Abstract
A phase 1 study of a recombinant mutant protective antigen (rPA) vaccine was conducted in 186 healthy adults aged 18 to 45 years. Volunteers were randomized to receive one of three formulations of rPA (formalin treated, alum adsorbed, or both), in 10- or 20-μg dosages each, or the licensed vaccine, AVA. Three injections were given at 2-month intervals and a 4th 1 year after the 3rd. Vaccinees were examined at the clinic once following each injection, at 48 to 72 h postinjection. Adverse reactions were recorded in diaries for 7 days. Sera were collected before each injection and 1 week after the 1st, 2 weeks after the 3rd and 4th, and 1 year after the 4th. Serum anti-PA IgG was assayed by enzyme-linked immunosorbent assay (ELISA) and toxin neutralization assay (TNA). All formulations at both dosages were safe and immunogenic, inducing booster responses, with the highest antibody levels following the 4th injection (354 to 732 μg/ml). The lowest levels were induced by the formalin-only-treated rPA; there was no statistical difference between levels induced by alum-adsorbed and formalin-treated/alum-adsorbed rPA or by the two dosages. The antibody levels declined in all groups during the 1-year intervals after the 3rd and 4th injections but less so during the 2nd year, after the 4th injection (fold decreases were 10 to 25 versus 3.4 to 7.0, P < 0.001). There were too few AVA recipients for statistical comparisons, but their antibody levels followed those of rPA. Anti-rPA measured by ELISA correlated with TNA titers (r = 0.97). These data support studying alum-adsorbed rPA in children.
INTRODUCTION
Bacillus anthracis, the causative agent of anthrax, is a Gram-positive, spore-forming bacterium commonly found in soil. Two components, anthrax toxin and a capsular polypeptide, are essential for its virulence (13). Two B. anthracis plasmids control the synthesis of these factors: pXO1 for the toxin and pXO2 for the capsule. Anthrax toxin conforms to the AB model of toxin. The B (binding) subunit is designated protective antigen (PA). The A (active) subunit is composed of two polypeptides designated lethal factor (LF), a metalloprotease, and edema factor (EF), an adenylate cyclase. Serum antibodies to PA confer immunity to anthrax in humans and in laboratory animals (4, 27). The active principle of the licensed anthrax vaccine adsorbed (AVA) is its PA (26). Animal studies and two clinical trials provide the basis for considering a vaccine containing only PA to be effective against anthrax.
The recombinant PA (rPA) used in this study was mutated to remove two sites that are highly susceptible to proteolysis. This was intended to facilitate production of the protein in a homogeneous, intact state, by limiting the protein's susceptibility to proteases secreted into the supernatant of the producer B. anthracis strain. Decreased protease susceptibility may also help stabilize the final vaccine product against trace amounts of protease in the final product. This protein may therefore not experience the reported stability issues that impacted a previous candidate vaccine, also produced from B. anthracis (1). The two sites altered are the “furin site,” residues RKKR at positions 164 to 167, which were changed to SNKE, and residues FF at positions 314 to 315, which were deleted. Removal of the RKKR sequence also prevents the PA from forming an oligomer that is responsible for pore formation and toxin action. Pore formation is highly unlikely to occur even with native PA when it is bound to alum and (in some formulations) treated with formaldehyde, but elimination of the pore-forming ability eliminates this hypothetical possibility of forming a toxic entity.
AVA is prepared from the cell-free filtrate of a mutant strain of B. anthracis, V770-NP1-R, that expresses PA and does not induce antibodies to LF or EF (26). Primary vaccination consisted until recently of 3 subcutaneous injections at 0, 2, and 4 weeks, with booster injections at 6, 12, and 18 months and then annually (6).
MATERIALS AND METHODS
Clinical protocol.
The study (Clinical Trial registration number 00114621) was approved by the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (protocol 04-CH-0283), the U.S. Food and Drug Administration (BB IND 11154), and the Institutional Review Board of Georgetown University (protocol 2003-080). The study was randomized and partially blinded; formalin-only-treated rPA was a clear fluid, distinguishable from alum-adsorbed or formalin-treated/alum-adsorbed rPA. The latter two formulations were indistinguishable. The dosages were blinded. AVA was in vials distinguishable from rPA vials.
