Abstract
Multitarget genotyping of the etiologic agent Mycobacterium avium subsp. paratuberculosis is necessary for epidemiological tracing of paratuberculosis (Johne's disease). The study was undertaken to assess the informative value of different typing techniques and individual genome markers by investigation of M. avium subsp. paratuberculosis transmission between wild-living red deer and farmed cattle with known shared habitats. Fifty-three M. avium subsp. paratuberculosis type II isolates were differentiated by short sequence repeat analysis (SSR; 4 loci), mycobacterial interspersed repetitive-unit–variable-number tandem-repeat analysis (MIRU-VNTR; 8 loci), and restriction fragment length polymorphism analysis based on IS900 (IS900-RFLP) using BstEII and PstI digestion. Isolates originated from free-living red deer (Cervus elaphus) from Eifel National Park (n = 13), six cattle herds living in the area of this park (n = 23), and five cattle herds without any contact with these red deer (n = 17). Data based on individual herds and genotypes verified that SSR G2 repeats did not exhibit sufficient stability for epidemiological studies. Two common SSR profiles (without G2 repeats), nine MIRU-VNTR patterns, and nine IS900-RFLP patterns were detected, resulting in 17 genotypes when combined. A high genetic variability was found for red deer and cattle isolates within and outside Eifel National Park, but it was revealed only by combination of different typing techniques. Results imply that within this restricted area, wild-living and farmed animals maintain a reservoir for specific M. avium subsp. paratuberculosis genotypes. No host relation of genotypes was obtained. Results suggested that four genotypes had been transmitted between and within species and that one genotype had been transmitted between cattle herds only. Use of multitarget genotyping for M. avium subsp. paratuberculosis type II strains and sufficiently stable genetic markers is essential for reliable interpretations of epidemiological studies on paratuberculosis.
INTRODUCTION
Mycobacterium avium subsp. paratuberculosis is the causative agent of paratuberculosis (Johne's disease), which appears worldwide in domestic and wild ruminants. Infected cattle herds were considered to be the source of infection for wild ruminants grazing on the same contaminated pastures (19, 27, 32). M. avium subsp. paratuberculosis was detected in a wide range of wild-living ruminants other than cattle, sheep, goats, and farmed deer (20), including different species of deer, mouflon, camelids, bison, and moose, and in nonruminant wildlife (6, 7). A valuable tool for epidemiological tracing is genotyping of M. avium subsp. paratuberculosis organisms originating from animals that suffer from paratuberculosis. Different molecular typing techniques have been used in a variety of studies worldwide for M. avium subsp. paratuberculosis genotyping (see references 1, 3, 4, 5, 21, 26, 33, 34, 36, 37, 38, and 43, as well as many others). An important step in the history of M. avium subsp. paratuberculosis differentiation was the detection of the two main M. avium subsp. paratuberculosis groups, type I (predominantly isolated from sheep) and type II (also called cattle type, with a broad host range), as well as type III (intermediate type, predominantly isolated from sheep and goats), which is closely related to type I (5, 9, 34). In order to differentiate M. avium subsp. paratuberculosis and in order to identify epidemiological linkages more specific than these main groups, sophisticated subtyping techniques exhibiting an adequate discriminatory power were demanded. Over a long period, restriction fragment length polymorphism analysis based on the insertion sequence IS900 (IS900-RFLP) and macrorestriction analysis using pulsed-field gel electrophoresis (PFGE), used as fingerprint methods, were the techniques mainly used for M. avium subsp. paratuberculosis subtyping. However, these tools are technically laborious and require large quantities of DNA. PCR- and sequence-based methods, such as mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) analysis and short sequence repeat (SSR) analysis, were developed to reduce the technical effort and increase the reproducibility of the results (1, 36). Altogether, however, individual typing methods do not reveal a sufficient discriminatory power for exhaustive epidemiological studies, especially concerning the M. avium subsp. paratuberculosis type II group. Due to a lack of correlation between typing results (21, 33, 37), interpretations with regard to epidemiological linkages depend on the typing method used. On the other hand, the discriminatory power was markedly improved when typing techniques were combined (21, 35, 37).
The major aim of the current study was to prove a proposed strategy of strain discrimination by a successive arrangement of MIRU-VNTR, SSR, and IS900-RFLP analyses (37) and to find out if IS900-RFLP could be replaced without producing epidemiological misinterpretations. Additionally, the informative value of individual genome markers was verified by detection of M. avium subsp. paratuberculosis transmission between wild-living red deer and farmed cattle within a geographically restricted area. The new approach of this study was to select a unique strain panel based on detailed previous knowledge about possible epidemiological associations (shared habitats) between the studied M. avium subsp. paratuberculosis isolates.
MATERIALS AND METHODS
Origin of bacterial isolates.
A total of 53 M. avium subsp. paratuberculosis isolates, collected between 2004 and 2006, were included in the present study. Thirteen M. avium subsp. paratuberculosis strains were recovered from wild-living red deer (Cervus elaphus) in the Eifel region which were shot down in two distinct forest districts (A and B) of Eifel National Park (Fig. 1). These animals were examined for paratuberculosis during hunting season because paratuberculosis had been diagnosed in fallen deer before. The diagnosis was based on characteristic intestinal lesions of granulomatous enteritis and lymphadenitis and on isolation of M. avium subsp. paratuberculosis from tissue and feces.
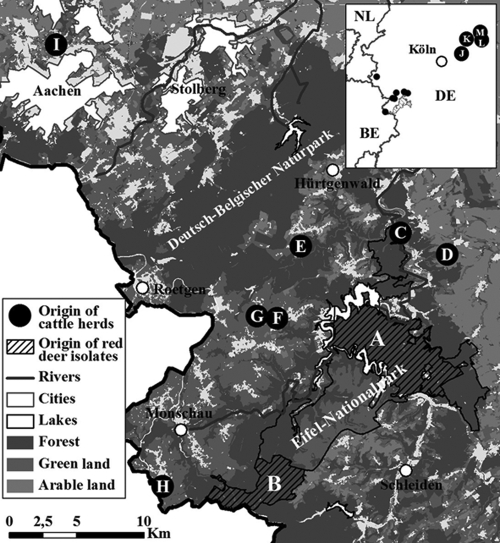
Fig 1.
