Abstract
Campylobacter jejuni, one of the most common causes of human gastroenteritis, is a thermophilic and microaerophilic bacterium. These characteristics make it a fastidious organism, which limits its ability to survive outside animal hosts. Nevertheless, C. jejuni can be transmitted to both humans and animals via environmental pathways, especially through contaminated water. Biofilms may play a crucial role in the survival of the bacterium under unfavorable environmental conditions. The goal of this study was to investigate survival strategies of C. jejuni in mono- and mixed-culture biofilms. We grew monoculture biofilms of C. jejuni and mixed-culture biofilms of C. jejuni with Pseudomonas aeruginosa. We found that mono- and mixed-culture biofilms had significantly different structures and activities. Monoculture C. jejuni biofilms did not consume a measurable quantity of oxygen. Using a confocal laser scanning microscope (CLSM), we found that cells from monoculture biofilms were alive according to live/dead staining but that these cells were not culturable. In contrast, in mixed-culture biofilms, C. jejuni remained in a culturable physiological state. Monoculture C. jejuni biofilms could persist under lower flow rates (0.75 ml/min) but were unable to persist at higher flow rates (1 to 2.5 ml/min). In sharp contrast, mixed-culture biofilms were more robust and were unaffected by higher flow rates (2.5 ml/min). Our results indicate that biofilms provide an environmental refuge that is conducive to the survival of C. jejuni.
INTRODUCTION
Campylobacter jejuni is one of the most common causes of human bacterial gastroenteritis in developed countries (6, 19). Several epidemiological studies reported that the incidence of campylobacter infections in humans has been increasing and that contaminated water and undercooked poultry products are common vehicles of transmission (9, 35, 36). C. jejuni has been isolated from environmental waters, including ground, river, pond, and drinking water (1, 18, 28, 37, 43). It has been suggested that the presence of biofilms in water distribution systems is responsible for the colonization of the bacteria in poultry flocks (10, 21, 29, 45) and that C. jejuni can persist in these aquatic environments (4). Thus, biofilms likely represent an important reservoir for C. jejuni.
Biofilms consist primarily of microbial cells and extracellular polymeric substances (EPS) and often grow on surfaces submerged in an aquatic environment (7, 41). Biofilms modulate their physical and chemical environment, resulting under conditions that are distinct from planktonic conditions (38, 44). C. jejuni is able to survive in both monospecies and mixed-culture biofilms outside the host (11, 16, 22, 39), and this ability is clearly a public health concern (8, 17, 26).
A number of factors, including bacterial strain, surface type, temperature, shear stress (quantified by shake rate), and oxygen and nutrient concentrations, can affect C. jejuni biofilm structure and dynamics (16, 30, 31). Reeser et al. (2007) reported that a reduction in biofilm formation was observed in both flaAB and luxS mutants of C. jejuni compared to their wild-type strains (30). Hanning et al. (2008) showed that C. jejuni had longer survival times in biofilms at 32°C than in biofilms at 10°C (11). In most cases, C. jejuni biofilms were grown under static conditions (on glass coverslips, in glass test tubes, and in 24-well plates). These growth conditions are significantly different from those found in the water channels where C. jejuni biofilms have been observed (16, 29, 30). Therefore, it is critical to investigate C. jejuni biofilm growth under dynamic conditions and in mixed and monoculture conditions.
The goals of this research were as follows: (i) to compare the structures and activities of mono- and mixed-culture C. jejuni biofilms, (ii) to test C. jejuni viability and culturability in these biofilms, and (iii) to quantify the structure of C. jejuni biofilm grown under flow. A flow cell was used to grow and image the biofilms. For mixed-culture biofilms, C. jejuni and Pseudomonas aeruginosa were used because P. aeruginosa has been found to cooccur with C. jejuni (13). The structure of the biofilms was monitored using digitized images taken daily. At the end of the experiment, dissolved oxygen concentration profiles were measured. The biofilms were imaged to quantify live/dead cells, and the culturability of C. jejuni was tested. Finally, we changed the flow rate and monitored the biofilm structure to provide information about the effect of hydrodynamics on biofilm structure and to generate information on how C. jejuni behaves under increasing flow rates.
