Abstract
ESAT-6 system 1 (ESX-1)-mediated secretion in Mycobacterium tuberculosis is dependent on proteins encoded by the cotranscribed espA-espC-espD gene cluster. While the roles of EspA and EspC with respect to the ESX-1 secretion system have been actively investigated, the function of EspD remains unknown. We show that EspD is secreted by M. tuberculosis, but unlike EspA and EsxA, its export does not exclusively require the ESX-1 system. Evidence for stabilization of cellular levels of EspA and EspC by EspD is presented, and depletion of EspD results in loss of EsxA secretion. Site-directed mutagenesis of EspD reveals that its role in the maintenance of cellular levels of EspA in M. tuberculosis is distinct from its facilitation of EsxA secretion. The same mutagenesis experiments have also shown that secretion of EspD is not required for the secretion of EsxA. Our findings highlight a critical and complex role for EspD in modulating the ESX-1 secretion system in M. tuberculosis.
INTRODUCTION
Mycobacterium tuberculosis requires the ESX-1 (ESAT-6 system 1) type 7 protein secretion system to export EsxA (Esat-6), EsxB (CFP-10), and EspB proteins, as well as for host cell entry, phagosome escape, and intercellular spread (2, 26). The M. tuberculosis ESX-1 system is encoded by several genes in the esx-1 locus, the functional components of which begin with eccA1 (rv3868) and include mycP1 (rv3883c) (2, 3). In this regard, genetic studies have helped identify genes in the esx-1 locus crucial for ESX-1-mediated secretion and virulence. Deleting the entire esx-1 locus or some of the individual genes therein blocks secretion and attenuates M. tuberculosis in macrophages and in animal models of infection (3, 5, 14–16, 21, 22, 27). Despite identification of these genes, our understanding of the actual mechanistic details by which individual ESX-1 components mediate secretion remains limited.
The espA-espC-espD (rv3616c-rv3615c-rv3614c) gene cluster is unlinked to esx-1, yet it is essential for ESX-1-dependent protein secretion and M. tuberculosis virulence (11, 17). EspA and EspC, encoded by the first two genes in this cluster, are cosecreted with EsxA and EsxB (11, 17). It was recently reported that replacing the sole cysteine residue in EspA blocks its ability to dimerize via disulfide bonding without affecting EsxA secretion (13). Although the precise mechanism remains poorly understood, the inability of EspA to dimerize also compromises M. tuberculosis cell wall integrity and attenuates the bacillus in mice despite normal EsxA secretion (13). EspC, a potent T-cell stimulator, engages in multiprotein complex formation with several ESX-1 components to ensure proper targeting of substrates to the secretion apparatus for translocation (8, 20). The carboxy terminus of EspC, although important for the initial targeting, is insufficient for the subsequent complex formation, suggesting that different domains in EspC are important for secretion (8). Several studies have shown that espA, espC, and espD are coregulated and likely cotranscribed. For example, mutations in PhoP, the response regulator of a two-component regulatory system, and in EspR, a DNA-binding protein, reduce the transcription of all three genes (12, 23, 30). On the other hand, deleting M. tuberculosis crp, which encodes the cAMP receptor protein (CRP), results in the transcriptional derepression of espA, espC, and espD (24). In addition, M. tuberculosis cells exposed to acidic pH in vitro and within the acidified phagosome of macrophages tend to increase espA, espC, and espD transcription by similar orders of magnitude (10, 25). Consistent with this notion of cotranscription, complementation of an M. tuberculosis espC transposon insertion mutant with the entire espA-espC-espD cluster is necessary to restore wild-type virulence and ESX-1 function (17). The characterization of EspA and EspC has been ongoing (8, 13, 20). To date, however, little is known about the function of EspD.
In this study, we sought to understand the function of EspD within the context of the espA-espC-espD cluster and its involvement with the ESX-1 secretion apparatus. Our findings underscore a critical role for EspD in ESX-1 protein secretion via a complex mode of action.
MATERIALS AND METHODS
Enzymes and reagents.
Restriction endonucleases and DNA-modifying enzymes were purchased from New England BioLabs (Ipswich, MA). High-fidelity Pfu polymerase used for all PCRs was purchased from Promega (Madison, WI). Custom oligonucleotides were synthesized by Microsynth (Balgach, Switzerland). 7H9 and 7H11 media, albumin-dextrose-catalase (ADC), and oleic acid-albumin-dextrose-catalase (OADC) were purchased from Becton Dickinson (Franklin Lakes, NJ). All other chemicals and reagents were purchased from Sigma-Aldrich (St. Louis, MO). Site-directed mutagenesis (SDM) was performed using the QuikChange site-directed mutagenesis kit purchased from Stratagene (La Jolla, CA).
Bacterial strains and growth conditions.
M. tuberculosis Erdman and H37Rv strains were grown in 7H9 broth (supplemented with 0.2% glycerol, 10% ADC, and 0.05% Tween 80) or on 7H11 agar (supplemented with 0.5% glycerol, 10% OADC). M. tuberculosis Erdman Tn5370 transposon insertion mutants (espA::Tn, espC::Tn, and strain 36-72) generated as described elsewhere (7) were grown in the presence of hygromycin (50 μg/ml). The recombineering method of van Kessel and Hatfull (29) was used to generate a hygromycin-marked 290-bp deletion of the 399-bp espR (rv3849) gene of M. tuberculosis H37Rv. Upstream and downstream regions of homology were amplified by PCR from genomic DNA using primers PRNS_40F (5′-GGGGTACCGGCCTGCTGTTCCTGCTGTT-3′) and PRNS_40R (5′-GGTCTAGAGATCACCTCCGCGGAGGTAT-3′) for the upstream region and primers PRNS_41F (5′-GGAAGCTTCGAAGGGATCGACGCTTAGT-3′) and PRNS_41R (5′-GGACTAGTAGCCGAGAAACCGTCAGCAA-3′) for the downstream region and cloned into the pYUB854 plasmid using Acc65I and XbaI for the upstream region and HindIII and SpeI for the downstream region to make pNS56 (restriction sites of primer sequences are italicized). This was linearized using Acc65I and SpeI and transformed into M. tuberculosis H37Rv, which had previously been transformed with pJV48, and selection was made as described by van Kessel and Hatfull (29). The mutant was verified by genomic DNA microarray analysis and qPCR. Escherichia coli TOP10 (Invitrogen, Carlsbad, CA) used for routine cloning was grown on Luria-Bertani agar or broth. When needed, kanamycin was used at a final concentration of 25 μg/ml for M. tuberculosis and at 50 μg/ml for E. coli.
Qualitative and quantitative RT-PCR.
