Abstract
An Escherichia coli library comprising 8,424 strains incorporating gene fragments of the equol-producing bacterium Slackia sp. strain NATTS was constructed and screened for E. coli strains having daidzein- and dihydrodaidzein (DHD)- metabolizing activity. We obtained 3 clones that functioned to convert daidzein to DHD and 2 clones that converted DHD to equol. We then sequenced the gene fragments inserted into plasmids contained by these 5 clones. All of the gene fragments were contiguous, encoding three open reading frames (ORF-1, -2, and -3). Analysis of E. coli strains containing an expression vector incorporating one of the orf-1, -2, or -3 genes revealed that (i) the protein encoded by orf-1 was involved in the conversion of cis/trans-tetrahydrodaidzein (cis/trans-THD) to equol, (ii) the protein encoded by orf-2 was involved in the conversion of DHD to cis/trans-THD, and (iii) the protein encoded by orf-3 was involved in the conversion of daidzein to DHD. ORF-1 had a primary amino acid structure similar to that of succinate dehydrogenase. ORF-2 was presumed to be an enzyme belonging to the short-chain dehydrogenase/reductase superfamily. ORF-3 was predicted to have 42% identity to the daidzein reductase of Lactococcus strain 20-92 and belonged to the NADH:flavin oxidoreductase family. These findings showed that the daidzein-to-equol conversion reaction in the Slackia sp. NATTS strain proceeds by the action of these three enzymes.
INTRODUCTION
Soybean isoflavones and their derivatives have been reported to prevent sex hormone-dependent diseases, such as prostate cancer, breast cancer, menopausal disorders, premenstrual syndrome, and osteoporosis (3, 9, 10, 16, 21, 30). The isoflavone equol is expected to prevent hormone-dependent diseases, such as prostate cancer, because of its ability to bind to dihydrotestosterone and its high capacity to bind to estrogen receptor β; moreover, it is the most potent antioxidant of all the isoflavones (1, 2, 5, 17, 23).
To date, several bacteria capable of producing equol have been isolated from human or animal feces (18–20, 29, 31). Many of these strains are suggested to first metabolize daidzein as a substrate to dihydrodaidzein (DHD) and to then metabolize DHD to equol. Recently, daidzein reductase, which converts daidzein to DHD, has been purified from the equol-producing Lactococcus strain 20-92 (25). On the other hand, it has been suggested that, in the Eggerthella strain Julong 732, DHD is converted to equol by the production of cis/trans-tetrahydrodaidzein (cis/trans-THD) as an intermediate metabolite (13, 14). These studies have therefore suggested that daidzein is converted to equol via DHD and cis/trans-THD. However, the details of the enzymes involved in the production of equol from daidzein, and of the genes encoding them, remain largely unknown.
We have recently isolated Slackia sp. strain NATTS, which has potent daidzein-to-equol conversion ability, from healthy human feces (26). This strain has a more potent daidzein-equol conversion activity than the other equol-producing strains previously reported (26). This paper identifies the genes in Slackia sp. strain NATTS responsible for the daidzein-to-equol conversion reaction and examines the function of the enzymes encoded by such genes.
MATERIALS AND METHODS
Bacteria, culture medium, and plasmid.
Slackia sp. strain NATTS was cultured on modified Gifu anaerobic medium (GAM) agar (Nissui Pharmaceutical Co., Ltd., Tokyo, Japan) supplemented with 1% (wt/vol) glucose. Escherichia coli JM109 (TaKaRa Bio, Osaka, Japan) was cultured on Luria-Bertani (LB) medium. For construction of the genomic library of Slackia sp. strain NATTS, the plasmids pUC19 (TaKaRa Bio) and pQE30Xa (Qiagen, Valencia, CA) as the expression vectors for the recombination enzymes were used. The amount of ampicillin added to the E. coli culture was 100 μg/ml.
Construction of genomic library.
Chromosomal DNA was purified from Slackia sp. strain NATTS as previously reported (26). Purified chromosomal DNA was partially digested with MboI (Toyobo, Osaka, Japan), and the resulting partially digested DNA and pUC19 completely digested with MboI were ligated by using a ligation convenience kit (NIPPON GENE Co., Ltd., Tokyo, Japan). pUC19 into which the genome fragment had been inserted was transformed into E. coli JM109 to yield recombinants.
