Abstract
We investigated the antiviral activity of nanosized copper(I) iodide (CuI) particles having an average size of 160 nm. CuI particles showed aqueous stability and generated hydroxyl radicals, which were probably derived from monovalent copper (Cu+). We confirmed that CuI particles showed antiviral activity against an influenza A virus of swine origin (pandemic [H1N1] 2009) by plaque titration assay. The virus titer decreased in a dose-dependent manner upon incubation with CuI particles, with the 50% effective concentration being approximately 17 μg/ml after exposure for 60 min. SDS-PAGE analysis confirmed the inactivation of the virus due to the degradation of viral proteins such as hemagglutinin and neuraminidase by CuI. Electron spin resonance (ESR) spectroscopy revealed that CuI generates hydroxyl radicals in aqueous solution, and radical production was found to be blocked by the radical scavenger N-acetylcysteine. Taken together, these findings indicate that CuI particles exert antiviral activity by generating hydroxyl radicals. Thus, CuI may be a useful material for protecting against viral attacks and may be suitable for applications such as filters, face masks, protective clothing, and kitchen cloths.
INTRODUCTION
Influenza virus is a communicable pathogen that typically spreads through humans, birds, and swine. Although avian influenza viruses rarely infect humans, since 2003 several cases of humans infected by avian influenza (H5N1) with high lethality have been reported in Asia and the Middle East. Avian and human influenza viruses can simultaneously infect swine, occasionally resulting in the genetic hybridization of these two strains. In fact, the influenza virus of swine origin spread worldwide in the 2009 pandemic (H1N1) was a genetically hybridized mutant of swine, avian, and human influenza viruses (17). In addition, the transmission of influenza viruses is very rapid due to the spread of worldwide transportation networks. Thus, additional measures to inhibit the spread of viral infection are urgently necessary.
To prevent viral infection, there is a strong need to develop antiviral products in addition to antibacterial products. There are few materials that have been reported to inactivate viruses. For example, titanium dioxide (TiO2) is an antiviral material that has been used as a coating agent. Under UV light, TiO2 generates hydroxyl radicals (·OH) (9), and this results in the degradation of organic compounds. However, there are some limitations regarding the use of TiO2, including the requirement of UV irradiation and the degradation of the base material.
On the other hand, copper has long been used as an antibacterial material (1), and several copper compounds have been reported to exhibit viral inactivation. Yamamoto et al. reported the inactivation of bacteriophages by copper in 1964 (25). More recently, the inactivation of avian influenza virus by copper metal (18) and divalent copper ions (Cu2+) (11) and the inactivation of human immunodeficiency virus (HIV) by copper ions (20) and copper oxide (2, 3) have been reported. Because copper metal and copper oxide are stable compounds, they can easily be used in antiviral applications. However, the external appearance of products treated with these materials is invariably altered because of their brown and black color.
To address this problem, we focused on another copper compound, copper(I) iodide (CuI), which is white and has greater stability in air and water than other cuprous halides, and therefore can be applied to products such as filters, face masks, protective clothing, and kitchen cloths. However, the antiviral properties of CuI have not been investigated. Generally, the characteristics of fine particles with sizes in the nanometer range differ significantly from those in bulk form. The vast increase in specific surface area by scaling down fine particles to the nanoscale markedly changes their original physical and chemical characteristics. Therefore, this study investigated the antiviral activity of nanosized CuI particles against pandemic (H1N1) 2009 influenza virus.
MATERIALS AND METHODS
Preparation of nanosized CuI particles and CuI suspension.
CuI (Nihon Kagaku Sangyo Co., Ltd., Tokyo, Japan) particles having a median particle diameter (D50) of 5.84 μm were pulverized in a NanoJetmizer NJ-100B (Aishin Nano Technologies Co., Ltd., Saitama, Japan), a superfine mill with a high-pressure jet stream, until the D50 reached 160 nm (see Fig. S1 in the supplemental material). These particle sizes were measured with a CILAS 1064L particle size analyzer (CILAS, Orleans, France). CuI particles then were suspended in phosphate-buffered saline (PBS; Wako Pure Chemical Industries, Ltd., Osaka, Japan) at 10 mg/ml, stirred for 15 min, and diluted to the desired concentrations with PBS.