Map of Eifel National Park and Aachen (large map) and the area northeast of Cologne (Köln, inset map) in North Rhine-Westphalia showing locations of regional origin of Mycobacterium avium subsp. paratuberculosis isolates from wild-living red deer (Cervus elaphus) and cattle. Areas between the forest districts A and B and areas north and northeast of district A belong to the national park and are freely accessible for red deer, including the adjacent green and arable lands. The six cattle herds C to H were situated in neighborhoods adjacent to Eifel National Park (2 to 6 km), cattle herd I was 35 km away from the park, and cattle herds J, K, L, and M were 120 km away from the park.
It was recorded that red deer continuously changed their habitat, crossing roads and rivers around the entire Eifel National Park. Hence, wild-living red deer were observed to share grazing areas with livestock and to graze fertilized pastures contaminated by bovine slurry in areas around the park. Twenty-three bovine isolates originating from six herds (C, D, E, F, G, and H) kept in areas near the A and B forest districts, as shown in Fig. 1, were included in this investigation. Additionally, 17 bovine isolates originating from five herds located 40 km (herd I) and 120 km (herds J, K, L, and M) away from Eifel National Park were studied. The cattle were red and black Holstein Friesian dairy cattle.
The investigated isolates originated from intestinal necropsy samples (red deer) and feces (cattle) of animals with suspected paratuberculosis.
Culture method.
Isolates had been cultured using Herrold's egg yolk medium (HEYM) supplemented with mycobactin J (Allied Monitor, Inc., Fayette, MO) for up to 9 months. One colony was then subcultivated using the same medium. M. avium subsp. paratuberculosis was identified by Ziehl-Neelsen staining of acid-fast bacilli, mycobactin J dependence, and a long growth time, as well as by detection of IS900 using PCR (12). Cross-contamination by other M. avium subspecies was excluded by a lack of insertion element IS1245 using PCR (15). A subculture was established using Middlebrook 7H9 broth containing oleic acid-albumin-dextrose-catalase (OADC) and mycobactin J, followed by mass cultivation on modified Lowenstein-Jensen medium and Stonebrink medium (Bioservice Waldenburg, Waldenburg, Germany) supplemented by mycobactin J.
Genotyping. (i) Preparation of DNA for genotyping.
DNA isolation was obtained by the standardized cetyltrimethylammonium bromide (CTAB) method (41). Briefly, the DNA was solubilized with lysozyme, sodium dodecyl sulfate (SDS)/proteinase K, and CTAB solution, precipitated with isopropanol, washed with alcohol, and then dissolved in aqua bidest. The DNA concentration was measured by a spectrophotometer (Nanodrop ND-1000; Peqlab, Germany).
(ii) SSR typing.
Short sequence repeat (SSR) sequencing was performed according to the procedure of Amonsin et al. (1). Four SSR loci were chosen: loci 1, 2, 8, and 9 (G1, G2, GGT, and TGC repeats, respectively). As a modification, a specific polymerase (Herculase II Fusion DNA polymerase; Agilent Technologies) was used for amplification of G1 and G2 repeats to minimize the risk of polymerase slippage at poly(G) motifs. The products were separated by agarose gel electrophoresis (1% agarose standard electro endosmosis [EEO]; SERVA, Heidelberg, Germany) and purified with the QIAquick gel extraction kit (Qiagen). Sequencing of the purified material was performed by external sequencing companies.
(iii) MIRU-VNTR typing.
For mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) typing, eight target regions throughout the M. avium subsp. paratuberculosis genome, loci 292, X3, 25, 47, 3, 7, 10, and 32, were investigated by specific PCRs as described by Thibault et al. (36). Primers, PCR conditions, and PCR product analysis were applied according to the above-mentioned publications, with slight modifications according to the procedure of Möbius et al. (21). Results, the numbers of tandem repeats, were arranged in the same order as mentioned above or designated according to the INRA Nouzilly MIRU-VNTR patterns (36).
(iv) IS900-RFLP analysis.
IS900-RFLP analysis, including the preparation of an IS900-specific digoxigenin (DIG)-labeled DNA probe, was carried out as described in detail by Möbius et al. (21). Briefly, purified genomic DNA (2 μg) was digested by two enzymes, BstEII and PstI (New England BioLabs, Frankfurt am Main, Germany). Fragments were separated by agarose gel electrophoresis and, together with an external molecular size marker in three lanes (both edges and middle) of each gel, transferred to a Hybond-N nylon membrane (Amersham Biosciences, Little Chalfont, United Kingdom) by vacuum blotting, heat fixed (2 h, 80°C), and hybridized at 68°C with the DIG-labeled probe overnight. After washing steps using different concentrations of SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) buffers, the restricted DNA was visualized as specific band patterns with the help of the CSPD {disodium 3-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclo[3.3.1.13,7]decan}-4-yl)phenyl phosphate} detection system (Roche Diagnostics) and subsequent exposure to Hyperfilm ECL (GE Healthcare, Freiburg, Germany).
BstEII–IS900-RFLP patterns were designated according to Pavlik et al. (26), Cnew1 (Cn1) was designated according to Möbius et al. (21), and Cnew2 (Cn2) and Cnew3 (Cn3) were novel patterns. PstI–IS900-RFLP patterns—using the probe sequence recommended within the standardized IS900-RFLP protocol (26)—were designated according to Whipple et al. (42) and Möbius et al. (21).
(v) Discrimination value of typing.
The following formula (17a) was used for the calculation of the numerical index for the discriminatory power of the combination of typing techniques:
DI is the discriminatory index. N is the total number of epidemiologically unrelated strains. S is the total number of distinct patterns discriminated by the respective typing method. nj is the number of epidemiologically unrelated strains belonging to the jth pattern.
All red deer isolates were considered epidemiologically related. Apart from that, the DI was calculated regardless of possible epidemiological relations between the cattle herds and the red deer.