Screening of daidzein metabolism-related genes.
A total of 8,424 recombinants into which the genome fragment had been inserted were inoculated onto 1 ml of GAM broth containing 100 μg/ml ampicillin and 100 μM daidzein (Fujicco Co., Ltd., Osaka, Japan) or DHD (Toronto Research Chemicals Inc., Ontario, Canada); the broth was then cultured at 37°C for 24 h under anaerobic conditions. Isoflavone was extracted from each culture medium and quantified by high-performance liquid chromatography (HPLC). Extraction and quantification of isoflavone were performed as previously described (26). Briefly, 100 μl diethyl ether was added to 200 μl medium, and the mixture was centrifuged at 1,000 × g for 10 min. Then, the upper layer was dehydrated thoroughly at 40°C under a stream of nitrogen gas, and the precipitate was dissolved in 100 μl of 80% (vol/vol) methanol. After filtration, the filtrate was analyzed by HPLC under the following conditions: apparatus, LC Module 1 (Waters Corp., Milford, MA); column, YMC-Pack CN (Y.M.C. Co., Kyoto, Japan). Known amounts of daidzein (Fujicco Co.), DHD (Toronto Research Chemicals Inc.), THD (Apin Chemicals Limited, Abingdon, United Kingdom), and equol (Extrasynthése S.A., Genay, France) were used as isoflavone standards.
Determination and analysis of DNA sequences.
For cycle sequencing PCR, an ABI PRISM BigDye Terminator version 3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA) was used. The 20-μl reaction mixture contained 1 μl of purified plasmid (30 ng) extracted from E. coli, 1.6 μl BigDye Terminator premix, and 8.0 pmol M13R or M13F primer (TaKaRa Bio). Cycle sequencing PCR was performed at an initial denaturation at 96°C for 1 min, followed by 25 cycles of denaturation at 96°C for 10 s, annealing at 50°C for 5 s, and extension at 60°C for 4 min. The cycle sequencing PCR products were purified by ethanol precipitation, and the precipitate was dissolved in 15 μl of Hi-Di formamide (Applied Biosystems) and sequenced by using an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems). The nucleotide sequences were analyzed with the bio-informatics software program Genetyx version 9 (Genetix Co., Ltd., Tokyo, Japan). For analysis of nucleotide sequences and amino acid sequences, DDBJ-BLAST (http://www.ddbj.nig.ac.jp/), BPROM (SoftBerry), GeneMark version 2.5 (http://opal.biology.gatech.edu/GeneMark/), FindTerm (SoftBerry), and PSORT (http://psort.ims.u-tokyo.ac.jp/) were used.
Analysis of expression of open reading frame 1 (ORF-1), ORF-2, and ORF-3.
Plasmids pQESL-1, -2, and -3, into which the orf-1, orf-2, and orf-3 genes had been inserted, were generated by using the following procedures. Using as a template genomic DNA extracted from strain NATTS, full-length orf-1, orf-2, and orf-3 genes were amplified by PCR with the following primer sets containing a BamHI digestion site: orf-1 gene, 5′-ATGGCCGAATTCGATGTTG-3′ (ORF1-F) and 5′-GGGGGATCCTAGTATGGGCGAAACCGTT-3′ (ORF1-R-BamHI); orf-2 gene, 5′-ATGACTACCATTCCTAAGCTCAAGG-3′ (ORF2-F) and 5′-GGGGGATCCTACTCAATTTCGCCCTGCATAG-3′ (ORF2-R-BamHI); and orf-3 gene, 5′-ATGCAGCACGCGAAATACCC-3′ (ORF3-F) and 5′-GGGGGATCCTAGATCATGCGCGCAACC-3′ (ORF3-R-BamHI). Each of the amplified products completely digested with BamHI was ligated to the StuI and BamHI sites of pQE30Xa (Qiagen) to generate plasmids pQESL-1, -2, and -3, each of which was transformed into E. coli JM109 to yield recombinants. Each recombinant was subjected to shaking culture at 37°C for 2 h on 3 ml of LB medium; the medium was then supplemented with isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 1 mM and subjected to another shaking culture at 37°C. The culture solution was centrifuged at 10,000 × g at 4°C for 10 min, and the resulting recombinant cells were suspended in 500 μl of 50 mM phosphate buffer (pH 7.0). The suspension and 0.3 g of glass beads (diameter, 0.1 mm; BioSpec Products, Inc., Bartlesville, OK) were added to a 2-ml tube, which was shaken violently with ShakeMaster AUTO (Biomedical Science, Tokyo, Japan) for 15 min. The resulting homogenate was centrifuged at 12,000 × g at 4°C for 10 min, and the supernatant was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
RESULTS
Identification of daidzein-metabolizing enzyme genes in Slackia sp. strain NATTS.
