Abstract
The protozoan parasites Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii are pathogens that are resistant to a number of environmental factors and pose significant risks to public health worldwide. Their environmental transmission is closely governed by the physicochemical properties of their cysts (Giardia) and oocysts (Cryptosporidium and Toxoplasma), allowing their transport, retention, and survival for months in water, soil, vegetables, and mollusks, which are the main reservoirs for human infection. Importantly, the cyst/oocyst wall plays a key role in that regard by exhibiting a complex polymeric coverage that determines the charge and hydrophobic characteristics of parasites' surfaces. Interaction forces between parasites and other environmental particles may be, in a first approximation, evaluated following the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory of colloidal stability. However, due to the molecular topography and nano- to microstructure of the cyst/oocyst surface, non-DVLO hydrophobic forces together with additional steric attractive and/or repulsive forces may play a pivotal role in controlling the parasite behavior when the organism is subjected to various external conditions. Here, we review several parameters that enhance or hinder the adhesion of parasites to other particles and surfaces and address the role of fast-emerging techniques for mapping the cyst/oocyst surface, e.g., by measuring its topology and the generated interaction forces at the nano- to microscale. We discuss why characterizing these interactions could be a crucial step for managing the environmental matrices at risk of microbial pollution.
INTRODUCTION
The protozoan parasites Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii are major pathogens able to survive in both aquatic and terrestrial environments in order to infect a wide range of vertebrate hosts occupying very different ecological niches (34). Their environmental transmission poses significant risks to human health. Giardia cysts and Cryptosporidium and Toxoplasma oocysts are typically acquired by consuming waters or foods that are inadequately treated to kill or to remove the parasites (29, 59–61, 95, 96). Resulting infections are among the most prevalent parasitic diseases worldwide. Giardia and Cryptosporidium are responsible for gastrointestinal diseases causing mild to severe diarrhea (11, 109), whereas Toxoplasma infections may lead to birth defects and severe neurological and ocular diseases, depending on the parasite and host genetic backgrounds (77, 85).
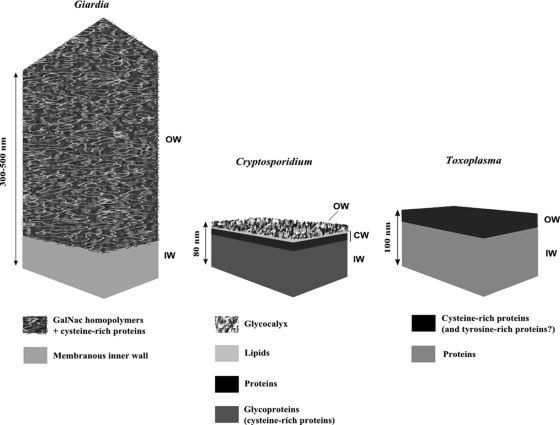
The environmental impact of these parasites is closely related to their extended survival in contrasting climatic conditions and disinfection processes (5, 60, 63) and to their ability to interact with other organic or nonorganic particles. The latter phenomenon governs their transport, retention and/or release, and survival in the transition from land to sea (1, 2, 82, 98–100). The cyst/oocyst wall plays a key role by forming a highly resistant barrier to a large set of physicochemical stressors and by, at the same time, exhibiting surface properties involved in parasite-particle interactions (7, 23, 30, 101). Though the biochemical composition and molecular architecture of their respective outer walls differ greatly (14, 47, 57, 64, 76, 89, 91) (Fig. 1), these three parasites could undergo similar surface interactions with their surrounding world due to their biophysical features (Table 1). Such interactions may be described in a first approximation using prediction models of colloidal stability and attractive and/or repulsive forces (110). Importantly, interaction forces depend on the chemistry and topography of the macromolecules at the parasite surface, on their hydrophobicity and electric charge, and on external physicochemical conditions, such as the ionic composition of the surrounding medium and organic contamination, which can also contribute to the promoting or hindering of parasite adhesion (21, 23, 56, 67–69, 74, 110). First measuring interaction forces and understanding their origin and then controlling them, therefore, appear to be critical in regulating the fate of parasites in the different aquatic and telluric environments and consequently their transmission to animals and humans.
Fig 1.
Schematic drawing of the walls of the Giardia cyst and the Cryptosporidium and Toxoplasma oocysts. OW, outer wall; CW, central wall; IW, inner wall.
Table 1.
