Abstract
Considering the increase in the consumption of yeasts as human probiotics, the aim of this study was to broadly investigate the beneficial properties of the lactic yeast Kluyveromyces marxianus (formerly Kluyveromyces fragilis) B0399. Several potential probiotic traits of K. marxianus B0399 were investigated by using in vitro assays, including adhesion and immune modulation, and the effect of the administration of 107 CFU/day of K. marxianus B0399 on the composition and metabolic activity of the human intestinal microbiota was investigated in a 3-stage continuous-culture system simulating the human colon. We demonstrated that this strain was highly adhesive to human enterocyte-like Caco-2 cells and modulated the immune response, inducing proinflammatory cytokines in peripheral blood mononuclear cells (PBMCs). In the presence of inflammatory stimulation with lipopolysaccharide (LPS), K. marxianus B0399 provoked decreases in the levels of production of proinflammatory cytokines in PBMCs and Caco-2 cells, thus ameliorating the inflammatory response. Furthermore, K. marxianus B0399 impacted the colonic microbiota, increasing the bifidobacterial concentration in the stages of the colonic model system simulating the proximal and transverse colon. The amounts of the short-chain fatty acids acetate and propionate also increased following yeast supplementation. Finally, K. marxianus B0399 was found to induce a decrease of the cytotoxic potential of the culture supernatant from the first stage of the colonic model system. The effects of K. marxianus B0399 on adhesion, immune function, and colonic microbiota demonstrate that this strain possesses a number of beneficial and strain-specific properties desirable for a microorganism considered for application as a probiotic.
INTRODUCTION
Increasing evidence is substantiating the utilization of beneficial microbes in functional foods, dairy products, or other dietary supplements aimed at maintaining and promoting human health (23). The consumer market for probiotic foods is >1.4 billion Euros, with an estimated annual growth of ∼7 to 8% for the period of 2008 to 2013 (41), and in particular, up to 20% of fermented dairy products contain probiotics (48). Probiotics have been demonstrated to exert health-promoting effects through several proposed mechanisms, including short-chain fatty acid (SCFA) production, the enhancement of the barrier function of the intestinal epithelium, the suppression of the growth and binding of pathogenic bacteria, and alterations of the immune activity of the host (1, 45). Furthermore, probiotics can alter colonic fermentation and stabilize symbiotic microbiota (42), improving the dynamic interplay between the resident bacterial community and the host.
Adhesion to the intestinal epithelium is an important requisite for allowing probiotics to modulate the immune system. Since the adhesion ability is strongly strain dependent, an evaluation of this characteristic is required as a selection criterion for novel probiotics (8). Probiotics can interact with mucosa-associated lymphoid tissues and bind to epithelial surface receptors, inducing humoral and cellular immune responses. The establishment and maintenance of a well-balanced ratio between pro- and anti-inflammatory cytokines are crucial for human health. Therefore, study of the dynamic cytokine modulation elicited by a microorganism represents a hot topic in the selection of novel probiotic strains. A wide strain-specific variation in the immune responses stimulated by probiotics has been described, and several in vitro cell models have been developed to evaluate their immunomodulatory effects (12). Even if these cellular models lack the complexity of the human immune system, they aid in elucidating the mechanisms involved in different means of bacterial sensing by human colonocytes and immunocompetent cells (4).
Functional foods commonly contain specific strains of lactic acid bacteria (LAB), belonging mainly to the genus Bifidobacterium or Lactobacillus. Less frequently used organisms are strains of Propionibacterium freudenreichii, bacilli, or yeasts (48). Kluyveromyces marxianus (formerly Kluyveromyces fragilis) is a lactic yeast isolated from different dairy products, mainly kefir (5, 15, 24). While the importance of this species in food development and fermentation is well documented, characterizations of its putative probiotic activities have been very limited (27, 38).
Due to the interest of the food industry in the selection of novel candidate probiotic strains, we evaluated for the first time the probiotic potential of K. marxianus B0399, a strain isolated from whey and curds of cow's milk and deposited at the Belgian Coordinated Collection of Microorganisms (BCCM) (accession number MUCL 41579). This strain is of particular interest for several reasons: (i) it is included in the European Food Safety Authority (EFSA) list of qualified presumption of safety (QPS) biological agents added to food and feed (13), (ii) it is included in different functional foods currently marketed in several countries, and (iii) it is capable of survival during gastric transit, maintaining its vitality and fermentation capacity (34).
In this study, K. marxianus B0399 was assessed for its ability to adhere to the human enterocyte-like Caco-2 cells. Furthermore, we evaluated its capacity to modulate the production of 27 immune mediators (cytokines, chemokines, and growth factors) in Caco-2 cells and peripheral blood mononuclear cells (PBMCs). Finally, the effect of the daily administration of 107 CFU of K. marxianus B0399 on the fecal microbiota of 2 individuals affected by irritable bowel syndrome (IBS) constipation was investigated by using a continuous-culture system simulating the human colon. IBS patients are generally considered an appropriate study group to support claims on gastrointestinal discomfort intended for the general population (14). The 3-stage continuous-culture colonic model system used in this study provides a controlled environment that can be maintained in a steady state and that simulates the complexity and diversity of the microbiota. Therefore, it represents a useful tool for monitoring the ecology and the metabolic activities of colonic microbiota in relation to different external perturbations (2, 28, 29). The main bacterial groups of the human intestinal microbiota, the production of SCFAs, principal end products of gut bacterial metabolism, and the cytotoxic activity of the fermentation supernatants were evaluated during the study.
MATERIALS AND METHODS
Culture conditions for K. marxianus B0399.
K. marxianus B0399 was routinely grown aerobically at 37°C in MV2 broth (40 g liter−1 lactose, 20 g liter−1 casein, 7.5 g liter−1 peptone, 1.5 g liter−1 yeast extract). The ability of K. marxianus B0399 to survive under the conditions of the colonic model was assessed by incubating 7.0 log CFU ml−1 of the actively growing culture in complex colonic model growth medium (CMGM) (29) at 37°C under anaerobic conditions for 24 h.
The resistance of the yeast strain in an environment simulating the upper gastrointestinal tract was further evaluated in vitro, as previously described by Maragkoudakis and colleagues (30). Briefly, an actively growing culture was harvested (10,000 × g for 5 min at 4°C) and washed twice in phosphate-buffered saline (PBS). Resistance to the environmental conditions of the stomach was assessed by resuspending the cell pellet (final concentration, 6.0 to 7.0 log CFU ml−1) in 0.1 mol liter−1 PBS adjusted with HCl to pH 2 containing pepsin (3 mg ml−1; Sigma-Aldrich, St. Louis, MO) and by evaluating the viable colony counts after 3 h of incubation at 37°C. Bile salt tolerance was tested by assessing colony viability after 3 h of incubation in MV2 broth supplemented with 0.3% (wt/vol) Oxgall (Sigma).