Viruses and cells.
Influenza virus A/California/07/09 (H1N1) pdm (A/H1N1pdm) was supplied by the National Institute of Infectious Diseases (NIID) in Japan to the Chiba University Graduate School of Medicine. Virus was propagated four times in embryonated chicken eggs, and infectious allantoic fluid then was harvested, followed by further propagation in Madin-Darby canine kidney (MDCK) cells. MDCK cells were supplied by the Bio-Medical Science Association of Japan.
MDCK cells were cultured as monolayers in minimum essential medium (MEM; MP Bio Japan K.K., Tokyo, Japan) supplemented with 10% fetal calf serum (Australian origin; Gibco, Carlsbad, CA), 1.1 g/liter sodium hydrogen carbonate (NaHCO3; Wako Pure Chemical Industries, Ltd.), and 0.006% kanamycin (Wako Pure Chemical Industries, Ltd.) (22). Kanamycin was selected in this study because it is superior to penicillin G-streptomycin in terms of antibacterial spectrum and cytotoxicity. MDCK cells were plated at a density of 1 × 105 cells/ml in a 150-cm2 flask and were cultured for 72 h. Cells then were washed with MEM containing 1.1 g/liter NaHCO3 and 0.006% kanamycin (1× MEM), and 1× MEM was added to the flask, followed by culture at 37°C in a CO2 incubator. After 1 h, medium was removed and 5 ml of virus suspension (104 PFU/ml) was added, and this was further incubated for 1 h. The medium containing viruses was removed, and 1× MEM containing 2.5 μg/ml trypsin (from bovine pancreas; MP Bio Japan K.K.) was added. After incubation at 34°C for 48 h, the culture supernatant was collected by centrifugation at 5,000 rpm for 10 min and was stored at −80°C. The virus titer was determined to be 2.8 × 106 PFU/0.1 ml by plaque formation in monolayers of MDCK cells as described below.
Virus titration by plaque assay.
The plaque assay was performed as follows (22). Ten-mg/ml CuI suspensions in PBS were prepared and were serially diluted with PBS from 1 to 1,000 μg/ml. One hundred μl of CuI suspension and 100 μl of diluted virus suspension (approximately 105 PFU/0.1 ml) were mixed and incubated with shaking for 1 h at 25°C. After incubation, 800 μl of soybean-casein digest with lecithin and polysorbate 80 medium (SCDLP; Wako Pure Chemical Industries, Ltd.) was added, followed by vortexing. Aliquots of supernatant were used as virus samples.
Confluent monolayers of MDCK cells in six-well tissue culture plates (Sumilon; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) were prepared, and the medium was removed and washed with 3 ml of MEM. Cells were inoculated with 100 μl of virus sample. After incubation for 60 min at 34°C, cells were washed with MEM and overlaid with MEM containing 1.1 g/liter NaHCO3, 0.01% DEAE-dextran (GE Healthcare Japan Co., Tokyo, Japan), 1 μg/ml trypsin, and 0.7% noble agar (Funakoshi Co., Ltd., Tokyo, Japan). After incubation for 48 h at 34°C under 5% CO2 in air, cells were fixed with 10% formaldehyde (Wako Pure Chemical Industries, Ltd.) in PBS for 4 h, and the agar overlay was removed by flooding with tap water. Attached cells were stained with 0.038% methylene blue (Wako Pure Chemical Industries, Ltd.), and plaques were counted and calculated.
SDS-PAGE.
Concentrated virus suspension was prepared as follows. Virus stock (1.8 × 1010 PFU) was concentrated by centrifugation (21,500 × g, 2.5 h) and resuspended in 2 ml of 10% glucose in PBS. A sucrose step gradient (20 and 60% glucose in PBS) was overlaid with virus suspension, followed by centrifugation (21,500 × g, 2.5 h). The virus layer between 20 and 60% glucose was recovered and used as the virus sample (1.1 × 1010 PFU/ml). Twelve microliters of virus suspension (1.3 × 108 PFU) was mixed with 12 μl of CuI suspension (1 to 1,000 μg/ml). After 1 h, 8 μl of 4× SDS sample buffer (0.2 M Tris-HCl [pH 6.8], 8% SDS [Wako Pure Chemical Industries, Ltd.], 40% glycerol [Sigma-Aldrich Japan Co., Ltd., Tokyo, Japan.], and 0.8% bromophenol blue [Wako Pure Chemical Industries, Ltd.]) was added to the sample, followed by vortexing and boiling for 5 min. The same amount of protein sample (7 μl/lane) was loaded onto the gels (4 to 12% bis-Tris gel; 15 wells; 1.0 mm thickness; Novex; Invitrogen, Carlsbad, CA) together with a molecular weight marker (Novex sharp prestained marker; Invitrogen), and the gels were electrophoresed at 200 V for 1 h. Subsequently, protein bands were stained with a CBB (Coomassie brilliant blue)-based stain, SimplyBlue SafeStain (Invitrogen), and were washed with distilled water for visualization.