The study was conducted at Georgetown University Hospital, Washington, DC, and at the Clinical Center (CC), NIH, Bethesda, MD. Participants were healthy volunteers of either sex, 18 to 45 years of age. Excluded were subjects with abnormal liver or renal function, hepatitis B and/or hepatitis C, HIV infection, history of anthrax, or previous anthrax vaccination.
After signing the informed consent, eligible volunteers were randomized to 1 of 7 groups, receiving one of 3 rPA formulations at 2 dosages each and AVA. When AVA became unavailable, new volunteers were randomized to 6 groups. The randomization schemes were prepared and kept by the Pharmacy Development Section, Pharmacy Department, CC, NIH, and given only to the members of the Data and Safety Monitoring Board. The vaccine groups were as follows: 1 (10 μg) and 2 (20 μg), alum adsorbed; 3 (10 μg) and 4 (20 μg), formalin treated/alum adsorbed; 5 (10 μg) and 6 (20 μg), formalin only treated; and 7, AVA.
Volunteers were injected intramuscularly with the assigned vaccine at 0, 2, 4, and 16 months. Vaccination was postponed for a week for those with an intercurrent infection. Blood samples were taken before the 1st injection and 1 week after the 1st, 2 months after the 1st (i.e., pre-2nd), 2 months after the 2nd (pre-3rd), 2 weeks after the 3rd, 1 year after the 3rd (pre-4th), 2 weeks after the 4th, and 1 year after the 4th.
A history and physical were taken before each injection. A negative urine pregnancy test within 24 h of injection was required for female volunteers. Vaccinees were observed at the clinic for 30 min after injection and given a diary form and instructions for measuring and recording temperature and local and systemic symptoms at 6 h after injection and daily for 7 days. The vaccinees were also examined at the clinic one time following each injection, between 48 and 72 h postinjection. Liver function tests were repeated 1 week after the 1st injection. Telephone inquiries about their health were made every 3 months by study nurses during the 24-month period from the 3rd injection through the year following the 4th.
Vaccines.
The investigational vaccines were composed of an rPA mutated at several sites to increase its resistance to proteolysis (18, 21, 24). The protein, previously designated PA-SNKE-ΔFF-E308D, is antigenically identical to the PA of B. anthracis toxin (21). This rPA was produced in the sporulation-deficient BH445 strain, which is cured of both pXO1 and pXO2. rPA was extracted from the culture supernatant of the mutant B. anthracis strain and purified by hydrophobic interaction chromatography, anion-exchange chromatography, and gel filtration (21). The appropriate formulations used 0.035% formalin and 0.75 mg Alhydrogel (Brenntag-Biosector, Frederiksund, Denmark) per dose. The vaccines were bottled and stored at the Pharmaceutical Development Section, Pharmacy Department, CC, NIH.
Serum antibody assays. (i) ELISA.
Anti-rPA IgG was assayed by enzyme-linked immunosorbent assay (ELISA) (20) with the following modification: PA antibodies were detected using monoclonal antibody (MAb) HP6043 anti-human IgG followed by alkaline phosphatase-labeled rat anti-mouse IgG (2, 23). C. P. Quinn, Centers for Disease Control and Prevention, provided the PA standard, controls, and the ELISA protocol.
(ii) Anthrax TNA.