Eukaryotic cell culture conditions.
Human enterocyte-like Caco-2 cells were grown in Dulbecco's modified Eagle medium (DMEM) supplemented with 1.5 g liter−1 glucose, 10% heat-inactivated fetal calf serum (Cambrex, Walkersville, MD), 1% nonessential amino acids (Sigma), penicillin (50 IU ml−1), and streptomycin (50 μg ml−1) at 37°C in an atmosphere of 5% CO2. The growth medium was changed to fresh medium without the addition of antibiotics for the last 24 h of incubation prior to the performance of the immunoassay and the adhesion assays.
Human colon adenocarcinoma HT29 cells were grown in DMEM supplemented with 10% (wt/vol) fetal bovine serum, penicillin (50 IU ml−1), and streptomycin (50 μg ml−1) at 37°C in an atmosphere of 5% CO2.
PBMCs were isolated from healthy volunteers by density gradient centrifugation (Lymphoprep; Nycomed Pharma, Oslo, Norway). Cells were resuspended in RPMI 1640 culture medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% (wt/vol) fetal bovine serum (Thermo Fisher Scientific Inc., Waltham, MA) and 0.23 mmol liter−1 sodium pyruvate solution (Sigma). PBMCs (106 cells ml−1) were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Evaluation of adhesion of K. marxianus B0399 cells to Caco-2 cells by qPCR.
The adhesion of K. marxianus B0399 cells to Caco-2 cells was evaluated by the quantification of Caco-2 cell-bound microorganisms via quantitative PCR (qPCR), as reported previously by Candela and colleagues (6). Stationary-phase-grown cells of the yeast and bacterial strains were washed and resuspended at a cell density of approximately 8 log CFU ml−1 in DMEM. Caco-2 cells were washed with DMEM, and 1 ml of DMEM containing the yeast-bacterial suspension was added. After incubation for 1.5 h at 37°C under a humidified atmosphere, unattached yeast or bacteria were removed by washing the monolayers four times with sterile PBS. After the detachment of Caco-2 cells from the plastic surface by treatment (15 min at 37°C) with 200 μl trypsin-EDTA (Cambrex) per well, Caco-2 cells and adhesive yeast or bacteria were transferred into a 1.5-ml reaction tube. To quantify microbial cells by qPCR, cell suspensions were boiled for 5 min, and after mixing, an aliquot of 20 μl was transferred into a 0.2-ml reaction tube and incubated for 10 min at room temperature with 3.8 μl of trypsin inhibitor solution (type I-S from soybean, at 1 mg ml−1 in H2O). Strongly adhesive enterotoxigenic Escherichia coli strain H10407 and mildly adhesive Leuconostoc mesenteroides strain C5 were used as reference bacterial strains. The quantification of the reference bacterial strains was performed with E. coli species-specific primer set ECO-1/ECO-2 (47) and LAB-specific PCR primer set Bact-0011f/Lab-0677r (20), whereas yeast-specific primer set NL1/LS2 (8) was used to quantify K. marxianus B0399 cells. qPCR was performed with a LightCycler instrument (Roche, Mannheim, Germany), and a SYBR green I fluorophore was used to correlate the amount of PCR product with the fluorescence signal. The quantification of bacterial and yeast DNAs was carried out by using standard curves made from known concentrations of genomic DNA from the reference bacterial strains and K. marxianus B0399. The experimental protocol consisted of the following programs: (i) a starting preincubation step at 95°C for 10 min; (ii) amplification for 30 cycles of 4 steps each at a temperature transition rate of 20°C s−1, consisting of denaturation at 95°C for 15 s, annealing at 63°C (Bact-0011f/Lab-0677r) or 60°C (ECO-1/ECO-2 and NL1/LS2) for 25 s, extension at 72°C for 30 s, and fluorescence acquisition at 85°C (Bact-0011f/Lab-0677r and NL1/LS2) or 88°C (ECO-1/ECO-2) for 5 s; and (iii) melting-curve analysis consisting of heating at 20°C s−1 to 95°C, cooling at 20°C s−1 to 60°C with a 15-s hold, and then heating at 0.2°C s−1 to 99°C. Chromosomal DNAs of the strains used as standards were extracted by using a DNeasy tissue kit (Qiagen, Hilden, Germany) and were serially diluted from 106 to 103 CFU μl−1. The data reported represent mean values obtained in 3 to 5 independent experiments. Each experiment was performed in duplicate.
Immunoassay.
K. marxianus B0399 cells, corresponding to a concentration of 1 × 106 CFU ml−1, were applied to confluent Caco-2 cells or PBMCs (adjusted to a final concentration of 1 × 106 CFU ml−1) and incubated at 37°C for 24 h. Unstimulated cells were used as a negative control, whereas lipopolysaccharide (LPS) (1 μg ml−1; Sigma) was used to stimulate eukaryotic cells. After incubation, supernatants from Caco-2 cell and PBMC cultures were collected, centrifuged at 400 × g for 15 min at 4°C, and used to determine levels of several immune mediators by using a multiplexed bead immunoassay. Caco-2 cells and PBMCs were checked for viability by trypan blue exclusion.
The concentrations of 27 immune mediators (Table 1) were measured by using the Human Ultrasensitive Cytokine 27-plex antibody bead kit (Bio-Rad, Los Angeles, CA). The assays were performed with 96-well filter plates, as previously described (46). The concentrations of the samples were estimated from the standard curve by using a fifth-order polynomial equation and are expressed as picograms per milliliter after adjusting for the dilution factor (Bio-Plex Manager software, version 5.0). Samples below the detection limit of the assay were recorded as zero, while samples above the upper limit of quantification of the standard curves were assigned the highest value of the curve. The intra-assay coefficient of variation (CV) averaged 17%. Experiments were performed in triplicate. For each single determination, 50 beads were read, and the standard deviation was calculated.
Table 1.
Immune mediators evaluated in this study
| Immune mediators evaluateda | Chemical class |
|---|---|
| IL-1β, IL-1Ra, IL-2, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12(p70), IL-13, IL-15, IL-17, IFN-γ, TNF-α |
Cytokines |
| MCP-1, MIP-1α, MIP-1β, RANTES, eotaxin, IL-8, IP-10 | Chemokines |
| PDGF-BB, FGF basic, G-CSF, GM-CSF, VEGF | Growth factors |
MCP-1, monocyte chemotactic protein 1; RANTES, regulated upon activation, normal T-cell expressed and secreted; PDGF-BB, platelet-derived growth factor BB; FGF, fibroblast growth factor; G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte-macrophage colony-stimulating factor; VEGF, vascular endothelial growth factor.