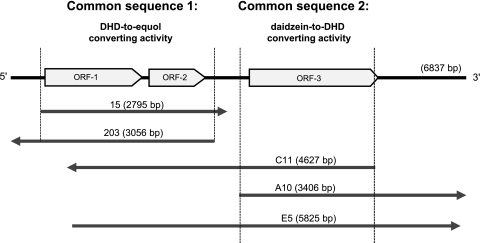
The E. coli library comprising 8,424 strains incorporating Slackia sp. strain NATTS genomic fragments was screened for in vivo daidzein-to-DHD conversion activity and DHD-to-equol conversion activity. Three clones with daidzein-to-DHD conversion activity (clones A-10, C-11, and E-5) and two clones (clones 15 and 203) with DHD-to-equol conversion activity were identified (Table 1). These enzyme activities were not observed in E. coli strains harboring only pUC19. The gene sequences of the Slackia sp. strain NATTS gene fragments inserted into the five E. coli strains obtained were decoded. Each strain carried an independent gene fragment, and clones 15 and 203 had a common sequence (common sequence 1) (Fig. 1). Also, clones A10, C11, and E5 had a common sequence (common sequence 2) (Fig. 1). Furthermore, clones 15, 203, C11, and E5 had a common domain. This suggested that the daidzein-metabolizing enzyme genes (daidzein-to-DHD- and DHD-to-equol-converting enzyme genes) were present as a series of clusters on the Slackia sp. strain NATTS genome.
Table 1.
Daidzein- or DHD-metabolizing properties in Escherichia coli A10, C11, E5, 15, and 203
| Clone and plasmid | Substrate (100 μM) | Isoflavone concn (μM)a |
Daidzein-to-DHD or DHD-to-equol % conversion | ||
|---|---|---|---|---|---|
| Daidzein | DHD | Equol | |||
| A10 | Daidzein | 5.4 ± 0.4 | 95.9 ± 4.5 | ND | 94.7 |
| DHD | NT | NT | NT | ||
| C11 | Daidzein | 3.9 ± 1.0 | 95.6 ± 1.3 | ND | 96.1 |
| DHD | NT | NT | NT | ||
| E5 | Daidzein | 3.9 ± 1.0 | 95.6 ± 1.3 | ND | 96.1 |
| DHD | NT | NT | NT | ||
| 15 | Daidzein | 92.4 ± 1.0 | ND | ND | |
| DHD | ND | 53.7 ± 0.7 | 40.5 ± 1.4 | 43.0 | |
| 203 | Daidzein | 90.8 ± 2.0 | ND | ND | |
| DHD | ND | 63.0 ± 0.4 | 21.3 ± 0.7 | 25.2 | |
| pUC19 | Daidzein | 106.1 ± 1.9 | ND | ND | |
| DHD | ND | 107.6 ± 1.6 | ND | ||
Data are expressed as means and standard deviations. NT, not tested; ND, not detected.
Fig 1.
Organization of the genetic fragment in clones expressing daidzein-to-DHD- and DHD-to-equol-converting activity, and cloning of fragments involved in the deduced open reading frames.
Characteristics of genes and amino acid sequences of daidzein-metabolizing enzymes.
