Abstract
The intestinal ecosystem is balanced by dynamic interactions between resident and incoming microbes, the gastrointestinal barrier, and the mucosal immune system. However, in the context of inflammatory bowel diseases (IBD), where the integrity of the gastrointestinal barrier is compromised, resident microbes contribute to the development and perpetuation of inflammation and disease. Probiotic bacteria have been shown to exert beneficial effects, e.g., enhancing epithelial barrier integrity. However, the mechanisms underlying these beneficial effects are only poorly understood. Here, we comparatively investigated the effects of four probiotic lactobacilli, namely, Lactobacillus acidophilus, L. fermentum, L. gasseri, and L. rhamnosus, in a T84 cell epithelial barrier model. Results of DNA microarray experiments indicating that lactobacilli modulate the regulation of genes encoding in particular adherence junction proteins such as E-cadherin and β-catenin were confirmed by quantitative reverse transcription-PCR (qRT-PCR). Furthermore, we show that epithelial barrier function is modulated by Gram-positive probiotic lactobacilli via their effect on adherence junction protein expression and complex formation. In addition, incubation with lactobacilli differentially influences the phosphorylation of adherence junction proteins and the abundance of protein kinase C (PKC) isoforms such as PKCδ that thereby positively modulates epithelial barrier function. Further insight into the underlying molecular mechanisms triggered by these probiotics might also foster the development of novel strategies for the treatment of gastrointestinal diseases (e.g., IBD).
INTRODUCTION
The gastrointestinal tract harbors a complex microbial ecosystem containing bacteria with both harmful and beneficial effects on host physiology (2, 23). Usually most members of the intestinal microbiota are commensals and/or probiotic bacteria that contribute to immune development and digestion and, furthermore, interfere with incoming pathogens (5, 47). This microbiota is engaged in a continuous cross talk with the host and maintains a balanced relationship between gut microbes, intestinal epithelial cells (IECs), and the immune responses of the host (27, 30). However, under pathological conditions—such as in the context of inflammatory bowel diseases (IBD)—this balance can be disturbed and the integrity of the gastrointestinal barrier can be compromised. In these cases, resident microbes contribute to the development and perpetuation of inflammation and disease (6, 15, 25, 32, 35).
Probiotic bacteria (also called probiotics), such as certain lactobacilli, are defined as “live microorganisms that, which when administered in adequate amounts, confer a health benefit on the host” (10). Probiotic lactobacilli reveal health benefits that appear to be based on three constitutional effects which contribute to their protective function (26, 36, 42): (i) restoration of microbial homeostasis by microbe-microbe interactions and pathogen inhibition (for example, see references 21, 28, and 43), (ii) strengthening of epithelial barrier function (38, 40, 53, 54), and (iii) modulation of immune responses (11, 17, 26).
For the maintenance of barrier integrity, the apical junctional complex (AJC), incorporating adherence junctions (AJ) and tight junctions (TJ), plays an important role. This complex is essential for cell proliferation, tissue differentiation, and regulation of paracellular transport. The assembly of TJ between epithelial cells requires the prior formation of AJ, and thus, alterations of the E-cadherin-dependent AJ also affect TJ formation (53, 54). The major transmembrane protein of AJ is E-cadherin, which belongs to the family of Ca2+-dependent adhesion proteins (1, 16, 29), is directly associated with β-catenin. This in turn provides a link to cytosolic actin, which is directly involved in AJ complex biogenesis (19, 34). In addition, several protein kinase C (PKC) isoforms have been localized close to the AJC. Little is known about the molecular mechanisms underlying the regulation of junctional dynamics by different PKCs, but it has been postulated that activation of distinct PKC isoforms differentially affects the maintenance of barrier function (45, 46). Meanwhile, 10 PKC isoforms have been identified and grouped in three distinct subtypes: conventional (cPKC) isozymes (α, βI, βII, and γ), novel (nPKC) isozymes (δ, ε, η, and θ), and atypical (aPKC) isozymes (ζ and ι/λ) (46). However, expression of PKC isoforms appears to be cell and tissue specific. Hence, only a subset of five PKC isoforms (α, βII, δ, ε, and ζ) is expressed in T84 human colonic adenocarcinoma epithelial cells (T84 cells) (44).
Here, we investigated the influence of four distinct lactobacilli on epithelial barrier integrity by utilizing confluent human T84 cell monolayers as a model system. Alterations in transepithelial resistance (TER) following incubation with probiotic lactobacilli were monitored on-line and taken as a measure of changes in the integrity of cellular barriers. We show that epithelial barrier function is modulated by Gram-positive probiotic lactobacilli via their effect on the adherence junction protein E-cadherin. In addition, incubation with lactobacilli differentially influences the phosphorylation status of adherence junction proteins and of PKC isoforms such as PKCδ, thereby positively modulating E-cadherin expression. Interestingly, the four lactobacillus species affected AJ differently, indicating that the probiotic effects are species specific.
(This study was conducted by S. Hummel and K. Veltman in partial fulfillment of the requirements for a Ph.D. from Westfälische Wilhelms-Universität Münster, Münster, Germany.)
MATERIALS AND METHODS
Bacterial strains and tissue culture.
L. acidophilus (PZ 1041), L. gasseri (PZ 1160), L. fermentum (PZ 1162), and L. rhamnosus (PZ 1121) were obtained from Pharma-Zentrale (Herdecke, Germany) as typical probiotic lactobacilli. PZ 1041 was isolated from the feces of a healthy person (14). PZ 1160 and PZ 1162 were donated by the Ardeypharm Collection of Strains (ACS) and have been investigated for their defensin-inducing properties (52). PZ 1121 is identical to the L. rhamnosus GG strain (ATCC 53103). The probiotic effects of these strains are well documented (for example, see references 13, 14, and 52). To maintain pH values in a physiological range and to condition the lactobacilli for incubation with T84 cells, overnight cultures of lactobacilli were grown in MRS broth (Merck, Darmstadt, Germany) buffered with 2.5% Na2HPO4 · 2H2O to pH 7.0 at 37°C and 5% CO2 without shaking. Overnight cultures of E. coli Nissle 1917 (referred to here as Nissle) (Pharma-Zentrale, Herdecke, Germany) and the prototype enteropathogenic E. coli (EPEC) strain E2348/69 were grown in Luria-Bertani broth at 37°C. Epithelial T84 cell monolayers were incubated with lactobacilli at a multiplicity of infection (MOI) of 1.5 due to the rapid production of lactic acid by lactobacilli, which negatively affects the epithelial cells. E. coli strains were applied at an MOI of 10.