Total mRNA was extracted from exponentially growing M. tuberculosis cells using TRIzol (Invitrogen, Carlsbad, CA). Purified mRNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI) according to the manufacturer's instructions. Treated mRNA was checked for complete removal of contaminating genomic DNA by PCR before proceeding to the reverse transcription step. cDNA from mRNA was generated using the Superscript III first-strand synthesis system (Invitrogen, Carlsbad, CA) and random hexamers. Primer pairs 1 (5′-TACGACCTTCTGGGGATTGG-3′ and 5′-ACAACCCGTCGATAGCCTTG-3′) and 2 (5′-CGAATCTGTGGCGATCACTC-3′ and 5′-CTTCTTCCGGCGATGGTAAC-3′) were used to amplify portions encompassing the coding sequence and intergenic regions between espA and espC and between espC and espD, respectively, from the cDNA generated as described above. Quantitative reverse transcriptase PCR (RT-PCR) assessing transcription of espA and espC, normalized to sigA, was done using the following primer pairs: espA (5′-TACGACCTTCTGGGGATTGG-3′ and 5′-ACGATCGAGGTCTGCCAGTT-3′), espC (5′-CGAATCTGTGGCGATCACTC-3′ and 5′-ACAACCCGTCGATAGCCTTG-3′), and sigA (5′-GAGATCGGCCAGGTCTACGGCGTG-3′ and 5′-CTGACATGGGGGCCCGCTACGTTG-3′).
Plasmid vector construction and M. tuberculosis strain generation.
Oligonucleotides for the construction of plasmid vectors used in this study are listed in Table 1 and described below.
Table 1.
List of oligonucleotides used for the construction and manipulation of plasmids in this study
| Name | Sequence (5′ to 3′)a | Purpose |
|---|---|---|
| espACD-Fwd | CAGAAGCTTCCATCGTCGGTTTTCGTC | PCR and construction of pMDespACD for ectopic expression of EspA, EspC, and EspD in M. tuberculosis |
| espACD-Rev | CGCCTGCAGATTAATGCTGAGCCGCGAAT | PCR and construction of pMDespACD for ectopic expression of EspA, EspC, and EspD in M. tuberculosis |
| espA-Fwd | CAGAAGCTTCGGTTTTCGTCGCCTTATCA | PCR and construction of pMDespA for ectopic expression of EspA only in M. tuberculosis |
| espA-Rev | CGCCTGCAGCGCTGGCTAGCAATGGATTT | PCR and construction of pMDespA for ectopic expression of EspA only in M. tuberculosis |
| espD-Fwd | GCAGAATTCGTGGACTTGCCCGGAAATGACTTT | PCR and construction of pMVespD for ectopic expression of EspD only in M. tuberculosis |
| espD-Rev | CGCAAGCTTTCACCATGGATCGCTCTCGTCGTCTGG | PCR and construction of pMVespD for ectopic expression of EspD only in M. tuberculosis |
| espDstop | ATTCCGAAGGGACGGTGGACTTGCCCTGAAATGACTTTGACAGCAACGATTTCGA | Insertion of a stop codon (in bold italics) in espD of pMDespACD to generate pMDespACDstop |
| espCFLAG | GCGCAATGCTAAACGGAAGGGACACGATCAATGGACTACAAGGACGATGACGACAAGGGAATGACGGAAAACTTGACCGTCCAGCCCGAG | Insertion of an amino-terminal FLAG epitope (underlined and italic) in espC of pMDespACD and pMDespACDstop to generate pMDespACFLAGD and pMDespACFLAGDstop |
| EspD_W19R | CCGTGGATCTCCGGGGTGCCGACGG | Site-specific mutagenesis and truncations in EspD for codon replacements (in bold italics) in espD of pMDespACD |
| EspD_W27R | GCGCGGAGGGCCGGACTGCGGATCC | Site-specific mutagenesis and truncations in EspD for codon replacements (in bold italics) in espD of pMDespACD |
| EspD_P31A | GGACTGCGGATGCGATTATTGGCGT | Site-specific mutagenesis and truncations in EspD for codon replacements (in bold italics) in espD of pMDespACD |
| EspD_R138A | CAGATGAACACGCCGTCGCACTGCT | Site-specific mutagenesis and truncations in EspD for codon replacements (in bold italics) in espD of pMDespACD |
| EspD_W150R | TGGGCGAAACCCGGGGGTTACCATC | Site-specific mutagenesis and truncations in EspD for codon replacements (in bold italics) in espD of pMDespACD |
| EspD_Δ30CT | GGTTACCATCGTAGGAAGAAGCCGC | Site-specific mutagenesis and truncations in EspD for codon replacements (in bold italics) in espD of pMDespACD |
| EspD_Δ16CT | TGTTCGCGACGTGATACAGCGACGA | Site-specific mutagenesis and truncations in EspD for codon replacements (in bold italics) in espD of pMDespACD |
| EspD_Δ8CT | ATTGTCCAGCATAAGACGACGAGAG | Site-specific mutagenesis and truncations in EspD for codon replacements (in bold italics) in espD of pMDespACD |
Modified codons are in bold italics, the FLAG sequence is underlined and italicized, and restriction sites are in italics without boldface or underlining.
(i) pMDespACD and derivatives.
For ectopic, espA promoter-driven expression of EspA, EspC, and EspD in M. tuberculosis, the espA-espC-espD cluster, including 568 bp upstream of the espA start codon, was PCR amplified from M. tuberculosis H37Rv cosmid IE118 (1) with primers espACD-Fwd and espACD-Rev (Table 1) that added 5′ HindIII (Table 1, italics) and 3′ PstI (Table 1, italics) sites. The PCR product generated was digested, purified, and ligated to the mycobacterial E. coli shuttle vector pMD31 (9) to obtain pMDespACD, which confers kanamycin resistance. For ectopic expression in M. tuberculosis of EspA and EspC only, a stop codon was site-specifically engineered 12 nucleotides downstream of the espD start codon in pMDespACD, using the QuikChange site-directed mutagenesis kit and the 55-bp espDstop oligonucleotide (Table 1; stop codon in bold italics) with its antisense strand. To express FLAG-tagged EspC in M. tuberculosis, nucleotides encoding a single FLAG epitope were incorporated in-frame at the 5′ end of the espC gene in pMDespACD and pMDespACDstop using the QuikChange kit and the 90-bp oligonucleotide espCFLAG (Table 1; FLAG sequence is underlined and italicized) with its antisense strand to give pMDespACFLAGD and pMDespACFLAGDstop. Site-directed mutagenesis of espD in pMDespACD for the expression of EspD amino acid variants and truncation mutants in M. tuberculosis was performed using the QuikChange kit and corresponding oligonucleotides with their antisense strands (Table 1; modified codons are in bold italics).
(ii) pMDespA.
The espA gene was PCR amplified along with 561 nucleotides upstream of the translational start codon from cosmid IE118 with espA-Fwd and espA-Rev primers (Table 1) that added 5′ HindIII (Table 1, italics) and 3′ PstI (Table 1, italics) sites. The PCR product was digested, purified, and ligated into pMD31 to obtain pMDespA for ectopic, espA promoter-driven expression of EspA only in M. tuberculosis.
(iii) pMVespD.
For ectopic expression of EspD from the Hsp60 promoter in pMV261 (28), espD was PCR amplified from cosmid IE118 with espD-Fwd and espD-Rev primers (Table 1) that added 5′ EcoRI (Table 1, italics) and 3′ HindIII (Table 1, italics) sites. The fragment was digested, purified, and ligated in-frame with Hsp60 into pMV261 to obtain pMVespD, which confers kanamycin resistance. It should be noted that expression from pMVespD results in a larger EspD protein containing the first 12 amino acids of Hsp60 at its amino terminus.
