Abstract
The global distribution of brucellosis and high incidence in certain areas of the world warrant the development of a safer and efficacious vaccine. For the past 10 years, we have focused our attention on the development of a safer, but still highly protective, live attenuated vaccine for human and animal use. We have demonstrated the safety and protective efficacy of the vaccine candidates 16MΔvjbR and S19ΔvjbR against homologous and heterologous challenge in multiple immunocompetent animal models, including mice and deer. In the present study, we conducted a series of experiments to determine the safety of the vaccine candidates in interferon regulatory factor-1-knockout (IRF-1−/−) mice. IRF-1−/− mice infected with either wild-type Brucella melitensis 16M or the vaccine strain Brucella abortus S19 succumb to the disease within the first 3 weeks of infection, which is characterized by a marked granulomatous and neutrophilic inflammatory response that principally targets the spleen and liver. In contrast, IRF-1−/− mice inoculated with either the 16MΔvjbR or S19ΔvjbR vaccine do not show any clinical or major pathological changes associated with vaccination. Additionally, when 16MΔvjbR- or S19ΔvjbR-vaccinated mice are challenged with wild-type Brucella melitensis 16M, the degree of colonization in multiple organs, along with associated pathological changes, is significantly reduced. These findings not only demonstrate the safety and protective efficacy of the vjbR mutant in an immunocompromised mouse model but also suggest the participation of lesser-known mechanisms in protective immunity against brucellosis.
INTRODUCTION
Brucellosis, a bacterial disease caused by members of the genus Brucella, is an important zoonosis of nearly worldwide distribution (22). In humans, the disease is characterized by episodes of fever, arthritis, endocarditis, and osteomyelitis (22). In animals, the most common manifestations are abortions and infertility, which result in extensive economic losses (28). To date, five out of the nine known Brucella species are capable of infecting humans, with the most pathogenic being Brucella melitensis, followed by Brucella suis and Brucella abortus. These three species have been classified as biological warfare agents by the Centers for Disease and Control and Prevention (CDC) due to their low infectivity dose when aerosolized and substantial capacity to cause a chronic debilitating illness (9). Vaccination against brucellosis has been integral for disease prevention in animals; however, the currently licensed vaccine strains for veterinary usage, such as S19, RB51, or Rev 1, are unacceptable for human use due to residual virulence that can result in the development of the disease (24, 27). To date, a safe and protective vaccine for use against human brucellosis is still needed.
Although many novel DNA and subunit vaccines against brucellosis are being developed, the live attenuated vaccines confer the greatest level of protection due to their ability to generate more persistent memory responses than nonliving preparations (7, 24). This protection is observed not only for Brucella vaccines but also for other infectious pathogens as well (18, 21, 30). At least 10 live attenuated vaccines are currently licensed in the United States (USDA website).
In the past, classic attenuation processes were somewhat empirical and unpredictable, which raised multiple concerns regarding safety, including reversion to wild-type virulence and vaccine shedding. Both of these adverse events pose a risk to vaccinated or even unvaccinated individuals with impaired immunity. Advances in molecular biology have led to the identification of genes that are essential for bacterial replication and persistence in vivo and in vitro. On the basis of this knowledge, our laboratory has designed a rational live attenuated vaccine for brucellosis via deletion of the vjbR gene (BMEII1116), which encodes the luxR-like quorum-sensing-related transcriptional regulator. VjbR is required for adequate VirB expression, which is essential for survival in phagocytes and virulence in mice (32). The vjbR mutants of B. melitensis and B. abortus are highly attenuated in mice and macrophages while conferring high levels of protective immunity, making such mutants ideal vaccine candidates (2, 3).
In the present study, we conducted a series of experiments designed to provide additional evidence of the safety and efficacy of the vaccine candidates 16MΔvjbR and S19ΔvjbR by demonstrating their performance in the interferon regulatory factor-1-knockout (IRF-1−/−) mouse model, in which mice are known to succumb to wild-type infection (16).
MATERIALS AND METHODS
Mice.
Six- to 8-week old, female B6.129s2-Irf1tm1Mak/J mice were obtained from the Jackson Laboratories (Bar Harbor, ME) and housed under specific-pathogen-free conditions and acclimated for 2 weeks prior to infection. All experimental procedures and animal care were performed in compliance with the institutional animal care guidelines.
Bacterial strains.
Strains used in these experiments include B. melitensis 16MΔvjbR (engineered for a previous study and used as a vaccine candidate) (35), S19ΔvjbR (engineered for a previous study and used as a vaccine candidate) (2), B. abortus S19 (NVSL, Ames, IA), the virulent strain Brucella melitensis 16M biovar 1 (originally obtained from ATCC and reisolated by this lab from an aborted goat fetus) (2, 12), and the wild-type strain Brucella abortus 2308 obtained from an aborted fetus by B. Deyoe (NADC). Bacteria were grown on tryptic soy agar (TSA; Difco, Becton Dickinson) or Farrell's medium (TSA supplemented with Brucella selective supplement [Oxoid Limited] at 37°C with 5% [vol/vol] CO2).
To prepare organisms for animal infections, the Brucella strains were harvested from the surface of the plates after 3 days of incubation using phosphate-buffered saline (PBS), pH 7.2 (Gibco). The bacteria were resuspended to a final concentration of 1 × 106 CFU/mouse on the basis of optical density readings using a Klett meter and a standardized light-scattering curve. Actual viable counts were confirmed retrospectively by serial dilution, plating, and enumeration.
Measurement of body temperature.