Cell culture and antibodies.
The human colonic adenocarcinoma epithelial cell line T84 (European Collection of Cell Cultures [ECACC; Salisbury, United Kingdom], passage 10 to 26) was grown in a humidified incubator at 37°C in DMEM (Dulbecco's modified Eagle medium) Ham's F-12 (PAA Laboratories, Cölbe, Germany) supplemented with 10% FCS and antibiotics (penicillin and streptomycin, 50 μg/ml each). Cells were passaged and cultured in cell culture flasks and on membrane filters. On Transwell filters (6.5-mm diameter; polycarbonate membrane; 0.4-μm pore size; Costar, Corning, NY), T84 cells were seeded at density of 5 × 105/insert. T84 cell monolayer medium was changed every other day. Specific antibodies to E-cadherin, ZO-2, PKCδ, PKCζ, phosphorylated β-catenin (P-β-catenin), and GAPDH antibodies were purchased from Santa Cruz Biotechnology (Heidelberg, Germany), and phospho-Ser/Thr came from ECM Biosciences (Versailles, KY) or from Biotrend (Köln, Germany). Anti-tubulin antibodies were obtained from Sigma-Aldrich (St. Louis).
Transepithelial electrical resistance (TER).
Beginning approximately 10 days after seeding on Transwell filters, the T84 monolayer confluence and barrier properties were documented by measuring TER before and after apical exposure to bacteria using an automated cell monitoring system. After reaching confluence, the filters were inserted into the appropriate wells of the cellZscope unit for real-time on-line TER monitoring (nanoAnalytics, Münster, Germany) according to Karczewski et al. (22) and Rempe et al. (39). The cellZscope is able to monitor transepithelial impedance (ohmic resistance and capacitance) under physiological conditions without affecting the cellular barrier under investigation. In this study, TER was used as a readout system to monitor the effect of probiotic bacteria on barrier function.
RNA extraction, cRNA, and cDNA preparation.
Total RNA was extracted using an RNeasy minikit (Qiagen, Hilden, Germany), and cRNA was prepared as follows. RNA was isolated from cells grown in tissue culture flasks that had been incubated or not with the specific lactobacillus strain. Total RNA samples were used to generate biotinylated cRNA targets according to the Affymetrix microarray suite 4.0 user guide. Enzymes were supplied by Invitrogen (Breda, The Netherlands) and Roche (Mannheim, Germany). The oligo(dT) primer containing a T7 RNA polymerase promoter was purchased from Ambion (Huntington, United Kingdom). Labeled cRNA was prepared by using a microarray target synthesis kit and biotin-labeled UTP from Roche (Mannheim, Germany) and hybridized to hu U133A 2.0 chip sets (representing 12,000 human DNA sequences; Affymetrix, Santa Clara, CA) according to the supplier's instructions.
cDNA synthesis from total RNA was performed using a Transcriptor reverse transcriptase kit (Roche Molecular Biochemicals, Mannheim, Germany).
Hybridization and scanning of DNA microarrays.
DNA array experiments were performed using hu U133A 2.0 Affymetrix DNA arrays to analyze the mRNA profiles of T84 cells incubated for 30, 60, 120, or 180 min with lactobacilli (L. gasseri and L. fermentum) and of untreated T84 cells (18). Hybridization and staining were done according to the Affymetrix microarray suite 5.0 user guide. Quality control and data analysis were performed using GeneData Expressionist software. For this setup, we considered a 1.8-fold difference in gene expression in log-log plots to be meaningful when epithelial cell monolayers with and without the addition of bacteria were compared. Results were derived from two independent biological repeats.
Confirmation of gene expression data by quantitative real-time PCR.
The results of DNA array experiments were confirmed for selected genes associated with barrier function by quantitative RT-PCR employing the LightCycler amplification and detection system (Roche Molecular Biochemicals, Mannheim, Germany). Gene-specific primer pairs synthesized by Eurofins MWG GmbH (Ebersberg, Germany) were designed by using the Universal ProbeLibrary (Roche, Mannheim, Germany). As an internal reference, expression of the hypoxanthine phosphoribosyl-transferase I gene (HPRT I) as a low-abundance housekeeping gene was also monitored. Data were analyzed with LightCycler quantification software (version 3.5; Roche Molecular Biochemicals, Mannheim, Germany). Sequences of primer pairs used for qRT-PCR experiments are listed in Table 1.
Table 1.
Sequences of primer pairs employed for qRT-PCR
| Primer | Sequence (5′→3′) |
|---|---|
| HPRT_for | TGATAGATCCATTCCTATGACTGTAGA |
| HPRT_rev | AGGACATTCTTTCCAGTTAAAGTTGAG |
| E-cadherin_for | GAAGTGTCCGAGGACTTTGG |
| E-cadherin_rev | CAGTGTCTCTCCAAATCCGATA |
| β-catenin_for | GCAGAGTGCTGAAGGTGCTA |
| β-catenin_rev | TCTGTCAGGTGAAGTCCTAAAGC |
| ZO-2_for | GAACGAGGCATCATCCCTAA |
| ZO-2_rev | CCAGCTTCTCGAAGAACCAC |
| Claudin 1_for | CCGTTGGCATGAAGTGTATG |
| Claudin 1_rev | CCAGTGAAGAGAGCCTGACC |
| Occludin_for | TTTGTGGGACAAGGAACACA |
| Occludin_rev | CAGGCGAAGTTAATGGAAGC |
Preparation of total protein cell lysates.
For the preparation of total cell lysates, M-PER mammalian protein extraction reagent (Pierce Biotechnology, Rockford, IL) was used. After incubation with lactobacilli, T84 cells were washed three times with Dulbecco's phosphate-buffered saline (D-PBS with CaCl2 and MgCl2; PAA, Cölbe, Germany), and 350 μl (250 to 500 μl/60 mm2 surface area) of M-PER mammalian protein extraction reagent containing Halt protease inhibitor single-use cocktail (10 μl/ml) (Pierce Biotechnology, Rockford) was added. The material was removed from cell culture flasks with a cell scraper and centrifuged for 10 min at 14,000 × g, and supernatants were collected and stored at −80°C.
Subcellular fractionation.