MATERIALS AND METHODS
Bacterial strains and inoculation.
C. jejuni strain NCTC11168 and P. aeruginosa strain PAO1 were used in this study. C. jejuni NCTC11168 was cultured on Mueller-Hinton (MH) agar (catalog no. 211825; Difco) supplemented with 7% defibrinated sheep blood (catalog no. DSB100; Hemostad) under microaerobic conditions at 42°C and incubated for 48 h. Microaerobic conditions were established using CampyGen (CN0025A; Oxoid, England) in an anaerobic jar. After 48 h of incubation, a loop of C. jejuni colony was removed from the agar plate and transferred to 100 ml of MH broth (catalog no. 275730; Difco). C. jejuni was grown overnight at 42°C under microaerobic conditions on a shaker (200 rpm) in MH broth. The reactor was inoculated with 6 ml (optical density at 600 nm [OD600] ≅ 0.5) of this culture, aseptically, via needle and syringe, through the line in which the growth medium entered the reactor.
A loop of P. aeruginosa colony was removed from the tryptic soy agar (TSA) (catalog no. 236950; Difco) and transferred to 100 ml of tryptic soy broth (TSB) (catalog no. 211825; Difco), where it was incubated overnight at room temperature under aerobic conditions on a shaker (200 rpm). Six milliliters (OD600 ≅ 0.5) of P. aeruginosa and 6 ml (OD600 ≅ 0.5) of C. jejuni were mixed. The biofilm reactor was inoculated with 6 ml of this mixture.
Biofilms and growth conditions.
MH broth was used to grow both mono- and mixed-culture biofilms. We used a simplified flat-plate flow reactor (Fig. 1A) that was placed on top of an inverted microscope for imaging. This custom-built reactor was designed to allow the quantification of biofilm structure and microelectrode measurements while the nutrient solution in the reactor was continuously recycled using a mixing chamber (see reference 23, p. 417). Prior to inoculation, the reactor and additional components were autoclaved at 121°C for 15 min. The reactor was initially filled with growth medium and then inoculated. After inoculation, we waited 4 h for the cells to attach to the surface, after which the medium was recycled at 0.75 ml/min and the system was fed continuously (0.1 ml/min). The recycle chamber was aerated (Fig. 1A) to allow more oxygen to be introduced into the growth medium.
Fig 1.
(A) The flat-plate flow reactor used to grow the biofilm; (B) the experimental layout used for microelectrode measurements.
Quantifying biofilm structure.
The digitized biofilm images were collected from the bottom of each biofilm and were used to calculate areal porosity, which is the ratio of the void area in a biofilm to the area of the total field of view. A lower areal porosity value indicates a higher biomass coverage on the surface. We used custom software developed by our research group, ISA-2, to calculate areal porosities automatically (see reference 23, p. 294). This software is provided elsewhere (23), and we used MATLAB software with the image analysis toolbox to calculate areal porosities. When we plot the average and standard deviation from the average against an increased number of images, we find that the average and the standard deviation typically become asymptotic for >10 images. Consequently, we used 20 images to ensure accurate estimates.
Measuring dissolved oxygen concentration profiles.
A custom-made dissolved oxygen (DO) microelectrode was used to measure the DO concentration in the biofilms (Fig. 1B). The DO microelectrode is an amperometric sensor in which oxygen diffuses through a silicone rubber membrane and is reduced to water at a polarized cathode (see reference 23, p. 232 to 249). A tapered platinum wire tip was plated with gold and used as the cathode. The outer case was made of a tapered Pasteur pipette, and the tip of the DO microelectrode was covered with silicone rubber. A custom-made silver/silver chloride reference electrode was inserted, and the DO microelectrode was filled with the electrolyte (0.3 M K2CO3, 0.2 M KHCO3, and 1 M KCl). DO microelectrodes were calibrated in air-saturated water and in a saturated Na2SO3 solution. The working electrode was polarized at −0.8 VAgAg/Cl with an HP 4140B pA meter/DC voltage source device. The microelectrodes were moved using a Mercury Step stepper motor controller (PI M-230.10S part no. M23010SX, PI; Physik Instrument, Auburn, MA 01501), and their movement was controlled with custom software (Microprofiler). Data were recorded on a laptop computer using a Measurement Computing USB-1608FS device (Measurement Computing Corporation, Norton, MA).