Implantable temperature transponders (IPTT-300) and a handheld reader (DAS-7007; Bio Medic Data Systems, DE) were used according to the manufacturer's instructions. Briefly, the mouse-specific transponders were preprogrammed with individual number identifiers before they were implanted into each individual mouse. One week prior to inoculation with the Brucella strains, the transponders were injected into the subcutaneous tissue along the left lateral aspect of the flank, using the insertion device included with the microchips. Prior to inoculation, the basal temperature was monitored for 3 days for each individual mouse. Once mice were inoculated, the temperature was monitored for the first 30 days and for 1 week following challenge in those individuals that survived past the initial 30 days. Measurement of the temperature was achieved by holding the handheld detector approximately 5 to 10 cm from the implanted chip. Temperature was monitored once or twice daily, always at the same time of the day. A temperature drop below 32°C was chosen as an endpoint indicating bacterial sepsis.
Evaluation of virulence of Brucella ΔvjbR mutant in IRF-1−/− mice.
Six groups of five mice each were injected intraperitoneally with 1 × 106 CFU/mouse with either (i) B. melitensis 16M, (ii) B. melitensis 16MΔvjbR, (iii) B. abortus 2308, (iv) B. abortus S19, (v) B. abortus S19ΔvjbR, or (vi) PBS. Mice were monitored daily for clinical evidence of disease. Any infected animal with substantial reduced mobility, abnormal postural changes, ruffled coat, and a temperature below 32°C was considered a mortality and immediately euthanized via carbon dioxide asphyxiation. At 30 days postinoculation, a selection of animals that survived the infection for the first month were also euthanized to determine the bacterial colonization and associated pathological findings. At each time point, spleens and livers were collected, weighed, and homogenized in 1 ml of peptone saline. Serial dilutions were prepared, and 100-μl aliquots of the different dilutions were plated in duplicate onto TSA or Farrell's medium. Levels of infection were expressed as the mean ± standard error (SE) of the individual log number of CFU/organ (see Fig. 8 and 9).
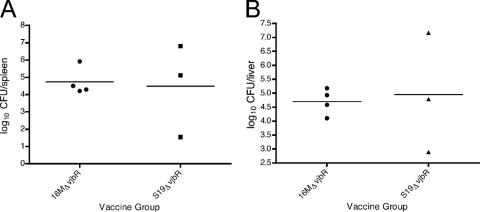
Fig 8.
16M recovery from spleens and livers 1 week postchallenge in IRF-1−/− mice. Groups of four mice were vaccinated with 1 × 106 of either 16MΔvjbR or S19ΔvjbR for 8 weeks prior to challenge. Data are expressed as log10 number of CFU/organ in the spleens (A) or livers (B).
Fig 9.
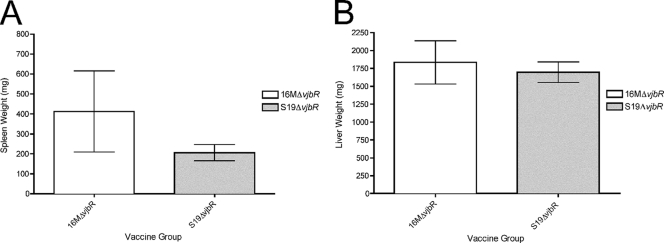
Spleen (A) and liver (B) weights of IRF-1−/− mice vaccinated with either 16MΔvjbR or S19ΔvjbR and challenged intraperitoneally with 16M. Data represent the average of 4 mice per group.
Evaluation of protective efficacy of vjbR mutants in IRF-1−/− mice.
Groups of four female IRF-1−/− mice were vaccinated intraperitoneally with 1 × 106 CFU of either B. melitensis 16MΔvjbR or B. abortus S19ΔvjbR. These mice were monitored for any sign of clinical disease, including body temperature changes as described above. At 8 weeks postvaccination, mice were challenged intraperitoneally with 5 × 106 CFU of B. melitensis wild-type 16M/mouse. At 1 week postchallenge, animals were euthanized by CO2 asphyxiation, and the spleens and livers were removed, weighed, and homogenized in 1 ml of peptone saline. Serial dilutions were prepared, and 100-μl portions were plated onto TSA plates. Levels of infection were expressed as the mean log number of CFU/organ ± SE recovered from mice.
Evaluation of histological changes in IRF-1−/− mice inoculated with the different Brucella strains.
Multiple organs, including spleen, liver, heart, kidney, lung, intestine, lymph nodes, and brain, from Brucella-infected mice were collected at death, 30 days postinoculation, or 1 week postchallenge, placed in 10% buffered formalin, paraffin embedded, and stained with hematoxylin and eosin. Histological changes between groups were assessed by a board-certified veterinary pathologist.
Statistical analysis.
Bacterial burdens from mutant clearance as well as efficacy studies were expressed as the mean number of CFU ± SE and presented graphically as the log10 number of Brucella CFU recovered per organ. Culture-negative organs were assigned a value of 4 CFU, which is below the limit of detection of 5 CFU/organ. Spleen weight data from challenge were plotted as the mean spleen weight (in mg) ± SE. A log-rank analysis (Mantel-Cox test) using GraphPad Prism software was used to determine the significance of survival curves among mice.
RESULTS
Temperature changes in IRF-1−/− mice infected with the different Brucella strains.