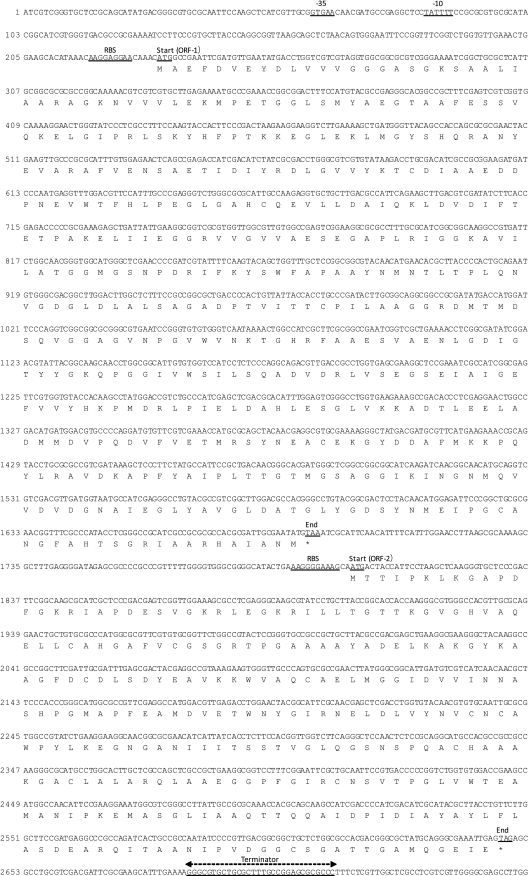
The orf-1 gene consisted of 1,458 nucleotides, with a promoter (−35 and −10), and a ribosome-binding site being located upstream and the terminator downstream (Fig. 2). The orf-1 gene encoded a polypeptide of 486 amino acids and a calculated molecular mass of 51.8 kDa. The orf-2 gene consisted of 846 nucleotides, with a ribosome-binding site being located upstream and the terminator downstream (Fig. 2). The orf-2 gene encoded a polypeptide of 282 amino acids and a calculated molecular mass of 29.4 kDa. Because no promoter region was found in the region between the orf-1 and orf-2 genes (108 bp), it was inferred that orf-1 and orf-2 were transcribed as polycistronic mRNA. The orf-3 gene consisted of 1,935 nucleotides, with −35, −10, and a ribosome-binding site located upstream and the terminator downstream. The orf-3 gene was therefore inferred to be transcribed as monocistronic mRNA. The orf-3 gene encoded a polypeptide of 644 amino acids and a calculated molecular mass of 70.1 kDa (Fig. 3). A homology search of the orf-1 gene products showed that these products were similar (32% to 35% identical) to the ORF annotated as a succinate dehydrogenase derived from several bacteria (Fig. 4). Analysis of the secondary structure of the amino acids suggested that ORF-1 was hydrophilic and was localized in the cytoplasmic compartment.
Fig 2.
Nucleotide sequences and deduced amino acids of components of DHD-to-equol-converting enzymes (ORF-1 and -2). The putative promoter, ribosome-binding site (RBS), terminator site, and start and stop codons are shown as underlined letters.
Fig 3.
Nucleotide sequences and deduced amino acids of the daidzein-to-DHD-converting enzyme (ORF-3). The putative promoter, ribosome-binding site (RBS), terminator site, and start and stop codons are shown as underlined letters.
Fig 4.
Sequence alignment of ORF-1 (A; accession no. AB646272) from Slackia sp. strain NATTS, succinate dehydrogenase (B; accession no. ZP_07333219) from Desulfovibrio fructosovorans, putative flavoprotein subunit of a reductase (C; accession no. ZP_03828868) from Pectobacterium carotovorum subsp. brasiliensis PBR1692, fumarate reductase/succinate dehydrogenase flavoprotein domain protein (D; accession no. YP_003505338) from Denitrovibrio acetiphilus DSM 12809, and succinate dehydrogenase (E; accession no. YP_001951188) from Geobacter lovleyi SZ. Identical amino acid residues are indicated in black boxes, and three-quarter-matched amino acid residues are in gray boxes.
A homology search of the orf-2 gene products showed that these products were similar (33% to 36% identical) to the ORF annotated as short-chain dehydrogenase/reductase derived from several bacteria (Fig. 5). Analysis of the primary structure of the amino acids showed that amino acid regions 36 to 42 contained an NADH/NADPH binding motif (GXXXGXG). Analysis of the secondary structure of amino acids showed that ORF-2 was hydrophilic and localized in the cytoplasmic compartment. The orf-3 gene products were, as amino acid sequences, 42% identical to daidzein reductase derived from Lactococcus strain 20-92. Furthermore, the orf-3 gene products were similar (32% to 34% identical) to the ORF annotated as NADH/NADPH oxidoreductase derived from several bacteria (Fig. 6). Analysis of the primary structure of amino acids revealed the following in ORF-3: a putative 4Fe-4S iron-sulfur cluster motif (CXXCX3CX12C) containing cysteine at residue 4 in amino acid domains 343 to 363 and an NADH/NADPH binding motif (GXGXXG) in amino acid domains 390 to 395. In addition, an old yellow enzyme (OYE)-like flavin mononucleotide (FMN) binding domain sequence was found at the N-terminal domain of this protein. Analysis of the secondary structure of the amino acids showed that ORF-3 was hydrophilic and was localized in the cytoplasmic compartment.