All plasmid constructs were verified by DNA sequencing. Wild-type M. tuberculosis Erdman strain, espA transposon insertion (espA::Tn, Hygr), and espC transposon insertion (espC::Tn, Hygr) mutants were grown to mid-logarithmic phase of growth prior to electroporation of the desired plasmids by using standard procedures (31). Transformants harboring pMD31 and pMV261 constructs were selected on Middlebrook 7H11 agar plates containing kanamycin.
Protein preparation for immunoblots.
M. tuberculosis starter cultures, grown in complete 7H9 broth to late logarithmic phase of growth (optical density at 600 nm [OD600] of ∼0.8 to 1), were used to inoculate Sauton's medium supplemented with 0.05% Tween 80, at a starting OD600 of 0.05 to 0.1. Cells were grown to mid-logarithmic phase of growth (OD600 of 0.6 to 0.7), centrifuged, washed once with phosphate-buffered saline (PBS), resuspended in Sauton's liquid medium without Tween 80, and grown further at 37°C with agitation for 4 or 5 days. Cultures were harvested by centrifugation to obtain culture filtrates and cell pellets. Culture filtrates were filtered sequentially through 0.4- and 0.2-μm-pore-size filters to remove M. tuberculosis cells and concentrated in Vivaspin columns with 5-kDa-molecular-weight-cutoff membranes (Sartorius Stedim Biotech GmbH, Goettingen, Germany). Cell lysates were prepared by resuspending cell pellets in lysis buffer (PBS containing Roche protease inhibitor cocktail tablets) and bead beating with 100-μm glass beads and were clarified by centrifugation. Total protein concentration in all preparations was determined using bicinchoninic acid (BCA) assays with bovine serum albumin as the standard.
Production of antibodies.
A truncated version of EspA, consisting of only the last 100 amino acids from the carboxy terminus, and full-length EspD were expressed in E. coli BL21/pLysS cells (Invitrogen, Carlsbad, CA) as amino terminus hexahistidine-tagged proteins. The recombinant proteins were purified by nickel column chromatography followed by size exclusion chromatography to near homogeneity. Purified EspD was used to produce EspD-specific polyclonal antibodies in rats. Purified truncated EspA and synthetic EspC peptides were used to produce polyclonal antibodies in rabbits (Eurogentec S.A., Seraing, Belgium). Rabbit polyclonal antibodies against FLAG epitope were purchased (Invitrogen, Carlsbad, CA). Mouse monoclonal antibodies against GroEL2 and SodA were obtained through the Tuberculosis Vaccine Testing and Research Materials Program of Colorado State University.
Immunoblotting.
For immunoblot analysis, indicated amounts of total protein from concentrated culture filtrates or cell lysates were resolved in NuPAGE 4 to 12% bis-Tris gels (Invitrogen, Carlsbad, CA) under reducing conditions and transferred to nitrocellulose membranes. Membranes were blocked with TBS-milk (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, and 5% nonfat milk powder) and incubated overnight with the desired primary antibody diluted in TNT-BSA (20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 0.05% Tween 20, and 1% BSA fraction V) at 4°C. Membranes were washed with TNT, incubated with the appropriate secondary antibody in TNT-BSA, washed again with TNT, and developed. GroEL2 was used as a lysis control for culture filtrates and as a loading control for cell lysates. ESX-1-independent secreted proteins Mpt64 and SodA were probed as the loading control for culture filtrates. Immunoblots were quantified using open-source ImageJ version 1.44i (http://rsb.info.nih.gov/ij) image processing software. Relative densities of samples/test peaks were obtained from dividing the percent values of samples/test (calculated by ImageJ) by the percent values of the wild-type standard (also calculated by ImageJ). Relative densities of samples/test are indicated as a percentage of the wild-type standard as shown in Table 2.
Table 2.
Summary of phenotypes associated with M. tuberculosis espA::Tn strains expressing EspD variants
| Plasmid | EsxA secretion | Relative EspA level (%)a | Relative EspD level (%)b | EspD secretion |
|---|---|---|---|---|
| pMD31 | No | 0 | 0 | No |
| pMDespACD | Yes | 100 | 100 | Yes |
| pMDespACDstop | No | 33 | 0 | No |
| pMDespACDW19R | Yes | 98 | 59 | No |
| pMDespACDW27R | Yes | 100 | 46 | No |
| pMDespACDP31A | Yes | 65 | 66 | Yesd |
| pMDespACDR138A | No | 72 | 5 | UNc |
| pMDespACDW150R | Yes | 44 | 64 | No |
| pMDespACDΔ8CT | Yes | 92 | 84 | Yes |
| pMDespACDΔ16CT | Yes | 80 | 17 | UN |
| pMDespACDΔ30CT | No | 41 | 19 | UN |
Indicated as a percentage of espA::Tn plus pMDespACD expressing wild-type EspA.
Indicated as a percentage of espA::Tn plus pMDespACD expressing wild-type EspD.
UN, unknown, either below detection limits or failure to secrete (see the text).
Secreted but at a lower level compared to espA::Tn plus pMDespACD expressing wild-type EspD. Data are representative of results from three independent experiments.
RESULTS
espA, espC, and espD are cotranscribed.
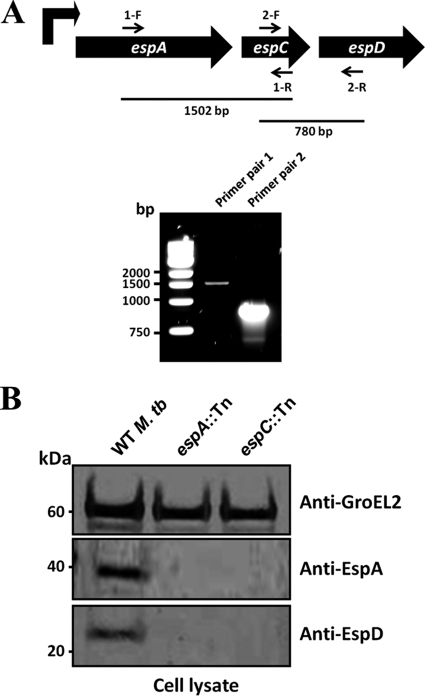
Previous studies suggest that espA, espC, and espD are cotranscribed. RT-PCR experiments confirmed this notion in yielding the expected DNA fragments spanning the intergenic regions between espA and espC, as well as espC and espD, from the total mRNA of M. tuberculosis Erdman (Fig. 1A). Furthermore, immunoblotting with EspD-specific antibodies revealed that M. tuberculosis transposon mutants, espA::Tn and espC::Tn, fail to express EspD (Fig. 1B), most likely due to downstream polar effects from the transposon insertions in the respective genes. These data together confirm that the genes in the espA-espC-espD cluster are cotranscribed into a single mRNA molecule and subsequently cotranslated.
Fig 1.
EspA, EspC, and EspD coexpression analysis. (A) Reverse transcriptase PCR analysis of total RNA from wild-type M. tuberculosis. The genetic arrangement of the espACD cluster, primer annealing sites, and expected sizes of the PCR products are shown. (B) Immunoblots of cell lysates (5 μg/well) from wild-type M. tuberculosis Erdman, espA::Tn, and espC::Tn strains cultured for 5 days. Antibodies used are indicated.