Anthrax lethal-toxin-neutralizing assay (TNA) was performed as described previously (7). PA and LF (100 ng/ml for each component) were prepared in Dulbecco's modified Eagle's medium (DMEM), and sera were diluted serially into the toxin mixture and incubated for 1 h at 37°C. Each serum was assayed in triplicate. Toxin-serum mixtures were then transferred to RAW264.7 macrophage cells in 96-well plates and incubated for 6 h, and cell viability assessed by incubation with MTT [3-(4,5-dimethylthiazo-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma, St. Louis, MO) at a final concentration of 0.5 mg/ml for 60 min. The medium was aspirated, the insoluble pigment (formazan) produced by viable cells dissolved in 0.5% (wt/vol) SDS, 25 mM HCl, in 90% (vol/vol) isopropanol, and the optical density (570 nm) measured. The effective serum concentration inducing 50% neutralization (EC50) was calculated with Prism software (Graphpad Software, Inc., San Diego, CA) and expressed as the reciprocal. TNAs were performed on 25 sera from recipients of all rPA formulations, collected after the 3rd and 4th injections. Five sera were assayed twice, and all assays were performed in a blinded fashion.
Statistical analysis.
The chi square test or Fisher's exact test was used to compare proportions. Anti-rPA IgG levels were expressed as geometric means (GM) and compared by the two-sided t test. The antibody declines of the first and second 1-year follow-ups were compared by the signed rank test. The correlation between anti-PA IgG levels (ELISA) and of toxin-neutralizing antibodies (TNA) was assessed by Spearman rank correlation. The threshold for statistical significance was a value of <0.05.
RESULTS
Study participants.
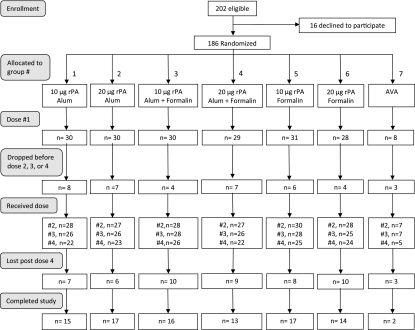
A total of 202 volunteers were enrolled from November 2004 to March 2008 (Fig. 1). Sixteen withdrew before the 1st injection; 186 (90 males and 96 females), 93 each from Georgetown University Hospital and the Clinical Center, NIH, comprised the study group. Their ages ranged from 18.2 to 45.9 years (median, 24.9). Another 39 withdrew after they were injected, 11 after the 1st, 9 after the 2nd, and 19 after the 3rd injection, none because of adverse reactions. An additional 52 volunteers were lost to the 1-year follow-up after the 4th injection.
Fig 1.
Anthrax rPA conjugate vaccine study flow chart.
Vaccination.
One hundred eighty-six volunteers received the 1st injection of the vaccine formulations, 175 the 2nd, 166 the 3rd, and 147 the 4th. The median intervals were 58 days between the 1st and 2nd and the 2nd and 3rd injections and 372 days between the 3rd and 4th injections. The number of volunteers injected in each rPA vaccine group ranged from 28 to 30 for the 1st injection, 27 to 30 for the 2nd, 25 to 28 for the 3rd, and 22 to 25 for the 4th. Because AVA became unavailable early during the study, enrollment into this group was stopped at 8 volunteers; 5 completed 4 injections. All volunteers received the assigned vaccine at each injection.
Vaccine safety.
There were no serious adverse events related to vaccination; 96% of vaccinees returned the diaries (Table 1). The highest temperature and maximal local reactions reported by each vaccinee during the 7 days were used for analysis.
Table 1.
Adverse reactions after vaccination
| Category | No. of vaccinees per injection |
|||
|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | |
| Total vaccinees | 186 | 175 | 166 | 147 |
| Vaccinees who returned diaries | 181 | 165 | 162 | 140 |
| Temperature ≥ 99.6°F (range, 99.6–100.4°F) | 1 | 1 | 4 | 3 |
| Redness ≥ 5 cm | 2 | 1 | 4 | 5 |
| Swelling ≥ 2.5 cm | 1 | 2 | 5 | 7 |
| Pain at injection site | ||||
| Mild | 41 | 37 | 38 | 32 |
| Moderate | 8 | 5 | 5 | 9 |
| Severe | 0 | 0 | 0 | 1 |
| Nausea | ||||
| Light | 9 | 7 | 3 | 5 |
| Moderate | 2 | 2 | 0 | 0 |
| Severe | 0 | 0 | 0 | 0 |
| Vomiting (all mild) | 2 | 0 | 1 | 0 |
| Stomachache | ||||
| Mild | 5 | 3 | 1 | 4 |
| Moderate | 2 | 2 | 0 | 1 |
| Severe | 0 | 0 | 0 | 0 |
| Headache | 1 | 0 | 0 | 1 |
Fever and local reactions were not statistically different between the 2 dosage groups or among the 3 formulations. A temperature of 99.6 to 100.4°F was reported by 9 vaccinees: 6 received alum-adsorbed, 2 formalin-only-treated, and 1 formalin-treated/alum-adsorbed rPA. All but one were single events. One volunteer had a temperature of 99.8 to 100.1°F for 3 days.
