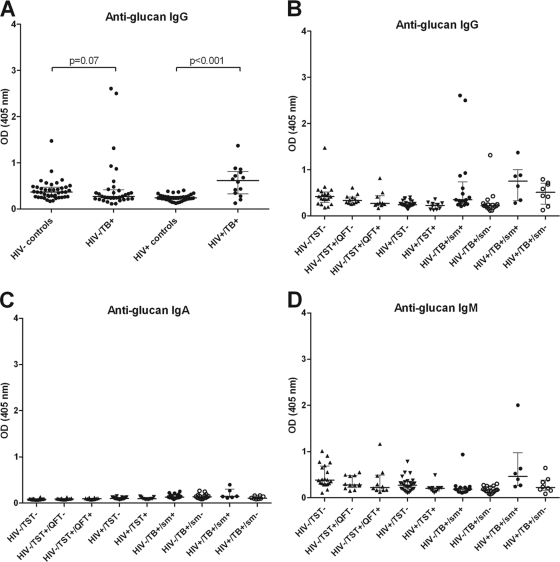
Ab responses to glucan.
Ab responses to glucan isolated from the BCG strain Pasteur were determined. In TB cases, IgG responses to glucan were significantly lower than those to AM (P < 0.0001), but these correlated significantly with one another (r = 0.49; P < 0.001). IgG responses were slightly higher in TB cases than in controls, a difference that was significant for HIV+ subjects (Fig. 5A). There was no significant difference among TB cases according to AFB smear results (Fig. 5B). IgA responses to glucan were low in all groups (Fig. 5C), and IgM responses did not differ significantly between the groups (Fig. 5D).
Fig 5.
Antibody responses to glucan isolated from the BCG strain Pasteur in tuberculosis (TB) patients and controls stratified by HIV status. (A) IgG responses to glucan in TB patients and controls stratified by HIV status. (B through D) IgG (B), IgA (C), and IgM (D) responses to glucan in HIV− and HIV+ controls further categorized by tuberculin skin test (TST) and QuantiFERON test (QFT) results and in HIV− and HIV+ TB patients further categorized by sputum smear (sm) results for AFB. The Mann-Whitney U test was used to test for statistical significance in two-group comparisons.
Ab responses to LAM.
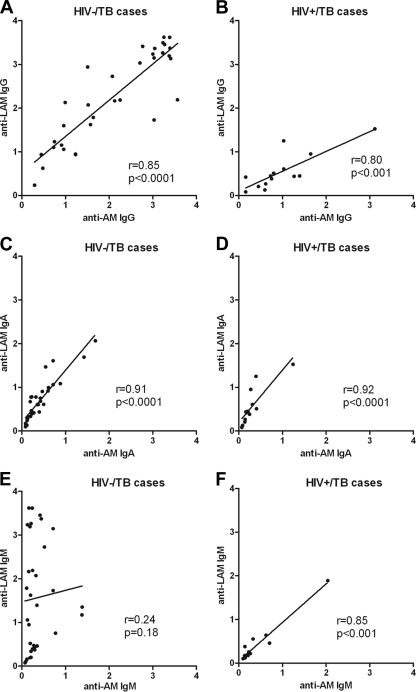
Among both HIV− and HIV+ subjects, IgG responses to LAM were significantly higher in TB cases than in controls (P, <0.0001 and 0.02, respectively). As was observed with AM, IgG responses to LAM were significantly higher in HIV− TB patients than in HIV+ TB patients (P < 0.001). However, in contrast to AM, no significant differences in IgG responses to LAM were seen between smear-positive and smear-negative TB patients. In both TB cases and controls, IgG responses to LAM correlated strongly and significantly with those to AM regardless of HIV status (Fig. 6A and B). In TB cases, IgA responses to LAM also showed a spectrum similar to those for AM, and these responses correlated strongly with one another regardless of HIV status (Fig. 6C and D). IgM responses to LAM were particularly high in HIV− smear-positive TB patients and significantly higher than in HIV− smear-negative TB patients (P < 0.0001). They were also significantly higher than IgM responses to AM (P < 0.0001). While IgM responses to LAM did not correlate with those to AM in HIV− TB cases (Fig. 6E), these responses correlated strongly and significantly with one another in HIV+ TB cases (Fig. 6F).