Following incubation with bacteria, T84 cell monolayers were washed three times with ice-cold D-PBS (with CaCl2 and MgCl2; PAA, Cölbe, Germany). For subcellular fractionation, approximately 6 × 106 cells were scraped into 700 μl of ice-cold lysis buffer A (20 mM Tris-HCl [pH 7.5], 250 mM sucrose, 4 mM EDTA, 2 mM EGTA, 1 mM Na3VO4 containing Halt protease single use cocktail and Halt phosphatase inhibitor; Pierce Biotechnology, Rockford, Ill). The cells were lysed on ice using a Branson ultrasonifier (Branson Ultrasonics, Danbury, CT). Lysates were ultracentrifuged for 30 min at 50,000 × g at 4°C. Supernatants were collected as the cytosolic fraction. The pellets were washed with lysis buffer A and resuspended in 500 μl buffer B (buffer A with 0.5% Triton X-100 and Halt protease single-use cocktail as well as Halt phosphatase inhibitor [Pierce Biotechnology]) and incubated on ice for 40 min. Afterwards, samples were centrifuged for 30 min at 50,000 × g and 4°C. Supernatants were collected as the membrane fraction. Lysates were stored at −80°C.
Western blotting experiments.
For denaturation, proteins in whole-cell lysates and the cytosolic and membrane fractions were added to sodium dodecyl sulfate (SDS) sample buffer and heated at 95°C for 5 min. Proteins were separated using a 10% SDS-gel, electrophoretically transferred to polyvinylidene difluoride (PVDF) membranes, and stained for ZO-2, E-cadherin, P-β-catenin, PKCζ, PKCδ, GAPDH (Santa Cruz Biotechnology, Santa Cruz, CA), and anti-α-tubulin (Sigma-Aldrich, St. Louis, MO) using specific antibodies.
Stripping of PVDF membranes and detection of protein phosphorylation.
For the identification of specific phosphorylation of junction-associated proteins in membrane and cytosolic fractions of T84 cells, following separation by SDS-polyacrylamide gel electrophoresis and Western blotting the blots were first incubated with antibodies to phospho-Ser/Thr and developed as described above. Subsequently, the membranes were stripped to remove primary and secondary antibodies to restore the accessibility of membrane-bound proteins. For stripping, the blots were incubated in 0.2 M glycine (pH 2.5) and incubated for 10 s in a microwave (1.0 kW). After shaking in T-TBS solution (0.05% Tween 20, 150 mM NaCl, 10 mM Tris [pH 7.4]) for 15 min, the blots were again washed twice for 15 min in TBS (10 mM Tris, 150 mM NaCl [pH 7.4]). Then the second round of incubation with specific antibodies and detection was performed.
Statistical analysis.
Statistical analysis was performed by using Student's t test.
Microarray data accession numbers.
The results of the microarray experiments have been deposited in the EMBL-EBI ArrayExpress database (EBI ArrayExpress accession no. E-MEXP-3445).
RESULTS
Increase of transepithelial electrical resistance (TER) indicates the reinforcement of barrier function following incubation with lactobacilli.
As previously shown by us and others (21, 31, 54), probiotics strengthen the barrier function of epithelial cells. Therefore, we measured the effect of four different lactobacillus species on the transepithelial electrical resistance of T84 cell barriers grown on Transwell filters. We incubated T84 cell barriers for 4 to 5 h with L. fermentum, L. gasseri, L. acidophilus, or L. rhamnosus (Fig. 1A and B). Bacteria were added to the apical chamber of a Transwell filter unit, and the TER was continuously measured for up to 7 to 8 h by cellZscope technology and compared with that of untreated T84 monolayers. We observed a strong increase in TER after incubation with lactobacilli, indicating the ability of lactobacilli to reinforce epithelial barriers. As a negative control and to further validate the Transwell model system for epithelial barriers, we also incubated T84 monolayers with the prototype enteropathogenic E. coli EPEC strain E2348/69, which induced a sharp decline of TER (Fig. 1C), confirming published results (51).
Fig 1.
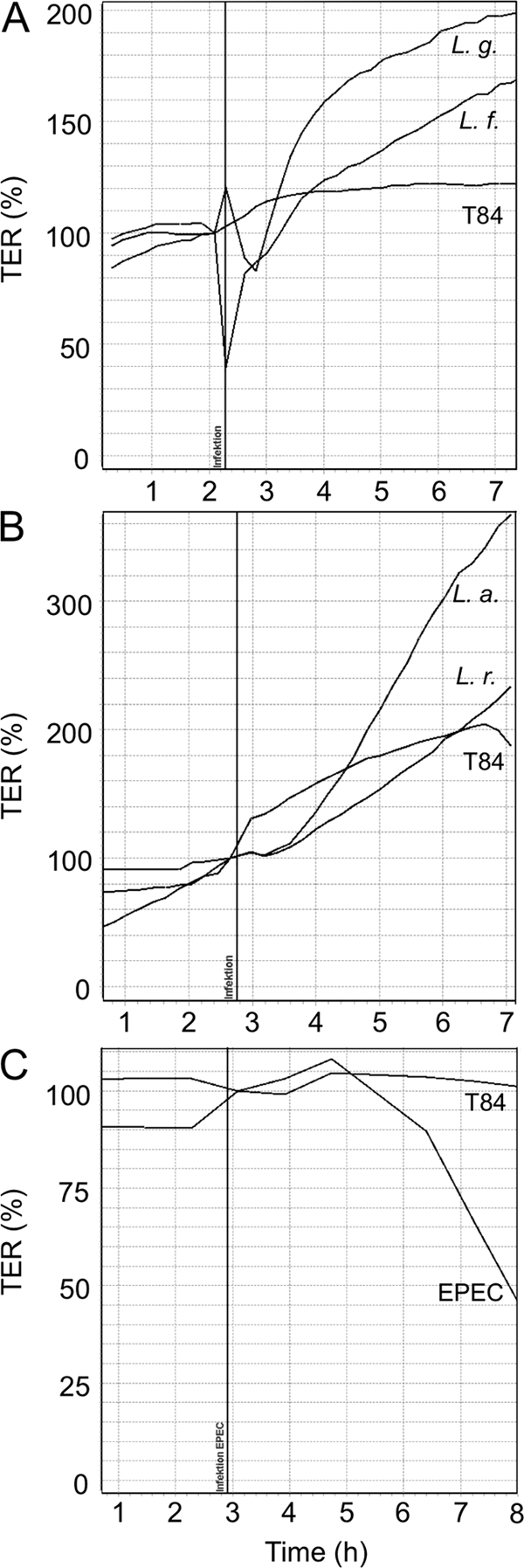
Monitoring changes of the transepithelial electrical resistance (TER) of T84 cell monolayers following incubation with lactobacilli and the enteropathogenic E. coli (EPEC) strain E2348/69. T84 cell monolayers were incubated with L. fermentum (L. f.) (A), L. gasseri (L. g.) (A), L. acidophilus (L. a.) (B), L. rhamnosus (L. r.) (B), and the enteropathogenic E. coli strain E2348/69 (EPEC) (C). T84 cells were grown on Transwell filter units, and the TER of untreated T84 cell monolayers (T84) and of T84 cell monolayers incubated with the different bacteria was measured. TER measurements were performed online using the cellZscope set-up (NanoAnalytics, Münster, Germany). The vertical line indicates the time when bacteria were added. TER values of untreated T84 cell monolayers were set to 100%.