Swelling of ≥2.5 cm at the injection site was noted by 15 vaccinees (5 to 9 cm in 8 and 10 to 13 cm in 2); one lasted 7 days, and the rest subsided within 3 days. The frequency of swelling increased from 0.6% after the 1st injection to 5% after the 4th (P = 0.01).
Pain at the injection site was more frequent among recipients of alum-adsorbed rPA than of formalin-only-treated rPA: 34.4% in groups 1 and 2, 28.7% in groups 3 and 4, 14.5% in groups 5 and 6, and 48.1% in group 7 (P < 0.001). No difference was found between recipients of alum-adsorbed rPA with or without formalin treatment (34% versus 29%) or between recipients of alum-adsorbed rPA and AVA.
A single episode of vomiting without other systemic reactions was reported by 3 participants, at 6 h, 2 days, and 6 days postinjection. No other systemic reactions were reported. No abnormal laboratory tests were found 1 week after the first injection.
Serum anti-rPA IgG.
Prevaccination antibody levels were low and similar among the groups (Table 2). Outstanding was one volunteer with a high prevaccination anti-rPA IgG level of 180 μg/ml. He was a frequent hunter since age 12, with no history of anthrax or vaccination. He received the first 2 injections of 10 μg formalin-treated rPA (group 5) and withdrew from the study 2 months later. His anti-PA IgG level rose to 423 μg/ml 1 week after the 1st injection and to 1,199 μg/ml prior to the 2nd injection, then declined to 748 μg/ml 2 months later. He reported no adverse reactions.
Table 2.
Anti-PA IgG levels
| rPA vaccine formulation or AVA group | Group | μg/dose | GM anti-PA IgG (no. of subjects)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-1st | 1 wk post-1st | 2 mo post-1st (pre-2nd) | 2 mo post-2nd (pre-3rd) | 2 wk post-3rd | 1 yr post-3rd (pre-4th) | 2 wk post-4th | ≥1 yr post-4th | ||||
| Alum adsorbed | 1 | 10 | 1.64 (30) | 1.67 (30) | 6.96 (27) | 60.5 (25) | 159 (25) | 13.8 (21) | 525 (22) | 112 (15) | |
| 2 | 20 | 0.99 (29) | 0.92 (30) | 2.73 (27) | 50.3 (26) | 150 (26) | 7.92 (23) | 732 (21) | 105 (17) | ||
| Formalin treated/alum adsorbed | 3 | 10 | 1.29 (30) | 1.75 (29) | 8.99 (28) | 63.8 (27) | 165 (26) | 10.2 (25) | 355 (23) | 99.3 (16) | |
| 4 | 20 | 0.57 (29) | 0.74 (28) | 5.74 (28) | 47.8 (25) | 134 (24) | 14.3 (22) | 442 (18) | 89.7 (13) | ||
| Formalin only treated | 5 | 10 | 1.39 (31) | 1.17 (30) | 1.36 (31) | 13.8 (29) | 52.8 (28) | 5.25 (24) | 124 (21) | 25.2 (17) | |
| 6 | 20 | 0.54 (28) | 0.70 (28) | 1.22 (26) | 17.2 (25) | 117 (24) | 4.60 (23) | 188 (22) | 36.1 (14) | ||
| AVA, this studyb | 7 | 0.21 (8) | 0.36 (7) | 2.31 (6) | 35.8 (6) | 152 (6) | 2.76 (5) | 409 (4) | 85.9 (2) | ||
| Pre-1st [0 mo] | Post-3rd [2 mo] | Pre-4th [6 mo] | Post-4th [6.5 mo] | Pre-6th [18 mo] | Post-6th [18.5 mo] | ||||||
| AVA, U.S. militaryc (time from start [mo]) | 1.83 (246) | 59.9 (129) | 24.6 (84) | 158 (84) | 92.4 (33) | 277 (33) | |||||
Levels (μg/ml) were measured by ELISA.