To determine possible temperature fluctuations due to the inoculation with the vaccine candidates, mice were monitored daily pre- and postinoculation for up to 30 days using implantable temperature transponders. Basal preinoculation temperatures were in the range of 35°C to 37.8°C. Mice inoculated with either the 16MΔvjbR or S19ΔvjbR mutant did not show a statistically significant fluctuation in the temperature at any time point (Fig. 1E and C). Temperatures postinoculation were in the range of 35.4°C to 37.8°C (Fig. 1). In contrast, temperature changes were evident in several mice infected with either B. melitensis 16M, B. abortus 2308, or B. abortus S19 and were characterized by a marked episode of hypothermia prior to death (Fig. 1A, B, and D). There was no detectable evidence of fever at any of the time points evaluated.
Fig 1.
Temperature profiles from infected immune-incompetent mice. IRF-1−/− mice were implanted with transponders and monitored daily over a 33-day period following inoculation with 1 × 106 CFU/mouse of either 2308 (A), S19 (B), S19ΔvjbR (C), 16M (D), or 16MΔvjbR (E). The dotted lines denote the day of inoculation; temperature profiles to the left of the dotted lines denote basal body temperatures prior to inoculation. Each symbol represents an individual mouse tracked for 30 days postinoculation.
Survival of Brucella ΔvjbR mutants in IRF-1−/− mice.
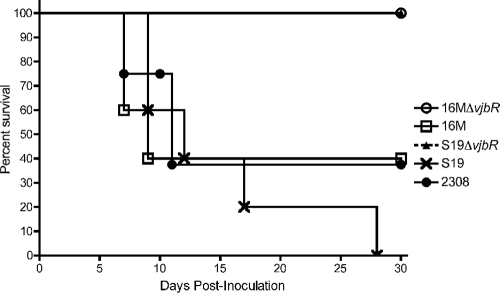
To determine the effect of the vjbR gene deletion in an immunocompromised mouse model, IRF-1−/− mice were infected intraperitoneally with 1 × 106 CFU/mouse of either B. melitensis 16MΔvjbR, B. abortus S19ΔvjbR, B. melitensis 16M, or B. abortus 2308 or S19. At 5 days postinoculation, mice that received the B. melitensis 16M strain started showing clinical evidence of disease, characterized by lethargy, ruffled coat, hunched posture, hypothermia, and anorexia (Fig. 2). By 10 days postinoculation, 60% of mice had succumbed to the infection (Fig. 3). Similarly, mice inoculated with B. abortus 2308 exhibited signs of illness by day 7, and 50% of mice were euthanized by day 11 postinoculation due to imminent deterioration (Fig. 3). Interestingly, mice vaccinated with the vaccine strain S19 also elicited clinical signs of illness, but at a time point later than that observed with the virulent strains, i.e., at day 10 postinoculation, with a 100% mortality rate by day 28 (Fig. 3). In contrast, 100% of mice vaccinated with either the 16MΔvjbR or S19ΔvjbR mutant exhibited no signs of disease (P < 0.0001), and 100% survived beyond day 30 postinoculation (Fig. 3). Two animals from the groups inoculated with B. melitensis 16M and B. abortus 2308 exhibited no signs of disease beyond day 30 postinoculation. These data clearly demonstrate that vjbR mutants are less virulent in immunocompromised mice.
Fig 2.
Health checks of immune-incompetent mice. Differences in behavior of IRF-1−/− mice infected with 1 × 106 CFU/mouse of either 16MΔvjbR (A) or 16M (B) were noticeable by day 10 postinoculation. Note the abnormal posture and ruffled coat in the mouse inoculated with the wild-type strain.
Fig 3.
Survival of IRF-1−/− mice infected with 1 × 106 CFU/mouse of either 2308, S19, S19ΔvjbR, 16M, or 16MΔvjbR. Mice inoculated with ΔvjbR vaccine candidates survived longer than mice inoculated with either 16M (P < 0.005), 2308 (P < 0.005), or S19 (P < 0.005).
Evaluation of bacterial colonization in IRF-1−/− mice.
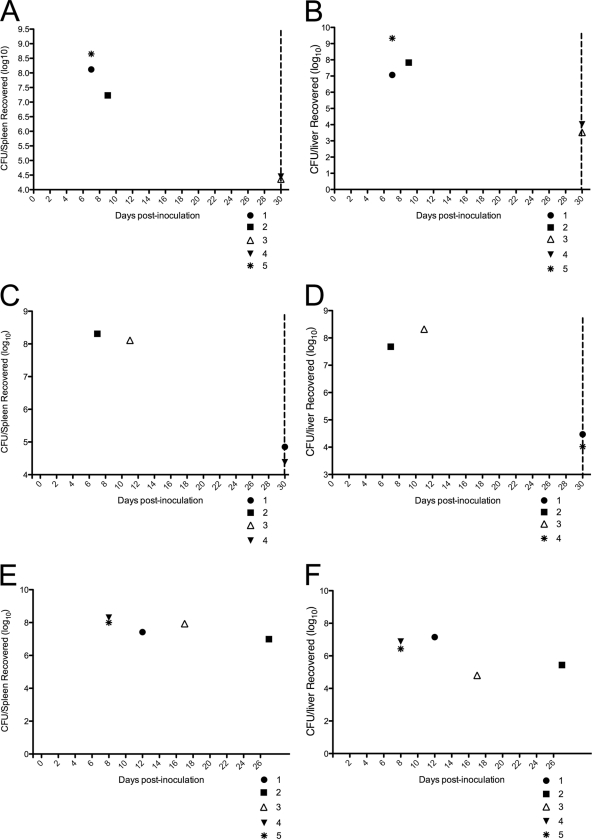
In addition to the signs of illness described above, bacterial colonization in selected tissues was determined in an effort to identify any correlation between death and the degree of colonization. Spleens and livers from animals that succumbed to the infection at the different time points were harvested, weighed, and homogenized in PBS, and the bacterial burden was determined by growth on solid medium. All mice inoculated with B. melitensis 16M that died within the first 2 weeks of infection (Fig. 3) had an overwhelming bacterial burden in the spleens (7.07, 7.83, and 9.33 log10 CFU/spleen) and livers (8.12, 8.65, and 7.23 log10 CFU/liver) (Fig. 4A and B). Animals that survived the initial 30 days without signs of disease demonstrated a reduced but still high level of bacterial colonization in the spleens (4.36 and 4.45 log10 CFU/spleen) and livers (3.52 and 4.02 log CFU/liver). Similar degrees of colonization were evident in mice that were inoculated with B. abortus 2308. Mice that died within the first 2 weeks of infection had an extremely elevated bacterial burden in the spleens (8.11 