RESULTS
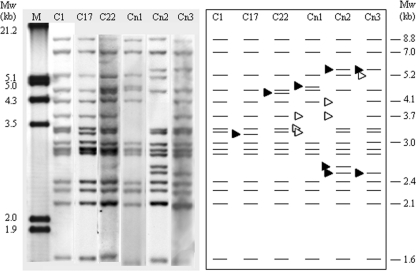
All 53 M. avium subsp. paratuberculosis isolates were identified to belong to the cattle group designated M. avium subsp. paratuberculosis type II by the following characteristics: growth after 6 to 12 weeks, nonpigmented creamy-colored colonies, smooth colony morphology, and BstEII-RFLP patterns (Fig. 2) with C (cattle) type characteristic bands at 8.8, 7.0, and 1.6 kb that were unlike those of the S (sheep) and I (intermediate) RFLP pattern types (26) detected for M. avium subsp. paratuberculosis type I (34) and M. avium subsp. paratuberculosis type III (9) isolates, respectively. Typing results of SSR, MIRU-VNTR, and IS900-RFLP analyses are summarized in Table 1, together with the origins of the isolates and the genotypes I to V that were determined in more than one herd. The results give an overview of the natural diversity of M. avium subsp. paratuberculosis in a defined area. The individual markers of typing techniques contributed differently to this genetic diversity. Within the SSR typing, G2 repeats (locus 2) exhibited very high diversity compared to that of G1 repeats (locus 1), GGT repeats (locus 8), and TGC repeats (locus 9); seven alleles (9G to 15G) were detected for G2 repeats, compared to one or two for the others. Results at SSR locus 2 were handled with care because of strong stutter peak effects discussed recently by Thibault et al. (37). The numbers of different alleles at each of the studied MIRU-VNTR loci ranged between only one allele (at loci 3 and 10), two alleles (at loci X3, 25, 7, and 32), three alleles (at locus 47), and four alleles (at locus 292). IS900-RFLP resulted in six BstEII patterns (C1, C17, C22, Cnew1, Cnew2, and Cnew3), five PstI patterns (P1, P3, P7, P8, and P10), and nine combined patterns. Hence, two new profiles which have never been published before were found by IS900-RFLP and BstEII digestion (Cnew2 and Cnew3) (Fig. 2). Using MIRU-VNTR, one novel allele with only one repeat was detected at MIRU-VNTR locus 292.
Fig 2.
IS900-RFLP profiles of Mycobacterium avium subsp. paratuberculosis isolates detected within the present study after digestion with BstEII. The numbers above the lanes are the IS900-RFLP type designations. The profiles C2, C17, and C22 were designated according to Pavlik et al. (26), and Cn1 (Cnew1) was designated according to Möbius et al. (21). Profiles Cn2 and Cn3 (Cnew2 and Cnew3) have never been published before. The arrowheads in the schematic diagram show the differences between type C1 and the other detected profiles. The black arrowheads designate additional or lightly shifted bands; the white arrowheads mark the absence of individual bands within the specific patterns. Lane M represents the molecular weight marker III (Roche Diagnostics). The numbers on the right side of the schematic patterns indicate the sizes of the reference bands, according to Overduin et al. (25).
Table 1.
Molecular characteristics of 53 Mycobacterium avium subsp. paratuberculosis isolates originating from different hosts within and outside Eifel National Park and assumed cross-species and intraspecies Mycobacterium avium subsp. paratuberculosis transmissions
| Geographic region (distance from park [km]) | Regional origin/herda | Host animal | No. of isolates | Strain no.b | SSR genotypec | MIRU-VNTR genotyped | INMV no.e | IS900-RFLP type after digestion withf: |
Genotype transmittedg | |
|---|---|---|---|---|---|---|---|---|---|---|
| BstEII | PstI | |||||||||
| Eifel National Park | A | Red deer | 1 | 05A0159 | 7–[10]–4–4 | 42332128 | 19 | C1 | P3 | I* |
| 1 | 06A0820 | 7–[11]–4–4 | 42332128 | 19 | C1 | P3 | I* | |||
| 2 | 06A0821, 06A0822 | 7–[10]–5–5 | 32332228 | 2 | Cn1 | P7 | II* | |||
| B | Red deer | 3 | 06A0815, 06A0818, 06A1285 | 7–[10]–5–5 | 32332228 | 2 | Cn1 | P7 | II* | |
| 1 | 06A0816 | 7–[10]–4–4 | 32332228 | 2 | C17 | P8 | III* | |||
| 1 | 06A0817 | 7–[11]–4–4 | 32332228 | 2 | C17 | P8 | III* | |||
| 1 | 06A0819 | 7–[12]–4–4 | 32332228 | 2 | C17 | P8 | III* | |||
| 1 | 06A1280 | 7–[10]–4–4 | 42332228 | 1 | C17 | P8 | IV* | |||
| 1 | 06A0813 | 7–[11]–4–4 | 42342228 | C17 | P8 | |||||
| 1 | 06A0814 | 7–[11]–4–4 | 32332228 | 2 | C22 | P7 | ||||
| C | Cattle | 1 | 06A0826 | 7–[13]–4–4 | 42332128 | 19 | C1 | P3 | I* | |
| 1 | 06A0830 | 7–[10]–5–5 | 32332228 | 2 | Cn1 | P7 | II* | |||
| 1 | 06A1107 | 7–[11]–4–4 | 42332128 | 19 | Cn2 | P3 | ||||
| D | Cattle | 1 | 06A1108 | 7–[10]–4–4 | 42332128 | 19 | Cn3 | P3 | ||
| 1 | 05A2674 | 7–[11]–4–4 | 42332128 | 19 | Cn3 | P3 | ||||
| E | Cattle | 1 | 06A1100 | 7–[10]–4–4 | 42332228 | 1 | C17 | P8 | IV* | |
| 1 | 06A1097 | 7–[15]–4–4 | 32332228 | 2 | C1 | P1 | V | |||
| F | Cattle | 1 | 06A0997 | 7–[09]–4–4 | 32332228 | 2 | C17 | P8 | III* | |
| 1 | 06A0993 | 7–[10]–4–4 | 32332228 | 2 | C17 | P8 | III* | |||
| 3 | 06A0996, 06A1264, 06A1265 | 7–[11]–4–4 | 32332228 | 2 | C17 | P8 | III* | |||
| 2 | 06A0990, 06A0994 | 7–[12]–4–4 | 32332228 | 2 | C17 | P8 | III* | |||
| 2 | 06A1263, 06A0995 | 7–[13]–4–4 | 32332228 | 2 | C17 | P8 | III* | |||
| 1 | 06A0991 | 7–[14]–4–4 | 32332228 | 2 | C17 | P7 | ||||
| 1 | 06A0998 | 7–[12]–4–4 | 32332228 | 2 | C1 | P1 | V | |||
| G | Cattle | 2 | 06A1268, 06A1271 | 7–[10]–4–4 | 42332128 | 19 | C1 | P3 | I* | |
| 1 | 06A1270 | 7–[10]–4–4 | 42332228 | 1 | C17 | P8 | IV* | |||
| H | Cattle | 1 | 05A3269 | 7–[10]–4–4 | 32332228 | 2 | C1 | P1 | V | |
| 1 | 05A3271 | 7–[11]–4–4 | 32332228 | 2 | C1 | P1 | V | |||
| Aachen (40) | Ih | Cattle | 1 | 04A1398 | 7–[9]–4–4 | 42332228 | 1 | C17 | P8 | IV* |