Fig 5.
Sequence alignment of ORF-2 (F; accession no. AB646272) from Slackia sp. strain NATTS; short-chain dehydrogenase/reductase (G; accession no. YP_003508312) from Meiothermus ruber DSM 1279; short-chain dehydrogenase/reductase (H; accession no. YP_001683547) from Caulobacter sp. K31; putative dehydrogenase (I; accession no. YP_002871585) from Pseudomonas fluorescens SBW25; and short-chain dehydrogenase/reductase (J; accession no. YP_003607655) from Burkholderia sp. CCGE1002. Identical amino acid residues are indicated in black boxes, and three-quarter-matched amino acid residues are in gray boxes. Consensus amino acid residues of the putative NADH/NADPH binding motif are indicated by underlining.
Fig 6.
Sequence alignment of ORF-3 (K; accession no. AB646272) from Slackia sp. strain NATTS, daidzein reductase (L; accession no. BAJ22678) from Lactococcus garvieae strain 20-92, NADH oxidase (M; accession no. ZP_06113274) from Clostridium hathewayi DSM 13479, NADH:flavin oxidoreductase/NADH oxidase (N; accession no. ZP_07547992) from Thermoanaerobacter wiegelii Rt8.B1, and NADH oxidase (O; accession no. P32382) from Thermoanaerobacter brockii. Identical amino acid residues are indicated in black boxes, and three-quarter-matched amino acid residues are in gray boxes. Consensus amino acids of the putative NADH/NADPH binding motif and the 4Fe-4S iron-sulfur cluster motif are indicated by underlining and broken underlining, respectively.
Analysis of expression of recombinant daidzein-metabolizing enzymes.
In the E. coli strains into which pQESL-1, pQESL-2, and pQESL-3 had been introduced, SDS-PAGE confirmed that a protein with a molecular mass corresponding to the size of each gene was expressed (Fig. 7). The recombinants in which protein expression was induced with IPTG were cultured at 37°C for 18 h in the presence of 100 μM daidzein or DHD under anaerobic conditions. Only the E. coli strains harboring pQELS-3 had daidzein-to-DHD conversion activity; DHD-to-equol conversion activity was observed in the copresence of the E. coli strain incorporating pQESL-1 and the E. coli strain incorporating pQESL-2 (Table 2). cis/trans-THD was detected both in the culture of the E. coli strain incorporating pQESL-2 and in the coculture of the E. coli strain incorporating pQESL-1 and the E. coli strain incorporating pQESL-2.
Fig 7.
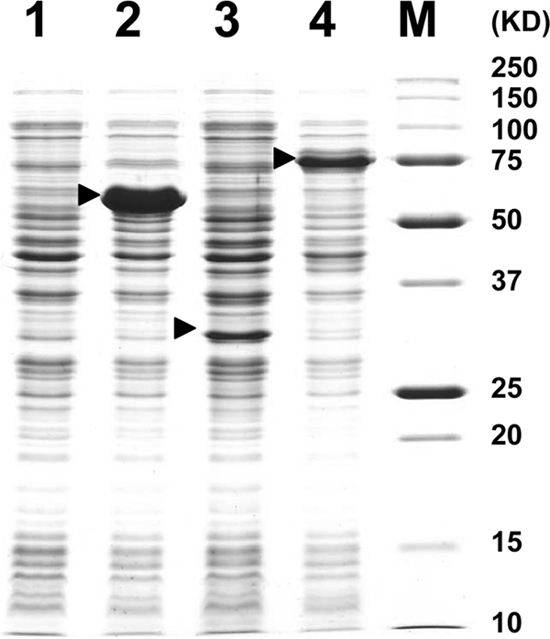
SDS-PAGE (12.5%) analysis of cell extracts from recombinant enzymes. 1, cell extract from pQE30Xa/E. coli JM109; 2, cell extract from pQESL-1/E. coli JM109; 3, cell extract from pQESL-2/E. coli JM109; 4, cell extract from pQESL-3/E. coli JM109; M, Precision Plus protein standard (Bio-Rad, Richmond, CA). Recombinant enzymes are indicated by arrows.