As expected, EspA was detected in the cell lysate of wild-type M. tuberculosis but not in the espA::Tn mutant (Fig. 1B). In agreement with previous observations, we also did not detect EspA in the cell lysate of the espC::Tn mutant (Fig. 1B) (8). Considering that the espA gene is encoded upstream of espC, this finding suggests that EspC and/or EspD deficiency impacts normal EspA protein levels.
EspD is not cosecreted with EsxA in M. tuberculosis.
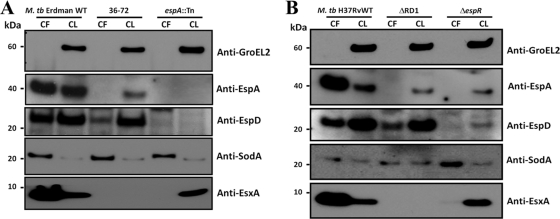
EspA and EspC are cosecreted with EsxA and EsxB (11, 17). To test if this is also true for EspD, culture filtrates and cell lysates of the wild-type M. tuberculosis Erdman strain and two isogenic transposon insertion mutants (36-72 and espA::Tn) were systematically immunoblotted for EspA, EspD, and EsxA. Genotyping of strain 36-72 revealed that the transposon had inserted 41 bp downstream of eccCb1 and 102 bp upstream of pe35 in the esx-1 locus. Deletion of pe35 has been shown to render M. tuberculosis incapable of expressing PPE68, EsxB, and EsxA (5). Therefore, insertion of the transposon in the pe35 promoter in strain 36-72 was expected to disrupt its transcription and, as we subsequently confirmed, to block EsxA expression (Fig. 2A). As expected, EspD was detected in the cell lysates of wild-type M. tuberculosis Erdman and strain 36-72 but not the espA::Tn mutant (Fig. 2A). Interestingly, EspD was detected in the culture filtrates of wild-type M. tuberculosis Erdman and strain 36-72, albeit at lower levels than in the wild-type strain (Fig. 2A). In contrast, EspA was not secreted by strain 36-72, and we noticed strikingly lower levels of EspA in the cell lysate of this mutant than that in the wild-type strain (Fig. 2A). EsxA was detected in the culture filtrate and cell lysate of wild-type M. tuberculosis Erdman but not in strain 36-72 (Fig. 2A). Consistent with published data, EsxA was not secreted by the espA::Tn mutant (Fig. 2A) (11, 13). GroEL2, a cytosolic protein, was not detected in the culture filtrates of all three strains by immunoblotting (Fig. 2A). This indicates that the cells from these cultures had not undergone significant lysis; therefore, leakage of cytosolic EspD does not explain its presence in these short-term culture filtrates of the wild-type M. tuberculosis Erdman strain or strain 36-72.
Fig 2.
EspD expression and secretion in M. tuberculosis. Immunoblots of total culture filtrates (10 μg/well) and total cell lysates (5 μg/lane) of the wild-type M. tuberculosis Erdman strain and transposon mutants 36-72 and espA::Tn (A) and wild-type M. tuberculosis H37Rv, isogenic ΔRD1, and ΔespR strains (B), cultured in Sauton's medium for 4 days. Antibodies used are indicated. CF, total culture filtrate; CL, total cell lysate. Results shown are representative of results from five independent experiments for each M. tuberculosis background.
To verify that these observations were not specific to the M. tuberculosis Erdman genetic background, the culture filtrates and cell lysates of wild-type M. tuberculosis H37Rv, isogenic ESX-1-deficient ΔRD1 (15), and ΔespR mutants were also analyzed. Like the M. tuberculosis Erdman strains, EspD was detected in the cell lysates of all three strains, although less EspD was detected in the ΔespR mutant, consistent with EspR being an activator of espA, espC, and espD transcription (Fig. 2B) (23). We also found EspD to be secreted by both the wild-type strain and ΔRD1 mutant, although slightly less protein was detected for the mutant (Fig. 2B). In contrast, EspD was either not secreted or was below the detection limit in the culture filtrate of the ΔespR mutant (Fig. 2B). EspA was not secreted by the ΔRD1 mutant as previously reported (11), and strikingly lower levels of the protein were observed in the cell lysate of the mutant than in that of the wild-type strain (Fig. 2B). Like EspD, we could not detect EspA in the culture filtrate and observed smaller amounts of the protein in the cell lysate of the ΔespR mutant (Fig. 2B). EsxA was expressed and secreted by wild-type M. tuberculosis H37Rv but not by the ΔRD1 mutant (Fig. 2B). Consistent with published data, EsxA was expressed but not secreted by the ΔespR mutant (Fig. 2B) (23). GroEL2 was not detected in the culture filtrates of all three strains; therefore, we conclude that cell lysis is not responsible for EspD in the culture filtrates of the wild-type H37Rv strain or the ΔRD1 mutant (Fig. 2B). It is not presently known why the culture filtrates of strain 36-72 and the ΔRD1 strain contain less EspD than their wild-type counterparts and also why cellular levels of EspA are lower in these two mutants. Nevertheless, these combined observations clearly indicate that EspD, unlike EspA, is not cosecreted with EsxA, although mutations in the esx-1 locus appear to affect its secretion. These observations also suggest that some components of the ESX-1 system may be necessary for the stability of EspA.
Additionally, EsxA and EspA levels in culture filtrates appear to be higher than or equal to levels in the cell lysates of wild-type M. tuberculosis Erdman and H37Rv strains. In contrast, EspD levels in the same culture filtrates appear lower than in the cell lysates of wild-type strains of both M. tuberculosis genetic backgrounds, suggesting that EspD is not as abundantly secreted as EsxA or EspA.
EspD deficiency results in decreased cellular levels of EspA and EspC.
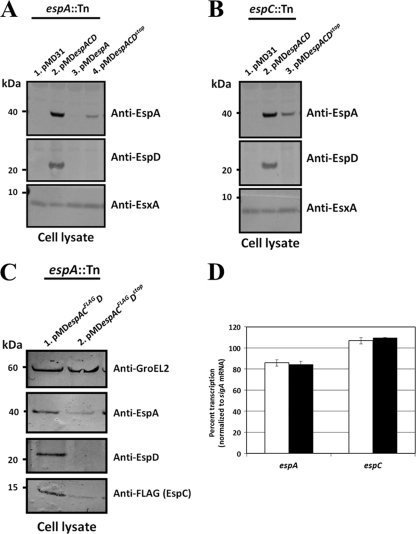
Based on the finding that the espA::Tn and espC::Tn mutants fail to express EspD, we exploited these strains to interrogate the function of espD as part of the espA-espC-espD gene cluster. To this end, cell lysates of espA::Tn and espC::Tn mutants harboring pMDespACD and its derivatives were immunoblotted with EspD-specific antibodies. EspD protein was detected only in cell lysates of the espA::Tn mutant with pMDespACD but not in the same strain harboring pMD31 (vector control), pMDespA, or pMDespACDstop (Fig. 3A). Likewise, EspD was detected only in the espC::Tn mutant harboring pMDespACD but not when pMD31 or pMDespACDstop were present (Fig. 3B).