and 8.31 log CFU/spleen) and livers (7.68 and 8.32 log10 CFU/liver) (Fig. 4C and D). Animals that survived the initial 30 days of infection had a reduced but still significant bacterial burden in both organs (Fig. 4). Interestingly, S19-inoculated animals also demonstrated an overwhelming infection, with approximately 7 log units present in the spleens and livers, even at 28 days postinoculation (Fig. 4E and F). Reduced splenic colonization by the 16MΔvjbR or S19ΔvjbR strain was evident by 30 days postinoculation, and only 20 CFU was obtained from the spleen from each mouse (Fig. 4). Increased splenic colonization by the 16M, 2308, or S19 strain correlated with increased splenic and hepatic weights (Fig. 5), suggesting an increased inflammatory response to the infection that could be confirmed only by histopathology. The overwhelming amount of bacteria in the tissues from animals inoculated with 16M, 2308, or S19 along with the clinical signs indicates a severe septicemic process as the cause of death in these animals.
Fig 4.
Bacterial colonization in the spleens and livers in terminally ill IRF-1−/− mice inoculated with 1 × 106 CFU/mouse of either 16M (A and B), 2308 (C and D), or S19 (E and F). The dotted lines denote 30 days postinfection, and animals that did not succumb to the infection prior to this time point were euthanized to determine the extent of bacterial colonization. Data are expressed as log10 number of CFU/organ.
Fig 5.
Spleen and liver weights in terminally ill IRF-1−/− mice inoculated with 1 × 106 CFU/mouse of either 2308 (A), S19 (B), or 16M (C). Each point represents an individual mouse and the spleen or liver weight at the time of death. The dotted lines represent the average normal weight of each organ for naïve animals.
Evaluation of gross and histopathologic changes in IRF-1−/− mice inoculated with different Brucella strains.
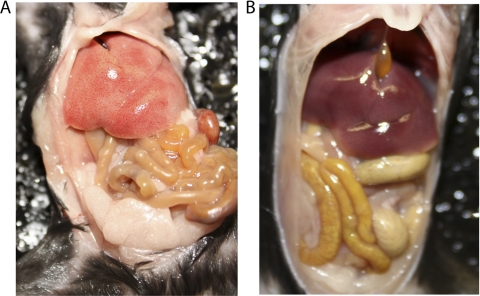
To determine whether gross and histologic changes were observed in IRF-1−/− inoculated with the different Brucella strains, a full necropsy as well as histopathologic examination was performed in all animals that succumbed to the infection and at 30 days postinfection. Gross changes were most prominent in the liver and spleen of B. melitensis 16M-inoculated mice, followed by B. abortus 2308- and S19-inoculated mice. All the livers from 16M-, 2308-, and, to a lesser degree, S19-infected mice were pale and enlarged (Fig. 6). The spleens from all mice inoculated with 16M and, to a lesser degree, 2308 and S19 mice were pale, markedly enlarged, and friable. Approximately 20% of mice inoculated with 16M or 2308 had a moderate amount of yellow to clear, gelatinous exudate within the abdominal cavity, consistent with ascites. The 16M- and 2308-inoculated animals that survived the initial 30 days postinfection had similar lesions but to a lesser extent. None of the animals that were inoculated with either the 16MΔvjbR or S19ΔvjbR strain exhibited any gross pathological changes (Fig. 6).
Fig 6.
Gross pathological changes observed at day 30 in mice infected with 1 × 106 CFU/mouse of either 16M (A) or 16MΔvjbR (B). Note the markedly enlarged and pale liver and spleen in the mouse inoculated with 16M. The abdominal cavity in the mouse inoculated with the 16MΔvjbR was unremarkable.
Histopathologic evaluation of the spleens from mice inoculated with either B. melitensis 16M, B. abortus 2308, or B. abortus S19 exhibited similar changes, which consisted of a marked histiocytic to granulomatous splenitis with multiple foci of splenic necrosis (Fig. 7) that in some instances completely obliterated the normal tissue architecture. These changes were more prominent in mice that died more acutely. By 30 days, the degree of inflammatory response was mildly reduced but was still very pronounced. Changes in the liver in 16M-, 2308-, and S19-inoculated mice consisted of a multifocal, random neutrophilic hepatitis at the earlier time points that progressed to a histiocytic infiltrate with epithelioid cells and microgranulomas at the later stages of the infection (Fig. 7). Other changes were evident in multiple organs and included a neutrophilic to lymphohistiocytic infiltrate in multiple lymph nodes, lung, brain, kidney, intestine, and heart (in 2 mice, 1 inoculated with 2308 and 1 inoculated with 16M), indicating that these mice died from a severe septicemic process. None of the animals that received the vaccine candidates and examined at 30 days postinoculation had any evidence of the Brucella-associated pathological findings mentioned above (Fig. 7).