Fig 6.
Correlations between IgG responses to arabinomannan (AM) and lipoarabinomannan (LAM) in tuberculosis (TB) patients stratified by HIV status. (A and B) Correlations between IgG responses to AM and LAM in HIV− (A) and HIV+ (B) TB patients. (C and D) Correlations between IgA responses to AM and LAM in HIV− (C) and HIV+ (D) TB patients. (E and F) Correlations between IgM responses to AM and LAM in HIV− (E) and HIV+ (F) TB patients. The Spearman rank correlation test was used to test for statistical significance.
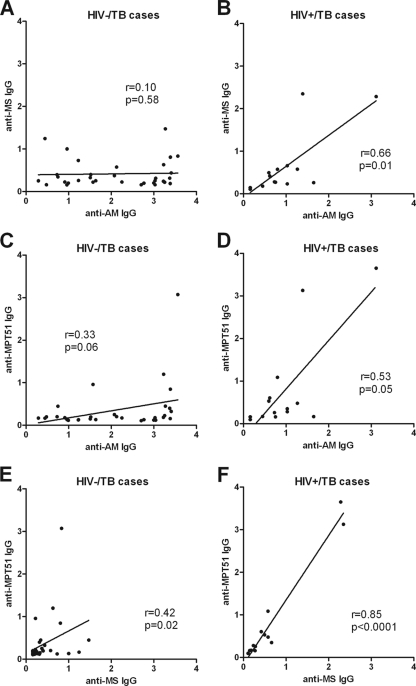
Ab responses to MS and MPT51.
IgG responses to MS and MPT51 were lower overall than IgG responses to AM (Fig. 7A to D). IgG responses to both MS and MPT51 were significantly higher in TB cases than in controls regardless of HIV status (P < 0.001). In accordance with our prior studies, Ab responses to MPT51 were higher in HIV+ than in HIV− TB cases, a difference that almost reached statistical significance (P = 0.06), while Ab responses to MS did not differ significantly between these two groups (2). In contrast to IgG responses to AM, no statistically significant difference was seen between smear-positive and smear-negative TB cases. IgG responses to MS or MPT51 did not correlate significantly with those to AM in HIV− TB cases (Fig. 7A and C). In contrast, the correlation between IgG responses to MS and AM was significant, and that for anti-MPT51 and anti-AM IgG was almost significant, in HIV+ TB cases (Fig. 7B and D). Similarly, the correlation between IgG Ab responses to MS and MPT51 was much stronger in HIV+ than in HIV− TB cases (Fig. 7E and F). IgA and IgM responses to MS and MPT51 were relatively low in all groups (data not shown).
Fig 7.
Correlations between IgG responses to arabinomannan (AM), malate synthase (MS), and MPT51 in tuberculosis (TB) patients stratified by HIV status. (A and B) Correlations between IgG responses to AM and MS in HIV− (A) and HIV+ (B) TB patients. (C and D) Correlations between IgG responses to AM and MPT51 in HIV− (C) and HIV+ (D) TB patients. (E and F) Correlations between IgG responses to MS and MPT51 in HIV− (E) and HIV+ (F) TB patients. The Spearman rank correlation test was used to test for statistical significance.
DISCUSSION
To our knowledge, this is the first study analyzing the spectrum of Ab responses to the mycobacterial capsular polysaccharide antigens AM and glucan in a large number of TB cases and controls categorized by HIV status. We found significantly higher Ab responses to AM in TB patients than in controls with and without LTBI, regardless of HIV status. These results complement data from a recent study showing significantly higher Ab responses to AM in a limited number of HIV− smear-positive Indian TB patients than in HIV− TST− controls (27). In our TB patients, IgG responses to AM were significantly higher than IgA and IgM responses. This is consistent with data on Ab responses to other antigens in TB and is likely due to the fact that TB is predominantly a reactivation disease (reviewed in reference 44). The IgG responses to AM isolated from a BCG strain and the IgG responses to AM from an M. tuberculosis strain correlated highly and significantly with one another, strongly suggesting that the same AM epitopes are recognized in these two strains. Of note is the wide spectrum of IgG responses to AM in our control groups, with some especially high titers in a few HIV− TST− and HIV− TST+ QFT− individuals. In addition, we observed significantly higher Ab responses in healthy HIV− TST+ QFT− individuals than in healthy HIV− TST+ QFT+ individuals. A positive QFT result is indicative of “true” LTBI, while a positive TST with a negative QFT result is more likely due to prior BCG vaccination and/or exposure to nontuberculous mycobacteria. All of our HIV− TST+ controls had a history of BCG vaccination, and significantly increased IgG responses to LAM have been reported in patients after BCG vaccination (3). Furthermore, conjugate vaccines with AM have resulted in modest protection of mice against M. tuberculosis infection (15, 18). Thus, the high levels of Abs to AM in some controls could be due to cross-reactivity or could potentially have some protective function against infection with M. tuberculosis.