Three-stage continuous-culture colonic model system.
The three-stage continuous-culture model of the human colon was comprised of 3 glass fermentors of increasing working volumes, simulating the proximal (vessel 1 [V1], 280 ml), transverse (V2, 300 ml), and distal (V3, 320 ml) colon. The 3 fermentors connected in series were kept at 37°C; the pH was stably maintained at pH 5.5 (V1), pH 6.2 (V2), and pH 6.8 (V3); and anaerobic conditions were ensured by continuous sparging with O2-free N2. V1 was fed with CMGM (29) by means of a peristaltic pump.
Human fecal samples were collected on site, kept in an anaerobic cabinet (10% H2, 10% CO2, 80% N2), and used within a maximum of 15 min after collection. This experiment was carried out in duplicate, using fecal samples from two different volunteers suffering from IBS constipation. None of the volunteers had received antibiotics or probiotics for at least 3 months before sampling. A 1:5 (wt/wt) fecal dilution in anaerobic PBS (0.1 mol liter−1 PBS [pH 7.4]) was prepared, and the samples were homogenized in a stomacher (Seward, Worthing, United Kingdom) for 2 min. Each stage of the colonic model was inoculated with 100 ml fecal slurry. The total system transit time was set at 72 h, according to the mean retention time for adults suffering from IBS constipation. Following inoculation, the colonic model was run as a batch culture for a 24-h period in order to stabilize bacterial populations prior to the initiation of the medium flow. After 24 h (time zero), the medium flow was initiated, and the system was run for 8 full volume turnovers to allow steady state to be achieved (steady state 1 [SS1]). Taking into account the operating volume (900 ml) and the retention time (72 h) of the colonic model system, 3 × 107 to 5 × 107 CFU of actively growing K. marxianus B0399 was added daily to V1. The yeast strain was added to the system as described above for a further 8 volume turnovers, after which SS2 was achieved. Each steady state was confirmed by sampling on three consecutive days for SCFAs and fluorescence in situ hybridization (FISH) analyses. Samples for FISH were immediately fixed in 4% paraformaldehyde as previously described (32). Samples for high-performance liquid chromatography (HPLC) and cytotoxicity analysis were centrifuged, and supernatants were frozen immediately, whereas cell pellets were resuspended in PBS-glycerol (1:1) and stored at −20°C prior to DNA extraction.
Evaluation of colonic microbiota composition by FISH, PCR-DGGE, and qPCR.
The concentrations of the main intestinal bacterial groups in samples from the colonic model system were evaluated by FISH, as previously described by Martín-Peláez and colleagues (32). The probes used are reported in Table 2 and were commercially synthesized and 5′ labeled with Cy3 fluorescent dye (Sigma).
Table 2.
Oligonucleotide probes used in this study for FISH analysis
| Target genus or group | Probe | Sequence (5′–3′) | Pretreatmentc | Hybridization-washing temp (°C) |
|---|---|---|---|---|
| Most bacteria | EUB338a | GCTGCCTCCCGTAGGAGT | None | 46–48 |
| Most bacteria | EUB338IIa | GCAGCCACCCGTAGGTGT | None | 46–48 |
| Most bacteria | EUB338IIIa | GCTGCCACCCGTAGGTGT | None | 46–48 |
| Atopobium, Collinsella, Olsenella, and Eggerthella spp.; Cryptobacterium curtum; Mycoplasma equigenitalium and Mycoplasma elephantis | Ato291 | GGTCGGTCTCTCAACC | None | 50–50 |
| Most Bacteroides sensu stricto and Prevotella spp., all Parabacteroides spp., Barnesiella viscericola, and Odoribacter splanchnicus | Bac303 | CCAATGTGGGGGACCTT | None | 46–48 |
| Most Bifidobacterium spp. | Bif164 | CATCCGGCATTACCACCC | None | 50–50 |
| Most of the Deltaproteobacteria and most of the Gemmatimonadetes | DELTA495ab | AGTTAGCCGGTGCTTCCT | 35% formamide | 50–50 |
| Some of the Deltaproteobacteria | DELTA495bb | AGTTAGCCGGCGCTTCCT | 35% formamide | 50–50 |
| Some of the Deltaproteobacteria | DELTA495cb | AATTAGCCGGTGCTTCCT | 35% formamide | 50–50 |
| Most members of Clostridium cluster XIVa; Syntrophococcus sucromutans, Bacteroides galacturonicus, Bacteroides xylanolyticus, Lachnospira pectinoschiza, and Clostridium saccharolyticum | Erec482 | GCTTCTTAGTCARGTACCG | None | 50–50 |
| Faecalibacterium prausnitzii and related sequences | Fprau655 | CGCCTACCTCTGCACTAC | None | 58–58 |
| Most Lactobacillus, Leuconostoc, and Weissella spp.; Lactococcus lactis; all Vagococcus, Enterococcus, Melissococcus, Tetragenococcus, Catellicoccus, Pediococcus, and Paralactobacillus spp. | Lab158 | GTATTAGCAYCTGTTTCCA | Lysozyme | 50–50 |
| Most members of Clostridium cluster I; all members of Clostridium cluster II; Clostridium tyrobutyricum; Adhaeribacter aquaticus and Flexibacter canadensis (family Flexibacteriaceae); Eubacterium combesii (family Propionibacteriaceae) | Chis150 | TTATGCGGTATTAATCTYCCTTT | None | 50–50 |
| Clostridium cluster IX | Prop853 | ATTGCGTTAACTCCGGCAC | None | 50–50 |
| Roseburia subcluster | Rrec584 | TCAGACTTGCCG(C/T)ACCGC | None | 50–50 |
These probes were used together in equimolar concentrations.
These probes were used together in equimolar concentrations.
Lysozyme treatment consisted of 100 U (20 μl of a 1-mg ml−1 solution of 50,000 U mg−1 protein).