Quantifying the effect of flow on biofilm structure.
To test how the flow rate affects the C. jejuni biofilm structure, after 4 h of initial attachment we operated the reactors at a flow rate of 0.75 ml/min. After the areal porosity reached a pseudo-steady state (∼ 2 h), we collected digitized images and then varied the flow rate (0.75, 1, and 2.5 ml/min). Areal porosities were quantified following the procedures described above.
Viability of C. jejuni and live/dead imaging of biofilms.
At the end of the experiment, the biofilm reactor was opened in a laminar flow chamber. The biofilm was removed in a sterilized tube and vortexed for 1 min to homogenize the sample. Biofilm samples (100 μl) were collected from each reactor using a sterilized micropipette. To enumerate and determine the viability of the C. jejuni cells, a 10-fold dilution series of the biofilm sample was made in phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 10 mM NaH2PO4, 2 mM KH2PO4, pH 7.4). Aliquots (10 μl) were taken from each dilution tube and plated on modified charcoal cefoperazone deoxycholate agar (mCCDA) (CM739; Oxoid) with an antibiotic supplement that contains cefoperazone and amphotericin B (SR155E; Oxoid). The petri plates were incubated under microaerobic conditions at 42°C for 48 to 72 h until visible colonies formed, and CFU were counted (27).
We used a Live/Dead BacLight bacterial viability kit (Molecular Probes Inc., Eugene, OR) to determine the viability of C. jejuni in biofilms according to the differential cellular uptake of two different stains. The Live/Dead BacLight bacterial viability kit contains two separate solid dyes (components A [SYTO 9] and B [propidium iodide]). A dye solution was prepared by dissolving the contents of components A and B in separate vials containing 2.5 ml of filter-sterilized deionized water (2× solution). These separate solutions were blended (1:1) and used for biofilm staining. The final concentration of the dye solution was 6 μM SYTO 9 stain and 30 μM propidium iodide. Biofilm samples were placed in a sterile petri plate and stained immediately by adding ∼200 μl of stain to the biofilm surface. The stain was added gently to the edge of the top surface without disturbing the biofilm. The petri plate was then covered with a cover dish and incubated for 20 to 30 min at room temperature to obtain the desired staining in the absence of light. At the end of the staining time, the biofilm sample was rinsed gently with filtered 0.9% NaCl to remove excess stain. Finally, the biofilms were kept in petri plates containing 0.9% NaCl to protect cell integrity until the CLSM study. The stained biofilms were imaged using a CSLM (Carl Zeiss LSM510) using a 60×/1.4-numerical-aperture (NA) oil lens.
RESULTS
Biofilm structures.
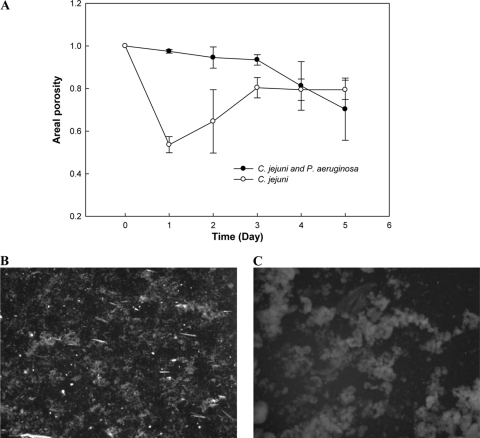
As expected, the areal porosity of mixed-culture biofilms decreased with time, demonstrating the growth of biofilms over time (Fig. 2A). In contrast, the areal porosities of the monoculture biofilms increased after day 1, indicating detachment of the biofilms. The areal porosity of the C. jejuni biofilms was ∼0.5 after inoculation, but it increased over time, which is only possible if the detachment rate is greater than the biofilm growth rate. The areal porosity of the monoculture biofilms increased until day 3, at which point any growth of biofilm was equivalent to the detachment. At day 5, the C. jejuni biofilms were generally composed of small colonies (Fig. 2B), while the mixed-culture biofilms were composed of large cell clusters (Fig. 2C). Statistical analysis (t test calculator; GraphPad) showed that the differences in areal porosity values for 5-day-old biofilms were statistically significant (P < 0.0001), and the biofilm images appeared different by visual inspection. Interestingly, only 4-day-old biofilms showed a difference in areal porosity values that was not statistically significant (P = 0.52). We observed a very similar pattern when the monoculture was incubated at 37°C. These results demonstrate that mixed-culture biofilms expand over time while monoculture biofilms detach until the biofilm structure reaches a pseudo-steady state.