AVA, anthrax vaccine adsorbed.
Data from Singer et al. (23).
No significant differences were found between responses to the 10- and 20-μg dosages or between the alum- (groups 1 and 2) and formalin-treated/alum-adsorbed formulations (groups 3 and 4).
There was no change in the GM antibody level 1 week after the 1st injection. Prior to the 2nd injection, groups 1 to 4 (alum-adsorbed rPA with or without formalin treatment) showed 3- to 10-fold increases in IgG antibody levels (P < 0.02), and groups 5 and 6 (formalin-only-treated rPA) showed no significant response (P = 0.4 for group 5, and P = 0.07 for group 6).
Prior to the 3rd injection, all but groups 5 and 6 had significant increases in antibody levels (P < 0.002), reaching GM levels of 48 to 64 μg/ml in recipients of all alum-adsorbed vaccines. Groups 5 and 6, who received the formalin-only-treated rPA, had the lowest levels. Two weeks after the 3rd injection, all but group 5 had significant antibody level rises of 2.6- to 6.8-fold; GM levels were 117 to 165 μg/ml (P < 0.004). One year after the 3rd injection, the antibody levels declined in all groups by 10- to 25-fold. A 4th injection at that time induced significant rises in all groups (P < 0.001); GM levels in recipients of the alum-adsorbed vaccines ranged from 354 to 732 μg/ml. Again, the lowest levels, 124 and 188 μg/ml, were in groups 5 and 6. After an additional 1 to 2 years, antibody levels declined in all groups but to a lesser degree than during the 1-year interval after the 3rd injection, 10- to 25-fold versus 3.4- to 7.0-fold (P < 0.001). At that time, the GM IgG level of all alum-adsorbed rPA vaccinees was 101 μg/ml.
There were too few AVA recipients for statistical comparisons, but the trend of their antibody levels was similar to that of the rPA vaccinees.
In a previous study of U.S. army recruits' responses to AVA injected according to the then-recommended schedule at 0, 2, and 4 weeks and 6, 12, and 18 months, GM levels of ∼60 μg/ml were measured at 6 weeks from the start, after 3 injections of AVA (23). Similar GM levels were found in our current study after 2 injections of alum-adsorbed rPA, but at 4 months. At 18.5 months, following the 4th injection of rPA, recipients of the alum-adsorbed vaccines achieved GM levels of 354 to 732 μg/ml, compared to 277 μg/ml at the same time interval but after the 6th injection of AVA in army recruits. No differences in antibody levels between male and female volunteers were found.
Toxin neutralization.
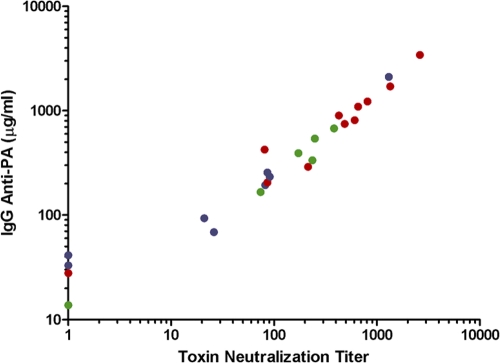
TNA was performed blindly on 25 serum samples selected from those taken from the six groups of rPA recipients after the 3rd and 4th injections, with antibody levels ranging from 13.8 to 3,422 μg/ml (median, 336 μg/ml). Five samples were assayed as 2 independent blinded samples and their values averaged. The correlation coefficient between antibody levels measured by ELISA and by TNA was 0.97 (Spearman rank correlation) (Fig. 2).