The dynamics of the yeast population during the study were assessed by PCR-denaturing gradient gel electrophoresis (PCR-DGGE) and qPCR. Frozen samples recovered from the colonic model system were thawed, and aliquots (250 μl) were processed for DNA extraction as previously described by Maccaferri and colleagues (28). Approximately 250 nucleotides of the 5′-end region of the 26S rRNA gene were amplified by PCR using the yeast-universal primers NL1 (or GC-clamped NL1 for PCR-DGGE) and LS2, according to methods described previously by Cocolin and colleagues (8). The PCR-DGGE experimental protocol was slightly modified by performing annealing at 56°C for 25 s and extension at 72°C for 30 s, in order to prevent the cross-amplification of bacterial DNA. Band identities were confirmed by a comparison of the position in the gel length with those of reference yeast DNAs as well as by band excision, reamplification, and sequencing. qPCR was performed, as described above, by using standard curves made from known concentrations of the genomic DNA of K. marxianus B0399 in order to quantify modifications in the concentrations of yeasts during the experiment.
Determination of short-chain fatty acid concentrations by HPLC.
Samples from each vessel of the colonic model system were centrifuged at 13,000 × g for 10 min to remove bacterial cells and any particulate material. SCFA (acetate, propionate, and butyrate) and lactic acid concentrations were determined by HPLC on an Aminex HPX-87H column (300 by 7.8 mm; Bio-Rad). Degassed 5 mM H2SO4 was used as the eluent at a flow rate of 0.6 ml min−1 and at an operating temperature of 50°C. Organic acids were detected by UV at a wavelength of 220 nm. Sample quantification was carried out by using calibration curve standards for lactate, acetate, propionate, and butyrate at concentrations of 12.5, 25, 50, 75, and 150 mM. An internal standard of 20 mM 2-ethylbutyric acid was included in the samples and external standards.
Modulation of growth of HT29 cells by K. marxianus B0399.
The influence of colonic model supernatants, recovered before and after the administration of K. marxianus B0399, on the growth and survival of the HT29 human colon carcinoma cell line was determined by using a growth curve assay, as previously described by Maccaferri and colleagues (28). Results are expressed as the EC50, which represents the effective concentration of colonic model supernatants resulting in a 50% reduction of the cell number under the specified cell culture and treatment conditions compared to the growth of untreated cells.
Statistical analysis.
All data were analyzed by one-way analysis of variance (ANOVA), using Tukey's posttest analysis when the overall P value of the experiment was below the value of significance (P < 0.05). An additional paired t test was applied in order to assess the significance of results of single pairs of data. Analyses were performed by using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA).
RESULTS
Adhesion of K. marxianus B0399 cells to Caco-2 cells.
The ability of K. marxianus B0399 cells to adhere to Caco-2 cells was evaluated by qPCR. Notably, K. marxianus B0399 cells showed an adhesion value of 4.13 × 103 cells/100 Caco-2 cells, whereas the reference bacterial strains L. mesenteroides C5 (a mildly adhesive bacterial strain) and E. coli H10407 (a strongly adhesive bacterial strain) showed adhesion values of 7.61 × 102 and 10.56 × 104 cells/100 Caco-2 cells, respectively. According to criteria described previously by Candela and colleagues (6), who defined strongly adhesive strains as those with more than 40 cells adhered to 1 Caco-2 cell, K. marxianus B0399 can be classified as a strongly adhesive strain.
Immunomodulatory activity of K. marxianus B0399 toward PBMCs and Caco-2 cells.
The ability of K. marxianus B0399 to modulate the secretion of 27 immune mediators in PBMCs and Caco-2 cells was tested. Unstimulated cells were used as a negative control, while LPS-stimulated cells were used as a positive control.
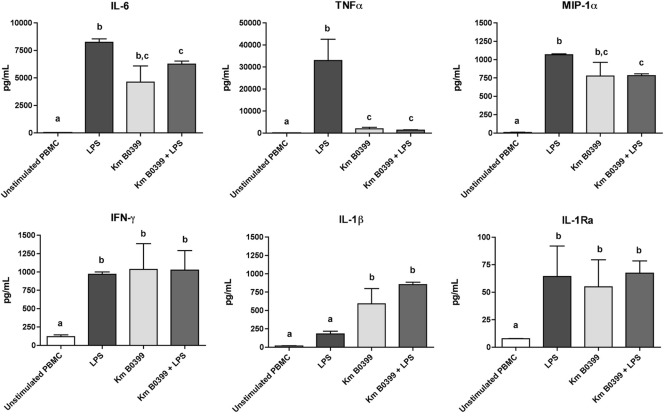
The incubation of PBMCs with K. marxianus B0399 cells provoked marked increases in the concentrations of the proinflammatory cytokines interleukin-1β (IL-1β), IL-6, gamma interferon (IFN-γ), macrophage inflammatory protein 1α (MIP-1α), and tumor necrosis factor alpha (TNF-α) and a moderate yet significant increase in the concentration of the anti-inflammatory cytokine IL-1 receptor antagonist (IL-1Ra). Conversely, when LPS was used to trigger an inflammatory response, coincubation with K. marxianus B0399 elicited significant decreases in the concentrations of the proinflammatory cytokines TNF-α, ΙL-6, and MIP-1α, whereas the concentration of IL-1β was significantly increased. No significant variations in the concentrations of IFN-γ and IL-1Ra were detected after coincubation (Fig. 1).
Fig 1.
Levels of immune mediators secreted by PBMCs after stimulation with LPS, stimulation with K. marxianus B0399 (Km B0399), and costimulation with LPS and the yeast strain (Km B0399 + LPS). Measurements were performed in triplicate. Results are means (picograms of immune mediators per milliliter of culture supernatant) ± standard errors of the means (SEM). Bars not sharing a common letter are significantly different at a confidence level of a P value of <0.05.
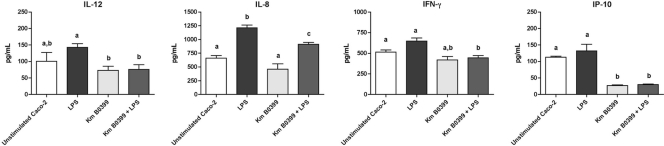
The incubation of Caco-2 cells with K. marxianus B0399 provoked a significant decrease in the level of secretion of the proinflammatory chemokine IFN-γ-induced protein 10 (IP-10). When K. marxianus B0399 was coincubated with LPS, it induced a significant decrease in the levels of secretion of the proinflammatory cytokines IP-10, IL-8, IL-12, and IFN-γ (Fig. 2).
Fig 2.
Levels of immune mediators secreted by Caco-2 cells whose concentrations significantly changed after stimulation with K. marxianus B0399 and costimulation with LPS and the yeast strain. Measurements were performed in triplicate. Results are means (picograms of immune mediators per milliliter of culture supernatant) ± SEM. Bars not sharing a common letter are significantly different at a confidence level of a P value of <0.05.