Table 2.
Daidzein- or DHD-metabolizing properties of clones
| Plasmid | Substrate (100 μM) | Isoflavone concn (μM)a |
Daidzein-to-DHD or DHD-to-equol % conversion | |||
|---|---|---|---|---|---|---|
| Daidzein | DHD | cis/trans-THD | Equol | |||
| pQESL-1 | DHD | ND | 97.4 ± 4.1 | ND | ND | |
| pQESL-2 | DHD | ND | 43.2 ± 5.7 | 53.6 ± 1.4 | ND | |
| pQESL-1 and pQESL-2 | DHD | ND | 38.7 ± 1.6 | 7.3 ± 1.9 | 53.0 ± 1.2 | 53.5 |
| pQESL-3 | Daidzein | 1.4 ± 2.4 | 94.5 ± 1.0 | ND | ND | 98.6 |
| pQE30Xa | Daidzein | 100.5 ± 3.6 | ND | ND | ND | |
| DHD | ND | 122 ± 3.3 | ND | ND | ||
Data are expressed as means and standard deviations. ND, not detected.
DISCUSSION
We showed that, in Slackia sp. NATTS strain, the daidzein-to-equol conversion reaction proceeded by the action of a series of three enzymes: ORF-3 of the Slackia sp. NATTS strain was responsible for daidzein-to-DHD conversion activity, and ORF-1 and ORF-2 were responsible for cis/trans-THD-to-equol and DHD-to-cis/trans-THD conversion activity, respectively. Furthermore, the genes encoding these three enzymes were present collectively in a specific region of the genome. As far as we are aware, this is the first evidence showing that this series of enzymes involved in daidzein-to-equol conversion reaction, as well as the genes encoding them, have been identified in a single strain. On an amino acid sequence level, ORF-3, which was responsible for the daidzein-to-DHD conversion reaction in Slackia sp. NATTS strain, was highly homologous to the daidzein reductase (25) derived from Lactococcus strain 20-92 (Fig. 6). ORF-3, like daidzein reductase, contains a 4Fe-4S iron-sulfur cluster motif comprising 4 cysteine residues, an NADH/NADPH binding motif, and an OYE-like FMN binding domain, suggesting that both of these enzymes belong to the NADH:flavin oxidoreductase family (4, 6), with similar reaction mechanisms.
However, whereas daidzein reductase derived from Lactococcus is presumed to be a membrane protein, ORF-3 was presumed to be a cytoplasmic protein, suggesting that their locations are different. Lactococcus and Slackia are bacteria belonging to different phyla; the former is found mainly in fish and animal milk and is rarely isolated from human (7, 24, 27, 28), whereas the latter inhabits the human intestines (11, 19, 22, 26). At present, although the phyletic evolution of these enzyme genes is unknown, the interbacterium propagation of the genes and their physiological roles in the bacteria are of interest.
This is the first report of the identification of 2 enzymes (ORF-1 and ORF-2) that catalyze the cis/trans-THD-to-equol and DHD-to-cis/trans-THD conversion reactions and their genes. Through a homology search of amino acid sequences, ORF-1 of Slackia sp. strain NATTS was shown to have a primary structure similar to that of the succinate dehydrogenase and fumarate reductase/succinate dehydrogenase flavoprotein domain protein derived from several bacteria, such as Desulfovibrio fructosovorans JJ and Geobacter lovleyi SZ (Fig. 4) However, these previously reported enzymes were among those found in a recent genomic sequencing, and only their functions have so far been inferred (8, 15). In addition, since the alignment analyses of the amino acid sequences of these enzymes and ORF-1 identified no consensus sequences, it is likely that the ORF-1 identified is a novel one involved in the metabolism of cis/trans-THD. On the other hand, our homology search and analysis of the primary structure of amino acids suggested that ORF-2 belongs to the short-chain dehydrogenase/reductase (SDR) superfamily (Fig. 5). The SDR family is a group of enzymes that catalyzes the oxidation-reduction reactions of steroids, cofactors, carbohydrates, lipids, aromatic compounds, and amino acids, using NADPH as an electron donor or acceptor (12). Until now, it was not known that the enzymes belonging to this family are involved in cis/trans-THD production; we have therefore suggested for the first time that this family is responsible for cis/trans-THD production.