| 1 | 04A1382 | 7–[10]–4–4 | 42332228 | 1 | C17 | P8 | IV* | |||
| 2 | 04A1384, 04A1387 | 7–[9]–4–4 | 42332128 | 19 | C1 | P10 | ||||
| 2 | 04A1381, 05A3258 | 7–[10]–4–4 | 42332128 | 19 | C1 | P10 | ||||
| 1 | 04A1383 | 7–[10]–4–4 | 43332128 | 19 | C1 | P3 | ||||
| 1 | 05A2669 | 7–[11]–4–4 | 43332128 | 19 | C1 | P3 | ||||
| 1 | 05A2670 | 7–[12]–4–4 | 43332128 | 19 | C1 | P3 | ||||
| Bergischer Kreis, northeast of Cologne (120) | J | Cattle | 1 | 05A0158 | 7–[11]–4–4 | 42332228 | 1 | C1 | P1 | |
| K | Cattle | 1 | 05A3268 | 7–[9]–4–4 | 12322226 | new | C1 | P1 | ||
| 1 | 05A3267 | 7–[10]–4–4 | 12322226 | new | C1 | P1 | ||||
| 1 | 05A3265 | 7–[10]–4–4 | 12522226 | new | C1 | P1 | ||||
| 1 | 05A3263 | 7–[12]–4–4 | 12322126 | new | C1 | P1 | ||||
| L | Cattle | 1 | 05A2760 | 7–[10]–5–5 | 32332228 | 2 | Cn1 | P7 | II* | |
| M | Cattle | 1 | 05A2765 | 7–[12]–4–4 | 32332228 | 2 | C1 | P1 | V | |
| 1 | 05A2763 | 7–[12]–4–4 | 22522228 | 12 | C1 | P1 | ||||
Regional origins of red deer/cattle herds are shown in detail in Fig. 1.
Strain number within the strain collection of the Friedrich-Loeffler-Institut in Jena, Germany.
Short sequence repeat genotypes were indicated by the numbers of G1 and G2 repeats (loci 1 and 2, respectively), GGT repeats (locus 8), and TGC repeats (locus 9) according to Amonsin et al. (1). Genotypes are listed in the format 1-[2]-8-9, with the number of repeats of each locus in the place of the locus number. G2 repeats (locus 2) were enclosed in brackets because of polymerase slippage during genotyping and the suspicion that they are not conserved enough for epidemiological studies.
Number of tandem repeats at MIRU-VNTR loci 292, X3, 25, 47, 3, 7, 10, and 32, according to Thibault et al. (36). The first number in the 8-digit genotype corresponds to the number of repeats at locus 292, the second number corresponds to the number of repeats at locus X3, etc.
Designation according to the INRA Nouzilly MIRU-VNTR nomenclature (36) for MIRU-VNTR patterns.
After digestion with BstEII, IS900-RFLP types C1, C17, and C22 were designated according to the nomenclature of Pavlik et al. (26) and Cn1 was designated according to Möbius et al. (21). Two novel profiles were detected (Cn2 and Cn3). After digestion with PstI, types P1 and P3 were designated according to the nomenclature of Whipple et al. (42) and types P7, P8, and P10 were designated according to Möbius et al. (21).
Genotypes which were detected in more than one herd or forest district and assumed to show cross-species (additionally designated by an asterisk) and intraspecies Mycobacterium avium subsp. paratuberculosis transmission were designated with roman numerals. Results of SSR G2 repeats were excluded.
Nine isolates from a total of 15 included in a herd previously examined by Möbius et al. (21) without SSR genotyping were again subcultivated and genotyped, and the isolates were included in the current study as part of a herd originating from North Rhine-Westphalia but far from Eifel National Park.
Concerning the investigation of epidemiological linkages, a comparison of the results indicated that separate analysis of individual typing methods leads to quite different relationships between the investigated isolates (Table 1). SSR analysis using four SSR loci resulted in genotypes that did not correlate with the nine detected MIRU-VNTR types or the nine IS900-RFLP profiles. One exception was the genotype 7-[10]-5-5/3233228/Cn1-P7, in which the SSR profile 7-[10]-5-5 was detected together with the IS900-RFLP pattern Cn1-P7. There were several matches between IS900-RFLP patterns and specific MIRU-VNTR patterns in which IS900-RFLP analysis revealed a higher discriminatory power, but IS900-RFLP type C1-P1 was also subdivided into six MIRU-VNTR genotypes (Table 2).
Table 2.
Individual genotypes subdivided by a second typing method
| First typing method | Genotype | No. of subdivisions | Second typing method | RFLP types or MIRU-VNTR profiles |
|---|---|---|---|---|
| MIRU-VNTR | 23322228 | 5 | RFLP | C1-P1, C17-P7, C17-P8, Cn1-P7, C22-P7 |
| 42332128 | 4 | RFLP | C1-P3, Cn2-P3, Cn3-P3, C1-P10 | |
| RFLP | C1-P1 | 6 | MIRU-VNTR | 42332228, 32332228, 22522228, 12322226, 12522226, 12322126 |
The combined SSR, MIRU-VNTR, and IS900-RFLP typing resulted in a more sufficient high-resolution differentiation of M. avium subsp. paratuberculosis strains and provided the basis for interpretation of typing data in the present study.
Within individual herds, a number of isolates which differed only in the number of SSR G2 repeats, exhibiting between 9 and 15 G2 repeats, were found (within cattle herds D, F, H, I, and K and red deer from forest B). The same applies to isolates with genotypes I*, III*, IV*, and V (Table 1). All other typing data exhibited identical results for these specific isolates. These results confirm the assessment of Thibault et al. (37) that SSR alleles with more than 11 repeats at loci 1 and 2 cannot be read reliably. The variable results at locus 2 were based on data of individual herds and also included 9 and 10 G2 repeats, suggesting that during the long infection time, the M. avium subsp. paratuberculosis genome changed in this region within relatively short periods of time. However, besides the suspicion of slippage during M. avium subsp. paratuberculosis replication or amplification for sequence analysis at loci with a high number of single nucleotide repeats, it is supposed that G2 alleles are not sufficiently conserved for epidemiological studies. Therefore, results were analyzed without the SSR G2 repeats.