Lactobacilli specifically influence the expression of adherence junction-associated proteins.
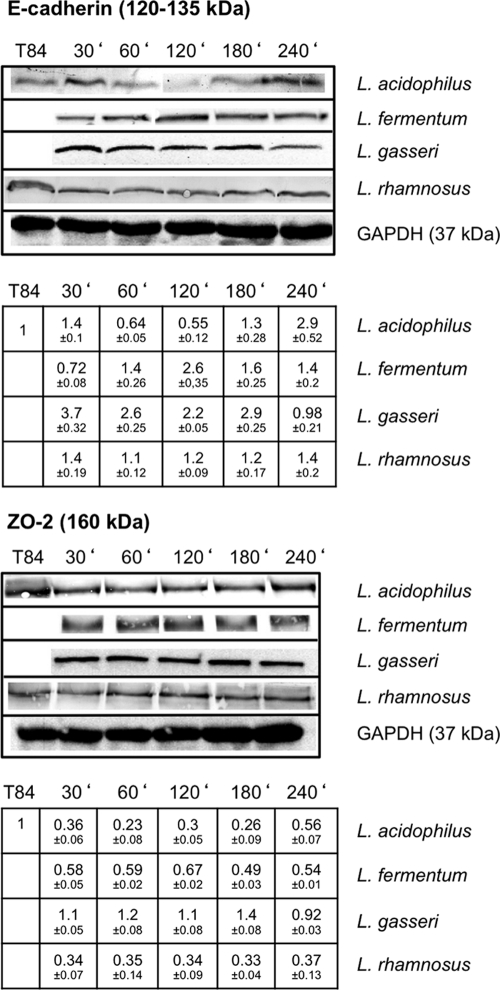
Searching for genes that might be modulated in T84 cells by incubation with lactobacilli and that might directly affect TER and, hence, also the integrity of the epithelial barrier, we performed DNA microarray analysis using hu U133A 2.0 Affymetrix arrays (see the EMBL-EBI ArrayExpress database). During incubation of T84 cells with L. fermentum and L. gasseri for up to 3 h we particularly observed upregulation of transcription of two genes encoding the major adherence junction proteins β-catenin and E-cadherin. Alterations in transcription identified by DNA array analysis were confirmed for L. gasseri and L. fermentum by quantitative real-Time PCR. However, quantification of β-catenin transcription by qRT-PCR revealed only minor changes. Therefore, we focused our main interest on the effects of lactobacilli on E-cadherin (Fig. 2). Interestingly, L. rhamnosus had almost no effect on E-cadherin transcription and in this respect turned out to be strikingly different from L. acidophilus, L. fermentum, and L. gasseri. In comparison with the effects on epithelial barriers of the Gram-negative probiotic E. coli Nissle 1917, which induced an upregulation of the tight-junction-associated protein ZO-2, thereby increasing TER (54), only minor effects of L. gasseri and L. rhamnosus on the regulation of ZO-2 (Fig. 2) could be observed. L. acidophilus even reduced expression of ZO-2, while L. fermentum had no effect. Similar results were obtained for claudin 1 and occludin (data not shown). In turn, E-cadherin expression is not affected by Nissle but was found to be downregulated by EPEC strain E2348/69. Western blot analysis of whole T84 cell lysates confirmed the changes in E-cadherin expression induced by incubation with L. acidophilus, L. fermentum, and L. gasseri. In contrast, no significant changes of ZO-2 expression during lactobacilli incubation were observed (Fig. 3).
Fig 2.