Fig 2.
(A) Average areal porosity over time (± SD) for biofilms formed by mono- and mixed cultures at 25°C and at 0.75 ml/min (n = 3 replicates). The areal porosity of mixed-culture biofilms decreased over time, demonstrating the growth of biofilms over time. (B) Five-day-old monoculture biofilm. (C) Five-day-old mixed-culture biofilm. The size of images B and C is 1,200 μm by 1,000 μm.
Dissolved oxygen concentration profiles in both biofilms.
The DO concentration was almost zero in the mixed-culture biofilms, showing consumption of all of the oxygen in the biofilms (Fig. 3). The oxygen concentration in the recycle stream and at the inlet of the reactor was near the saturation concentration (∼7.8 mg/liter). Thus, all the oxygen delivered to the biofilm reactor was consumed by the mixed-culture biofilms. The DO concentration was approximately 7.8 mg/liter in monoculture biofilms, and this concentration was almost constant within the biofilm. The same measurements were repeated with the biofilms grown at 37°C, and identical results were found. The DO measurements of both biofilms indicate that C. jejuni in monoculture biofilms was not consuming oxygen.
Fig 3.
Representative dissolved oxygen concentration profiles in C. jejuni monoculture and C. jejuni plus P. aeruginosa mixed-culture biofilms. In both cases the measurements were performed in the middle parts of large clusters, where the biofilms were approximately 400 μm thick. Repeated measurements at different locations showed the same profiles.
Effect of flow rate on C. jejuni biofilm structures.
An increased flow rate increased areal porosity for the monoculture, and the higher flow rate corresponded to higher shear stress (see reference 23, p. 80 to 82) (Fig. 4). Statistical analysis (t test calculator; Graphpad) showed that the differences in areal porosities when biofilms were grown at 0.75 ml/min were not significant (P = 0.16). For biofilms grown at higher flow velocities, the differences in areal porosities were statistically significant (P < 0.0001). When we increased flow rates above 2.5 ml/min, we found that the areal porosity was approximately 1 (results not shown). This demonstrates that for flow velocities above 2.5 ml/min, the cells were detached. When we ran the same tests using a mixed culture, we found that areal porosity decreased slightly (∼0.02 unit) (Fig. 4), consistent with a limited impact of the flow rate on mixed-culture biofilms. The results in Fig. 4 demonstrate that C. jejuni biofilms can persist at low shear stress or flow rates but not at higher flow rates, whereas mixed-culture biofilms remain stable when shear stress is increased.
Fig 4.
Average areal porosity of C. jejuni biofilms (± SD) relative to the flow rate in the reactor. A lower porosity corresponds to a more developed biofilm.
Viability and culturability of C. jejuni in mono- and mixed-culture biofilms.
We found that after 5 days of biofilm growth, C. jejuni was not viable in monoculture biofilms. In contrast, C. jejuni found in mixed-culture biofilms averaged 3 × 105 CFU/ml. When we imaged live/dead cells in monoculture C. jejuni biofilms, we found that most of the cells appeared to be alive (Fig. 5). Nevertheless, we could not culture C. jejuni from monoculture biofilms, suggesting that C. jejuni monocultures enter a “viable but not culturable” (VBNC) state when maintained as a monoculture.
Fig 5.

Confocal images of C. jejuni in monoculture biofilms (5 days) stained for cell viability, containing a mixture of living (green) and dead (red) bacteria (magnification, ×60).