Physicochemical characteristics of the environmentally resistant stages of Giardia duodenalis, Cryptosporidium spp., and Toxoplasma gondii
| Characteristic | Giardia duodenalis cyst | Cryptosporidium oocyst | Toxoplasma gondii oocyst | References |
|---|---|---|---|---|
| Size (μm) | 7–10 × 5 | 3.8–6.3 × 4.6–8.4a | 10 × 12 | 11, 60, 109 |
| Wall | ||||
| Thickness (nm) | 300–500 | 50–80 | ∼100 | 14, 37, 57 |
| Number of layers | 2 | 3 | 2 | |
| Outer wall thickness (nm) | 250 | 8 | 30 | |
| Surface biochemistry | Matrix of filamentous GalNAc homopolymer and Cys-rich proteins | Glucose-rich glycocalyx | Possible polymeric cross-links of Cys- and/or Tyr-rich proteins | 14, 37, 57, 64, 76, 103 |
| Zeta potential (mV) | −33.5 in distilled H2O at pH 6.4 | −25.0 in deionized H2O (conductivity, 83.9 μS cm−1 at pH 6.5) | −43.7 in ultrapure H2O (conductivity, 4 μS cm−1 at pH 6.7) | 30, 88, 101 |
| Specific gravity | 1.013–1.117 | 1.009–1.08 | 1.050–1.100 | 22, 32, 55, 78, 83, 120 |
| Settling velocity (μm s−1) | 0.84–1.4 | 0.35–1.31 | Not reported | 22, 78, 121 |
Depending on the species.
In this review, we describe the different parameters contributing to the interactions between the environmentally resistant stages of Giardia, Cryptosporidium, and Toxoplasma and other particles and point out the importance of an accurate characterization of underlying forces to better predict parasite distribution through the environment and therefore prevent their transmission to humans.
SURFACE CHEMISTRY OF THE CYST/OOCYST WALL
The biochemical nature of the macromolecules at the cyst/oocyst surface inherently contributes to the interactions between the parasites and their environment. Thanks to the combination of powerful imaging analysis techniques, such as confocal laser-scanning microscopy and immunoelectron microscopy, and chemical methods, e.g., gas chromatography-mass spectrometry, the list of described macromolecules composing the cyst/oocyst wall surface has been recently extended (57, 64, 76, 91).
The quadranucleate cysts of Giardia duodenalis form in the intestinal lumen of the infected host via a complex multifactorial process (70). The cyst wall is 300 to 500 nm thick, consists mainly of a surface filamentous layer (Fig. 1), and is built with materials that originate from encystation-specific secretory vesicles appearing in the encysting parasites (44, 64). The biochemical composition and structural arrangement of the filamentous layer consist of a dense network of curled fibrils of N-acetylgalactosamine measuring ∼10 nm in diameter (13). These fibrils are closely associated with certain wall proteins called cyst wall proteins (CWP) (13). Four major proteins have been identified in the cyst wall. They include CWP1 to CWP3, which harbor N-terminal leucine-rich repeats together with a C-terminal cysteine-rich region, and a fourth, belonging to the family of cysteine-rich non-variant-specific surface proteins of Giardia (25). In addition, an epidermal growth factor-like cyst protein has been shown to be involved in the cyst wall formation, in partnership with the non-variant-specific surface protein (16). The thick filamentous outer layer of the cyst wall has been shown to be fully impermeable to water-soluble substances, enhancing the survival of cysts in water and their resistance to disinfectants (5).
The four infective sporozoites of Cryptosporidium are protected by a complex multilayer wall 50 to 80 nm thick that forms while the oocyst develops in the intestinal cells of the infected hosts (47, 57, 89). The oocyst wall of Cryptosporidium is built mainly with materials released sequentially by different subsets of specific organelles found in the cytoplasm of the fertilized macrogamete, the so-called wall-forming bodies (103). The current proposed model shows an inner layer of glycoproteins and a central lipid-protein layer covered by an outer glucose-rich glycocalyx (12, 57, 87) (Fig. 1). High-molecular-weight cysteine-rich proteins, namely, Cryptosporidium oocyst wall proteins (COWPs), are thought to form extensive disulfide bridges and, consequently, matrices in the inner layer, which chiefly provides the overall mechanical strength of the oocyst wall (103, 108). The glycocalyx, decorating the wall structure and facing the surrounding medium, provides at the same time immunogenicity and potential attachment possibilities (57, 87). The outcomes of physical and chemical treatments of the oocyst wall indicate that the glycocalyx is delicate and highly susceptible to disinfectants, such as sodium hypochlorite, and to conservative agents like formalin (42, 47).