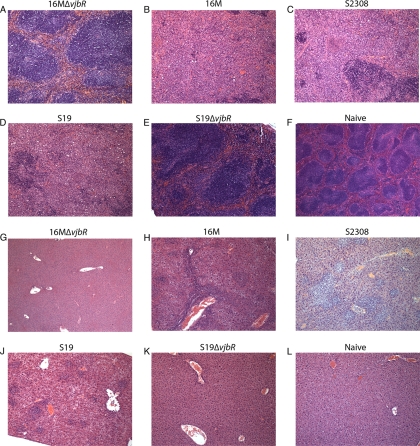
Fig 7.
Microscopic changes observed in the spleens (A to F) and livers (G to L) of IRF-1−/− mice infected with either 16MΔvjbR (A and G), 16M (B and H), 2308 (C and I), S19 (D and J), or S19ΔvjbR (E and K) or naïve mice (F and L). Note the complete obliteration of the normal splenic parenchyma and replacement with a histiocytic infiltrate in 16M-, 2308-, and S19-inoculated mice (B, C, and D). None of the mice infected with the vjbR mutants exhibited significant changes in the spleen. All livers inoculated with 16M, 2308, or S19 (H, I, and J) exhibited multifocal, random neutrophilic and histiocytic infiltrates. The livers from ΔvjbR mutant-infected mice are unremarkable.
Evaluation of immune protection provided by 16MΔvjbR and S19ΔvjbR in IRF-1−/− mice against 16M challenge.
In order to determine the vaccination efficacy elicited by either the 16MΔvjbR or S19ΔvjbR mutant, the level of protection provided by the vaccine candidate was evaluated against intraperitoneal B. melitensis 16M wild-type challenge at 8 weeks postinoculation. The 8-week time point was chosen because by 4 weeks postvaccination both vaccine candidates were already cleared from the host (data not shown). At 1 week postchallenge (9 weeks postvaccination), there was a marked decrease in the splenic and hepatic bacterial loads (4-log-unit reduction) from the mice vaccinated with the 16MΔvjbR mutant as well as from the animals that received the S19ΔvjbR mutant (Fig. 8). This is only an observation, and it is solely based on the previous finding (Fig. 3A and B) that at 1 week postinfection the 16M infection was overwhelming (9 log units). Reduced bacterial loads correlated with decreased splenic and hepatic weights, which indicated a reduced inflammatory response (Fig. 9). This indicates that the vaccine candidates were able to induce partial protective immunity in IRF-1−/− mice.
Evaluation of temperature changes in mice vaccinated with the vjbR mutants and challenged intraperitoneally.
To determine temperature fluctuations due to the challenge dose, mice were monitored daily for 1 week postchallenge using transplantable temperature transponders. None of the mice showed a statistically significant fluctuation in temperature at any time point (Fig. 10).
Fig 10.
Temperature profiles collected over a 1-week period from IRF-1−/− mice vaccinated with 1 × 106 CFU/mouse of either S19ΔvjbR or 16MΔvjbR and challenged at 8 weeks postvaccination with 1 × 106 CFU/mouse of wild-type B. melitensis 16M.
Evaluation of gross and histologic findings in mice vaccinated with the vjbR mutants and challenged with wild-type strain 16M.
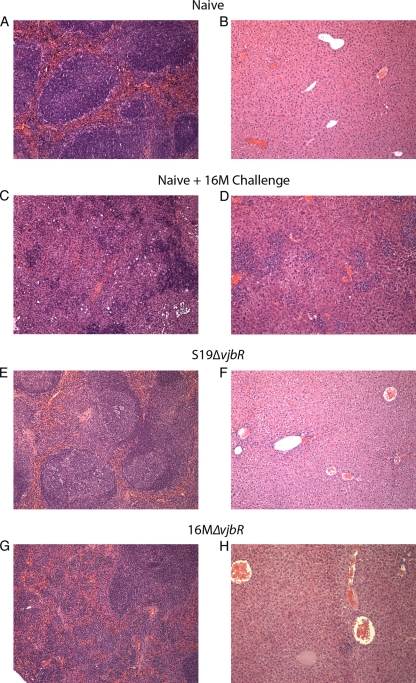
In order to determine the gross and microscopic changes associated with wild-type challenge, a full necropsy and microscopic evaluation of the spleen, liver, kidneys, heart, lung, intestine, and brain were performed. Gross findings were most obvious in naïve but challenged mice and consisted of pale, enlarged livers and spleens. In vjbR-vaccinated mice, the only consistent finding was a mild to moderate increase in the spleen size (Fig. 9). Histopathologic evaluation of the spleens revealed a markedly reduced to absent inflammatory response compared to nonvaccinated but challenged animals and mainly consisted of a mild and multifocal neutrophilic to granulomatous hepatitis. In contrast, the nonvaccinated but challenge mice had a marked neutrophilic to granulomatous inflammatory response with areas of hepatocellular degeneration and necrosis (Fig. 11). There were no changes observed in the lung, brain, heart, kidneys, or intestine in vjbR-vaccinated mice (data not shown).
Fig 11.
Microscopic changes observed in the spleens (A, C, E, and G) or livers (B, D, F, and H) of IRF-1−/− mice vaccinated with either S19ΔvjbR (E and F) or 16MΔvjbR (G and H) and challenged at 8 weeks postvaccination with 1 × 106 CFU/mouse of wild-type B. melitensis 16M. Results for naïve but challenged mice (C and D) and naïve mice (A and B) are presented for comparison. Note the marked reduction in the inflammatory response in both the spleen and liver in animals that received the ΔvjbR mutants.