DISCUSSION
Biofilm formation.
Several epidemiological studies recently reported that C. jejuni was responsible for outbreaks of waterborne infections in humans (15, 17, 26). It is important to determine the bacterial characteristics, such as biofilm formation, that affect the ability of the bacteria to survive outside a host in aquatic environments. A detailed knowledge of C. jejuni biofilm structures under dynamic conditions similar to those of its natural environment may help to engineer water systems to limit C. jejuni exposure.
In the present study, biofilm formation by C. jejuni was investigated under flow conditions after 4 h of initial attachment rather than under static conditions. We found that C. jejuni monoculture grows as a biofilm regardless of the temperature tested (25°C or 37°C). Furthermore, C. jejuni forms a sparse monoculture biofilm when grown at a flow rate of 0.75 ml/min. When the flow rate increased above 2.5 ml/min, C. jejuni detached from the surface. At the end of the fifth day, we could not culture C. jejuni despite the fact that the bacterial cells in the biofilms appeared viable based on a commercial live/dead stain. C. jejuni is known to enter a VBNC state in which the bacterial cells cannot be detected by conventional culture methods but retain their morphology and remain viable when exposed to unfavorable conditions (20, 25, 40). The presence of VBNC cells has been clearly shown using different molecular methods, such as fluorescence-based methods and ethidium monoazide/real-time PCR, but there is no consensus as to how investigators can accurately determine bacterial viability (14, 14, 25). We used a fluorescence-based technique that distinguishes live cells from dead ones based on the presence of an intact cytoplasmic membrane. This method is rapid and not labor intensive compared to the other methods. He and Chen (2010) reported that the plate count method can recover only culturable cells and that BacLight staining may provide a better compromise in the detection of viable cells of C. jejuni (12). Our results are consistent with previously published results according to which Campylobacter spp. can enter a VBNC state consistent with failure to grow on standard bacteriological media.
Sanders et al. (2007) also showed that C. jejuni enters the VBNC state in biofilms (34). Bacteria in the VBNC state can be resuscitated by laboratory animal challenge and mucin treatment (2, 3, 5, 33). Although the significance of this state in the transmission of C. jejuni infection is uncertain, it has been reported that the development of the VBNC state could be a survival strategy in aquatic environments (22, 42, 45). Our results show that the survival of C. jejuni in the VBNC state in monoculture biofilm is likely.
The dissolved oxygen concentration profiles show that the C. jejuni monoculture biofilms were not consuming oxygen and the oxygen concentration was near the saturation level. This is consistent with the physiology of C. jejuni and the possibility that oxygen stress triggers entry into a VBNC state, at which point the bacteria do not multiply and the biofilm becomes static (Fig. 2A). It has also been previously reported that Campylobacter species enter the VBNC state accompanied by changes in cell morphology and in cellular activity under adverse environmental conditions, such as starvation, unsuitable temperatures, excess oxygen concentration, and elevated osmotic pressure (24, 25).
Effect of flow rate on C. jejuni biofilm formation.
Some researchers have reported that C. jejuni forms biofilms on a microplate under aerobic conditions and stagnant culture conditions (30, 31, 34). Growing biofilms under stagnant conditions protects C. jejuni against shear stress and detachment (30, 31, 34) and may permit the formation of microaerophilic conditions conducive to C. jejuni growth. Rollins and Colwell (1986) reported that C. jejuni entered the VBNC state more rapidly when left for incubation in microcosms subjected to shaking than in stagnant microcosms; this is consistent with the higher oxygen concentrations in shake flasks (32). Joshua et al. (2006) reported that C. jejuni bacteria did not attach to surfaces when they were grown in a shaker at a moderate rate; however, when the shaking rate was low, they observed that C. jejuni developed into a biofilm (16). They also found that at higher flow rates, C. jejuni did not form biofilms in a Robbins device (16). This is consistent with our findings that C. jejuni biofilms can persist at a low flow rate but cannot survive at higher flow rates.
Mixed-culture biofilms as a survival strategy.