The main IgG subclass elicited by AM was IgG2, a finding in agreement with a prior study (27) and consistent with the known observation that this subclass predominates in human Ab responses to polysaccharide antigens (10, 31, 41). HIV infection can be associated with an impaired IgG2 subclass response, and significantly lower IgG2 responses to LAM have been found in HIV+ than in HIV− TB patients (7). The significantly higher IgG2 response, and thus the higher overall IgG response, to AM in our HIV− TB patients than in our HIV+ TB patients is in agreement with these findings.
The finding of significantly higher IgG titers in smear-positive than in smear-negative TB patients was unexpected. Although higher titers of Abs to mycobacterial proteins have been found in smear-positive than in smear-negative TB patients from regions of endemicity (reviewed in reference 44), our group found no differences in Ab responses to the mycobacterial proteins MS and MPT51 in U.S. TB patients, who, in general, are diagnosed at earlier disease states (2). However, in a prior study, we did find significantly higher Ab responses to those proteins in Indian than in U.S. TB patients, in agreement with other studies demonstrating higher titers of Abs to proteins in advanced versus earlier states of disease (2, 21). By comparing data generated simultaneously from the same U.S. TB patients, the results of this study suggest that Ab responses to AM are a more sensitive indicator of mycobacterial burden than Ab responses to the mycobacterial proteins, especially in HIV− TB patients. This hypothesis is further supported by the significant correlation between AM detection and CFU in the lungs of M. tuberculosis-infected mice and by the associated proportional increase in titers of Ab to AM (39).
Ab responses to glucan were significantly lower than Ab responses to AM in TB patients, although responses to these two polysaccharides correlated strongly and significantly with one another. Even though Ab responses to M. tuberculosis polysaccharide fractions containing glucan have been detected in some TB patients (5), and mice infected with M. tuberculosis mount Ab responses to glucan (40), our data suggest that glucan has limited immunogenicity in humans. We hypothesize that this limited immunogenicity could be explained by the structural resemblance of glucan to glycogen, a ubiquitous intracellular polysaccharide (12, 23).
Overall, the differences in the profiles of Abs to mycobacterial antigens between HIV− and HIV+ TB patients were remarkable. HIV− TB patients had a more heterogeneous IgG subclass response to AM, with no significant correlation between the subclasses. In contrast, HIV+ TB patients had a more homogeneous IgG subclass response, with significant correlations between anti-AM IgG2 and IgG3 as well as IgG1 and IgG3. In a similar manner, IgG responses to AM did not correlate with IgG responses to the mycobacterial protein MS or MPT51 in HIV− TB patients, while they correlated significantly in HIV+ TB patients. Even the correlation between IgG responses to the proteins MS and MPT51 was much stronger and more significant in HIV+ than in HIV− TB patients. To our knowledge, Ab responses to mycobacterial polysaccharides and proteins have not been correlated before. However, studies have documented the heterogeneity of Ab responses to proteins in HIV− TB cases, which might be explained by the broad range of clinical presentations at the time of sample acquisition (9, 21, 24, 34, 35). Despite a predominant IgG2 response, variations in IgG subclass titers to LAM were associated with different clinical manifestations of leprosy (11). It is conceivable that several factors in our HIV− TB patients contributed to the lack of significant correlation between AM and either MS or MPT51: (i) the stronger IgG responses to AM than to MS and MPT51, (ii) the differences in IgG subclass responses, and (iii) the heterogeneity of IgG responses to mycobacterial proteins. In contrast, the impaired IgG2 response coupled with the mostly IgG1 associated hypergammaglobulinemia observed in HIV infection could explain the significant correlation of IgG subclass responses to AM in our HIV+ TB patients (19, 22). Since IgG responses to proteins are driven mostly by IgG1 and IgG3, while those to polysaccharides are due more to IgG2 (10, 31, 41, 43), the same argument could be made for the more homogenous Ab response to polysaccharide and protein antigens in HIV+ TB patients. Our findings suggest that TB serology is relatively more complex in immunocompetent TB patients than in HIV+ TB patients and that evaluation of IgG subclasses in HIV− TB cases could provide additional serodiagnostic information.