Fig 3.
EspA, EspC, and EspD expression in EspD-proficient and -deficient M. tuberculosis strains. Immunoblots of total cell lysates (5 μg/lane) of the espA::Tn mutant transformed with pMD31 (vector control), pMDespACD, pMDespA, and pMDespACDstop (A), the espC::Tn mutant transformed with pMD31 (vector control), pMDespACD, and pMDespACDstop (B), and the espA::Tn mutant transformed with pMDespACFLAGD and pMDespACFLAGDstop (C) cultured in Sauton's medium for 5 days. Antibodies used are indicated. Results shown are representative of three independent experiments. (D) Quantitative RT-PCR of espA and espC transcription in EspD-proficient (white bars) and -deficient (black bars) strains. Data shown are means ± standard deviations of results from triplicate experiments.
To investigate the consequences of EspD deficiency alone, we measured the expression of EspA and EspC in the M. tuberculosis strains described above by immunoblotting. Approximately 5.7-fold more EspA protein was detected in cell lysates of the espA::Tn mutant with pMDespACD than in those of the same strain harboring pMDespACDstop (Fig. 3A). In contrast, EspA could not be detected in espA::Tn mutants with pMD31 or pMDespA (Fig. 3A). Likewise, approximately 3.3-fold more EspA protein was detected in cell lysates of the espC::Tn mutant with pMDespACD than in cell lysates of the same strain harboring pMDespACDstop (Fig. 3B). Consistent with our earlier immunoblot results (Fig. 1B), EspA could not be detected in the espC::Tn mutant transformed with pMD31 (Fig. 3B).
Immunization of rabbits with EspC peptides failed to produce suitable polyclonal antibodies. As an alternative, cell lysates of espA::Tn mutants harboring either pMDespACFLAGD or pMDespACFLAGDstop were immunoblotted for EspA and FLAG-tagged EspC expressed therein. Approximately 5.5-fold and 2.5-fold more EspA and FLAG-tagged EspC, respectively, were detected in the EspD-proficient strain than in the EspD-deficient strain (Fig. 3C). The smaller amount of EspA protein detected in EspD-deficient strains is not due to decreased transcription, as quantitative RT-PCR showed no differences in espA and espC transcript levels between EspD-proficient and -deficient strains (Fig. 3D). Additionally, the differences in EspA and EspC protein levels between all EspD-proficient and EspD-deficient M. tuberculosis strains were not growth rate dependent, as we did not observe any growth differences between these strains in 7H9 broth, on 7H11 agar, and in Sauton's medium (see Fig. S1A and B in the supplemental material).
The capacity of EspD to stabilize cellular EspA and EspC levels led us to consider direct protein-protein interactions as a possible explanation. Therefore, coimmunoprecipitation of EspA, EspC, and EspD with EspA, FLAG, and EspD antibodies was attempted from cell lysates of the espA::Tn mutant harboring pMDespACFLAGD. Although we were able to precipitate target proteins with the corresponding antibodies, we saw no evidence of coimmunoprecipitation of the other two proteins with the target protein. Likewise, efforts to pull down epitope-tagged EspA with EspC and EspD in E. coli strains coexpressing all three proteins provided no evidence of interactions between them.
These combined results clearly indicate that EspD deficiency in M. tuberculosis markedly reduces the cellular levels of both EspA and EspC proteins, although the molecular basis of this remains unknown.
EspC and EspD are both required for the maintenance of adequate cellular levels of EspA in M. tuberculosis.
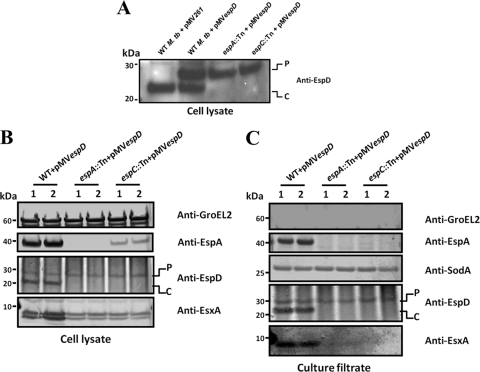
The results of the preceding experiments show that EspD is necessary to maintain wild-type cellular levels of both EspA and EspC in M. tuberculosis. To discern whether EspA requires only EspC, which in turn needs EspD, or if it requires both EspC and EspD, we compared levels of EspA produced in wild-type M. tuberculosis, espA::Tn, and espC::Tn strains transformed with the episomal plasmid pMVespD. The constitutive Hsp60 promoter driving the production of EspD from pMVespD results in the synthesis of a larger EspD isoform bearing the first 12 amino acids of Hsp60 at its amino terminus. Hence, EspD proteins of two sizes were detected in the lysates of wild-type M. tuberculosis/pMVespD—one produced from pMVespD (labeled P) and the other from the chromosomally borne gene (labeled C) (Fig. 4A and B). A control strain harboring pMV261 produces only EspD from the chromosomal gene (Fig. 4A). Only the larger EspD isoform (labeled P) was detected in the wild-type, espA::Tn, and espC::Tn strains harboring pMVespD (Fig. 4A and B). Ectopic expression of EspD alone in the espC::Tn mutant resulted in the appearance of detectable EspA protein (Fig. 4B) where none could be seen before (Fig. 1B and 3B). More importantly, however, this did not restore EspA in the espC::Tn mutant to the wild-type level, which was 6-fold higher in comparison (Fig. 4B, lanes 1 and 2). This indicates that maintenance of wild-type levels of EspA in M. tuberculosis requires both EspC and EspD.
Fig 4.
Expression and secretion analysis of M. tuberculosis strains constitutively expressing EspD. Immunoblots of total cell lysates (20 μg/lane) of the wild-type M. tuberculosis Erdman strain transformed with pMV261 and the wild-type M. tuberculosis Erdman, espA::Tn, and espC::Tn strains transformed with pMVespD (A) and total cell lysates (5 μg/lane) (B) and culture filtrates (10 μg/lane) (C) of wild-type M. tuberculosis Erdman, espA::Tn, and espC::Tn mutants transformed with pMVespD, cultured in Sauton's medium for 4 days. Antibodies used are indicated. “P” indicates the larger EspD isoform expressed from pMVespD, and “C” indicates native EspD expressed off the chromosomal allele. Two clones of each strain were analyzed, and blots shown are representative of results from three independent experiments.
Further analyses of the culture filtrates of these strains confirmed that EspA, EspC, and EspD are all essential for ESX-1 function. Only the wild-type strain harboring pMVespD exhibited EsxA and EspA secretion, whereas the espA::Tn and espC::Tn mutants with pMVespD did not (Fig. 4C). Also, the presence of the larger EspD isoform in the culture filtrates of espA::Tn and espC::Tn mutants indicates that EspD secretion does not require EspA and EspC expression or EsxA secretion (Fig. 4C).
Site-directed mutagenesis of EspD identifies amino acid residues important for EsxA secretion, EspA and EspD stability, and EspD secretion in M. tuberculosis.