The production of the other immune modulators by PBMCs and Caco-2 cells was not significantly modulated by K. marxianus B0399 under all the tested conditions (data not shown).
Survival of K. marxianus B0399 under simulated gastrointestinal conditions.
K. marxianus B0399 was confirmed to survive under simulated gastric conditions, since incubation for 3 h at pH 2 in the presence of pepsin provoked a moderate decrease of yeast viability, from an initial concentration of 6.90 log CFU ml−1 to a final value of 4.97 log CFU ml−1. Similarly, survival was maintained when the strain was incubated for 3 h with a physiological concentration of bile salts, with a slight decrease from 6.96 log CFU ml−1 to 6.63 log CFU ml−1. K. marxianus B0399 was further able to grow anaerobically in the colonic model system medium CMGM, reaching a final concentration of 8.38 log CFU ml−1 after 24 h (data not shown).
Impact of K. marxianus B0399 on the composition of the colonic microbiota.
The total yeasts populations in each vessel of the colonic model system before (SS1) and after (SS2) the daily administration of K. marxianus B0399 were evaluated by PCR-DGGE. PCR-DGGE, the sensitivity of which (∼105 yeast cells ml−1) was not sufficient to detect yeasts in the fermentation system before the intervention, confirmed the presence of a clear band corresponding to K. marxianus in V1 and V2 at SS2 (99% identity with the K. marxianus 13MCHS 26S rRNA gene [see the supplemental material]). Furthermore, qPCR analysis confirmed that the total yeast population in V1, V2, and V3 at SS1 was below the detection limit of the method (2.5 × 102 CFU ml−1), while at SS2, yeasts reached the following concentrations: 3.7 × 107 ± 0.9 × 107 CFU ml−1 in V1, 6.1 × 104 ± 0.6 × 104 CFU ml−1 in V2, and <2.5 × 102 CFU ml−1 in V3.
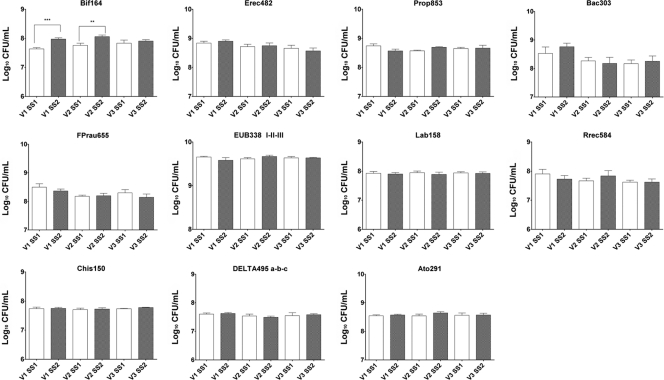
The concentrations of the main bacterial groups constituting the core of the human intestinal microbiota were assessed before and after supplementation with K. marxianus B0399 by FISH (Fig. 3). The administration of yeasts did not mediate any significant modification in the total bacterial counts (EUB338I, EUB338II, and EUB338III) during the intervention. FISH analysis showed that Clostridium cluster XIVa (Erec482) and cluster IX (Prop853) were the predominant bacterial groups in the colonic microbiota and that the addition of K. marxianus B0399 did not significantly influence (P > 0.05) their concentrations. A similar behavior was demonstrated for Bacteroides sp. (Bac303), Faecalibacterium prausnitzii (Fprau655), the subdominant lactic acid bacteria (Lab158), the Roseburia intestinalis-Eubacterium rectale group (Rrec584), Clostridium clusters I and II (Chis150), the Atopobium cluster (Ato291), and members of the Deltaproteobacteria (DELTA495a, DELTA495b, and DELTA495c), whose concentrations were stably maintained during the study.
Fig 3.
Bacterial groups detected by FISH in the culture broth recovered from each vessel (V1, V2, and V3) of the colonic model system before (SS1) and after (SS2) the daily administration of K. marxianus B0399. Results are reported as means of the data from two colonic models (log10 CFU/ml) ± SEM. For each colonic model, measurements were performed in triplicate at SS1 and SS2. ∗∗, P < 0.01; ∗∗∗, P < 0.001.
Notably, the administration of K. marxianus B0399 provoked a significant increase in the amounts of bacteria belonging to the health-promoting genus Bifidobacterium (Bif164) in the first and second stages of the colonic model system (7.57 to 7.96 log CFU ml−1 in V1 [P = 0.0004] and 7.78 to 8.12 log CFU ml−1 in V2 [P = 0.009]).
Impact of K. marxianus B0399 on production of SCFAs.
SCFAs (acetate, propionate, and butyrate) and lactic acid in the three different stages of the colonic model systems, at SS1 and SS2, were detected and quantified by HPLC.
The administration of K. marxianus B0399 induced a significant increase in the concentrations of acetate (64.58 to 76.02 mM; P = 0.02) and propionate (57.42 to 70.16 mM; P = 0.0005) over the course of the experiment. In particular, the concentration of acetate increased significantly in the first stage of the colonic model system, whereas the concentration of propionate increased significantly in the first and second stages of the colonic model system (Table 3). Conversely, no significant modification of the lactate and butyrate concentrations occurred.
Table 3.
Concentrations of short-chain fatty acids recovered in each colonic model system vessel before and after administration of K. marxianus B0399 as assessed by HPLCa
| Metabolite | Mean concn (mM) ± SEM |
|||||
|---|---|---|---|---|---|---|
| V1 |
V2 |
V3 |
||||
| Before B0399 administration (SS1) | After B0399 administration (SS2) | Before B0399 administration (SS1) | After B0399 administration (SS2) | Before B0399 administration (SS1) | After B0399 administration (SS2) | |
| Lactate | 3.32 ± 0.11 | 3.40 ± 0.09 | 0.49 ± 0.24 | 0.96 ± 0.85 | 0.0 ± 0.0 | 1.52 ± 0.96 |
| Acetate | 58.79 ± 5.78 | 60.94 ± 1.79b | 69.23 ± 3.13 | 79.67 ± 6.20 | 68.01 ± 2.14 | 80.70 ± 8.24 |
| Propionate | 47.45 ± 1.77 | 63.09 ± 2.33c | 55.51 ± 2.55 | 72.69 ± 5.76b | 69.31 ± 4.89 | 74.71 ± 2.69 |
| Butyrate | 49.93 ± 11.53 | 40.43 ± 3.29 | 52.04 ± 6.70 | 50.12 ± 6.85 | 62.97 ± 5.29 | 61.58 ± 2.05 |
For each sample, measurements were performed in triplicate. Results are means (mM) of the measurements in the two colonic models ± standard errors of the means.