The susceptibility of C. jejuni to environmental conditions outside the host has inspired a number of epidemiological studies focused on the survival mechanisms of the organism. In fact, one of the most studied mechanisms is the synergistic interaction between this bacterial species and the environment. Sanders et al. (2007) reported that C. jejuni cells might have enhanced persistence through attachment to preexisting biofilms of other bacteria in a water source (34). In addition, there are reports that C. jejuni can display multispecies biofilm formation in cases where surfaces are colonized primarily by different bacterial species (4, 11). Under aerobic conditions, such coexistence prolongs the survival of C. jejuni (13). Buswell et al. (1998) reported that the survival of C. jejuni was prolonged to almost twice as long in preestablished biofilms formed by autochthonous water microflora (4). Hanning (2008) indicated that the secondary attachment of C. jejuni to preestablished biofilms formed by bacteria isolated from poultry farms prolonged the survival of C. jejuni in external environments (11). Trachoo et al. (2002) reported that C. jejuni had enhanced attachment and survival when introduced onto a biofilm formed by Pseudomonas spp. (42). We determined that P. aeruginosa and C. jejuni formed multispecies biofilms concomitantly without any preestablished biofilm formation. The dissolved oxygen concentration in the mixed-culture biofilms at the end of the fifth day was approximately 0 mg/liter, demonstrating that most of the oxygen was consumed by the mixed-culture biofilm. We surmise that P. aeruginosa consumes oxygen and generates a favorable environment for C. jejuni growth and survival. We also expect that this coexistence prolongs the survival of C. jejuni in the natural environment. Similarly, Sanders (2007) reported that without any preestablished biofilm formation, under aerobic conditions a multispecies microcosm prolonged the survival of C. jejuni (34). Hilbert (2010) reported that C. jejuni had a longer survival time, despite oxygen stress, when cocultured with Pseudomonas spp. than when cocultured with other bacteria, including Proteus mirabilis, Citrobacter freundii, Micrococcus luteus, and Enterococcus faecalis (13).
In summary, our results show that monoculture C. jejuni cells attach to surfaces and develop monoculture biofilms under limited flow conditions. C. jejuni is not culturable from monoculture biofilms, and this is probably related to exposure to dissolved oxygen. C. jejuni is culturable from mixed-culture biofilms, in which dissolved oxygen is presumably consumed by biofilm partners through aerobic respiration. The C. jejuni biofilm structure is not robust in the presence of moving fluid, presumably because of sensitivity to sheer forces.
ACKNOWLEDGMENT
Tuba Ica acknowledges support from TUBITAK-BIDEB 2219 (The Scientific and Technological Research Council of Turkey).
Footnotes
Published ahead of print 16 December 2011
REFERENCES
- 1. Abulreesh HH, Paget TA, Goulder R. 2006. Campylobacter in waterfowl and aquatic environments: incidence and methods of detection. Environ. Sci. Technol. 40:7122–7131 [DOI] [PubMed] [Google Scholar]
- 2. Baffone W, et al. 2006. Campylobacter jejuni loss of culturability in aqueous microcosms and ability to resuscitate in a mouse model. Int. J. Food Microbiol. 107:83–91 [DOI] [PubMed] [Google Scholar]
- 3. Bovill RA, Mackey BM. 1997. Resuscitation of ‘non-culturable’ cells from aged cultures of Campylobacter jejuni. Microbiology 143:1575–1581 [DOI] [PubMed] [Google Scholar]
- 4. Buswell CM, et al. 1998. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl. Environ. Microbiol. 64:733–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cappelier JM, Minet J, Magras C, Colwell RR, Federighi M. 1999. Recovery in embryonated eggs of viable but nonculturable Campylobacter jejuni cells and maintenance of ability to adhere to HeLa cells after resuscitation. Appl. Environ. Microbiol. 65:5154–5157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. CDC 2006. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 States, United States, 2005. MMWR Morb. Mortal. Wkly. Rep. 55:392–395. [PubMed] [Google Scholar]