Altogether, 17 genotypes were found for this panel of isolates using a combination of results from the three typing techniques with a mutually additive DI value of 0.95 (Hunter and Gaston index). As shown in Table 1, identical patterns of isolates of different origin were designated by the “genotype transmitted” (I to V), which represents suspected epidemiological linkages. Transmitted genotype designations labeled with an asterisk represent genotypes which were possibly cross-species transmitted.
Genotype I*, one of the most common genotypes in Germany, was detected in red deer isolates originating from forest district A and in the adjoining cattle herds C and G, both only about 6 km away. Genotype II*, exhibiting an IS900-RFLP pattern not common in Germany, was also detected repeatedly in the entire red deer population tested (forest districts A and B), in the adjoining cattle herd C, and additionally in herd L, which was far away from Eifel National Park. The majority of isolates originating from cattle herd F showed genotype III*. This genotype was also found in 50% of the wild red deer isolates originating from the neighboring forest district B. Two red deer isolates exhibited a genotype very similar to III*, IV*; this genotype was also detected in herd G within Eifel National Park and in herd I from Aachen, Germany. Genotype 7-4-4/32332228/C22-P7 was only identified in wild-living red deer (forest district B). The most common genotype for cattle in Germany, genotype V (data not yet published), was detected in four cattle herds: herds E, F, and H around Eifel National Park and herd M, northeast of Cologne (Fig. 1). This genotype was not detected within the red deer isolates studied. Isolates from cattle herd I (Aachen) exhibited two individual genotypes not found within Eifel National Park or within the cattle isolates from the Bergischer Kreis district: genotypes 7-4-4/42332128/C1-P10 and 7-4-4/43332128/C1-P3. The MIRU-VNTR profiles of isolates from herd K subdivided the SSR/IS900-RFLP pattern 7-4-4/C1-P1 into three subtypes: 12322226, 12522226, and 12322126. These are unique genotypes. The genotype 7-4-4/22522228/C1-P1 of one isolate of herd M was also unique.
Consequently, only by combination of SSR (3 loci), MIRU-VNTR (7 loci), and IS900-RFLP (2 digestion enzymes) analysis was it possible to profoundly differentiate M. avium subsp. paratuberculosis isolates and to verify cross-species and intraspecies transmission of strains, reflecting the present epidemiological situation within a local area.
DISCUSSION
These are the first published genotyping data demonstrating M. avium subsp. paratuberculosis transmission between wildlife and livestock based on results of three typing techniques (SSR, MIRU-VNTR, and IS900-RFLP analysis) and previous knowledge about partially shared habitats. The major goal of the present study was to evaluate the informative value of these different typing techniques with regard to the reliability of epidemiological interpretations.
It was decided to investigate isolates originating from two hosts: wild-ranging red deer (Cervus elaphus) and farmed cattle living within a distinct area (Eifel National Park) and having possible epidemiological connections. Additionally, isolates originating from cattle in farms 40 km and 120 km away from the national park and, most likely, not associated with red deer were included. All investigated isolates belonged to the M. avium subsp. paratuberculosis type II group; therefore, for epidemiological tracing, genotyping methods had to further subtype isolates within this group. Methods targeting different structures within the M. avium subsp. paratuberculosis genome were used to obtain broad information about the individual isolates. According to Thibault et al. (37), the most cost-effective and efficient strategy for strain discrimination consists of using MIRU-VNTR typing first, followed by SSR typing; IS900-RFLP was proposed by these authors for the potential discrimination of the remaining clustered isolates and for optional confirmation of discriminated isolates. However, it has to be noticed that the combined analysis of SSR, MIRU-VNTR, and IS900-RFLP data leads to other and more sophisticated interpretations revealing epidemiological connections of M. avium subsp. paratuberculosis within the distinct area investigated in the current study.
From a total of 11 polymorphic short sequence repeats published by Amonsin et al. (1), the four polymorphic SSR loci 1, 2, 8, and 9 were selected as the most informative SSR markers (16, 37). These markers were investigated by other scientists for epidemiological studies. Motiwala et al. used solely SSR locus 1 (23); SSR loci 1 and 8 were used by Ghadiali et al. (13), Motiwala et al. (24), Corn et al. (6), and Sevilla et al. (33); three repeat loci (1, 2, and 8) were included in a combined typing study by El-Sayed et al. (11) and Möbius et al. (22); and four repeat loci (1, 2, 8, and 9) were used by Harris et al. (16). The variabilities of individual SSR loci were quite different depending on different hosts and the regional origins of the investigated isolates. Some authors found the highest allele diversity at SSR locus 1 (1, 16). In the current study, only a single allele with 7G repeats was detected at locus 1 for all isolates. Some studies documented that the informative value of some SSR structures has to be analyzed critically because their stability is in question. There are differences in stability depending on the kind of repeats: poly(GT) tracts are at least 10-fold more stable than poly(G) tracts (17), as suggested by the poly(G) repeats at SSR loci 1 and 2 in the current study. Independently of the cause, stutter peak effects during sequencing (37) and a high variability of markers, results for otherwise identical genotypes originating from the same herd with differences only at G2 repeats, suggest that SSR locus 2 is not a suitable target for epidemiological tracing. These results are supported by the study of El-Sayed et al. (11), in which M. avium subsp. paratuberculosis was differentiated by six SSR and MIRU-VNTR markers. Within this study, genotypes of some cattle isolates originating from individual farms (farms 6, 9, 10, 12, 14, and 15) exhibited diversity only at SSR locus 2.
Therefore, the present data were analyzed without SSR locus 2, resulting in only two SSR genotypes (7-4-4 and 7-5-5). These are common genotypes for cattle and deer isolates worldwide (1, 6, 16, 33, 36). However, tracking of M. avium subsp. paratuberculosis transmission by only SSR analysis was not possible using the present strain panel.
Three MIRU-VNTR profiles were most frequently found within Eifel National Park: 32332228 (67%; designated INMV 2), 42332128 (22%; INMV 19), and 42332228 (8%; INMV 1). These three profiles were detected for both red deer and cattle isolates and were predominantly found in Germany (data not yet published). In contrast, only one INMV 19 profile was found by Thibault et al. (36) within a strain panel of 183 M. avium subsp. paratuberculosis isolates originating from different hosts of 10 countries and exhibiting mostly profiles INMV 1 and INMV 2. Furthermore, within the current study, five individual patterns which had never been detected before by others were found for cattle isolates (42342228, 43332128, 1232226, 12522226, and 12322126).