Significant differences between SS1 and SS2 at a confidence level of a P value of <0.05.
Significant differences between SS1 and SS2 at a confidence level of a P value of <0.01.
Cytotoxic effects of colonic model supernatants.
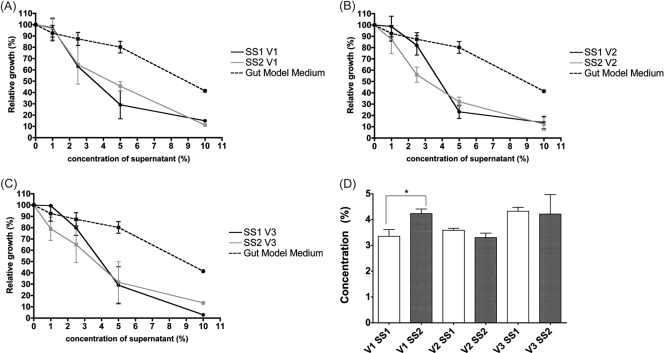
EC50 values were used to compare the effects of colonic model supernatants, before and after the administration of K. marxianus B0399, on HT29 cell growth (Fig. 4). No significant changes between EC50(SS1) and EC50(SS2) were found in the second and third stages of the colonic model system. Conversely, V1 colonic model supernatants after the administration of the yeast strain were significantly less cytotoxic than those at SS1 [EC50(SS1) of 3.35 versus EC50(SS2) of 4.23; P < 0.05].
Fig 4.
(A to C) Cytotoxic effect of supernatants recovered from vessel 1 (A), vessel 2 (B), and vessel 3 (C) of the colonic model system before (SS1) and after (SS2) the administration of K. marxianus B0399. Cytotoxicity was assessed by coincubating HT29 cells with increasing concentrations (0%, 1%, 2.5%, 5%, and 10%) of fermentation supernatants, followed by staining with DAPI (4′,6-diamidino-2-phenylindole). Results are expressed as means of relative HT29 cell growths (percent) of 2 colonic models ± SEM. For each colonic model, measurements were performed in triplicate. EC50 values were calculated from the growth curves shown in panels A, B, and C for SS1 and SS2. (D) Comparison of EC50 values at SS1 and SS2 for each vessel. ∗, P < 0.05.
DISCUSSION
In recent years, an evolving number of studies suggested that the administration of probiotics plays a role in the promotion of human health. In the present study, we assessed the probiotic potential of the food-grade yeast strain K. marxianus B0399, investigating a number of traits, such as (i) adhesion to the intestinal epithelium, (ii) modulation of the immune response, (iii) impact on the composition and fermentation potential of the human colonic microbiota, and (iv) modulation of the cytotoxicity of the microbiota metabolites.
Using Caco-2 cells, a largely accepted in vitro model, we demonstrated that K. marxianus B0399 is a strongly adhesive strain. It is noteworthy that the health-promoting effects of probiotic strains might be partly dependent on their persistence in the intestine and adhesion to mucosal surfaces (10).
A further important characteristic of potential probiotic candidates is the capacity to modulate the immune response of the host. In fact, a finely tuned balance between immune responses and tolerance to the gut microbiota is required at the edge of the colonic epithelium for preventing intestinal inflammation. Several in vitro and in vivo studies demonstrated two main effects of probiotics on host immunity: (i) strengthening the immunological barrier by stimulating the development and maintaining the state of alert of the innate and adaptive immune system, and (ii) decreasing the immune responsiveness to unbalanced inflammatory conditions. Both of these health-promoting activities are accomplished through an effective modulation of the balance of pro- and anti-inflammatory cytokine production (44). Many probiotic species have been demonstrated to share a relatively common immune pattern, such as a reduction in Th2 cytokines (i.e., IL-4, IL-5, IL-6, IL-10, and IL-13) or a shift toward Th1-mediated immunity (i.e., IL-2, TNF-α, and IFN-γ production). However, distinctive effects are often strain specific, and therefore, the assessment of the immune potential of novel probiotics is a challenging research area in food microbiology (9, 12). Nowadays, very little is known about the immune potential of the genus Kluyveromyces (38). In the present study, we evaluated the immunomodulatory potential of K. marxianus B0399 toward human PBMCs and Caco-2 cells. In PBMCs, K. marxianus B0399 induced the production of the proinflammatory cytokines IL-1β, TNF-α, IFN-γ, and IL-6, which are known to play a crucial role in host defense mechanisms. A similar overproduction of IL-6 and TNF-α was demonstrated for PBMCs exposed to well-established probiotic strains of lactobacilli, streptococci, Leuconostoc spp., and Bifidobacterium breve (18, 26, 43). Notably, when K. marxianus B0399 was coincubated with LPS, the concentrations of TNF-α and IL-6 decreased to values similar to those detected in yeast-stimulated PBMCs without LPS. These data are in agreement with previous findings which demonstrated that probiotic Lactobacillus rhamnosus and Lactobacillus gasseri strains were capable of diminishing the release of TNF-α, IL-6, and IFN-γ in LPS-stimulated macrophages and PBMCs in a different manner (33, 36). Interestingly, a similar behavior was also determined by using the in vitro model system of Caco-2 cells. In fact, in the presence of costimulation with K. marxianus B0399 and LPS, significant decreases in the concentrations of the proinflammatory cytokines IFN-γ and IL-12 and the chemokines IP-10 and IL-8 were demonstrated. In particular, a decreased level of production of IL-8 in response to inflammatory stimuli (LPS, TNF-α, IL-1β, and enteropathogenic bacteria) was described previously for a wide array of probiotic bacteria (7, 17, 25). Indeed, a massive and protracted release of IL-8 by colonocytes, associated with enteropathogenic infections, leads to persistent inflammation and epithelial barrier dysfunction (40).
The ability of K. marxianus strains to modulate the composition and the functional activity of the human intestinal ecosystem is poorly understood. In this perspective, we aimed at investigating how the administration of K. marxianus B0399 impacts the gut ecosystem using a three-stage colonic model system that simulates the human colon.
The administration of yeasts did not mediate any significant modification of the total bacterial counts or of the concentrations of the predominant and subdominant bacterial groups. Notably, the administration of K. marxianus B0399 provoked significant increases in levels of bacteria belonging to the health-promoting genus Bifidobacterium in the first and second stages of the colonic model system, which simulate the proximal and transverse colon. While the metabolic potential of K. marxianus in the human gut has not been fully explored, it was reported previously that this yeast can improve the growth and survival of bifidobacteria in complex food matrices (37). Indeed, it was described previously that LAB growth can be stimulated by vitamins or amino acids produced by yeasts (39). Furthermore, it cannot be excluded that a small fraction of the K. marxianus B0399 cells added to the colonic model partially autolyses, releasing polysaccharides such as glucan and mannan, the main constituents of the yeast cell wall. These polysaccharides can be converted into oligosaccharides, which are known to stimulate the growth of Bifidobacterium spp. in human and animal intestines (3).