ESR analysis.
Ten microliters of spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO; Labotec Co., Ltd., Hiroshima, Japan) was added to 390 μl of CuI suspension (0.5 mM) or copper(II) sulfate (CuSO4·5H2O) solution (0.5 mM), followed by mixing for 1 min. Radical adducts then were analyzed by electron spin resonance (ESR) spectroscopy. Measurement conditions for ESR (JES-FA-100; JEOL, Tokyo, Japan) throughout the experiments were the following: modulation width, 0.1 millitesla (mT); amplitude, 200; sweep time, 2 min; time constant, 0.1 s; and microwave power, 4 mW. Magnetic fields for the measurement of Cu2+ and ·OH were 280 to 350 mT and 325 to 345 mT, respectively.
Luminol chemiluminescence detection of reactive oxygen species (ROS).
CuI suspensions (10 mg/ml in Milli-Q water) or the same molar concentrations of CuSO4·5H2O were centrifuged (9,400 × g, 3 min), and supernatants were passed through a polytetrafluoroethylene filter (0.2 μm pore size). Samples (50 μl) were placed into tubes and set in the luminometer (TK-LP400; Tokken Inc., Chiba, Japan). When measurement started, 50 μl of horseradish peroxidase solution (Tokken Inc.) and 50 μl of luminol solution (Tokken Inc.) were automatically added to the sample tube, and luminol chemiluminescence intensity was recorded sequentially. Hydrogen peroxide and distilled water were used as positive and negative controls, respectively.
RESULTS
Inactivation of influenza virus by CuI.
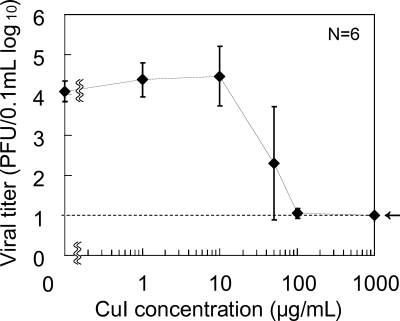
To test whether CuI shows antiviral effects, we first incubated A/H1N1pdm (105 PFU/0.1 ml) in CuI suspension and the virus titer was determined. Figure 1 shows the dose dependence of viral inactivation by CuI suspension in PBS from 0 to 1,000 μg/ml. A/H1N1pdm was incubated with CuI for 60 min at room temperature, and virus titers were measured by plaque assay. The virus titer was decreased by incubation with CuI in a dose-dependent manner with a 50% effective concentration (EC50) of approximately 17 μg/ml (0.0017% [wt/vol]). This indicates that CuI particles inactivate influenza virus.
Fig 1.
Inactivation of A/H1N1pdm (105 PFU/0.1 ml) with various concentrations of CuI suspensions in PBS after exposure for 60 min. The dotted line indicates the lower detection limit. Data are expressed as means ± standard deviations from two independent experiments (n = 6 samples per plot).