DISCUSSION
One of the biggest challenges for producing an effective Brucella vaccine is to develop an immunization strategy that can induce a robust and prolonged memory response while still being safe. In the case of brucellosis, live attenuated vaccines are considered to be the vaccine of choice due to their overall ability to induce the most potent and effective memory response (7, 24). Two clear examples of the success of the live attenuated vaccines are S19 and Rev 1, both of which induce a protective memory response in animals (15, 20, 27). Other clear examples of the importance of live attenuated vaccines in controlling diseases throughout the world include Mycobacterium bovis BCG against tuberculosis and the smallpox vaccine. While S19 and Rev 1 have been extremely valuable for the past 50 years, one of the main safety concerns is their virulence in humans. These vaccines were obtained empirically by serial passage or repeated passage in culture of an active organism, without identification of the molecular basis of attenuation (27). Due to the more stringent regulatory environment, it is unlikely that a live attenuated vaccine generated empirically would be approved for use, based on safety concerns that include the possibility of reversion to wild-type virulence. Therefore, the molecular basis for attenuation of a live vaccine needs to be both stable and well-defined, as is the case for multiple live attenuated Brucella vaccines under investigation (1, 2, 8, 13, 17, 23, 34, 36).
Our pursuit of the identity of genes essential for bacterial replication and persistence in vivo and in vitro led us to the generation of rationally designed, live attenuated vaccine candidates 16MΔvjbR and s19ΔvjbR. The LuxR-like transcriptional regulator VjbR has been shown to positively influence the expression of the type IV secretion system and flagellar genes, both of which are necessary for bacterial virulence and survival (2, 3, 6). Our previous results have demonstrated that the B. melitensis and B. abortus ΔvjbR mutant strains conferred, with a single dose, a strong protective immune response against wild-type challenge in the mouse model (2, 3). The protective efficacy was demonstrated by different parameters, such as reduced bacterial burden in the spleen, decreased pathology in the spleen and liver, and an overall reduction in the inflammatory response, evidenced by a reduction in splenomegaly. Additionally, ΔvjbR mutants exhibit decreased survival in macrophages compared with the vaccine strain S19 (2).
As mentioned above, safety is one of the main concerns and a major challenge in the development of live vaccines. In this work, we extended the safety studies of the vjbR vaccine candidate strains in order to provide enough assurance for its potential future use in humans. The first step was the development of an unmarked deletion mutant to prevent future rearrangements in the vjbR::Tn5 or vjbR::Kmr mutation since loss of either element could potentially restore the mutant to the virulent form during subcultivation. Additionally, removal of the antibiotic marker eliminates any potential risk associated with its use in humans. Another important concern regarding the safety of live vaccines is that primary or secondary exposure, i.e., exposure to bacteria shed by vaccine recipients, could potentially pose a risk to immunocompromised individuals. To address this question, we utilized the IRF-1−/− mouse model to determine the effect of the vaccine candidates in these mice. IRF-1−/− mice are a valuable means to test these candidates, since it has been shown over the last 10 years that a single intraperitoneal inoculation of wild-type Brucella melitensis or Brucella abortus at 1 × 106 CFU/mouse causes significant illness and death in these mice (16).
IRF-1 is a member of a multigene family of transcription factors that regulates an array of genes involved in cell differentiation and growth (19). Additionally, the transcription factor IRF-1 is involved in many aspects of innate and adaptive immune responses, including the differentiation of naïve CD4+ T cells into effector helper T cells (10, 11, 33). The T helper type 1 (TH1)-, T helper type 2 (TH2)-, and interleukin 17 (IL-17)-producing T helper (TH17) populations play a critical role in immunity and are characterized by their distinct patterns of cytokine production. TH1 cells mainly produce gamma interferon (IFN-γ) and mediate cellular immune responses to intracellular pathogens (26). TH2 cells typically produce IL-4 and regulate humoral immune responses to extracellular pathogens (26). It is known that IRF-1−/− mice have impaired TH1 responses to Brucella abortus 2308 and Brucella melitensis 16M (16). Although impairment of IL-12 production in IRF-1−/− macrophages has been considered the main cause of the imbalance in TH1 versus TH2 differentiation, the exact mechanism was unknown until recently, when it was demonstrated that the IRF-1 gene is essential for TH1 differentiation acting on the IL12rb1 promoter in CD4+ T cells (14). As a result, IRF-1−/− mice infected with the most virulent Brucella strains have an impaired IL-12 production and succumb to the infection within the first month of inoculation, favoring a TH1 over a TH2 response (16). Despite this, attenuated strains such as the vaccine strain RB51 are cleared from these mice without causing a marked inflammatory response (16). In these experiments, none of the ΔvjbR mutants, regardless of the background strain, caused death or any significant Brucella-associated pathological changes, confirming the reduced virulence of these strains. This is further supported by the lack of clinical signs of disease such as lethargy, anorexia, weight loss, abnormal posture, or temperature changes. To the authors' knowledge, this is the first time that telemetry monitoring of the body temperature in mice infected with Brucella spp. has been performed. Although it has been maintained for years that mice infected with Brucella spp. do not develop fever, temperature has never been monitored to detect possible fluctuations that can indicate disease. Previous investigations in rodents have demonstrated that mice are unlikely to recover from an infection by fungus or bacteria if there is a marked temperature drop (usually below 32 to 33°C) (29, 31). Interestingly, we were able to observe a similar drop in body temperature in animals that were obviously sick, and most importantly, once the temperature dropped below 32 to 33°C, the mice became terminally ill. Temperature measurement provides a useful and objective parameter of health, while interpretation of other clinical signs such as depression, piloerection, badly groomed coat, decreased locomotion, and abnormal posture may be relative and potentially subjective. Additionally, the severity of the symptoms may differ among individuals. It has long been recognized that septic patients can have variable thermoregulatory responses (5). Mice, rats, and guinea pigs routinely exhibit hypothermia in response to endotoxin challenge under normal laboratory conditions (4). The potential mechanisms of hypothermia related to septicemia are somewhat speculative, but probably, as tissue oxygen delivery decreases, maximal tissue oxygen extraction capability is exceeded, oxygen consumption declines, and heat production decreases. Other factors that might lower body temperature include malnutrition, cell senescence, and venous congestion causing decreased liver and or gastrointestinal metabolism.