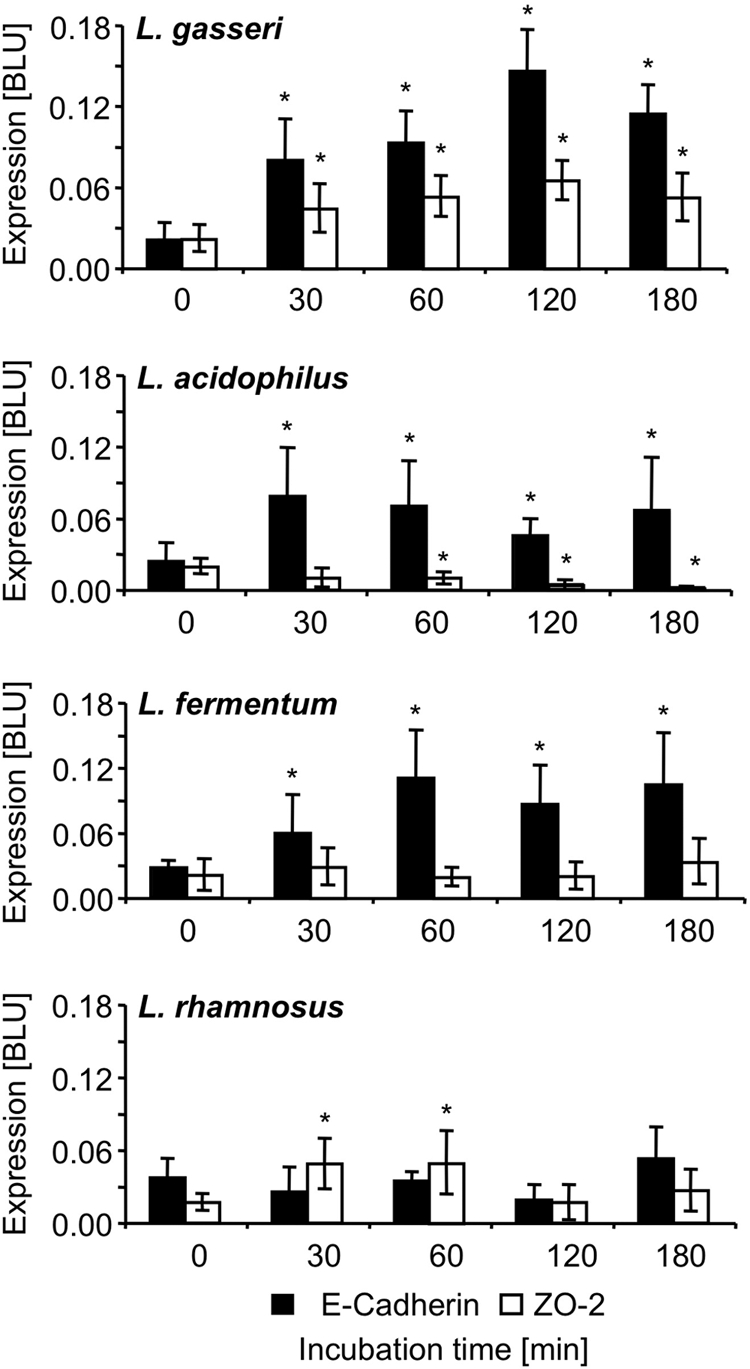
Impact of incubation of T84 cells with different lactobacilli on ZO-2 (TJ-associated protein) and E-cadherin (AJ-associated protein). T84 cells were incubated with L. gasseri, L. acidophilus, L. fermentum, and L. rhamnosus for 30, 60, 120, and 180 min. ZO-2 and E-cadherin mRNA expression was quantified using qRT-PCR (*, P ≤ 0.05). Bars represent standard deviations (SD).
Fig 3.
Western blot analysis of E-cadherin and ZO-2 expression following incubation of T84 cells with lactobacilli. T84 cells were incubated with L. acidophilus, L. fermentum, L. gasseri, and L. rhamnosus for 30, 60, 120, 180, and 240 min. Protein expression in T84 cells that were incubated with lactobacilli relative to that in untreated T84 cells is shown. Representative blots of experiments conducted at least three times are shown. Densitometry measurements, including SD, are given below the gels. The values for untreated T84 cells were set to 1.
Stabilization of the E-cadherin/β-catenin complex by activation of protein kinases.
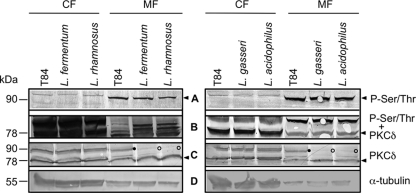
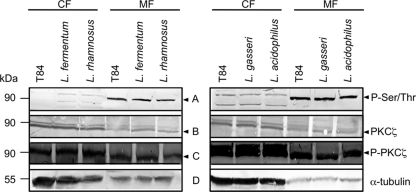
Previous results from our laboratory indicated the involvement of PKC Ser/Thr protein kinases in the modulation of cellular junctions by lactobacilli (53, 54). To further investigate whether the activity of specific PKC isoforms might be affected by the incubation with lactobacilli, we utilized Western blotting to detect alterations in the phosphorylation status of novel PKCδ and atypical PKCζ in the membrane and cytosolic fractions of T84 cells. Incubation with anti-P-Ser/Thr antibodies identified prominent bands (∼90 kDa) where P-PKCδ would be expected (Fig. 4A). However, as specific anti-PKCδ antibodies did not recognize these proteins following incubation with lactobacilli (Fig. 4C) but did recognize them with untreated T84 cells, we conclude that the anti-P-Ser/Thr antibodies reacted with a different protein(s) of similar size (Fig. 4B). Therefore, it appears that the incubation of T84 cells with lactobacilli reduces the abundance of PKCδ in membrane fractions. Furthermore, it has been shown that junctional permeability is increased by overexpression or activation of PKCδ in cultured renal epithelial cells as well as in Caco-2 epithelial cells (33, 45). Interestingly, atypical PKCζ is not at all affected by incubation with lactobacilli, as only the phosphorylated form could be detected in all cellular compartments of T84 cells (Fig. 5). PKCζ is the only isoform that localizes near the tight-junction complex (46). This corresponds to previous results showing that the Gram-negative strain Nissle inactivates PKCζ (53, 54). Our current evidence supports the notion that Gram-negative and Gram-positive probiotic bacteria appear to address different cellular junctions (50).
Fig 4.
Distribution and phosphorylation of PKCδ in T84 cells after incubation with lactobacilli. Following incubation of T84 cells with L. fermentum, L. rhamnosus, L. gasseri, and L. acidophilus for 2.5 h, cells were fractionated in cytosolic (CF) and membrane (MF) fractions. Following Western blotting, probing with anti-P-Ser/Thr antibodies, development, and documentation (A), the membranes were stripped and probed additionally with anti-PKCδ specific antibodies (B). T84 fractions were probed with anti-PKCδ antibodies (C). (D) α-Tubulin loading control. Following incubation with the different lactobacillus species, the abundance of P-PKCδ in the membrane fraction of T84 cells (•) is reduced (○). Relevant positions are indicated by an arrowhead. Representative blots from experiments performed at least three times are shown.
Fig 5.
Phosphorylation status of PKCζ in T84 cells after incubation with L. fermentum, L. rhamnosus, L. gasseri, and L. acidophilus for 2.5 h. Protein expression in T84 cells is depicted in comparison to that in T84 cells that were not incubated with lactobacilli. Probing and development was performed as described in the legend to Fig. 4. Representative blots of experiments repeated at least three times are shown. CF, cytosolic fraction; MF, membrane fraction. Relative positions are indicated by an arrowhead.
Enhanced phosphorylation of β-catenin.
As lactobacilli apparently exert their barrier-enforcing effect via modulation of adherence junction proteins, we were interested to see whether, in addition to the upregulation of E-cadherin expression, β-catenin as complex partner of E-cadherin might be directly affected. Following incubation with different lactobacillus species for 2.5 h, we therefore investigated whether the serine/threonine phosphorylation of β-catenin might be altered, as the active complex enables the link between the AJ and the cytoskeleton. Incubation with lactobacilli clearly increased P-β-catenin levels in the membrane fractions of T84 cells relative to the levels in untreated T84 cells. This effect was particularly pronounced following incubation with L. acidophilus and L. rhamnosus (Fig. 6).