Our analysis of the genes in Slackia sp. NATTS strain encoding the daidzein-to-equol conversion enzymes, and their genes, showed that the daidzein-to-equol conversion reaction proceeds by the action of three independent enzymes. These findings will lead to progress in enzymological studies of the daidzein-to-equol conversion reaction in enteric bacteria and in research on the ecology of equol-producing bacteria.
ACKNOWLEDGMENTS
We thank Takuya Takahashi, Katsuhisa Harada, Mitsuyoshi Kano (Yakult Central Institute for Microbiological Research), Kenji Oishi (Yakult Honsha European Research Center for Microbiology ESV), and Shigeyoshi Sakamoto (Yakult Honsha, Co., Ltd.) for their helpful discussions.
Footnotes
Published ahead of print 16 December 2011
REFERENCES
- 1. Akaza H, et al. 2004. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn. J. Clin. Oncol. 34:86–89 [DOI] [PubMed] [Google Scholar]
- 2. Akaza H, et al. 2002. Is daidzein non-metabolizer a high risk for prostate cancer? A case-controlled study of serum soybean isoflavone concentration. Jpn. J. Clin. Oncol. 32:296–300 [DOI] [PubMed] [Google Scholar]
- 3. Appt SE. 2004. Usefulness of the monkey model to investigate the role of soy in postmenopausal women's health. ILAR J. 45:200–211 [DOI] [PubMed] [Google Scholar]
- 4. Argyrou A, Blanchard JS. 2004. Flavoprotein disulfide reductases: advances in chemistry and function. Prog. Nucleic Acid Res. Mol. Biol. 78:89–142 [DOI] [PubMed] [Google Scholar]
- 5. Arora A, Nair MG, Strasburg GM. 1998. Antioxidant activities of isoflavones and their biological metabolites in a liposomal system. Arch. Biochem. Biophys. 356:133–141 [DOI] [PubMed] [Google Scholar]
- 6. Dym O, Eisenberg D. 2001. Sequence-structure analysis of FAD-containing proteins. Protein Sci. 10:1712–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eldar A, Goria M, Ghittino C, Zlotkin A, Bercovier H. 1999. Biodiversity of Lactococcus garvieae strains isolated from fish in Europe, Asia, and Australia. Appl. Environ. Microbiol. 65:1005–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Glasner JD, et al. 2008. Niche-specificity and the variable fraction of the Pectobacterium pan-genome. Mol. Plant Microbe Interact. 21:1549–1560 [DOI] [PubMed] [Google Scholar]
- 9. Ishiwata N, Melby MK, Mizuno S, Watanabe S. 2009. New equol supplement for relieving menopausal symptoms: randomized, placebo-controlled trial of Japanese women. Menopause 16:141–148 [DOI] [PubMed] [Google Scholar]
- 10. Jackman KA, Woodman OL, Sobey CG. 2007. Isoflavones, equol and cardiovascular disease: pharmacological and therapeutic insights. Curr. Med. Chem. 14:2824–2830 [DOI] [PubMed] [Google Scholar]
- 11. Jin JS, Kitahara M, Sakamoto M, Hattori M, Benno Y. 2010. Slackia equolifaciens sp. nov., a human intestinal bacterium capable of producing equol. Int. J. Syst. Evol. Microbiol. 60:1721–1724 [DOI] [PubMed] [Google Scholar]
- 12. Kavanagh KL, Jornvall H, Persson B, Oppermann U. 2008. Medium- and short-chain dehydrogenase/reductase gene and protein families: the SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell. Mol. Life Sci. 65:3895–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim M, et al. 2009. Stereospecific biotransformation of dihydrodaidzein into (3S)-equol by the human intestinal bacterium Eggerthella strain Julong 732. Appl. Environ. Microbiol. 75:3062–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim M, Marsh EN, Kim SU, Han J. 2010. Conversion of (3S,4R)-tetrahydrodaidzein to (3S)-equol by THD reductase: proposed mechanism involving a radical intermediate. Biochemistry 49:5582–5587 [DOI] [PubMed] [Google Scholar]