Given the lack of a significant difference between IgG responses to AM and LAM and the strong and highly significant correlation between these responses in our TB and control groups, it remains to be determined whether Abs are directed against the capsular AM or the arabinose-containing portion of LAM. Of note, in smear-positive HIV− TB patients, IgM responses to LAM were significantly higher than IgM responses to AM, and these responses did not correlate with each other, indicating that the IgM responses are directed to different epitopes in AM and LAM in HIV− TB cases. A recent study demonstrated that LAM, in addition to being a glycolipid cell wall antigen, is also abundantly present in membrane vesicles released by pathogenic mycobacteria and contributes to an inflammatory host response (30). Thus, it is conceivable that components contained in these vesicles elicit a humoral immune response that could contribute to ongoing stimulation of IgM responses in smear-positive HIV− TB. By studying the characteristics of the binding of four different murine monoclonal Abs (MAbs) to AM and other arabinose-containing fractions, Navoa et al. obtained data suggesting that AM and LAM share many epitopes (27). However, one of their MAbs, MAb 9d8, bound exclusively to capsular AM, indicating that parts of AM may be structurally different from LAM. To determine whether Ab responses in our TB patients are directed against the capsular AM or the AM-containing portion of LAM requires competition ELISAs with MAbs that are beyond the scope of this study.
Neither LAM nor AM is an M. tuberculosis-specific component of the mycobacterial cell wall or capsule, respectively. However, LAM displays species-specific heterogeneity at the nonreducing arabinan termini (4, 17, 20), and some studies suggest differences in the capsular composition of AM in different mycobacterial strains (15, 39). The highly significant correlation between IgG responses to BCG AM and M. tuberculosis AM in our study suggests that the same epitopes are recognized in these two strains. Thus, as seen for LAM-based TB serodiagnosis (49), cross-reactive Ab responses to AM from different mycobacterial strains could also limit the specificity of TB serodiagnosis with capsular polysaccharide antigens. Whether such cross-reactivity would reduce the potential clinical value of polysaccharide-based serodiagnostic tests for patients with respiratory diseases other than TB remains to be determined.
In accordance with prior studies, we found higher Ab responses to MPT51 in HIV+ than in HIV− TB cases, albeit at a smaller magnitude (P = 0.06), while, in contrast to the findings of our prior studies, the Ab responses to MS were not significantly different for those two groups. Possible explanations for the less pronounced differences in Ab responses to the two proteins between HIV+ and HIV− TB cases are (i) the higher number of TB cases in our prior study than in our present study, allowing for more statistical power, and (ii) comparison of different values via ΔODs (subtracting the mean of negative controls + 3 standard deviations) in our prior study, in contrast with the comparison of OD values in our present study (2). Larger studies with HIV+ and HIV− TB patients from regions where TB is endemic are needed to provide additional data on the difference in magnitude of Ab responses to the mycobacterial proteins MS and MPT51.