- 7. Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappinscott HM. 1995. Microbial biofilms. Annu. Rev. Microbiol. 49:711–745 [DOI] [PubMed] [Google Scholar]
- 8. Flanders JR, Yildiz FH. 2004. Biofilms as reservoirs for disease, p 314–331 In Ghannoum M, O'Toole GA. (ed), Microbial biofilms. ASM Press, Washington, DC [Google Scholar]
- 9. Friedman CR, et al. 2004. Risk factors for sporadic Campylobacter infection in the United States: A case-control study in FoodNet sites. Clin. Infect. Dis. 38:S285–S296 [DOI] [PubMed] [Google Scholar]
- 10. Gregory E, Barnhart H, Dreesen DW, Stern NJ, Corn JL. 1997. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 41:890–898 [PubMed] [Google Scholar]
- 11. Hanning I, Jarquin R, Slavik M. 2008. Campylobacter jejuni as a secondary colonizer of poultry biofilms. J. Appl. Microbiol. 105:1199–1208 [DOI] [PubMed] [Google Scholar]
- 12. He YP, Chen CY. 2010. Quantitative analysis of viable, stressed and dead cells of Campylobacter jejuni strain 81-176. Food Microbiol. 27:439–446 [DOI] [PubMed] [Google Scholar]
- 13. Hilbert F, Scherwitzel M, Paulsen P, Szostak MP. 2010. Survival of Campylobacter jejuni under conditions of atmospheric oxygen tension with the support of Pseudomonas spp. Appl. Environ. Microbiol. 76:5911–5917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inoue D, et al. 2008. Application of real-time polymerase chain reaction (PCR) coupled with ethidium monoazide treatment for selective quantification of viable bacteria in aquatic environment. Water Sci. Technol. 58:1107–1112 [DOI] [PubMed] [Google Scholar]
- 15. Jakopanec I, et al. 2008. A large waterborne outbreak of campylobacteriosis in Norway: the need to focus on distribution system safety. BMC Infect. Dis. 8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Joshua GWP, Guthrie-Irons C, Karlyshev AV, Wren BW. 2006. Biofilm formation in Campylobacter jejuni. Microbiology 152:387–396 [DOI] [PubMed] [Google Scholar]
- 17. Karagiannis I, et al. 2010. A waterborne Campylobacter jejuni outbreak on a Greek island. Epidemiol. Infect. 138:1726–1734 [DOI] [PubMed] [Google Scholar]
- 18. Kemp R, et al. 2005. Prevalence and genetic diversity of Campylobacter spp. in environmental water samples from a 100-square-kilometer predominantly dairy farming area. Appl. Environ. Microbiol. 71:1876–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kovats RS, et al. 2005. Climate variability and campylobacter infection: an international study. Int. J. Biometeorol. 49:207–214 [DOI] [PubMed] [Google Scholar]
- 20. Lazaro B, Carcamo J, Audicana A, Perales I, Fernandez-Astorga A. 1999. Viability and DNA maintenance in nonculturable spiral Campylobacter jejuni cells after long-term exposure to low temperatures. Appl. Environ. Microbiol. 65:4677–4681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee MD, Newell DG. 2006. Campylobacter in poultry: filling an ecological niche. Avian Dis. 50:1–9 [DOI] [PubMed] [Google Scholar]
- 22. Lehtola MJ, Pitkanen T, Miebach L, Miettinen IT. 2006. Survival of Campylobacter jejuni in potable water biofilms: a comparative study with different detection methods. Water Sci. Technol. 54:57–61 [DOI] [PubMed] [Google Scholar]
- 23. Lewandowski Z, Beyenal H. 2007. Fundamentals of biofilm research. CRC Press, Boca Raton, FL [Google Scholar]
- 24. Murphy C, Carroll C, Jordan KN. 2006. Environmental survival mechanisms of the foodborne pathogen Campylobacter jejuni. J. Appl. Microbiol. 100:623–632 [DOI] [PubMed] [Google Scholar]
- 25. Oliver JD. 2005. The viable but nonculturable state in bacteria. J. Microbiol. 43:93–100 [PubMed] [Google Scholar]
- 26. O'Reilly CE, et al. 2007. A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clin. Infect. Dis. 44:506–512 [DOI] [PubMed] [Google Scholar]