The wall of the sporulated oocyst of Toxoplasma encloses two sporocysts, each containing four infectious sporozoites (36, 104). As a conserved feature among Coccidia, the double-layered wall of the Toxoplasma oocyst forms a highly resistant and impermeable shell (3). Nonetheless, the outer layer can be stripped off rather easily with chemical treatments, so the robustness of the oocyst wall appears to be mainly due to its inner layer (76). The ∼100-nm-thick oocyst wall forms while the oocyst is still housed by the enterocytes of cats, the definitive hosts of the parasite (37). This structure is built with materials released sequentially by different types of wall-forming bodies present in the cytoplasm of the macrogamete/early stage oocyst (37). The oocyst wall of Toxoplasma (and of related coccidian parasites) is more than 90% proteins, with two identified types so far, namely, cysteine- and tyrosine-rich proteins (76) (Fig. 1). In Toxoplasma, three cysteine-rich oocyst wall proteins (OWP), TgOWP1 to -3, out of the seven encoded by the parasite genome have recently been characterized biochemically (91). They are structurally homologous to COWPs, and TgOWP3 localizes specifically in the outer layer. In contrast, tyrosine-rich proteins are small molecules that form protein-protein dityrosine cross-links responsible for the hardening and natural blue autofluorescence of oocysts under UV light (4). Such proteins have been identified in the oocyst and sporocyst walls of the members of the closely related Coccidia genus Eimeria (3) but not in the Toxoplasma oocyst, which, however, exhibits the same typical autofluorescence (73).
In conclusion, current models of the surface chemistry of the environmentally resistant stages of Giardia, Cryptosporidium, and Toxoplasma strongly suggest a wall covering made of complex polymeric matrices. This covering determines the charge and hydrophobic characteristics of parasites' surfaces, which are expected to generate and modulate electrostatic attractive and/or repulsive interactions with the surrounding particles (Table 1).
PARASITE-PARTICLE INTERACTIONS
Due to the size, shape, and electrical charges of the parasites, it is tempting to predict parasite adhesion following the Derjaguin-Landau-Verwey-Overbeek (DLVO) theory of colloidal stability (26, 72, 114). This theory takes into account the electrostatic repulsion between surface charges, which strongly depends on the ionic strength of the surrounding liquid, and electro-dynamic attractions due to London-van der Waals forces. At great distances, repulsion is less important than attraction, resulting in an overall attraction, whereas a repulsive barrier due to the glycocalyx must be overcome to reach irreversible adhesion when the interparticle distance becomes small enough. However, applied to the Cryptosporidium oocyst, parasite-silica interaction models do not closely fit the DVLO theory at separations of <35 nm because of the roughness of the oocyst surface and of the extension of the surface macromolecules from the surface into the electrical double layer (20). Thus, some other forces that are not included in the DLVO approximation may have an important role in parasite-particle interactions, notably hydrophobic and steric repulsion forces (19, 20, 23). At the nanoscale, adhesion strongly depends on the topography (surface roughness) and on the molecular coverage of the parasite surface by macromolecules creating potential attractive and/or repulsive forces. Surface properties can be investigated by using several physical methods (111), among which is atomic force microscopy (AFM). AFM gives valuable information about the surface topography by directly allowing imaging at nanometer-scale resolution and allows force measurements in physiological media, with unfixed samples (40, 118). AFM uses a nano-finger, at the extremity of a very soft, several-micrometer-long spring, to gently delineate the surface (imaging mode), to indent the object's surface by pressing on it and thus make measurements of the object's mechanical properties through its Young modulus (force mode, mechanics) (46, 80), or to probe the adhesion of surface molecules when decorated with suitable haptens and pull the lever off the surface until all built bridges are broken, allowing direct quantification of the force that those bridges can sustain (force mode, adhesion). Different variants of adhesion force measurements have been employed so far in cell biology (38, 65, 92–94, 107, 112). This technique is now fast evolving for the study of environmental pathogens (119). To date, AFM has been employed to observe the surface topography of the oocyst of Cryptosporidium parvum and measure its mechanical properties (8, 9, 18, 19). AFM images describe a rather rough landscape at the oocyst surface, while the measurements of its mechanical properties indicate that it is as hard as siliceous materials (18).