Stevenson et al. (35) investigated a widespread panel of isolates originating from different hosts from seven European countries (not including Germany) that were genotyped by PFGE, MIRU-VNTR analysis, and IS900-RFLP. Typing patterns of different methods within this study were not associated, corresponding to the results of Thibault et al. (36) and Möbius et al. (21). The most widely distributed MIRU-VNTR types were INMV 1 and INMV 2. Five of six investigated red deer isolates belonged to one of these two genotypes. They originated from the Czech Republic and The Netherlands. These results are comparable with results of the present study, where 10 out of 13 red deer isolates also exhibited INMV 1 or INMV 2.
MIRU-VNTR analysis data from different hosts (dairy cattle, goat, fallow deer, mouflon, and bullfighting cattle) from different locations in Spain indicated that within the M. avium subsp. paratuberculosis type II group, isolates originating from different animal species shared genotypes with cattle isolates, suggesting interspecies transmissions (4). Separate analysis of the current MIRU-VNTR data leads to the same assumption for the red deer and cattle isolates, not only within Eifel National Park but also for isolates originating from Aachen and Bergischer Kreis.
Some more-detailed information was obtained for the current strain panel by additional use of IS900-RFLP. In contrast to the predominant occurrence of IS900-RFLP BstEII type C1 for bovine isolates from France, The Netherlands (36), Germany (21), and the Czech Republic (26, 35) and for 12 red deer isolates originating from the Czech Republic (18), the predominant genotypes for wild-living red deer in Eifel National Park were C17 (38%) and Cnew1 (38%). Only two red deer isolates showed the C1 pattern. Three of six cattle herds from the national park region also exhibited the profile C17; in total, 47% of isolates from cattle and red deer within Eifel National Park exhibited this pattern, followed by 25% for C1 and 17% for Cn1. In contrast, 82% of the investigated cattle isolates outside Eifel National Park revealed the profile C1, subdivided by PstI digestion into C1-P1, C1-P3, and C1-P10.
Until now, the C17 pattern has been the predominant genotype only in Scotland but has also been detected in Germany, The Netherlands, and Norway (21, 31, 35). After a nearly total eradication of wild red deer in North Rhine-Westphalia during the Franco-German War, resettlement started 140 years ago. Animal trade with M. avium subsp. paratuberculosis-infected herds and cross-species transmission from cattle to red deer may have caused infection with the genotype C17 in this specific area in the past. Furthermore, the red deer population of Eifel National Park is part of the large population on both sides of the German-Belgian border. Migrations have been confirmed by means of direct observation and telemetry (2, 28, 29).
Isolates were characterized by 11 genomic markers in a combination of methods, three in SSR analysis and eight in MIRU-VNTR analysis, and two by fingerprint patterns. In total, 17 M. avium subsp. paratuberculosis genotypes were found. It was possible to detect several perfect matches between different isolates (all target sequences were identical). Six genotypes were identified in the studied red deer isolates (n = 13), indicating high M. avium subsp. paratuberculosis variability within wild-living red deer. However, the results indicate no species-specific genotypes. For five genotypes designated I* to V, intraspecies and cross-species transmission is presumed. Genotype III* and the very similar genotype IV*, exhibiting RFLP pattern C17-P8 and only differing at MIRU-VNTR locus 292, were predominantly found within Eifel National Park. A geographic relation is supposed for these genotypes. Genotypes I* and V are the most common genotypes in Germany (data not yet published); therefore, it is not surprising to find genotype I* in the red deer population as well as in cattle herds in Eifel National Park and genotype V in cattle herds from this area and in one cattle herd from the Bergischer Kreis district. Genotype II* represents a unique strain which is not common in Germany but which is notably found within Eifel National Park in bovine and red deer isolates as well as in one head of cattle in the Bergischer Kreis district.
Furthermore, 11 genotypes were detected only once within single cattle herds or the red deer population. Six of these 11 unique genotypes differed from the other genotypes in only one MIRU-VNTR marker or one IS900-RFLP pattern, suggesting a close relationship between these strains. Single targets within the genome change at different speeds or by chance (39, 40), and the selective pressure on the organisms can possibly differ.
In the current study, 13 mycobacteria isolates from wild-living red deer in Germany were isolated, differentiated, and confirmed as M. avium subsp. paratuberculosis for the first time. Based on the detailed knowledge about the origin of the M. avium subsp. paratuberculosis isolates, cross-species transmission of four M. avium subsp. paratuberculosis genotypes was suggested. Information on contaminated grassland leads to the assumption that M. avium subsp. paratuberculosis—distributed by bovine slurry—is the primary cause of infection of red deer in this area. There were specific genotypes found in Eifel National Park which were different from those detected in distant cattle herds and from the most common genotypes in Germany. We suggest that, within this restricted area, epidemiologically associated free-ranging and farmed animals maintain a reservoir for specific M. avium subsp. paratuberculosis genotypes.
Deutz and Spergser (10) investigated M. avium subsp. paratuberculosis-positive wild animals of different species in Austria on a large scale and supposed that M. avium subsp. paratuberculosis transmission had its origin in cattle, probably in contaminated pastures. The volume of contaminated manure produced by infected domestic ruminant livestock is much higher than the volume of contaminated matter from the few cases of shedding by free-ranging animals (6). In addition, transmission and infection in wild animals may occur during winter when the animals gather at feeding sites (14). Today's farm animals in general are considered to be a reservoir for paratuberculosis, as the prevalence of the disease is significantly higher in farmed deer than in free-living ruminants (8, 19). Ruminant or nonruminant wildlife species may, however, be of epidemiological significance as reservoir hosts (30) or disseminators for farms where M. avium subsp. paratuberculosis has just recently been eliminated or for M. avium subsp. paratuberculosis-free farms located in the geographic vicinity of farms with infected livestock (6). Depending on the density of populations in different regions of the world, both possibilities for M. avium subsp. paratuberculosis reservoirs must be considered and must be examined on a local scale.