The observation that smear-positive individuals had higher Ab responses to mycobacterial polysaccharide antigens may seem counterintuitive when considered in the context of recent data that some MAbs can protect against M. tuberculosis (reviewed in reference 14). A similar phenomenon has been observed with gamma interferon (IFN-γ) detection in TB vaccine studies of animal models (29). On the one hand, high IFN-γ levels correlated closely with the mycobacterial burden; on the other hand, high levels also correlated with protection against TB. However, in humans, some studies have shown decreased IFN-γ levels with advanced disease (6, 32). We caution against drawing inferences from the observation of high titers of Abs to AM in smear-positive TB patients, given that no information on the biological activity of these Abs is available. In fact, one can propose several possible explanations for this association that could serve as hypotheses for future studies. For example, higher Ab titers in smear-positive individuals may result from exposure to higher antigen loads in individuals with higher mycobacterial burdens. The finding of higher Ab titers could represent the humoral component of a stronger inflammatory response associated with cavitary disease and a higher mycobacterial load independent of any physiological function for these Abs. Alternatively, higher Ab concentrations could predispose to more-severe disease through prozone-like effects in vivo, as has been reported for mice (38). In this regard, the dose-response relationship for Ab efficacy against M. tuberculosis is poorly understood, and experience with other systems suggests that Abs that are protective at one concentration can be nonprotective or even deleterious at other concentrations (48).
In summary, our data suggest that the detection of IgG responses to AM could contribute to the serodiagnosis of TB and distinguish between smear-positive and smear-negative TB, especially for HIV− TB patients. This study also provides new and important insights into the differences in the profiles of Abs to mycobacterial antigens between HIV− and HIV+ TB patients. Our detailed evaluation adds valuable information on the heterogeneity of Ab responses in HIV− TB, contributing to better understanding of the complexity of TB serology in non-HIV-infected persons. In contrast, the homogeneity of Ab responses in HIV+ TB suggests a higher likelihood that an accurate serologic test for TB can be developed for HIV-infected persons.
ACKNOWLEDGMENTS
This work was supported by funds from the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) (AI-067665, to J.M.A.; AI-033774, AI-052733, and AI-033142, to A.C.) and National Heart, Lung, and Blood Institute (NHLBI) (HL-059842, to A.C.), the Center for AIDS Research (CFAR) at the Albert Einstein College of Medicine (AI-51519, to J.M.A.), the clinical Translational Science Institute (NCRR 1UL1RR029893) at the New York University School of Medicine, the Aeras TB Vaccine Foundation (to R.P.-R.), and the Food and Drug Administration (FDA) (1U18 FD004012/01, to J.M.A.).
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Achkar JM, Jenny-Avital E. 2011. Incipient and subclinical tuberculosis: defining early disease states in the context of host immune response. J. Infect. Dis. 204:S1179–S1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Achkar JM, et al. 2010. Antibodies against immunodominant antigens of Mycobacterium tuberculosis in subjects with suspected tuberculosis in the United States compared by HIV status. Clin. Vaccine Immunol. 17:384–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown RM, et al. 2003. Lipoarabinomannan-reactive human secretory immunoglobulin A responses induced by mucosal bacille Calmette-Guerin vaccination. J. Infect. Dis. 187:513–517 [DOI] [PubMed] [Google Scholar]
- 4. Chatterjee D, Lowell K, Rivoire B, McNeil MR, Brennan PJ. 1992. Lipoarabinomannan of Mycobacterium tuberculosis. Capping with mannosyl residues in some strains. J. Biol. Chem. 267:6234–6239 [PubMed] [Google Scholar]
- 5. Coates SR, et al. 1986. Identification of Mycobacterium tuberculosis antigens in Seibert fractions by immunoblotting. J. Clin. Microbiol. 24:126–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Condos R, Rom WN, Liu YM, Schluger NW. 1998. Local immune responses correlate with presentation and outcome in tuberculosis. Am. J. Respir. Crit. Care Med. 157:729–735 [DOI] [PubMed] [Google Scholar]