The administration of K. marxianus B0399 induced significant increases in the concentrations of acetate and propionate over the course of the experiment. SCFAs, the main end products of carbohydrate fermentation, have been demonstrated to play a pivotal role in the physiology and metabolism of the human colon. In particular, they provide energy for intestinal colonocytes and promote epithelial cell growth (22). Although the fermentation capability of K. marxianus to produce acetate has been described (16), the increase in the concentration of acetate is consistent with a yeast-mediated modification of the composition of the colonic microbiota, since Bifidobacterium spp. are principal producers of acetate (22). The relevant increase in the acetate concentration in the colonic model system represents a valuable endpoint of probiotic supplementation, since decreased levels of acetate and propionate have been correlated with gut metabolic profiles of patients affected by a variety of functional gastrointestinal disorders (21, 31).
Finally, we demonstrated that the administration of K. marxianus B0399 modulated a decrease in the cytotoxic potential of the culture supernatant from the first vessel of the colonic model system. Our results are in agreement with those reported in the literature, which showed that an alteration of the gut microbiota related to probiotic consumption may alter various parameters of fecal water activity by reducing toxicity (35). The aqueous phase of human feces contains bioactive compounds that are likely to interact with colonic epithelial cells. These compounds include potentially harmful components, such as bile acids, fatty acids, N-nitroso compounds, and heterocyclic amines, as well as compounds that are potentially beneficial, such as polyphenols and SCFAs (35). Indeed, the cytotoxicity of fecal water has been reported to be a risk biomarker, since several studies correlated the toxicity of this fecal fraction with a higher level of colonic cell proliferation and an increased risk of colon cancer (11, 19).
In conclusion, the effects of K. marxianus B0399 on adhesion, immune function, and the colonic microbiota demonstrate that this strain possesses a number of beneficial and strain-specific properties desirable for a microorganism considered for application as a probiotic.
Supplementary Material
ACKNOWLEDGMENT
P.C. is a member of the Research Department of Coop Italia, which supplied the yeast strain evaluated in this study.
Footnotes
Published ahead of print 9 December 2011
Supplemental material for this article may be found at http://aem.asm.org/.
REFERENCES
- 1. Aragon G, Graham DB, Borum M, Doman DB. 2010. Probiotic therapy for irritable bowel syndrome. Gastroenterol. Hepatol. 6:39–44 [PMC free article] [PubMed] [Google Scholar]
- 2. Bahrami B, Child MW, Macfarlane S, Macfarlane GT. 2011. Adherence and cytokine induction in Caco-2 cells by bacterial populations from a three-stage continuous-culture model of the large intestine. Appl. Environ. Microbiol. 77:2934–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Belem MAF, Lee BH. 1998. Production of bioingredients from Kluyveromyces marxianus grown on whey: an alternative. Crit. Rev. Food Sci. Nutr. 38:565–598 [DOI] [PubMed] [Google Scholar]
- 4. Boirivant M, Strober W. 2007. The mechanism of action of probiotics. Curr. Opin. Gastroenterol. 23:679–692 [DOI] [PubMed] [Google Scholar]
- 5. Bolla PA, de los Angeles Serradell M, de Urraza PJ, De Antoni GL. 2011. Effect of freeze-drying on viability and in vitro probiotic properties of a mixture of lactic acid bacteria and yeasts isolated from kefir. J. Dairy Res. 78:15–22 [DOI] [PubMed] [Google Scholar]
- 6. Candela M, et al. 2005. Real-time PCR quantification of bacterial adhesion to Caco-2 cells: competition between bifidobacteria and enteropathogens. Res. Microbiol. 156:887–895 [DOI] [PubMed] [Google Scholar]
- 7. Candela M, et al. 2008. Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int. J. Food Microbiol. 125:286–292 [DOI] [PubMed] [Google Scholar]
- 8. Cocolin L, Bisson LF, Mills DA. 2000. Direct profiling of the yeast dynamics in wine fermentations. FEMS Microbiol. Lett. 189:81–87 [DOI] [PubMed] [Google Scholar]
- 9. Collado MC, Surono IS, Meriluoto J, Salminen S. 2007. Potential probiotic characteristics of Lactobacillus and Enterococcus strains isolated from traditional dadih fermented milk against pathogen intestinal colonization. J. Food Prot. 70:700–705 [DOI] [PubMed] [Google Scholar]
- 10. Collado MC, Isolauri E, Salminen S, Sanz Y. 2009. The impact of probiotic on gut health. Curr. Drug Metab. 10:68–78 [DOI] [PubMed] [Google Scholar]
- 11. de Kok TM, van Maanen JM. 2000. Evaluation of fecal mutagenicity and colorectal cancer risk. Mutat. Res. 463:53–101 [DOI] [PubMed] [Google Scholar]
- 12. Delcenserie V, et al. 2008. Immunomodulatory effects of probiotics in the intestinal tract. Curr. Issues Mol. Biol. 10:37–54 [PubMed] [Google Scholar]
- 13. EFSA Panel on Biological Hazards 2010. Scientific opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed. EFSA J. 8:1944 [Google Scholar]
- 14. EFSA Panel on Dietetic Products Nutrition Allergies 2011. Guidance on the scientific requirements for health claims related to gut and immune function. EFSA J. 9:1984 [Google Scholar]
- 15. Farnworth ER. 2005. Kefir: a complex probiotic. Food Sci. Technol. Bull. 2:1–17 [Google Scholar]
- 16. Fonseca GG, Gombert AK, Heinzle E, Wittmann C. 2007. Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res. 7:422–435 [DOI] [PubMed] [Google Scholar]
- 17. Frick JS, et al. 2007. Identification of commensal bacterial strains that modulate Yersinia enterocolitica and dextran sodium sulfate-induced inflammatory responses: implications for the development of probiotics. Infect. Immun. 75:3490–3497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gaudana SB, Dhanani AS, Bagchi T. 2010. Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Br. J. Nutr. 103:1620–1628 [DOI] [PubMed] [Google Scholar]