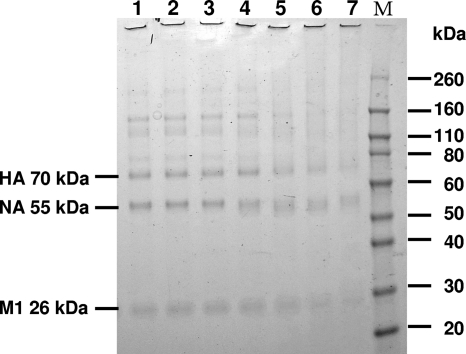
We next analyzed whether CuI modifies viral proteins such as hemagglutinin (HA) and neuraminidase (NA). Figure 2 shows the dose-dependent differences in the SDS-PAGE patterns of proteins in influenza virus from control and CuI-treated virus. These protein bands were derived from virus, as no protein bands were observed in the samples of CuI or PBS without virus (see Fig. S2 in the supplemental material). Protein bands were wider and showed lower intensity after the treatment with 1 μg/ml (lane 4) of CuI for 1 h, indicating the degradation of viral proteins by CuI.
Fig 2.
Degradative effects of CuI on A/H1N1pdm. The data represent SDS-PAGE gel results (CBB stain). Concentrations of CuI in PBS are the following: lane 1, 0 μg/ml (only PBS); lane 2, 0.01 μg/ml; lane 3, 0.1 μg/ml; lane 4, 1 μg/ml; lane 5, 10 μg/ml; lane 6, 100 μg/ml; lane 7, 1,000 μg/ml. M indicates the molecular mass marker. HA, hemagglutinin; NA, neuraminidase; M1, matrix protein.
Detection of oxygen radical formation by ESR spectroscopy.
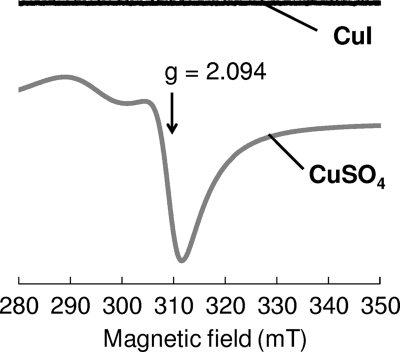
Generally, monovalent copper (Cu+) in an aqueous solution (Cu+aq) tends to be converted to metallic copper or Cu2+ by a disproportionation reaction. Therefore, the valence state of copper in CuI suspension was investigated by ESR spectroscopy. As shown in Fig. 3, the ESR spectrum of CuSO4·5H2O solution showed bands assigned to Cu2+, with the perpendicular g value (spectroscopic splitting factor) being 2.0. The g values of the bands were assigned to mononuclear Cu2+ complexes with four-oxygen ligation (23, 26). On the other hand, no ESR bands for Cu2+ were detected in the CuI suspension, indicating that most CuI exists as Cu+ in aqueous solution.
Fig 3.
ESR spectra for CuSO4·5H2O solution and CuI suspension in distilled water. Concentrations of CuI and CuSO4·5H2O were 20 mM. The g value of CuSO4·5H2O is indicated.
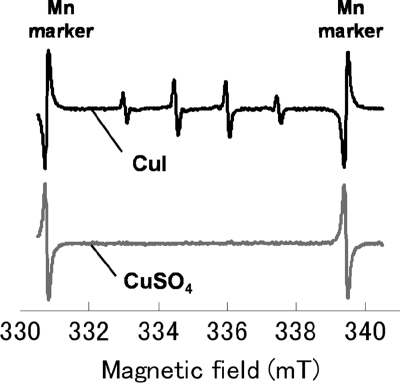
It has been reported that Cu+ in aqueous solution might behave as a catalyst in a Fenton-like reaction (16), leading to the generation of ·OH. ESR measurement using a spin trap DMPO, which is widely used in ESR spectroscopy, was performed to investigate whether CuI generates ·OH. Figure 4 shows the ESR spectra of the 0.5 mM CuI suspension and the 0.5 mM CuSO4·5H2O solution (Cu2+ source; control). The formation of DMPO-OH adduct from DMPO through the nucleophilic addition of water in the presence of metal ions (10) was blocked in this experiment by the addition of a chelating agent, EDTA. An equal volume of EDTA (1 mM) was added to the CuI suspension, and DMPO then was added. As shown in the figure, the CuI suspension showed a typical DMPO-OH signal with four peaks between the manganese markers (15). However, the CuSO4·5H2O solution did not show this signal, thus suggesting that Cu+ is involved in ·OH generation (Fig. 4).
Fig 4.
ESR spectra for CuSO4·5H2O solution and CuI suspension in the presence of DMPO and EDTA (1 mM). Concentrations of CuI and CuSO4·5H2O were 0.5 mM.