The EspD polypeptide consists of 184 amino acid residues. Bioinformatic analyses of the primary amino acid sequence of EspD provided no insight into its function. To define amino acid residues that might offer functional clues, we identified and selected for site-directed mutagenesis highly conserved residues found only in EspD and its orthologues from Mycobacterium bovis, Mycobacterium leprae, and Mycobacterium marinum. Consequently, M. tuberculosis espA::Tn mutants expressing variants of EspD from pMDespACD were generated and analyzed for EsxA secretion, EspA stability, and EspD stability and secretion. We verified that all test strains obtained grew at rates similar to those of the control strains. Confirming previous observations (13, 17), we found that EsxA secretion was restored only in the espA::Tn mutant harboring pMDespACD (here referred to as the wild-type strain), while espA::Tn mutants with pMD31 or pMDespACDstop failed to do so (Fig. 5A and Table 2).
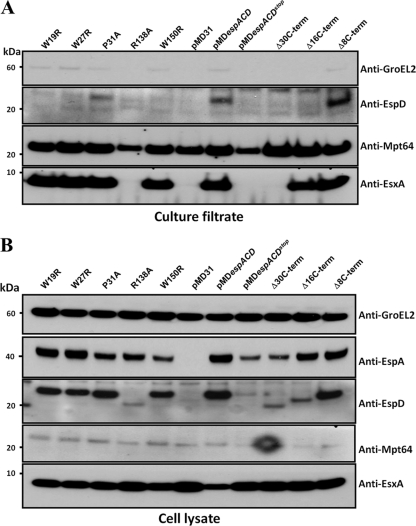
Fig 5.
Expression and secretion analysis of M. tuberculosis espA::Tn mutants producing single amino acid variants and C terminus truncations of EspD. Immunoblots of total culture filtrates (10 μg/lane) (A) and total cell lysates (5 μg/lane) (B) of M. tuberculosis espA::Tn mutants transformed with pMDespACD producing single amino acid variants and C terminus truncations of EspD, cultured in Sauton's medium for 4 days. Antibodies used are indicated. Mpt64 (an ESX-1-independent secreted protein) was used as a loading control. Results shown are representative of three independent experiments.
Over 20 espA::Tn mutant strains expressing variants of EspD were systematically evaluated, but only five strains exhibiting interesting phenotypes will be described here. Of the five, only one strain expressing EspDR138A displayed loss of EsxA secretion (Fig. 5A). Approximately 28% less EspA and 95% less EspD were also detected compared to expression in the wild-type strain (Fig. 5B and Table 2). Moreover, the EspDR138A protein produced in this strain migrated faster in SDS-PAGE gels than in the wild-type protein, indicating that the protein is likely processed upon expression (Fig. 5B). The mechanism underlying the processing and instability of this particular EspD variant is currently not understood. Notably, however, expression of EspDR138A in E. coli did not result in cleavage (J. M. Chen and S. T. Cole, unpublished data). This suggests that the posttranslational cleavage of EspDR138A may be specific to M. tuberculosis. Nevertheless, the minimal decrease in EspA levels exhibited by this ESX-1-defective strain suggests that the role played by EspD in maintaining EspA levels is distinct from its role in facilitating EsxA secretion. It also suggests that maintenance of wild-type levels of EspA can be achieved by low concentrations of EspD.
Although none of the other strains displayed defects in EsxA secretion, some of them did exhibit various cellular levels of EspA and EspD (Fig. 5B). Of these, three strains expressing tryptophan variants EspDW19R, EspDW27R, and EspDW150R also failed to secrete EspD (Fig. 5A). We found 41, 54, and 34% less EspD in strains producing EspDW19R, EspDW27R, and EspDW150R, respectively, than in the wild-type strain. Equivalent amounts of EspA protein were detected in strains expressing wild-type EspD, EspDW19R, and EspDW27R, except for the strain expressing EspDW150R, wherein 56% less EspA was detected (Fig. 5B and Table 2). Absence of EspD secretion by these strains suggests that tryptophan residues 19, 27, and 150 may be required for secretion. It is also possible that these strains fail to reach a threshold cellular concentration of EspD necessary for secretion, which could account for the unsuccessful translocation of EspD. Most importantly, however, these strains demonstrate that EspD expression intracellularly but not secretion is sufficient for EsxA secretion. In the case of the EspDW150R-producing strain, this tryptophan residue also appears to influence the maintenance of cellular levels of EspA. Restoration of normal EsxA secretion in spite of the reduction in cellular EspA levels to those seen in EspD-deficient strains suggests that EspA stability may not be as important for EsxA secretion and underscores a more critical role for EspD in this process. This observation is also in agreement with the notion that EspD plays distinct roles in facilitating EsxA secretion and EspA maintenance.
In addition to strains expressing the tryptophan variants described above, we also obtained a mutant expressing a proline variant of EspD (EspDP31A) that consistently displayed decreased EspD secretion. The levels of EspD in the culture filtrate of this strain were approximately 44% less than those in the wild-type strain (Fig. 5A and Table 2). This strain also displayed a small reduction in cellular EspA levels, with 35% less protein than that of the wild-type strain (Fig. 5B and Table 2).
C-terminal truncations of EspD impact EsxA secretion, EspA and EspD stability, and EspD secretion.
The carboxy termini of EsxB and EspC are essential for their secretion (8). To investigate if the carboxy terminus of EspD is also important for its secretion and function, we introduced premature stop codons into espD at sites 30, 16, and 8 amino acids from the carboxy terminus. The strain producing EspDΔ30CT, effectively truncated at proline 155, failed to secrete EsxA (Fig. 5A and Table 2). Furthermore, this strain exhibited significantly decreased cellular EspA levels with 59% less protein compared to that of the wild-type strain (Fig. 5B and Table 2). As expected, EspDΔ30CT migrated faster in SDS-PAGE gels (Fig. 5B). Moreover, the amount of cellular EspDΔ30CT detected was 81% less than that in the wild-type strain expressing full-length EspD (Fig. 5B and Table 2). Deletion of the last 30 amino acid residues of EspD likely results in a misfolded and inactive protein, thereby impacting both EspA stability and EsxA secretion.
Interestingly, the strain producing EspDΔ16CT, truncated at arginine 169, was able to secrete EsxA (Fig. 5A and B and Table 2). In addition, 83% less of a faster-migrating EspD protein was detected in this strain compared to that in the wild-type strain (Fig. 5B and Table 2). The phenotypes associated with the EspDΔ16CT-producing strain suggest that amino acid residues beyond arginine 169 are dispensable for maintaining EspA levels and EsxA secretion but important for EspD stability. The near-wild-type levels of EspA observed in this strain again suggest that EspA maintenance can be effectively achieved by low concentrations of EspD.
It should be noted that the faint immunoblot signals of EspDΔ30CT and EspDΔ16CT expressed in M. tuberculosis are indeed due to lower protein amounts and not due to decreased binding or recognition by the antibodies as a result of the truncations. When the same truncated variants were expressed in E. coli and immunoblotted, the signal intensities of these EspD variants were identical to that of the full-length protein (Chen and Cole, unpublished). Since the levels of both EspDΔ30CT and EspDΔ16CT in cell lysates were quite low and below the detection limit in the culture filtrate of the M. tuberculosis strains expressing these truncated variants, we are presently unable to gauge if the truncations affect secretion of EspD (Fig. 5A and Table 2).