To track M. avium subsp. paratuberculosis transmission within a distinct area, high-resolution genotyping was necessary. For our strain panel, this included a combination of MIRU-VNTR and IS900-RFLP analysis. SSR analysis was not helpful for our specific strain panel consisting of M. avium subsp. paratuberculosis type II isolates. Substitution of one method for another was not applicable. Comparison with other studies showed that the discriminatory powers of these three methods were different depending on the diversity of the origin of the isolates or the M. avium subsp. paratuberculosis type. The combination of methods extended the discriminatory power of typing considerably and resulted in more reasonable epidemiological links. We propose the use of loci 1 (assigning alleles with more than 9 repeats as >9), 7, 8, and 9 for further investigations with SSR analysis. For MIRU-VNTR analysis, the most useful typing markers are VNTR 292, X3, 25, 47, and 7. For comparable IS900-RFLP fingerprint patterns after using PstI, it is essential to apply a specific probe without a PstI digestion site (according to the standardized method) to prevent patterns with a doubled number of IS900 bands.
Results of our study support the thesis that the choice of techniques depends on the specific objective of the study: epidemiological tracing of infection within a selected area requires different approaches than does the analysis of worldwide M. avium subsp. paratuberculosis diversity. For worldwide data exchange, it is essential to reach an agreement about included typing markers.
ACKNOWLEDGMENTS
The study was funded by the German Ministry for Education and Research, Consortium ZooMAP, grant no. 01Kl 0755.
We thank Kerstin Steger, Danny Michel, Uta Brommer (IMP, Friedrich-Loeffler-Institut, Jena, Germany), and Petra Kranz (Friedrich-Loeffler-Institut, Wusterhausen, Germany) for their excellent technical assistance.
Footnotes
Published ahead of print 16 December 2011
REFERENCES
- 1. Amonsin A, et al. 2004. Multilocus short sequence repeat sequencing approach for differentiating among Mycobacterium avium subsp. paratuberculosis strains. J. Clin. Microbiol. 42:1694–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bresseler P. 1971. Das Rotwild in der Eifel. Wirtschafts- und Forstverlags Euting K.G., Straßenhaus, Germany. [Google Scholar]
- 3. Bull TJ, et al. 2003. Mycobacterial interspersed repetitive units (MIRU) differentiate Mycobacterium avium subspecies paratuberculosis from other species of the Mycobacterium avium complex. Mol. Cell. Probes 17:157–164 [DOI] [PubMed] [Google Scholar]
- 4. Castellanos E, et al. 2010. Molecular characterization of Mycobacterium avium subspecies paratuberculosis types II and III isolates by a combination of MIRU-VNTR loci. Vet. Microbiol. 144:118–126 [DOI] [PubMed] [Google Scholar]
- 5. Collins DM, Gabric DM, de Lisle GW. 1990. Identification of two groups of Mycobacterium paratuberculosis strains by restriction endonuclease analysis and DNA hybridization. J. Clin. Microbiol. 28:1591–1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corn JL, Manning EJ, Sreevatsan S, Fischer JR. 2005. Isolation of Mycobacterium avium subsp. paratuberculosis from free-ranging birds and mammals on livestock premises. Appl. Environ. Microbiol. 71:6963–6967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Daniels MJ, et al. 2003. Do non-ruminant wildlife pose a risk of paratuberculosis to domestic livestock and vice versa in Scotland? J. Wildl. Dis. 39:10–15 [DOI] [PubMed] [Google Scholar]
- 8. Davidson WR, Manning EJ, Nettles VF. 2004. Culture and serologic survey for Mycobacterium avium subsp. paratuberculosis infection among southeastern white-tailed deer (Odocoileus virginianus). J. Wildl. Dis. 40:301–306 [DOI] [PubMed] [Google Scholar]
- 9. de Juan L, Mateos A, Domínguez L, Sharp JM, Stevenson K. 2005. Genetic diversity of Mycobacterium avium subspecies paratuberculosis isolates from goats detected by pulsed-field gel electrophoresis. Vet. Microbiol. 106:249–257 [DOI] [PubMed] [Google Scholar]
- 10. Deutz A, Spergser J. 2009. Paratuberkulose bei Wildtieren–Verbreitung, klinische und postmortale Befunde. Rundsch. Fleischhygiene Lebensmittelüberwachung 61:12–15 [Google Scholar]
- 11. El-Sayed A, et al. 2009. Evaluation of three molecular methods of repetitive element loci for differentiation of Mycobacterium avium subsp. paratuberculosis (MAP). J. Microbiol. 47:253–259 [DOI] [PubMed] [Google Scholar]
- 12. Englund S, Ballagi-Pordány A, Bölske G, Johansson KE. 1999. Single PCR and nested PCR with a mimic molecule for detection of Mycobacterium avium subsp. paratuberculosis. Diagn. Microbiol. Infect. Dis. 33:163–171 [DOI] [PubMed] [Google Scholar]
- 13. Ghadiali AH, Strother M, Naser SA, Manning EJ, Sreevatsan S. 2004. Mycobacterium avium subsp. paratuberculosis strains isolated from Crohn's disease patients and animal species exhibit similar polymorphic locus patterns. J. Clin. Microbiol. 42:5345–5348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Glawischnig W, Steineck T, Spergser J. 2006. Infection caused by Mycobacterium avium subspecies avium, hominissuis, and paratuberculosis in free-ranging red deer (Cervus elaphus hippelaphus) in Austria, 2001–2004. J. Wildl. Dis. 42:724–731 [DOI] [PubMed] [Google Scholar]
- 15. Guerrero C, Bernosconi C, Burki D, Bodmer T, Telenti A. 1995. A novel insertion element from Mycobacterium avium, IS1245, is a specific target for analysis of strain relatedness. J. Clin. Microbiol. 33:304–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris NB, Payeur JB, Kapur V, Sreevatsan S. 2006. Short-sequence-repeat analysis of Mycobacterium avium subsp. paratuberculosis and Mycobacterium avium subsp. avium isolates collected from animals throughout the United States reveals both stability of loci and extensive diversity. J. Clin. Microbiol. 44:2970–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henderson ST, Petes TD. 1992. Instability of simple sequence DNA in Saccharomyces cerevisiae. Mol. Cell. Biol. 12:2749–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a. Hunter PR, Gaston MA. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kopecna M, et al. 2008. The wildlife hosts of Mycobacterium avium subsp. paratuberculosis in the Czech Republic during the years 2002–2007. Vet. Med. 53:420–426 [Google Scholar]