Detection of ROS by luminol chemiluminescence.
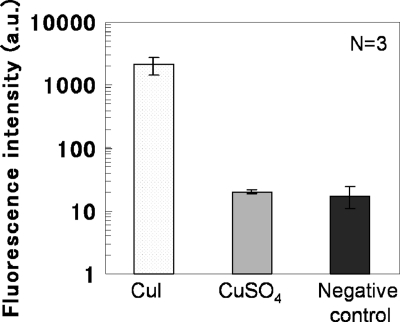
Luminol chemiluminescence was employed as another method for detecting ROS. In aqueous solution, luminol reacts with oxidants such as hydrogen peroxide (H2O2) and hydroxide (OH−), resulting in chemiluminescence emission. Therefore, the phenomenon generally is used for the detection of ROS. Figure 5 shows the chemiluminescence intensity of the luminol reaction in CuSO4·5H2O solution or supernatant of CuI suspension. The CuI particles, which interfere with the measurement of chemiluminescence due to scattering, were removed by centrifugation and filtration. Similarly to the ESR results, there were no differences in chemiluminescence intensity between the negative control (PBS) and CuSO4·5H2O solution, whereas a significant increase in luminol chemiluminescence was detected in the supernatant of the CuI suspension, thus confirming the generation of ROS in CuI suspension.
Fig 5.
Measurement of reactive oxygen species by luminol reaction in supernatant of a 10 mg/ml (52.5 mM) CuI suspension and 52.5 mM CuSO4·5H2O solution. Data are expressed as means ± standard deviations.
Inhibition of ·OH formation by NAC.
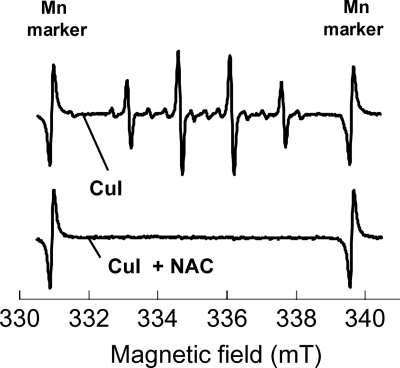
The results shown in Fig. 4 and 5 confirmed ·OH generation by CuI. To examine the consistency within these observations, we tested whether the ·OH formation associated with CuI is inhibited by a radical scavenger. Figure 6 shows the ESR spectra for the 0.5 mM CuI suspension and the CuI suspension in the presence of a radical scavenger, 250 μg/ml N-acetylcysteine (NAC). The ESR signal for DMPO-OH was observed in the CuI suspension, but it was scarcely detected in the CuI suspension with NAC or in the CuSO4·5H2O solution, indicating that CuI-induced ROS formation is inhibited by NAC.
Fig 6.
ESR spectra of 0.5 mM CuI suspension with or without radical scavenger NAC (2 mM).
DISCUSSION
In this study, we found for the first time that nanosized CuI particles have antiviral activity. It was estimated from our data that the EC50 of CuI particles against influenza viruses was 0.0017% (wt/vol) after treatment for 1 h. We also found that CuI particles possess the ability to generate ·OH derived from Cu+ (no ·OH was generated by Cu2+ from CuSO4·5H2O).
We further elucidated that ROS exert antiviral activity against influenza virus by degrading functional proteins, such as HA and NA. It is well known that ·OH oxidatively modifies proteins. It has been reported that ·OH produced covalently bound aggregate, whereas the combination of ·OH and O2− caused extensive protein fragmentation (8). As discussed below, Cu+ probably produces both ·OH and O2−, resulting in the degradation fragment of viral proteins as shown in the SDS-PAGE analysis. Yamamoto et al. reported that viral protein modification by myeloperoxidase resulted in antiviral activity (24). Thus, CuI appears to inactivate influenza viruses by degrading viral proteins. It has been reported that copper metal and copper oxide have antiviral activity; copper metal takes approximately 6 h to inactivate 104 influenza A virus particles (18). In the case of HIV-1, the virus is surrounded by a lipid bilayer with envelope proteins, similarly to influenza viruses. The 50% inhibitory concentration of copper oxide (a mixture of 70% Cu2O and 30% CuO) against HIV-1 is approximately 0.3% (wt/vol) after treatment for 2 h (2, 3).