Finally, the strain producing EspDΔ8CT, which is truncated at proline 177, did not exhibit any appreciable phenotypes. This indicates that unlike EsxB and EspC, the last 8 amino acids of EspD are dispensable for stability, for the maintenance of EspA levels, and for facilitating EsxA secretion.
DISCUSSION
The goal of our work was to elucidate the role of EspD within the espA-espC-espD cluster and in the facilitation of ESX-1-mediated secretion. Several studies clearly establish the unlinked espA-espC-espD gene cluster to be essential for ESX-1-mediated protein transport and virulence in M. tuberculosis (8, 11, 13, 17).
First, we have confirmed that espA, espC, and espD are indeed cotranscribed. Second, we have shown that EspD is also secreted by M. tuberculosis. However, unlike EspA and EspC, EspD is clearly not cosecreted with EsxA. Mutations in esx-1 appear to result in slightly decreased EspD levels in the culture filtrate, and we also observed noticeable reductions in EspA levels within the cytosol of these mutants. It is possible that one or more components of the ESX-1 system, absent from both the Erdman 36-72 mutant and H37Rv ΔRD1 mutant, stabilize EspA and aid in optimal EspD export. Nevertheless, to our knowledge EspD is the first example of an Esp in M. tuberculosis whose expression is critical for EsxA secretion but whose own export does not exclusively require ESX-1. EspD lacks amino-terminal signal sequences for the general secretion (SecA) pathway and the twin-arginine transport (TAT) system; thus, it seems likely that EspD utilizes neither of these pathways. We have experimentally ruled out secretion of EspD through the ESX-5 (encoded by rv1782 to rv1798) secretion apparatus (D. Bottai, J. M. Chen, and S. T. Cole, unpublished data). However, the possibility remains that EspD may yet require one (or more) of the other ESX systems found in M. tuberculosis, namely, systems ESX-2, ESX-3, and ESX-4.
Third, we provide formal evidence that maintaining cellular levels of EspA and EspC, both of which are essential for ESX-1 function, requires EspD. We have extended these findings to show that EspA maintenance requires both EspC and EspD, as we were unable to restore wild-type levels of the EspA protein by expressing EspD alone in the espC::Tn mutant, which retains the ability to produce EspA. Our findings are consistent with previous reports that EspA is more abundant only when coexpressed with EspC and EspD in an M. tuberculosis ΔespA ΔespC ΔespD strain (13) and that M. tuberculosis strains expressing truncated EspC proteins exhibit decreased levels of EspA and defective ESX-1 function (8). We have also confirmed that EspD deficiency does not affect transcription of espA and espC, and this is consistent with the finding that wild-type M. tuberculosis and espC::Tn strains synthesize similar levels of espA mRNA as determined by DNA microarray analyses (17). The possibility that mRNA secondary structures encoded within the espD gene mediate espA-espC-espD transcript stability also seems unlikely, because the EspD-deficient M. tuberculosis strains generated in this study retain the ability to make full-length espA-espC-espD transcripts, even though they are unable to synthesize the EspD protein. We initially hypothesized that EspD acts as a chaperone to both EspA and EspC through direct physical interaction. Despite considerable effort, we were unable to coimmunoprecipitate EspD with EspA or EspC from cell lysates of M. tuberculosis. Efforts to pull down EspA with EspC or EspD in E. coli strains coexpressing these proteins also did not provide evidence of interactions between these proteins. While it is possible that the interactions between EspA, EspC, and EspD are weak and transient and escape detection under the conditions tested, it is noteworthy that our inability to detect protein-protein interactions is consistent with results of yeast two-hybrid and coimmunoprecipitation experiments, which found no interactions between EspA, EspC, and EspD (8, 13, 17). Collectively, these points suggest that EspD-mediated stabilization occurs by other means. Alternative mechanisms underlying the capacity of EspD to maintain normal cellular levels of EspA and EspC include posttranscriptional stabilization of espA-espC-espD mRNA from degradation by EspD and/or posttranslational prevention of rapid EspA and EspC protein turnover. These hypotheses are currently being tested.
The use of site-directed mutagenesis and generation of truncation mutants have proved invaluable in dissecting the function of several proteins associated with the ESX-1 system. For instance, site-directed mutagenesis of EsxA has been instrumental in defining residues critical for secretion, formation of heterodimeric complexes with EsxB, virulence, and immunogenicity (4, 18, 19). Carboxy-terminal truncations of EsxB and EspC have helped identify interacting partners of these proteins necessary for their translocation and for optimal ESX-1 function (6, 8). Motivated by the utility of these approaches, we used site-directed mutagenesis to begin defining important residues and domains in EspD. Indeed, based on the characterization of M. tuberculosis espA::Tn strains expressing site-directed mutants of EspD, we are able to draw several important conclusions regarding EspD biology. (i) The two roles of EspD, namely, maintenance of cellular EspA levels and facilitation of EsxA secretion, are distinct, as evidenced by the phenotypes of M. tuberculosis espA::Tn strains producing EspDR138A and EspDW150R. In the former, there is a significant decrease in EspD stability and abrogation of EsxA secretion; however, EspA maintenance is not severely affected. In the latter, EspD stability is not significantly decreased, yet EspA cellular maintenance is reduced. In spite of this, EsxA secretion is unaffected. It is particularly noteworthy that this recurring theme of multifunctionality associated with different domains or amino acid residues within an ESX-1-associated protein is shared by EspA, EspC, and MycP1 (8, 13, 21). (ii) Unlike EspA and EspC, EsxA is not cosecreted with EspD. This conclusion is based on the restoration of EsxA secretion in the absence of EspD secretion in M. tuberculosis strains expressing EspDW19R, EspDW27R, and EspDW150R. This is in agreement with our findings that EspD secretion does not require ESX-1 functions, as indicated by strain 36-72 and the ΔRD1 mutant. This is also in agreement with our finding that EspD secretion does not require EspA and EspC expression, as we have seen in espA::Tn and espC::Tn mutants transformed with pMVespD. (iii) The ability to maintain wild-type levels of EspA (and EspC) does not require wild-type EspD protein concentrations, as demonstrated by the preservation of near wild-type levels of EspA even in strains with significantly diminished levels of EspDR138A and EspDΔ16CT. This is consistent with our proposal that EspD-mediated stabilization of EspA and EspC likely does not involve a conventional chaperone-effector-type interface utilizing direct protein-protein interactions with stringent stoichiometries. (iv) Unlike EsxB and EspC, the last 8 amino acid residues at the carboxy terminus of EspD are dispensable for its function and secretion.
Our findings with EspD contribute novel insights into its role but also raise new questions, such as how EspD is secreted and how it mediates the stabilization of EspA and EspC. The phenotypic characterization of M. tuberculosis strains expressing EspD variants revealed the existence of a highly complex and multifunctional aspect of EspD in M. tuberculosis biology. Ongoing efforts to dissect the consequences of these amino acid replacements and truncations and their effects on ESX-1 function will increase our understanding of protein secretion and virulence in M. tuberculosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank Philippe Busso (Cole lab, EPFL) for DNA sequencing and Debbie Hunt (MRC-NIMR) for assistance in generating the ΔespR mutant.