Fig 6.
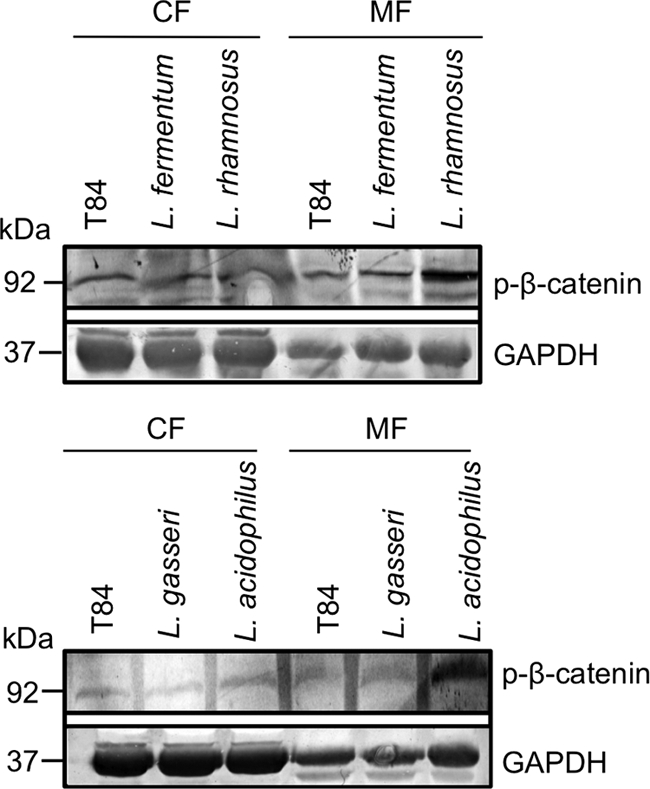
Phosphorylation of β-catenin after incubation of T84 cells with L. fermentum, L. rhamnosus, L. gasseri, and L. acidophilus for 2.5 h. Cellular fractionation in cytoplasmic (CF) and membrane (MF) fractions indicated a differentially enhanced expression of Ser/Thr-phosphorylated β-catenin in the membrane fractions of T84 cells induced by incubation with different lactobacilli.
DISCUSSION
Lactobacilli have long been associated with beneficial effects in the human intestine (for example, see references 4, 7, 8, 31, 48, and 49); however, the molecular mechanisms responsible for their effects on barrier functions and immune responses are still only poorly understood (5). Here, we investigated the effects of four lactobacillus species (L. acidophilus, L. fermentum, L. gasseri, and L. rhamnosus) on T84 monolayers serving as a model for the intestinal epithelial barrier. The increase of TER after incubation of T84 cell barriers with lactobacilli indicates that these Gram-positive probiotic bacteria reinforce the barrier function of epithelial cells. Continuous on-line monitoring of TER with the cellZscope for more than 7 h demonstrates that the effect is long lasting. To further confirm the validity of our epithelial barrier model, we employed the prototype enteropathogenic E. coli strain E2348/69 (Fig. 1C), which rapidly disrupts epithelial barriers and decreases TER following infection as a negative control.
Based on our previous results (54), where we showed that the Gram-negative strain E. coli Nissle 1917 enhances tight-junction integrity, we were interested to see whether Nissle might also affect AJ. However, incubation of T84 cells with Nissle did not alter expression of mRNA for E-cadherin (data not shown). In contrast, in this study we specifically demonstrated that incubation of T84 cells with Gram-positive probiotic lactobacilli modulated AJ, as indicated by alterations in the major component, E-cadherin (Fig. 2). Incubation of T84 cells with lactobacilli enhances the expression of E-cadherin at the mRNA and protein levels (Fig. 2 and 3), thereby contributing to barrier integrity as reflected by increasing TER. Interestingly, the influence of lactobacilli on the expression of the TJ-associated protein ZO-2 appears to be heterogeneous (Fig. 2 and 3). In this context, it is remarkable that the observed increase in TER induced by L. rhamnosus (PZ 1121) (Fig. 1) appears to be due to a different mechanism(s), as has been identified for the other three lactobacillus species investigated in this study. Consequently, the mechanisms leading to the barrier-enhancing effects of L. rhamnosus need to be further investigated in future studies.
The importance of PKC in regulating the stability of the junctional complex in epithelial cells and also distinct effects of specific isoforms have been amply documented, and it has been shown that the activity of PKC is largely regulated by differential phosphorylation (for example, see references 20 and 24). However, their specific contributions to barrier integrity are still only partially understood. PKCδ has been found to be crucial for cell-cell adhesion and modulation of AJ (9). Activation of PKCδ appears to be necessary for the binding of PKCδ to E-cadherin to induce cell scattering and disturbance of the AJ complex (45). Therefore, we were interested to see whether the observed effect of lactobacilli on barrier integrity via the modulation of AJ might also involve PKCδ. We showed that all four lactobacillus species reduced the abundance of PKCδ in the membrane fractions of T84 cells (Fig. 4). This indicates that the redistribution of membrane PKCδ by incubation with lactobacilli might be involved in enhancing barrier integrity. In contrast, in previous studies we demonstrated an inactivation of PKCζ after incubation with the Gram-negative strain Nissle. As only P-PKCζ is able to phosphorylate ZO-2 and only the phosphorylated form of ZO-2 can be removed from the TJ complex, inhibition of PKCζ activation results in stabilization of TJ. Interestingly, PKCζ appears not to be influenced by incubation of T84 cells with lactobacilli (Fig. 5). This supports the notion that Gram-positive lactobacilli and the Gram-negative strain Nissle target distinct signaling pathways, which in turn address different intercellular junctions.
The adherence junction protein β-catenin is a complex partner of E-cadherin and as such plays a key role in the maintenance of adherence junction complexes by linking the cytoskeleton and E-cadherin (12, 34). Several studies had already reported the enhancement of the E-cadherin/β-catenin complex through distinct phosphorylation of β-catenin by Ser/Thr kinases (29). Therefore, putative alterations in P-β-catenin were investigated in cytosolic and membrane fractions following incubation of T84 cells with lactobacilli. We found a lactobacillus-dependent enhanced phosphorylation of β-catenin in the membrane fractions of T84 cells, which is most pronounced after incubation with L. acidophilus and L. rhamnosus (Fig. 6). Increased phosphorylation of β-catenin would further strengthen the E-cadherin/β-catenin complex.