- 19. Machackova M, et al. 2004. Paratuberculosis in farmed and free-living wild ruminants in the Czech Republic (1999–2001). Vet. Microbiol. 101:225–234 [DOI] [PubMed] [Google Scholar]
- 20. Mackintosh CG, Griffin JF. 2010. Paratuberculosis in deer, camelids and other ruminants, p 179–187 In Behr MA, Collins DM. (ed), Paratuberculosis. CAB International, Wallingford, Oxfordshire, United Kingdom. [Google Scholar]
- 21. Möbius P, Luyven G, Hotzel H, Köhler H. 2008. High genetic diversity among Mycobacterium avium subsp. paratuberculosis strains from German cattle herds shown by combination of IS900 restriction fragment length polymorphism analysis and mycobacterial interspersed repetitive unit-variable-number tandem-repeat typing. J. Clin. Microbiol. 46:972–981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Möbius P, Fritsch I, Luyven G, Hotzel H, Köhler H. 2009. Unique genotypes of Mycobacterium avium subsp. paratuberculosis strains of type III. Vet. Microbiol. 139:398–404 [DOI] [PubMed] [Google Scholar]
- 23. Motiwala AS, et al. 2004. Molecular epidemiology of Mycobacterium avium subsp. paratuberculosis isolates recovered from wild animal species. J. Clin. Microbiol. 42:1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Motiwala AS, et al. 2005. Rapid detection and typing of strains of Mycobacterium avium subsp. paratuberculosis from broth cultures. J. Clin. Microbiol. 43:2111–21117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Overduin P, et al. 2004. Use of multilocus variable-number tandem-repeat analysis for typing Mycobacterium avium subsp. paratuberculosis.. J. Clin. Microbiol. 42:5022–5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pavlik I, et al. 1999. Standardisation of restriction fragment length polymorphism analysis for Mycobacterium avium subspecies paratuberculosis. J. Microbiol. Methods 38:155–167 [DOI] [PubMed] [Google Scholar]
- 27. Pavlik I, et al. 2000. Epidemiology of paratuberculosis in wild ruminants studied by restriction fragment length polymorphism in the Czech Republic during the period 1995–1998. Vet. Microbiol. 77:231–251 [DOI] [PubMed] [Google Scholar]
- 28. Petrak M. 1999. Habit use and game path–a key to an understanding of the interactions between red deer (Cervus elaphus L 1758) and their habitats, p 289–297 In Gerken B, Görner M. (ed), History, models and perspectives for the development of European landscapes with large herbivores. In Proceedings of an of the International Symposium on Development of European Landscapes with Large Herbivores, 21 to 23 April 1998, Neuhaus, Solling, Germany [Google Scholar]
- 29. Petrak M. 1999. Raumnutzung und Wildwechsel–Schlüssel zur Überlebensstrategie des Rothirsches (Cervus elaphus) und zu den Wechselbeziehungen zwischen Lebensraum und Wildbestand, p 289–297 In Gerken B, Görner M. (ed), Natur- und Kulturlandschaft. Höxter Verlag, Jena, Germany [Google Scholar]
- 30. Raizman EA, Wells SJ, Jordan PA, DelGiudice GD, Bey RR. 2005. Mycobacterium avium subsp.paratuberculosis from free-ranging deer and rabbits surrounding Minnesota dairy herds. Can. J. Vet. Res. 69:32–38 [PMC free article] [PubMed] [Google Scholar]
- 31. Reid HW, et al. 1996. Mycobacterial infections of free-living deer in Scotland, p 180–182 In Chiodini RJ, Hines ME, Collins MT. (ed), Proceedings of the Fifth International Colloquium on Paratuberculosis, Madison, WI. International Association for Paratuberculosis, Inc., East Providence, RI. [Google Scholar]
- 32. Riemann H, et al. 1979. Paratuberculosis in cattle and free-living exotic deer. J. Am. Vet. Med. Assoc. 174:841–843 [PubMed] [Google Scholar]
- 33. Sevilla I, et al. 2008. Comparative analysis of Mycobacterium avium subsp. paratuberculosis isolates from cattle, sheep and goats by short sequence repeat and pulsed-field gel electrophoresis typing. BMC Microbiol. 8:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stevenson K, et al. 2002. Molecular characterization of pigmented and nonpigmented isolates of Mycobacterium avium subsp. paratuberculosis.. J. Clin. Microbiol. 40:1798–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stevenson K, et al. 2009. Occurrence of Mycobacterium avium subspecies paratuberculosis across host species and European countries with evidence for transmission between wildlife and domestic ruminants. BMC Microbiol. 9:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thibault VC, et al. 2007. New variable-number tandem-repeat markers for typing Mycobacterium avium subsp. paratuberculosis and M. avium strains: comparison with IS900 and IS1245 restriction fragment length polymorphism typing. J. Clin. Microbiol. 45:2404–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thibault VC, et al. 2008. Combined multilocus short-sequence-repeat and mycobacterial interspersed repetitive unit-variable-number tandem-repeat typing of Mycobacterium avium subsp. paratuberculosis isolates. J. Clin. Microbiol. 46:4091–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Turenne CY, Collins DM, Alexander DC, Behr MA. 2008. Mycobacterium avium subsp. paratuberculosis and M. avium subsp. avium are independently evolved pathogenic clones of a much broader group of M. avium organisms. J. Bacteriol. 190:2479–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van Belkum A. 1999. The role of short sequence repeats in epidemiologic typing. Curr. Opin. Microbiol. 2:306–311 [DOI] [PubMed] [Google Scholar]
- 40. van Belkum A, Struelens M, de Visser A, Verbrugh H, Tibayrenc M. 2001. Role of genomic typing in taxonomy, evolutionary genetics, and microbial epidemiology. Clin. Microbiol. Rev. 14:547–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. van Soolingen D, Hermans PWM, de Haas PE, Soll DR, van Embden JDA. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578–2586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Whipple D, Kapke P, Vary C. 1990. Identification of restriction fragment length polymorphisms in DNA from Mycobacterium paratuberculosis. J. Clin. Microbiol. 28:2561–2564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Whittington R, Marsh I, Whitlock RH. 2001. Typing of IS1311 polymorphisms confirms that bison (Bison bison) with paratuberculosis in Montana are infected with a strain of Mycobacterium avium subsp. paratuberculosis distinct from that occurring in cattle and other domesticated livestock. Mol. Cell. Probes 15:139–145 [DOI] [PubMed] [Google Scholar]