Fig 2.
Correlation between toxin neutralization titers and anti-rPA IgG levels measured by ELISA. Red, alum-adsorbed-rPA recipients; green, formalin-treated/alum-adsorbed-rPA recipients; purple, formalin-only-treated-rPA recipients.
DISCUSSION
We evaluated the safety and immunogenicity of three formulations at two dosages each of a recombinant mutant PA vaccine in healthy adult volunteers, 18 to 45 years old. Low anti-PA IgG levels were measured in prevaccination sera, similar among all vaccine groups and U.S. army recruits (Table 2). The volunteers were injected three times 2 months apart, using the schedule recommended for primary routine vaccination of infants and children, and then 1 year later. AVA was included for comparison but stopped early due to unavailability of the vaccine. No serious adverse reactions were observed. Rare, minor local reactions and mild fever occurred among recipients of all rPA formulations and AVA, similar to the response to intramuscular injections of AVA reported by Morano et al. (15). All rPA formulations were immunogenic and induced high anti-rPA IgG levels with booster responses. A correlation coefficient of 0.97 was found between antibody levels measured by ELISA and by TNA.
The 10- and 20-μg dosages were chosen based upon the dosages of diphtheria and tetanus toxoids and many other investigational vaccines over the past 60 years. Alum is used as an adjuvant for many bacterial, viral, and allergenic injectables and was shown to exert a similar effect on rPA (5). The licensed vaccine, AVA, is alum adsorbed. Formalin treatment has been shown to increase the immunogenicity of diphtheria toxin and to confer hydrolytic stability to this and to other proteins (19). In mice, we found the lowest immunogenicity of rPA injected as a purified protein. The immunogenicity was enhanced by formalin treatment, further enhanced by alum adsorption, and most enhanced when both formalin treated and alum adsorbed (unpublished data). Adult human responses in this study were best to alum-adsorbed formulations and low to the formalin-treated rPA, with no added benefit of formalin treatment in addition to alum adsorption; alum-adsorbed rPA with or without formalin treatment induced similar and significant antibody level increases starting with the first injection.
Antibody responses elicited by the 10- and 20-μg dosages were not significantly different. Spore challenge studies in rabbits showed protection with PA antibody levels of 50 to 100 μg/ml (14, 16), but a protective level in humans is unknown. Even less can be surmised about the level needed to protect from a bioterrorist attack, making assessment of the responses to the different vaccines and schedules difficult.
In army recruits, the first postimmunization sera we obtained were drawn after the 3rd injection, at about 6 weeks from the start of AVA vaccination; a GM level of 60 μg/ml was found (23). Such GM levels were achieved in this study by 2 injections of alum-adsorbed rPA, measured 4 months from the start (sera after the 2nd injection were obtained only at 2 months postinjection, just before the 3rd injection; earlier responses to the 2nd injection were not measured); AVA at the then-recommended schedule induced a GM antibody level of 60 μg/ml earlier than the rPA at the schedule used in our study. After the 4-month interval, our alum-adsorbed vaccines achieved comparable if not higher antibody levels at all time intervals and with fewer injections than the AVA in the military. These results are also comparable to those achieved by Morano et al. in spite of different study designs (15).
The 1-year-decline slope in antibody levels was steeper during the 1st than during the 2nd year of follow-up, as was the experience with AVA in army recruits (17, 23). At the end of the 2nd year, after 4 injections, the GM level of all alum-adsorbed rPA recipients was about 100 μg/ml. This level and the slower decline during the 2nd year of follow-up may indicate that booster doses could be spaced at >1-year intervals.
The use of rPA as a vaccine has the advantage of it being a purified single protein, easy to control and standardize. The high correlation between the level of serum anti-rPA IgG (ELISA) and the results of bioassay of toxin neutralization could permit relying on ELISA to measure the serum antibody responses in the future.