- 27. Oyarzabal OA, Macklin KS, Barbaree JM, Miller RS. 2005. Evaluation of agar plates for direct enumeration of Campylobacter spp. from poultry carcass rinses. Appl. Environ. Microbiol. 71:3351–3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oyofo BA, Rollins DM. 1993. Efficacy of filter types for detecting Campylobacter jejuni and Campylobacter coli in environmental water samples by polymerase chain reaction. Appl. Environ. Microbiol. 59:4090–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pearson AD, et al. 1993. Colonization of broiler chickens by waterborne Campylobacter jejuni. Appl. Environ. Microbiol. 59:987–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Reeser RJ, Medler RT, Billington SJ, Jost BH, Joens LA. 2007. Characterization of Campylobacter jejuni biofilms under defined growth conditions. Appl. Environ. Microbiol. 73:1908–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reuter M, Mallett A, Pearson BM, van Vliet AHM. 2010. Biofilm formation by Campylobacter jejuni is increased under aerobic conditions. Appl. Environ. Microbiol. 76:2122–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rollins DM, Colwell RR. 1986. Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl. Environ. Microbiol. 52:531–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Saha SK, Saha S, Sanyal SC. 1991. Recovery of injured Campylobacter jejuni cells after animal passage. Appl. Environ. Microbiol. 57:3388–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sanders SQ, Boothe DH, Frank JF, Arnold JW. 2007. Culture and detection of Campylobacter jejuni within mixed microbial populations of biofilms on stainless steel. J. Food Prot. 70:1379–1385 [DOI] [PubMed] [Google Scholar]
- 35. Schlundt J, Toyofuku H, Jansen J, Herbst SA. 2004. Emerging food-borne zoonoses. Rev. Sci. Tech. 23:513–533 [DOI] [PubMed] [Google Scholar]
- 36. Sheppard SK, et al. 2009. Campylobacter genotypes from food animals, environmental sources and clinical disease in Scotland 2005/6. Int. J. Food Microbiol. 134:96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stanley K, Cunningham R, Jones K. 1998. Isolation of Campylobacter jejuni from groundwater. J. Appl. Microbiol. 85:187–191 [DOI] [PubMed] [Google Scholar]
- 38. Stoodley P, Sauer K, Davies DG, Costerton JW. 2002. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 56:187–209 [DOI] [PubMed] [Google Scholar]
- 39. Teh KH, Flint S, French N. 2010. Biofilm formation by Campylobacter jejuni in controlled mixed-microbial populations. Int. J. Food Microbiol. 143:118–124 [DOI] [PubMed] [Google Scholar]
- 40. Tholozan JL, Cappelier JM, Tissier JP, Delattre G, Federighi M. 1999. Physiological characterization of viable-but-nonculturable Campylobacter jejuni cells. Appl. Environ. Microbiol. 65:1110–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tolker-Nielsen T, Molin S. 2000. Spatial organization of microbial biofilm communities. Microb. Ecol. 40:75–84 [DOI] [PubMed] [Google Scholar]
- 42. Trachoo N, Frank JF, Stern NJ. 2002. Survival of Campylobacter jejuni in biofilms isolated from chicken houses. J. Food Prot. 65:1110–1116 [DOI] [PubMed] [Google Scholar]
- 43. Waage AS, Vardund T, Lund V, Kapperud G. 1999. Detection of small numbers of Campylobacter jejuni and Campylobacter coli cells in environmental water, sewage, and food samples by a seminested PCR assay. Appl. Environ. Microbiol. 65:1636–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wimpenny JWT, Peters A, Scourfield M. 1989. Modeling spatial gradients, p. 111–127 In Characklis WG, Wilderer PA. (ed), Structure and function of biofilms. Chichester, John Wiley, Chichester, United Kingdom [Google Scholar]
- 45. Zimmer M, Barnhart H, Idris U, Lee MD. 2003. Detection of Campylobacter jejuni strains in the water lines of a commercial broiler house and their relationship to the strains that colonized the chickens. Avian Dis. 47:101–107 [DOI] [PubMed] [Google Scholar]