Bacterial sepsis is defined as a clinical syndrome characterized by the active multiplication of bacteria that results in an overwhelming infection and a systemic inflammatory response. The pathophysiology of sepsis begins with the entry of the organism into the bloodstream, and it is characterized by simultaneous activation of inflammation and coagulation in response to the microbial insult (25). This inflammatory process is normally accompanied by activation of circulating and fixed phagocytic cells and by generation of proinflammatory and anti-inflammatory mediators. As the bacteria multiply, the response becomes uncontrolled and unregulated, exerting harmful systemic effects resulting in death. This is the case for IRF-1−/− mice inoculated with either Brucella melitensis 16M, Brucella abortus 2308, or Brucella abortus S19, but it was not the case for animals vaccinated with the ΔvjbR mutants. At different time points, terminally ill mice inoculated with the Brucella strains were euthanized to determine the extent of bacterial colonization prior to death. All moribund mice had an extremely high bacterial burden in the spleens and livers as well as a marked inflammatory response, evidenced by histopathology. An extremely high bacterial burden associated with a severe neutrophilic and granulomatous inflammatory response in multiple organs clearly indicates that these mice were unable to control the infection, leading to a systemic spread and death. In contrast, none of the animals inoculated with the vaccine candidates had evidence of an inflammatory response by 30 days postinfection, and 20 CFU was recovered from the spleens of only two of five animals at 30 days postinfection (data not shown), indicating that the ΔvjbR mutants are unable to colonize IRF-1−/− mice to the same extent as the wild-type organism or the vaccine strain S19. This provides further evidence of the increased safety of the ΔvjbR mutants.
The protective immunity induced in immunocompetent mice by vaccination with the ΔvjbR mutant strains has been previously evaluated (2, 3). The use of immunocompromised mouse models to determine induction of protective efficacy can be an important tool to elucidate other possible immune mechanisms that may play a role in adaptive immunity. IRF-1-knockout mice vaccinated with the vaccine candidates and challenged with the wild-type strain 16M survived, while they exhibited a significant reduction in bacterial colonization, reduced pathology, and no detectable evidence of clinical disease, including no temperature fluctuations after 1 week postinfection. Evidence of protection in the absence of IFN-γ signaling raises questions regarding the components of the innate and adaptive immunity that play a role in the control of the Brucella infection. Investigations are in progress to obtain an understanding between synergy and mutual interdependence between the different cellular immune responses as well as humoral immunity.
Our results extend our previous findings by demonstrating that considerable bacterial attenuation in immunocompromised individuals can be achieved if the vjbR gene is deleted. Future studies to determine the correlates of immune protection in immunocompetent and immunocompromised mice as well as toxicity studies are under way.
ACKNOWLEDGMENT
This work has been supported by grant NIAID/NIH 1U54AI057156-0100.
Footnotes
Published ahead of print 14 December 2011
REFERENCES
- 1. Adone R, et al. 2005. Protective properties of rifampin-resistant rough mutants of Brucella melitensis. Infect. Immun. 73:4198–4204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Gomez G, Rice-Ficht AC. 2009. The Brucella abortus S19 deltavjbR live vaccine candidate is safer than S19 and confers protection against wild-type challenge in BALB/c mice when delivered in a sustained-release vehicle. Infect. Immun. 77:877–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arenas-Gamboa AM, Ficht TA, Kahl-McDonagh MM, Rice-Ficht AC. 2008. Immunization with a single dose of a microencapsulated Brucella melitensis mutant enhances protection against wild-type challenge. Infect. Immun. 76:2448–2455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atwood RP, Kass EH. 1964. Relationship of body temperature to the lethal action of bacterial endotoxin. J. Clin. Invest. 43:151–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clemmer TP, et al. 1992. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. Crit. Care Med. 20:1395–1401 [DOI] [PubMed] [Google Scholar]
- 6. Delrue RM, et al. 2005. A quorum-sensing regulator controls expression of both the type IV secretion system and the flagellar apparatus of Brucella melitensis. Cell. Microbiol. 7:1151–1161 [DOI] [PubMed] [Google Scholar]
- 7. Ficht TA, Kahl-McDonagh MM, Arenas-Gamboa AM, Rice-Ficht AC. 2009. Brucellosis: the case for live, attenuated vaccines. Vaccine 27(Suppl 4):D40–D43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grillo MJ, et al. 2006. Increases of efficacy as vaccine against Brucella abortus infection in mice by simultaneous inoculation with avirulent smooth bvrS/bvrR and rough wbkA mutants. Vaccine 24:2910–2916 [DOI] [PubMed] [Google Scholar]