- 15. Kiss H, et al. 2010. Complete genome sequence of Denitrovibrio acetiphilus type strain (N2460). Stand. Genomic Sci. 2:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lukaczer D, et al. 2005. Clinical effects of a proprietary combination isoflavone nutritional supplement in menopausal women: a pilot trial. Altern. Ther. Health Med. 11:60–65 [PubMed] [Google Scholar]
- 17. Lund TD, et al. 2004. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol. Reprod. 70:1188–1195 [DOI] [PubMed] [Google Scholar]
- 18. Maruo T, Sakamoto M, Ito C, Toda T, Benno Y. 2008. Adlercreutzia equolifaciens gen. nov., sp. nov., an equol-producing bacterium isolated from human faeces, and emended description of the genus Eggerthella. Int. J. Syst. Evol. Microbiol. 58:1221–1227 [DOI] [PubMed] [Google Scholar]
- 19. Matthies A, Blaut M, Braune A. 2009. Isolation of a human intestinal bacterium capable of daidzein and genistein conversion. Appl. Environ. Microbiol. 75:1740–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minamida K, et al. 2008. Asaccharobacter celatus gen. nov., sp. nov., isolated from rat caecum. Int. J. Syst. Evol. Microbiol. 58:1238–1240 [DOI] [PubMed] [Google Scholar]
- 21. Mizunuma H, Kanazawa K, Ogura S, Otsuka S, Nagai H. 2002. Anticarcinogenic effects of isoflavones may be mediated by genistein in mouse mammary tumor virus-induced breast cancer. Oncology 62:78–84 [DOI] [PubMed] [Google Scholar]
- 22. Nagai F, Watanabe Y, Morotomi M. 2010. Slackia piriformis sp. nov. and Collinsella tanakaei sp. nov., new members of the family Coriobacteriaceae, isolated from human faeces. Int. J. Syst. Evol. Microbiol. 60:2639–2646 [DOI] [PubMed] [Google Scholar]
- 23. Rufer CE, Kulling SE. 2006. Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J. Agric. Food Chem. 54:2926–2931 [DOI] [PubMed] [Google Scholar]
- 24. Salama MS, Sandine WE, Giovannoni SJ. 1993. Isolation of Lactococcus lactis subsp. cremoris from nature by colony hybridization with rRNA probes. Appl. Environ. Microbiol. 59:3941–3945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shimada Y, et al. 2010. Cloning and expression of a novel NADP(H)-dependent daidzein reductase, an enzyme involved in the metabolism of daidzein, from equol-producing Lactococcus strain 20-92. Appl. Environ. Microbiol. 76:5892–5901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsuji H, Moriyama K, Nomoto K, Miyanaga N, Akaza H. 2010. Isolation and characterization of the equol-producing bacterium Slackia sp. strain NATTS. Arch. Microbiol. 192:279–287 [DOI] [PubMed] [Google Scholar]
- 27. Vela AI, et al. 2000. Phenotypic and genetic characterization of Lactococcus garvieae isolated in Spain from lactococcosis outbreaks and comparison with isolates of other countries and sources. J. Clin. Microbiol. 38:3791–3795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang CY, et al. 2006. Lactococcus garvieae infections in humans: possible association with aquaculture outbreaks. Int. J. Clin. Pract. [DOI] [PubMed] [Google Scholar]
- 29. Wang XL, Hur HG, Lee JH, Kim KT, Kim SI. 2005. Enantioselective synthesis of S-equol from dihydrodaidzein by a newly isolated anaerobic human intestinal bacterium. Appl. Environ. Microbiol. 71:214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Watanabe S, Terashima K, Sato Y, Arai S, Eboshida A. 2000. Effects of isoflavone supplement on healthy women. Biofactors 12:233–241 [DOI] [PubMed] [Google Scholar]
- 31. Yokoyama S, Suzuki T. 2008. Isolation and characterization of a novel equol-producing bacterium from human feces. Biosci. Biotechnol. Biochem. 72:2660–2666 [DOI] [PubMed] [Google Scholar]