The results of this study are in agreement with previous data suggesting a PKCδ/β-catenin complex, which has been indicated by double-staining and coimmunoprecipitation experiments (53). This is reminiscent of the PKCζ/ZO-2 complex after incubation with Nissle (54), which regulates the stability of the junctional complex (3). Also, differences between Gram-positive and Gram-negative probiotic bacteria in their impact on barrier function are apparent here.
We showed that Gram-positive lactobacilli reinforce the barrier function of gastrointestinal epithelial cells by stabilizing AJ by affecting E-cadherin expression and functionality as well as modulating the corresponding E-cadherin/β-catenin complex. This is in contrast to the mode of action of Gram-negative probiotics, as we demonstrated earlier (54). These findings confirm the important role of probiotic bacteria for the maintenance and restoration of barrier function and therefore for the therapy of IBD and other gastrointestinal disorders (31, 37, 41).
Further investigations on the pathways of the specific phosphoregulation and the activation process induced by probiotic lactobacilli are certainly needed to elucidate how these properties might be efficiently applied for therapeutic purposes such as inducing beneficial effects in or even preventing gastrointestinal disorders or infections by numerous pathogens. Additional insight into the molecular mechanisms exploited by probiotics to modulate barrier functions might foster even the development of novel strategies for the treatment of IBD like Crohn's disease and/or ulcerative colitis.
ACKNOWLEDGMENTS
This study was supported by grant SchMA2/027/08 of the Interdisciplinary Clinical Research Center (IZKF) of the Medical Faculty of the University of Münster and the Graduiertenkolleg GRK1409 funded by the Deutsche Forschungsgemeinschaft (DFG) at the University of Münster.
Footnotes
Published ahead of print 16 December 2011
REFERENCES
- 1. Angst BD, Marcozzi C, Magee AI. 2001. The cadherin super family: diversity in form and function. J. Cell Sci. 114:629–664 [DOI] [PubMed] [Google Scholar]
- 2. Artis D. 2008. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8:411–420 [DOI] [PubMed] [Google Scholar]
- 3. Baldwin TJ, et al. 1990. Protein phosphorylation by protein kinase C in HEp-2 cells infected with enteropathogenic Escherichia coli. Infect. Immun. 58:761–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baugher JL, Klaenhammer TR. 2011. Applications of omics tools to understanding probiotic functionality. J. Dairy Sci. 94:4753–4765 [DOI] [PubMed] [Google Scholar]
- 5. Bron PA, van Baarlen P, Kleerebezem M. 2011. Emerging molecular insight into the interaction between probiotics and the host intestinal mucosa. Nat. Rev. Microbiol. [Epub ahead of print.] doi:10.1038/nrmicro2690 [DOI] [PubMed] [Google Scholar]
- 6. Chassaing B, Darfeuille-Michaud A. 2011. The commensal microbiota and enteropathogens in the pathogenesis of inflammatory bowel diseases. Gastroenterology 140:1720–1728 [DOI] [PubMed] [Google Scholar]
- 7. Corthésy B, Gaskins HR, Mercenier A. 2007. Cross-talk between probiotic bacteria and the host immune system. J. Nutr. 137:781S–790S [DOI] [PubMed] [Google Scholar]
- 8. Dai C, Zhao D-H, Jiang M. 2012. VSL#3 probiotics regulate the intestinal epithelial barrier in vivo and in vitro via the p38 and ERK signaling pathways. Int. J. Mol. Med. 29:202–208 [DOI] [PubMed] [Google Scholar]
- 9. DeVries TA, Neville MC, Reyland ME. 2002. Nuclear import of PKCδ is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 21:6050–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. FAO/WHO 2001. Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Report of a Joint FAO/WHO Expert Consultation, Cordoba, Argentina. World Health Organization, Geneva, Switzerland [Google Scholar]
- 11. Galdeano CM, Perdigon G. 2006. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin. Vaccine Immunol. 13:219–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gooding JM, Yap KL, Ikura M. 2004. The cadherin-catenin complex as a focal point of cell adhesion and signalling: new insights from three-dimensional structures. BioEssays 26:497–511 [DOI] [PubMed] [Google Scholar]
- 13. Gorbach SL. 2000. Probiotics and gastrointestinal health. Am. J. Gastroenterol. 95(Suppl 1): S2–S4 [DOI] [PubMed] [Google Scholar]
- 14. Gordon H, Lewis B, Eales L, Brock JF. 1957. Dietary fat and cholesterol metabolism; fecal elimination of bile acids and other lipids. Lancet 273:1299–1306 [DOI] [PubMed] [Google Scholar]
- 15. Harrison OJ, Maloy KJ. 2011. Innate immune activation in intestinal homeostasis. J. Innate Immun. 3:585–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartsock A, Nelson WJ. 2008. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 1778:660–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hörmannsperger G, Haller D. 2010. Molecular crosstalk of probiotic bacteria with the intestinal immune system: clinical relevance in the context of inflammatory bowel disease. Int. J. Med. Microbiol. 300:63–73 [DOI] [PubMed] [Google Scholar]
- 18. Hummel S. 2010. Zielstrukturen der gastrointestinalen Barriere für Gram-positive probiotische Bakterien. Dissertation. Westfälische Wilhelms-Universität Münster, Münster, Germany [Google Scholar]
- 19. Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. 2005. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol. Biol. Cell 16:2636–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ivanov AI, Samarin SN, Bachar M, Parkos CA. 2009. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol. 10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson-Henry KC, Donato KA, Shen-Tu G, Gordanpour M, Sherman PM. 2008. Lactobacillus rhamnosus strain GG prevents enterohemorrhagic Escherichia coli O157:H7-induced changes in epithelial barrier function. Infect. Immun. 76:1340–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karczewski J, et al. 2010. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 298:G851–G859 [DOI] [PubMed] [Google Scholar]