Anthrax has been reported in children living in herding communities, and more children died from gastrointestinal anthrax than adults (11, 25). Children are expected to be as or more susceptible than adults to bioterrorism attacks. The level of safety achieved in adults by diphtheria and tetanus toxoids has been predictive of their effects in children and infants. Based upon this long and successful experience, we propose that the safety and immunogenicity of rPA in healthy adults predicts its effects in children. Accordingly, we plan to evaluate our alum-adsorbed rPA in children. We also plan on evaluating an rPA-γDPGA (poly-γ-d-glutamic acid; B. anthracis capsule) conjugate clinically (8, 22).
A new role for anthrax vaccine has evolved with the demonstration of anthrax toxin produced by strains of Bacillus cereus associated with systemic disease (9, 10). Recently, anthrax toxin-positive strains identified in hospitalized patients who have died were reported in several communities (3, 12, 28). One immediate step might be to immunize contacts of patients with this newly described B. cereus infection with an rPA vaccine.
ACKNOWLEDGMENTS
This research was supported by the Divisions of Intramural Research, NICHD and NIAID, National Institutes of Health.
We thank the volunteers for their participation in this study, Karen Dowdy, Terry McHugh, and Dolores Medina at the Clinical Center, NIH, Bethesda, MD, and Abby Panner and Henry Castro at the Immunology Center, Georgetown University Medical Center, Washington, DC, for their dedication and support of the project, and Patricia Moyer, Division of Epidemiology, Statistics and Prevention Research, NICHD, and James F. Troendle, National Heart, Lung and Blood Institute, for statistical assistance.
Footnotes
Published ahead of print 21 December 2011
REFERENCES
- 1. Anonymous 2007. US biodefence—shocking and awful. Nat. Biotechnol. 25:603. [DOI] [PubMed] [Google Scholar]
- 2. Ashkenazi S, et al. 1999. Safety and immunogenicity of Shigella sonnei and Shigella flexneri 2a O-specific polysaccharide conjugates in children. J. Infect. Dis. 179:1565–1568 [DOI] [PubMed] [Google Scholar]
- 3. Avashia SB, et al. 2007. Fatal pneumonia among metal workers due to inhalation exposure to Bacillus cereus containing Bacillus anthracis toxin genes. Clin. Infect. Dis. 44:414–416 [DOI] [PubMed] [Google Scholar]
- 4. Brachman PS, et al. 1962. Field evaluation of a human anthrax vaccine. Am. J. Public Health Nations Health 52:632–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campbell JD, et al. 2007. Safety, reactogenicity and immunogenicity of a recombinant protective antigen anthrax vaccine given to healthy adults. Hum. Vaccin. 3:205–211 [DOI] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention 2000. Use of anthrax vaccine in the United States. Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 49(RR-15):1–20 http://www.cdc.gov/mmwr/preview/mmwrhtml/rr4915a1.htm [PubMed] [Google Scholar]
- 7. Chen Z, et al. 2009. Potent neutralization of anthrax edema toxin by a humanized monoclonal antibody that competes with calmodulin for edema factor binding. Proc. Natl. Acad. Sci. U. S. A. 106:13487–13492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Z, et al. 2011. Pre- and postexposure protection against virulent anthrax infection in mice by humanized monoclonal antibodies to Bacillus anthracis capsule. Proc. Natl. Acad. Sci. U. S. A. 108:739–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoffmaster AR, et al. 2006. Characterization of Bacillus cereus isolates associated with fatal pneumonias: strains are closely related to Bacillus anthracis and harbor B. anthracis genes. J. Clin. Microbiol. 44:3352–3360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffmaster AR, et al. 2004. Identification of anthrax toxin genes in a Bacillus cereus associated with an illness resembling inhalation anthrax. Proc. Natl. Acad. Sci. U. S. A. 101:8449–8454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaya A, Tasyaran MA, Erol S, Ozkurt Z, Ozkan B. 2002. Anthrax in adults and children: a review of 132 cases in Turkey. Eur. J. Clin. Microbiol. Infect. Dis. 21:258–261 [DOI] [PubMed] [Google Scholar]