- 7. Da Costa CT, Khanolkar-Young S, Elliott AM, Wasunna KM, McAdam KP. 1993. Immunoglobulin G subclass responses to mycobacterial lipoarabinomannan in HIV-infected and non-infected patients with tuberculosis. Clin. Exp. Immunol. 91:25–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Daffé M, Etienne G. 1999. The capsule of Mycobacterium tuberculosis and its implications for pathogenicity. Tuber. Lung Dis. 79:153–169 [DOI] [PubMed] [Google Scholar]
- 9. Davidow A, et al. 2005. Antibody profiles characteristic of Mycobacterium tuberculosis infection state. Infect. Immun. 73:6846–6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deshaw M, Pirofski LA. 1995. Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clin. Exp. Immunol. 99:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhandayuthapani S, Izumi S, Anandan D, Bhatia VN. 1992. Specificity of IgG subclass antibodies in different clinical manifestations of leprosy. Clin. Exp. Immunol. 88:253–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dinadayala P, Sambou T, Daffé M, Lemassu A. 2008. Comparative structural analyses of the alpha-glucan and glycogen from Mycobacterium bovis. Glycobiology 18:502–508 [DOI] [PubMed] [Google Scholar]
- 13. Gagliardi MC, et al. 2007. Cell wall-associated alpha-glucan is instrumental for Mycobacterium tuberculosis to block CD1 molecule expression and disable the function of dendritic cell derived from infected monocyte. Cell. Microbiol. 9:2081–2092 [DOI] [PubMed] [Google Scholar]
- 14. Glatman-Freedman A. 2006. The role of antibody-mediated immunity in defense against Mycobacterium tuberculosis: advances toward a novel vaccine strategy. Tuberculosis (Edinb.) 86:191–197 [DOI] [PubMed] [Google Scholar]
- 15. Glatman-Freedman A, et al. 2004. Antigenic evidence of prevalence and diversity of Mycobacterium tuberculosis arabinomannan. J. Clin. Microbiol. 42:3225–3231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gounder C, et al. 2002. Field evaluation of a rapid immunochromatographic test for tuberculosis. J. Clin. Microbiol. 40:1989–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guerardel Y, et al. 2002. Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with α1,3-mannopyranose side chains. J. Biol. Chem. 277:30635–30648 [DOI] [PubMed] [Google Scholar]
- 18. Hamasur B, et al. 2003. Mycobacterium tuberculosis arabinomannan-protein conjugates protect against tuberculosis. Vaccine 21:4081–4093 [DOI] [PubMed] [Google Scholar]
- 19. Kekow J, Hobusch G, Gross WL. 1988. Predominance of the IgG1 subclass in the hypergammaglobulinemia observed in pre-AIDS and AIDS. Cancer Detect. Prev. 12:211–216 [PubMed] [Google Scholar]
- 20. Khoo KH, Dell A, Morris HR, Brennan PJ, Chatterjee D. 1995. Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J. Biol. Chem. 270:12380–12389 [DOI] [PubMed] [Google Scholar]
- 21. Kunnath-Velayudhan S, et al. 2010. Dynamic antibody responses to the Mycobacterium tuberculosis proteome. Proc. Natl. Acad. Sci. U. S. A. 107:14703–14708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lane HC, et al. 1983. Abnormalities of B-cell activation and immunoregulation in patients with the acquired immunodeficiency syndrome. N. Engl. J. Med. 309:453–458 [DOI] [PubMed] [Google Scholar]
- 23. Lemassu A, Daffé M. 1994. Structural features of the exocellular polysaccharides of Mycobacterium tuberculosis. Biochem. J. 297(Pt 2):351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lyashchenko K, et al. 1998. Heterogeneous antibody responses in tuberculosis. Infect. Immun. 66:3936–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McEllistrem MC. 2009. Genetic diversity of the pneumococcal capsule: implications for molecular-based serotyping. Future Microbiol. 4:857–865 [DOI] [PubMed] [Google Scholar]
- 26. Morris K. 2011. WHO recommends against inaccurate tuberculosis tests. Lancet 377:113–114 [DOI] [PubMed] [Google Scholar]
- 27. Navoa JA, et al. 2003. Specificity and diversity of antibodies to Mycobacterium tuberculosis arabinomannan. Clin. Diagn. Lab. Immunol. 10:88–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Riordan K, Lee JC. 2004. Staphylococcus aureus capsular polysaccharides. Clin. Microbiol. Rev. 17:218–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parra M, et al. 2009. Development of a murine mycobacterial growth inhibition assay for evaluating vaccines against Mycobacterium tuberculosis. Clin. Vaccine Immunol. 16:1025–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prados-Rosales R, et al. 2011. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Invest. 121:1471–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riesen WF, Skvaril F, Braun DG. 1976. Natural infection of man with group A streptococci. Levels; restriction in class, subclass, and type; and clonal appearance of polysaccharide-group-specific antibodies. Scand. J. Immunol. 5:383–390 [DOI] [PubMed] [Google Scholar]