J.M.C. is a recipient of postdoctoral fellowships from the Canadian Thoracic Society and the Canadian Institutes of Health Research. F.P. is a Swiss National Science Foundation MHV postdoctoral fellow. This study was supported by funding from the European Community's Seventh Framework Program (FP7/2007-2013) under grant agreement no. 201762 and from the Swiss National Science Foundation (31003A-125061). Antibodies against GroEL2 and SodA were received as part of the National Institutes of Health, National Institute of Allergy and Infectious Diseases, contract (no. HHSN266200400091c) entitled “Tuberculosis Vaccine Testing and Research Materials,” awarded to Colorado State University.
Footnotes
Published ahead of print 9 December 2011
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Bange FC, Collins FM, Jacobs WR., Jr 1999. Survival of mice infected with Mycobacterium smegmatis containing large DNA fragments from Mycobacterium tuberculosis. Tuber. Lung Dis. 79:171–180 [DOI] [PubMed] [Google Scholar]
- 2. Bitter W, et al. 2009. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 5:e1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bottai D, et al. 2011. ESAT-6 secretion-independent impact of ESX-1 genes espF and espG1 on virulence of Mycobacterium tuberculosis. J. Infect. Dis. 203:1155–1164 [DOI] [PubMed] [Google Scholar]
- 4. Brodin P, et al. 2005. Functional analysis of early secreted antigenic target-6, the dominant T-cell antigen of Mycobacterium tuberculosis, reveals key residues involved in secretion, complex formation, virulence, and immunogenicity. J. Biol. Chem. 280:33953–33959 [DOI] [PubMed] [Google Scholar]
- 5. Brodin P, et al. 2006. Dissection of ESAT-6 system 1 of Mycobacterium tuberculosis and impact on immunogenicity and virulence. Infect. Immun. 74:88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Champion PA, Stanley SA, Champion MM, Brown EJ, Cox JS. 2006. C-terminal signal sequence promotes virulence factor secretion in Mycobacterium tuberculosis. Science 313:1632–1636 [DOI] [PubMed] [Google Scholar]
- 7. Dhar N, McKinney JD. 2010. Mycobacterium tuberculosis persistence mutants identified by screening in isoniazid-treated mice. Proc. Natl. Acad. Sci. U. S. A. 107:12275–12280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DiGiuseppe Champion PA, Champion MM, Manzanillo P, Cox JS. 2009. ESX-1 secreted virulence factors are recognized by multiple cytosolic AAA ATPases in pathogenic mycobacteria. Mol. Microbiol. 73:950–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Donnelly-Wu MK, Jacobs WR, Jr, Hatfull GF. 1993. Superinfection immunity of mycobacteriophage L5: applications for genetic transformation of mycobacteria. Mol. Microbiol. 7:407–417 [DOI] [PubMed] [Google Scholar]
- 10. Fisher MA, Plikaytis BB, Shinnick TM. 2002. Microarray analysis of the Mycobacterium tuberculosis transcriptional response to the acidic conditions found in phagosomes. J. Bacteriol. 184:4025–4032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fortune SM, et al. 2005. Mutually dependent secretion of proteins required for mycobacterial virulence. Proc. Natl. Acad. Sci. U. S. A. 102:10676–10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frigui W, et al. 2008. Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog. 4:e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garces A, et al. 2010. EspA acts as a critical mediator of ESX1-dependent virulence in Mycobacterium tuberculosis by affecting bacterial cell wall integrity. PLoS Pathog. 6:e1000957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guinn KM, et al. 2004. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol. Microbiol. 51:359–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hsu T, et al. 2003. The primary mechanism of attenuation of bacillus Calmette-Guerin is a loss of secreted lytic function required for invasion of lung interstitial tissue. Proc. Natl. Acad. Sci. U. S. A. 100:12420–12425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lewis KN, et al. 2003. Deletion of RD1 from Mycobacterium tuberculosis mimics bacille Calmette-Guerin attenuation. J. Infect. Dis. 187:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. MacGurn JA, Raghavan S, Stanley SA, Cox JS. 2005. A non-RD1 gene cluster is required for Snm secretion in Mycobacterium tuberculosis. Mol. Microbiol. 57:1653–1663 [DOI] [PubMed] [Google Scholar]
- 18. Meher AK, Bal NC, Chary KV, Arora A. 2006. Mycobacterium tuberculosis H37Rv ESAT-6-CFP-10 complex formation confers thermodynamic and biochemical stability. FEBS J. 273:1445–1462 [DOI] [PubMed] [Google Scholar]
- 19. Meher AK, Lella RK, Sharma C, Arora A. 2007. Analysis of complex formation and immune response of CFP-10 and ESAT-6 mutants. Vaccine 25:6098–6106 [DOI] [PubMed] [Google Scholar]
- 20. Millington KA, et al. 2011. Rv3615c is a highly immunodominant RD1 (region of difference 1)-dependent secreted antigen specific for Mycobacterium tuberculosis infection. Proc. Natl. Acad. Sci. U. S. A. 108:5730–5735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ohol YM, et al. 2010. Mycobacterium tuberculosis MycP1 protease plays a dual role in regulation of ESX-1 secretion and virulence. Cell Host Microbe 7:210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pym AS, Brodin P, Brosch R, Huerre M, Cole ST. 2002. Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol. Microbiol. 46:709–717 [DOI] [PubMed] [Google Scholar]
- 23. Raghavan S, Manzanillo P, Chan K, Dovey C, Cox JS. 2008. Secreted transcription factor controls Mycobacterium tuberculosis virulence. Nature 454:717–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rickman L, et al. 2005. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol. Microbiol. 56:1274–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rohde KH, Abramovitch RB, Russell DG. 2007. Mycobacterium tuberculosis invasion of macrophages: linking bacterial gene expression to environmental cues. Cell Host Microbe 2:352–364 [DOI] [PubMed] [Google Scholar]
- 26. Simeone R, Bottai D, Brosch R. 2009. ESX/type VII secretion systems and their role in host-pathogen interaction. Curr. Opin. Microbiol. 12:4–10 [DOI] [PubMed] [Google Scholar]
- 27. Stanley SA, Raghavan S, Hwang WW, Cox JS. 2003. Acute infection and macrophage subversion by Mycobacterium tuberculosis require a specialized secretion system. Proc. Natl. Acad. Sci. U. S. A. 100:13001–13006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stover CK, et al. 1991. New use of BCG for recombinant vaccines. Nature 351:456–460 [DOI] [PubMed] [Google Scholar]
- 29. van Kessel JC, Hatfull GF. 2008. Mycobacterial recombineering. Methods Mol. Biol. 435:203–215 [DOI] [PubMed] [Google Scholar]
- 30. Walters SB, et al. 2006. The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol. Microbiol. 60:312–330 [DOI] [PubMed] [Google Scholar]
- 31. Wards BJ, Collins DM. 1996. Electroporation at elevated temperatures substantially improves transformation efficiency of slow-growing mycobacteria. FEMS Microbiol. Lett. 145:101–105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.