·OH is known as an ROS that induces damage in biological tissues and cells through its potent oxidation activity. There are numerous reports on virus inactivation by the photocatalytic properties of TiO2, which also generates ·OH by the oxidation of H2O (7, 12, 13, 21). On the other hand, Cu+ probably participates in a Fenton-like reaction to produce ·OH from hydrogen peroxide (16): Cu+aq + H2O2 → Cu2+aq + OH− + ·OH.
CuI, a monovalent copper compound, would be the source of Cu+ in this reaction. In addition, the copper-hydroperoxo complex formed through the reaction between Cu+ and H2O2 would cause DNA damage (19). Although no exogenous H2O2 was added in the present study, Cu+ probably reacts with O2 to generate O2− and subsequently H2O2 (5, 6). Under aerobic conditions, Cu+ is spontaneously oxidized back to Cu2+ with the subsequent formation of hydrogen peroxide via the superoxide intermediate (6): 2 Cu+ + 2O2 (aq) → 2 Cu2++ 2O2−, 2O2− + 2 H+ → H2O2 + O2, and Cu+ + H2O2 → Cu2+ + OH− + ·OH.
Similarly, Cu+ dissolved from CuI in water or Cu+ existing on the surface of CuI may be oxidized back to Cu2+ with the subsequent formation of hydrogen peroxide via the superoxide intermediate.
Another mechanism of viral inactivation by Cu+ is thought to be lipid peroxidation through the following reactions (4, 14): Cu+ + LOOH → Cu2+ + LO· + OH− and Cu2+ + LOOH → LOO· + H+.
As the envelope of influenza viruses is composed mainly of lipids, Cu+ may inactivate viruses by oxidizing lipids. In addition to oxidative damage by ·OH, the precise mechanisms of ·OH formation by CuI and lipid peroxide levels remain to be elucidated in future studies.
The activity of conventional TiO2 can be increased by nanoparticle processing, but this leads to the degradation of treated organic polymers. On the other hand, CuI shows sufficient ROS generation but has less activity than the photocatalyst TiO2. The properties of CuI will enable it to be applied to filters, masks, protective clothing, and dishcloths by mixing with or coating on polymer base materials without any breakdown of treated materials. In fact, we have successfully coated nonwoven fabrics with CuI particles without any discoloration while maintaining antiviral activity (unpublished data).
Supplementary Material
Footnotes
Published ahead of print 9 December 2011
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Borkow G, Gabbay J. 2005. Copper as a biocidal tool. Curr. Med. Chem. 12:2163–2175 [DOI] [PubMed] [Google Scholar]
- 2. Borkow G, Gabbay J. 2004. Putting copper into action: copper-impregnated products with potent biocidal activities. FASEB J. 18:1728–1730 [DOI] [PubMed] [Google Scholar]
- 3. Borkow G, Lara HH, Covington CY, Nyamathi A, Gabbay J. 2008. Deactivation of human immunodeficiency virus type 1 in medium by copper oxide-containing filters. Antimicrob. Agents Chemother. 52:518–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burkitt MJ. 2001. A critical overview of the chemistry of copper-dependent low density lipoprotein oxidation: roles of lipid hydroperoxides, α-tocopherol, thiols, and ceruloplasmin. Arch. Biochem. Biophys. 394:117–135 [DOI] [PubMed] [Google Scholar]
- 5. Cole AP, Root DE, Mukherjee P, Solomon EI, Stack TDP. 1996. A trinuclear intermediate in the copper-mediated reduction of O2: four electrons three coppers. Science 273:1848–1850 [DOI] [PubMed] [Google Scholar]
- 6. Cross JB, et al. 2003. Killing of Bacillus spores by aqueous dissolved oxygen, ascorbic acid, and copper ions. Appl. Environ. Microbiol. 69:2245–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cui H, et al. 2010. Photocatalytic inactivation efficiency of anatase nano-TiO2 Sol on the H9N2 avian influenza virus. Photochem. Photobiol. 86:1135–1139 [DOI] [PubMed] [Google Scholar]