- 32. Sahiratmadja E, et al. 2007. Dynamic changes in pro- and anti-inflammatory cytokine profiles and gamma interferon receptor signaling integrity correlate with tuberculosis disease activity and response to curative treatment. Infect. Immun. 75:820–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Samanich K, Belisle JT, Laal S. 2001. Homogeneity of antibody responses in tuberculosis patients. Infect. Immun. 69:4600–4609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Samanich KM, et al. 1998. Delineation of human antibody responses to culture filtrate antigens of Mycobacterium tuberculosis. J. Infect. Dis. 178:1534–1538 [DOI] [PubMed] [Google Scholar]
- 35. Samanich KM, et al. 2000. Serodiagnostic potential of culture filtrate antigens of Mycobacterium tuberculosis. Clin. Diagn. Lab. Immunol. 7:662–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sani M, et al. 2010. Direct visualization by cryo-EM of the mycobacterial capsular layer: a labile structure containing ESX-1-secreted proteins. PLoS Pathog. 6:e1000794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sartain MJ, Slayden RA, Singh KK, Laal S, Belisle JT. 2006. Disease state differentiation and identification of tuberculosis biomarkers via native antigen array profiling. Mol. Cell. Proteomics 5:2102–2113 [DOI] [PubMed] [Google Scholar]
- 38. Schwebach JR. 2002. The carbohydrate surface of M. tuberculosis: antigenicity and antibody immunity. Ph.D. thesis Albert Einstein College of Medicine, Bronx, New York [Google Scholar]
- 39. Schwebach JR, et al. 2001. Expression of a Mycobacterium tuberculosis arabinomannan antigen in vitro and in vivo. Infect. Immun. 69:5671–5678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwebach JR, et al. 2002. Glucan is a component of the Mycobacterium tuberculosis surface that is expressed in vitro and in vivo. Infect. Immun. 70:2566–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Siber GR, Schur PH, Aisenberg AC, Weitzman SA, Schiffman G. 1980. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N. Engl. J. Med. 303:178–182 [DOI] [PubMed] [Google Scholar]
- 42. Siev M, et al. 2011. Correlation between serum and plasma antibody titers to mycobacterial antigens. Clin. Vaccine Immunol. 18:173–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Skvaril F. 1986. IgG subclasses in viral infections. Monogr. Allergy 19:134–143 [PubMed] [Google Scholar]
- 44. Steingart KR, et al. 2009. Performance of purified antigens for serodiagnosis of pulmonary tuberculosis: a meta-analysis. Clin. Vaccine Immunol. 16:260–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Steingart KR, et al. 2007. Commercial serological antibody detection tests for the diagnosis of pulmonary tuberculosis: a systematic review. PLoS Med. 4:e202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steingart KR, et al. 2007. A systematic review of commercial serological antibody detection tests for the diagnosis of extrapulmonary tuberculosis. Postgrad. Med. J. 83:705–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stokes RW, et al. 2004. The glycan-rich outer layer of the cell wall of Mycobacterium tuberculosis acts as an antiphagocytic capsule limiting the association of the bacterium with macrophages. Infect. Immun. 72:5676–5686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Taborda CP, Casadevall A. 2001. Immunoglobulin M efficacy against Cryptococcus neoformans: mechanism, dose dependence, and prozone-like effects in passive protection experiments. J. Immunol. 166:2100–2107 [DOI] [PubMed] [Google Scholar]
- 49. Tessema TA, et al. 2002. Circulating antibodies to lipoarabinomannan in relation to sputum microscopy, clinical features and urinary anti-lipoarabinomannan detection in pulmonary tuberculosis. Scand. J. Infect. Dis. 34:97–103 [DOI] [PubMed] [Google Scholar]
- 50. Wanchu A, et al. 2008. Biomarkers for clinical and incipient tuberculosis: performance in a TB-endemic country. PLoS One 3:e2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. World Health Organization 2008. Laboratory-based evaluation of 19 commercially available rapid diagnostic tests for tuberculosis. World Health Organization on behalf of the Special Programme for Research and Training in Tropical Diseases, Geneva, Switzerland [Google Scholar]