- 19. Glinghammar B, Venturi M, Rowland IR, Rafter JJ. 1997. Shift from a dairy product-rich to a dairy product-free diet: influence on cytotoxicity and genotoxicity of fecal water—potential risk factors for colon cancer. Am. J. Clin. Nutr. 66:1277–1282 [DOI] [PubMed] [Google Scholar]
- 20. Heilig HG, et al. 2002. Molecular diversity of Lactobacillus spp. and other lactic acid bacteria in the human intestine as determined by specific amplification of 16S ribosomal DNA. Appl. Environ. Microbiol. 68:114–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huda-Faujan N, et al. 2010. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 4:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jacobs DM, Gaudier E, van Duynhoven J, Vaughan EE. 2009. Non-digestible food ingredients, colonic microbiota and the impact on gut health and immunity: a role for metabolomics. Curr. Drug Metab. 10:41–54 [DOI] [PubMed] [Google Scholar]
- 23. Jankovic I, Sybesma W, Phothirat P, Ananta E, Mercenier A. 2010. Application of probiotics in food products—challenges and new approaches. Curr. Opin. Biotechnol. 21:175–181 [DOI] [PubMed] [Google Scholar]
- 24. Jianzhong Z, Xiaoli L, Hanhu J, Mingsheng D. 2009. Analysis of the microflora in Tibetan kefir grains using denaturing gradient gel electrophoresis. Food Microbiol. 26:770–775 [DOI] [PubMed] [Google Scholar]
- 25. Kamada N, et al. 2008. Nonpathogenic Escherichia coli strain Nissle 1917 inhibits signal transduction in intestinal epithelial cells. Infect. Immun. 76:214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kekkonen RA, et al. 2008. Probiotic Leuconostoc mesenteroides ssp. cremoris and Streptococcus thermophilus induce IL-12 and IFN-γ production. World J. Gastroenterol. 14:1192–1203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumura H, Tanoue Y, Tsukahara M, Tanaka T, Shimazaki K. 2004. Screening of dairy yeast strains for probiotic applications. J. Dairy Sci. 87:4050–4056 [DOI] [PubMed] [Google Scholar]
- 28. Maccaferri S, et al. 2010. Rifaximin modulates the colonic microbiota of patients with Crohn's disease: an in vitro approach using a continuous culture colonic model system. J. Antimicrob. Chemother. 65:2556–2565 [DOI] [PubMed] [Google Scholar]
- 29. Macfarlane G, Macfarlane S, Gibson GR. 1998. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb. Ecol. 35:180–187 [DOI] [PubMed] [Google Scholar]
- 30. Maragkoudakis PA, et al. 2006. Probiotic potential of Lactobacillus strains isolated from dairy products. Int. Dairy J. 16:189–199 [Google Scholar]
- 31. Marchesi JR, et al. 2007. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J. Proteome Res. 6:546–551 [DOI] [PubMed] [Google Scholar]
- 32. Martín-Peláez S, et al. 2008. In vitro fermentation of carbohydrates by porcine faecal inocula and their influence on Salmonella Typhimurium growth in batch culture systems. FEMS Microbiol. Ecol. 66:608–619 [DOI] [PubMed] [Google Scholar]
- 33. Matsumoto S, et al. 2005. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. Clin. Exp. Immunol. 140:417–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mustacchi G, et al. 2010. Trial #130.1: verification of the capacity of colonization of the gastrointestinal tract in healthy subjects, after the utilization of the lactic yeast Kluyveromyces marxianus fragilis B0399, through examination of the feces. Turval, Udine, Italy: http://www.turval.com/research/humans_and_nutrition/ [Google Scholar]
- 35. Pearson JR, Gill CI, Rowland IR. 2009. Diet, fecal water, and colon cancer—development of a biomarker. Nutr. Rev. 67:509–526 [DOI] [PubMed] [Google Scholar]
- 36. Peña JA, Versalovic J. 2003. Lactobacillus rhamnosus GG decreases TNF-alpha production in lipopolysaccharide-activated murine macrophages by a contact-independent mechanism. Cell. Microbiol. 5:277–285 [DOI] [PubMed] [Google Scholar]
- 37. Rada V. 1997. Effect of Kluyveromyces marxianus on the growth and survival of bifidobacteria in milk. Folia Microbiol. 42:145–148 [DOI] [PubMed] [Google Scholar]
- 38. Romanin D, et al. 2010. Down-regulation of intestinal epithelial innate response by probiotic yeasts isolated from kefir. Int. J. Food Microbiol. 140:102–108 [DOI] [PubMed] [Google Scholar]
- 39. Roostita R, Fleet GH. 1996. Growth of yeasts in milk and associated changes to milk composition. Int. J. Food Microbiol. 31:205–219 [DOI] [PubMed] [Google Scholar]
- 40. Roselli M, Finamore A, Britti MS, Mengheri E. 2006. Probiotic bacteria Bifidobacterium animalis MB5 and Lactobacillus rhamnosus GG protect intestinal Caco-2 cells from the inflammation-associated response induced by enterotoxigenic Escherichia coli K88. Br. J. Nutr. 95:1177–1184 [DOI] [PubMed] [Google Scholar]
- 41. Saxelin M. 2008. Probiotic formulations and applications, the current probiotics market, and changes in the marketplace: a European perspective. Clin. Infect. Dis. 46:S76–S79 [DOI] [PubMed] [Google Scholar]
- 42. Spiller R. 2008. Probiotics and prebiotics in irritable bowel syndrome. Aliment. Pharmacol. Ther. 28:385–396 [DOI] [PubMed] [Google Scholar]
- 43. Timmerman HM, et al. 2007. Design of a multispecies probiotic mixture to prevent infectious complications in critically ill patients. Clin. Nutr. 26:450–459 [DOI] [PubMed] [Google Scholar]
- 44. Vanderpool C, Yan F, Polk DB. 2008. Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 14:1585–1596 [DOI] [PubMed] [Google Scholar]
- 45. Ventura M, et al. 2009. Genome-scale analyses of health-promoting bacteria: probiogenomics. Nat. Rev. Microbiol. 7:61–71 [DOI] [PubMed] [Google Scholar]
- 46. Vignali DA. 2000. Multiplexed particle-based flow cytometric assays. J. Immunol. Methods 243:243–255 [DOI] [PubMed] [Google Scholar]
- 47. Wang RF, Cao WW, Cerniglia CE. 1996. PCR detection and quantification of predominant anaerobic bacteria in human and animal fecal samples. Appl. Environ. Microbiol. 62:1242–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wassenaar TM, Klein G. 2008. Safety aspects and implications of regulation of probiotic bacteria in food and food supplements. J. Food Prot. 7:1734–1741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.