- 12. Klee SR, et al. 2006. Characterization of Bacillus anthracis-like bacteria isolated from wild great apes from Cote d'Ivoire and Cameroon. J. Bacteriol. 188:5333–5344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leppla SH, Robbins JB, Schneerson R, Shiloach J. 2002. Development of an improved vaccine for anthrax. J. Clin. Invest. 110:141–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Little SF, et al. 2004. Defining a serological correlate of protection in rabbits for a recombinant anthrax vaccine. 22:422–430 [DOI] [PubMed] [Google Scholar]
- 15. Morano N, et al. 2008. Effects of a reduced dose schedule and intramuscular administration of anthrax vaccine adsorbed on immunogenicity and safety at 7 months. JAMA 81:1532–1543 [DOI] [PubMed] [Google Scholar]
- 16. Pitt ML, et al. 2001. In vitro correlate of immunity in a rabbit model of inhalational anthrax vaccine. 19:4768–4773 [DOI] [PubMed] [Google Scholar]
- 17. Pittman PR, et al. 2006. Patterns of antibody response in humans to the anthrax vaccine adsorbed (AVA) primary (six-dose) series. Vaccine 24:3654–3660 [DOI] [PubMed] [Google Scholar]
- 18. Pomerantsev AP, et al. 2011. A Bacillus anthracis strain deleted for six proteases serves as an effective host for production of recombinant proteins. Protein Expr. Purif. 80:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Porro M, Saletti M, Nencioni L, Tagliaferri L, Marsili I. 1980. Immunogenic correlation between cross-reacting material (CRM197) produced by a mutant of Corynebacterium diphtheriae and diphtheria toxoid. J. Infect. Dis. 142:716–724 [DOI] [PubMed] [Google Scholar]
- 20. Quinn CP, et al. 2002. Specific, sensitive, and quantitative enzyme-linked immunosorbent assay for human immunoglobulin G antibodies to anthrax toxin protective antigen. Emerg. Infect. Dis. 8:1103–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ramirez DM, Leppla SH, Schneerson R, Shiloach J. 2002. Production, recovery and immunogenicity of the protective antigen from a recombinant strain of Bacillus anthracis. J. Ind. Microbiol. Biotechnol. 28:232–238 [DOI] [PubMed] [Google Scholar]
- 22. Schneerson R, et al. 2003. Poly(gamma-d-glutamic acid) protein conjugates induce IgG antibodies in mice to the capsule of Bacillus anthracis: a potential addition to the anthrax vaccine. Proc. Natl. Acad. Sci. U. S. A. 100:8945–8950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Singer DE, et al. 2008. Serum IgG antibody response to the protective antigen (PA) of Bacillus anthracis induced by anthrax vaccine adsorbed (AVA) among U.S. military personnel. Vaccine 26:869–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh Y, Klimpel KR, Arora N, Sharma M, Leppla SH. 1994. The chymotrypsin-sensitive site, FFD315, in anthrax toxin protective antigen is required for translocation of lethal factor. J. Biol. Chem. 269:29039–29046 [PubMed] [Google Scholar]
- 25. Sirisanthana T, Brown AE. 2002. Anthrax of the gastrointestinal tract. Emerg. Infect. Dis. 8:649–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Taft SC, Weiss AA. 2008. Neutralizing activity of vaccine-induced antibodies to two Bacillus anthracis toxin components, lethal factor and edema factor. Clin. Vaccine Immunol. 15:71–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turnbull PC, Leppla SH, Broster MG, Quinn CP, Melling J. 1988. Antibodies to anthrax toxin in humans and guinea pigs and their relevance to protective immunity. Med. Microbiol. Immunol. 177:293–303 [DOI] [PubMed] [Google Scholar]
- 28. Wright AM, et al. 2011. Rapidly progressive, fatal, inhalation anthrax-like infection in a human: case report, pathogen genome sequencing, pathology, and coordinated response. Arch. Pathol. Lab. Med. 135:1447–1459 [DOI] [PubMed] [Google Scholar]