- 23. Kim M, et al. 2010. Bacterial interactions with the host epithelium. Cell Host Microbe 8:20–35 [DOI] [PubMed] [Google Scholar]
- 24. Koizumi J, et al. 2008. Protein kinase C enhances tight junction barrier function of human nasal epithelial cells in primary culture by transcriptional regulation. Mol. Pharmacol. 74:432–442 [DOI] [PubMed] [Google Scholar]
- 25. Kosiewicz MM, Zimheld AL, Alard P. 2011. Gut microbiota, immunity, and disease: a complex relationship. Front. Microbiol. 2:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lebeer S, Vanderleyden J, De Keersmaecker SC. 2008. Genes and molecules of lactobacilli supporting probiotic action. Microbiol. Mol. Biol. Rev. 72:728–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lebeer S, Vanderleyden J, De Keersmaecker SC. 2010. Host interactions of probiotic surface molecules: comparison with commensals and pathogens. Nat. Rev. Microbiol. 8:171–184 [DOI] [PubMed] [Google Scholar]
- 28. Lee YK, Puong KY, Ouwehand AC, Salminen S. 2003. Displacement of bacterial pathogens from mucus and Caco-2 cell surface by lactobacilli. J. Med. Microbiol. 52(Pt. 10): 925–930 [DOI] [PubMed] [Google Scholar]
- 29. Lickert H, Bauer A, Kemler R, Stappert J. 2000. Casein kinase II phosphorylation of E-cadherin increases E-cadherin/β-catenin interaction and strengthens cell-cell adhesion. J. Biol. Chem. 275:5090–5095 [DOI] [PubMed] [Google Scholar]
- 30. Littman DR, Pamer EG. 2011. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe 10:311–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madsen K, et al. 2001. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580–591 [DOI] [PubMed] [Google Scholar]
- 32. Maloy KJ, Powrie F. 2011. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 474:298–306 [DOI] [PubMed] [Google Scholar]
- 33. Mullin JM, et al. 1998. Overexpression of protein kinase C-delta increases tight junction permeability in LLC-PK1 epithelia. Am. J. Physiol. 275:C544–C554 [DOI] [PubMed] [Google Scholar]
- 34. Nagafuchi A, Takeichi M. 1988. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO J. 7:3679–3684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nell S, Suerbaum S, Josenhans C. 2010. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat. Rev. Microbiol. 8:564–577 [DOI] [PubMed] [Google Scholar]
- 36. Oelschlaeger T. 2009. Mechanisms of probiotic actions—a review. Int. J. Med. Microbiol. 300:57–62 [DOI] [PubMed] [Google Scholar]
- 37. Otte JM, Cario E, Podolsky DK. 2004. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126:1054–1070 [DOI] [PubMed] [Google Scholar]
- 38. Parassol N, et al. 2005. Lactobacillus casei DN-114 001 inhibits the increase in paracellular permeability of enteropathogenic Escherichia coli-infected T84 cells. Res. Microbiol. 156:256–262 [DOI] [PubMed] [Google Scholar]
- 39. Rempe R, Cramer S, Huwel S, Galla HJ. 2011. Transport of poly(n-butylcyano-acrylate) nanoparticles across the blood-brain barrier in vitro and their influence on barrier integrity. Biochem. Biophys. Res. Commun. 406:64–69 [DOI] [PubMed] [Google Scholar]
- 40. Resta-Lenert S, Barrett KE. 2003. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sartor RB. 2005. Probiotic therapy of intestinal inflammation and infections. Curr. Opin. Gastroenterol. 21:44–50 [PubMed] [Google Scholar]
- 42. Shanahan F. 2006. Probiotics: promise, problems, and progress. Gastroenterol. Hepatol. Annu. Rev. 1:41–45 [Google Scholar]
- 43. Sherman PM, et al. 2005. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect. Immun. 73:5183–5188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Silva M, Song C, Nadeau WJ, Matthews JB, McCormick BA. 2004. Salmonella typhimurium SipA-induced neutrophil transepithelial migration: involvement of a PKC-α-dependent signal transduction pathway. Am. J. Physiol. Gastrointest. Liver Physiol. 286:G1024–G1031 [DOI] [PubMed] [Google Scholar]
- 45. Singh R, Lei P, Andreadis ST. 2009. PKC-δ binds to E-cadherin and mediates EGF-induced cell scattering. Exp. Cell Res. 315:2899–2913 [DOI] [PubMed] [Google Scholar]
- 46. Song JC, et al. 2001. Regulation of epithelial transport and barrier function by distinct protein kinase C isoforms. Am. J. Physiol. Cell Physiol. 281:C649–C661 [DOI] [PubMed] [Google Scholar]
- 47. Strober W. 2009. The multifaceted influence of the mucosal microflora on mucosal dendritic cell responses. Immunity 31:377–388 [DOI] [PubMed] [Google Scholar]
- 48. Sullivan Å, Nord CE. 2005. Probiotics and gastrointestinal disease. J. Intern. Med. 257:78–92 [DOI] [PubMed] [Google Scholar]
- 49. Szajewska H, Wanke M, Patro B. 2011. Meta-analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare-associated diarrhoea in children. Aliment. Pharmacol. Ther. 34:1079–1084 [DOI] [PubMed] [Google Scholar]
- 50. Veltman K, Hummel S, Cichon C, Sonnenborn U, Schmidt MA. 2011. Identification of specific miRNAs targeting proteins of the apical junctional complex that simulate the probiotic effect of E. coli Nissle 1917 on T84 cells. Int. J. Biochem. Cell Biol. [Epub ahead of print.] doi:10.1016/j.biocel.2011.11.006 [DOI] [PubMed] [Google Scholar]
- 51. Viswanathan VK, Weflen A, Koutsouris A, Roxas JL, Hecht G. 2008. Enteropathogenic E. coli-induced barrier function alteration is not a consequence of host cell apoptosis. Am. J. Physiol. Gastrointest. Liver Physiol. 294:G1165–G11670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wehkamp J, et al. 2004. NFκB- and AP-1-mediated induction of human β defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect. Immun. 72:5750–5758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zyrek AA. 2006. Effects of probiotic bacteria on intestinal epithelial cells with special emphasis on the barrier function. Dissertation. Westfälische Wilhelms-Universität Münster, Münster, Germany [Google Scholar]
- 54. Zyrek AA, et al. 2007. Molecular mechanisms underlying the probiotic effects of Escherichia coli Nissle 1917 involve ZO-2 and PKCδ redistribution resulting in tight junction and epithelial barrier repair. Cell. Microbiol. 9:804–816 [DOI] [PubMed] [Google Scholar]