- 8. Davies KJA. 1987. Protein damage and degradation by oxygen radicals. J. Biol. Chem. 262:9895–9901 [PubMed] [Google Scholar]
- 9. Fujishima A, Honda K. 1972. Electrochemical photolysis of water at a semiconductor electrode. Nature 238:37–38 [DOI] [PubMed] [Google Scholar]
- 10. Hanna PM, Chamulitrat W, Mason RP. 1992. When are metal ion-dependent hydroxyl and alkoxyl radical adducts of 5,5-dimethyl-1-pyrroline N-oxide artifacts? Arch. Biochem. Biophys. 296:640–644 [DOI] [PubMed] [Google Scholar]
- 11. Horie M, et al. 2008. Inactivation and morphological changes of avian influenza virus by copper ions. Arch. Virol. 153:1467–1472 [DOI] [PubMed] [Google Scholar]
- 12. Lee S, Nishiida K, Otaki M, Ohgaki S. 1997. Photocatalytic inactivation of phage Qβ by immobilized titanium dioxide mediated photocatalyst. Water Sci. Technol. 35:101–106 [Google Scholar]
- 13. Lee S, Nishida K, Ohgaki S. 1998. Inactivation of phage Qβ by 254nm UV light and titanium dioxide photocatalyst. J. Environ. Sci. Health 33:1643–1655 [Google Scholar]
- 14. Litter MI. 1999. Heterogeneous photocatalysis: transition metal ions in photocatalytic systems. Appl. Catal. B Environ. 23:89–114 [Google Scholar]
- 15. Madden KP, Taniguchi H. 2001. The role of the DMPO-hydrated electron spin adduct in DMPO-·OH spin trapping. Free Radic. Biol. Med. 30:1374–1380 [DOI] [PubMed] [Google Scholar]
- 16. Masarwa M, et al. 1988. Reactions of low-valent transition-metal complexes with hydrogen peroxide. Are they “Fenton-like” or not? 1. The case of Cu+aq and Cr2+aq. J. Am. Chem. Soc. 110:4293–4297 [Google Scholar]
- 17. Neumann G, Noda T, Kawaoka Y. 2009. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature 459:931–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Noyce JO, Michels H, Keevil CW. 2007. Inactivation of influenza A virus on copper versus stainless steel surfaces. Appl. Environ. Microbiol. 73:2748–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oikawa S, Kawanishi S. 1998. Distinct mechanisms of site specific DNA damage induced by endogenous reductants in the presence of iron(III) and copper(II). Biochim. Biophys. Acta 1399:19–30 [DOI] [PubMed] [Google Scholar]
- 20. Sagripanti JL, Lightfoote MM. 1996. Cupric and ferric ions inactivate HIV. AIDS Res. Hum. Retrovir. 12:333–337 [DOI] [PubMed] [Google Scholar]
- 21. Takehara K, et al. 2010. Inactivation of avian influenza virus H1N1 by photocatalyst under visible light irradiation. Virus. Res. 151:102–103 [DOI] [PubMed] [Google Scholar]
- 22. Tobita K. 1975. Permanent canine kidney (MDCK) cells for isolation and plaque assay of influenza B virus. Med. Microbiol. Immunol. 162:23–27 [DOI] [PubMed] [Google Scholar]
- 23. Tomita H, Goto T, Shimada S, Takahashi K. 1996. Solution spinning of a high-Tc oxide superconductor: 5. The influence of yttrium and barium ions on the poly(vinyl alcohol)-copper(II) complex. Polymer 37:1071–1077 [Google Scholar]
- 24. Yamamoto K, Miyoshi-Koshio T, Utsuki Y, Mizuno S, Suzuki K. 1991. Virucidal activity and viral protein modification by myeloperozidase: a candidate for defense factor of human polymorphonuclear leukocytes against influenza virus infection. J. Infect. Dis. 164:8–14 [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto N, Hiatr CW, Haller W. 1964. Mechanism of inactivation of bacteriophages by metals. Biochim. Biophys. Acta 91:257–261 [DOI] [PubMed] [Google Scholar]
- 26. Yokoi H, Kawata S, Iwaizumi M. 1986. Interaction modes between heavy metal ions and water-soluble polymers. 1. Spectroscopic and magnetic reexamination of the aqueous solutions of cupric ions and poly(vinyl alcohol). J. Am. Chem. Soc. 108:3358–3361 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.