- 9. Guihot A, Bossi P, Bricaire F. 2004. Bioterrorism with brucellosis. Presse Med. 33:119–122 (In French.) [DOI] [PubMed] [Google Scholar]
- 10. Honda K, Takaoka A, Taniguchi T. 2006. Type I interferon [corrected] gene induction by the interferon regulatory factor family of transcription factors. Immunity 25:349–360 [DOI] [PubMed] [Google Scholar]
- 11. Honda K, Taniguchi T. 2006. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat. Rev. Immunol. 6:644–658 [DOI] [PubMed] [Google Scholar]
- 12. Kahl-McDonagh MM, et al. 2006. Evaluation of novel Brucella melitensis unmarked deletion mutants for safety and efficacy in the goat model of brucellosis. Vaccine 24:5169–5177 [DOI] [PubMed] [Google Scholar]
- 13. Kahl-McDonagh MM, Ficht TA. 2006. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect. Immun. 74:4048–4057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kano S, et al. 2008. The contribution of transcription factor IRF1 to the interferon-gamma-interleukin 12 signaling axis and TH1 versus TH-17 differentiation of CD4+ T cells. Nat. Immunol. 9:34–41 [DOI] [PubMed] [Google Scholar]
- 15. Klesius PH, Kramer TT, Swann AI, Christenberry CC. 1978. Cell-mediated immune response after Brucella abortus S19 vaccination. Am. J. Vet. Res. 39:883–886 [PubMed] [Google Scholar]
- 16. Ko J, Gendron-Fitzpatrick A, Ficht TA, Splitter GA. 2002. Virulence criteria for Brucella abortus strains as determined by interferon regulatory factor 1-deficient mice. Infect. Immun. 70:7004–7012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magnani DM, Harms JS, Durward MA, Splitter GA. 2009. Nondividing but metabolically active gamma-irradiated Brucella melitensis is protective against virulent B. melitensis challenge in mice. Infect. Immun. 77:5181–5189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mallory RM, et al. 2010. Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials. PLoS One 5:e13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McElligott DL, et al. 1997. CD4+ T cells from IRF-1-deficient mice exhibit altered patterns of cytokine expression and cell subset homeostasis. J. Immunol. 159:4180–4186 [PubMed] [Google Scholar]
- 20. Nicoletti P. 1990. Vaccination against Brucella. Adv. Biotechnol. Processes 13:147–168 [PubMed] [Google Scholar]
- 21. Orme IM. 2006. Preclinical testing of new vaccines for tuberculosis: a comprehensive review. Vaccine 24:2–19 [DOI] [PubMed] [Google Scholar]
- 22. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Brucellosis. N. Engl. J. Med. 352:2325–2336 [DOI] [PubMed] [Google Scholar]
- 23. Parent MA, et al. 2007. Brucella abortus bacA mutant induces greater pro-inflammatory cytokines than the wild-type parent strain. Microbes Infect. 9:55–62 [DOI] [PubMed] [Google Scholar]
- 24. Perkins SD, Smither SJ, Atkins HS. 19 January 2010. Towards a Brucella vaccine for humans. FEMS Microbiol. Rev. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 25. Remick DG. 2007. Pathophysiology of sepsis. Am. J. Pathol. 170:1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Romagnani S. 2000. T-cell subsets (Th1 versus Th2). Ann. Allergy Asthma Immunol. 85:9–18 [DOI] [PubMed] [Google Scholar]
- 27. Schurig GG, Sriranganathan N, Corbel MJ. 2002. Brucellosis vaccines: past, present and future. Vet. Microbiol. 90:479–496 [DOI] [PubMed] [Google Scholar]
- 28. Seleem MN, Boyle SM, Sriranganathan N. 2010. Brucellosis: a re-emerging zoonosis. Vet. Microbiol. 140:392–398 [DOI] [PubMed] [Google Scholar]
- 29. Silva TM, Costa EA, Paixao TA, Tsolis RM, Santos RL. 2011. Laboratory animal models for brucellosis research. J. Biomed. Biotechnol. 2011:518323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sirard JC, Niedergang F, Kraehenbuhl JP. 1999. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol. Rev. 171:5–26 [DOI] [PubMed] [Google Scholar]
- 31. Soothill JS, Morton DB, Ahmad A. 1992. The HID50 (hypothermia-inducing dose 50): an alternative to the LD50 for measurement of bacterial virulence. Int. J. Exp. Pathol. 73:95–98 [PMC free article] [PubMed] [Google Scholar]
- 32. Sun YH, den Hartigh AB, Santos RL, Adams LG, Tsolis RM. 2002. virB-mediated survival of Brucella abortus in mice and macrophages is independent of a functional inducible nitric oxide synthase or NADPH oxidase in macrophages. Infect. Immun. 70:4826–4832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623–655 [DOI] [PubMed] [Google Scholar]
- 34. Vemulapalli R, et al. 2004. Enhanced efficacy of recombinant Brucella abortus RB51 vaccines against B. melitensis infection in mice. Vet. Microbiol. 102:237–245 [DOI] [PubMed] [Google Scholar]
- 35. Weeks JN, et al. 2010. Brucella melitensis VjbR and C12-HSL regulons: contributions of the N-dodecanoyl homoserine lactone signaling molecule and LuxR homologue VjbR to gene expression. BMC Microbiol. 10:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang X, Thornburg T, Walters N, Pascual DW. 2010. deltaznuAdeltapurE Brucella abortus 2308 mutant as a live vaccine candidate. Vaccine 28:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]