Any modification of the parasite surface chemistry may promote or hinder adhesion. In lab-scale experiments using a radial stagnation point flow system to investigate adhesion kinetics of Cryptosporidium oocysts and Giardia cysts, enzymatic treatments with proteinase K or pepsin have been found to seriously damage the outer layer of the parasites (69). While the surface glycocalyx of Cryptosporidium oocysts prevents their adhesion to quartz surfaces by imposing a steric repulsion, proteolytic enzymes that cause such a degradation naturally enhance their attachment to the very same surfaces (68, 69, 74). Under conditions close to natural field ones, dissolved ions and organic contaminants deeply impact the surface properties of the parasites. Dissolved calcium ions in solution tend to apparently diminish negative charges at the parasite surface, consequently abolishing repulsion and thus enhancing attachment of Cryptosporidium oocysts to sand grain surfaces (68). Using packed-bed bead columns to investigate the behavior of Cryptosporidium oocysts in granular porous media (as a model for sand), Kim et al. showed that increasing the ionic strength of the medium promotes parasite retention in conjunction with a low velocity of the solution flow (62). It has been shown, as might be expected, that parasites exhibit variation in their zeta potential when suspended in water-based solutions that differ in conductivity, pH, and dissolved organic carbon concentration. For instance, Toxoplasma oocysts are negatively charged and tend not to aggregate to other particles in freshwater solutions (ζ = −16.16 mV), while their global charge approaches neutral in higher-ionic-strength solutions that mimic the conditions encountered in estuarine water and seawater (ζ = −1.84 and −2.81 mV, respectively), thus leading to efficient parasite aggregation with other particles in these environments (101). According to different studies, the oocyst of Cryptosporidium parvum has an isoelectric point of 2.2 to 3.3, at which electrostatic repulsion forces are abolished (6, 19, 30, 53). In contrast, when placed in the presence of dissolved compounds from natural organic matters, parasites exhibit an absolute increase of their negative charges and of their hydrophobicity, possibly due to the adsorption of clays and humic and fulvic acids onto their surfaces. This may enhance transport rather than parasite sedimentation (24, 81, 82, 88, 101).
SURFACE INTERACTIONS DRIVE THE TRANSPORT AND SURVIVAL OF PARASITIC PROTOZOA
At the field scale, surface interactions critically affect the behavior of these pathogens and their distribution in terrestrial and aquatic environments. Transport of parasitic protozoa in soil follows the colloid filtration theory, suggesting that the size of these microorganisms control to a large extend their transport in granular media (45). However, the theory does not take into account the surface characteristics of the parasites, their viability, and their reversible interactions with soil grain surfaces, which promote or inhibit the terrestrial transport of parasitic protozoa (97). Soil physicochemical properties, e.g., mineralogy, natural organic matter content, and pH, critically affect the parasite-particle interactions and the mobilization behavior of Cryptosporidium oocysts (43, 52, 79, 81, 82). Spatial dissemination of excreted parasites depends also on local hydrodynamic forces and occurs mainly by leaching, typically following heavy rainfalls, leading to the possible entry of the parasites into water (39, 84). It has been shown that wetlands with established vegetation may efficiently retain parasites, while degraded habitats promote pathogen pollution of waters, with great impact on humans and animals (100, 106).
The ability of waterborne Giardia cysts and Cryptosporidium oocysts to settle contributes to either their transport in waters or their retention in sediments. Their respective settling velocity has been determined according to the Stokes law, taking into account the parasite diameter and its specific gravity (Table 1). The settling velocity of unattached parasites is relatively low (e.g., 0.35 μm s−1 for Cryptosporidium) (78); however, when attached to particles, the very same parasites settle faster (∼1.3 μm s−1) mainly due to an increase of the apparent diameter of the objects and of their specific gravity (49, 78). These parasites are likely to be associated with fecal or soil particles before entering rivers or water reservoirs, so settling may occur more or less efficiently depending on the size of particles, suggesting that some particle-attached parasites may travel along rivers (35). Also, aggregation of the parasites with particles does not preclude their survival in water beds for months or their redistribution from sediments due to local water turbulences (99). The latter phenomenon may cause recurrent parasitic contaminations, as observed in certain surface waters used for drinking (84, 98, 99). Overall, it must be considered that such interactions could be responsible for the amazing persistence of infective parasites in water and solid matrices, in conjunction with variations of local physical and chemical conditions (63).
If recent investigations on the molecular coverage and surface forces of Cryptosporidium, Giardia, and Toxoplasma parasites have provided important information on the parasite behavior at different spatial and time scales, they have also brought to light some limits of the technology used for working with protozoan cysts and oocysts. In particular, parasites used for imaging and for force-based and transport experiments should be carefully purified and stored in order to prevent any modification or loss of their macromolecular coverage caused by chemical agents or by disparities in parasite populations (6). As a consequence, bleach-sterilized, formalin-treated, or heat-inactivated parasites may exhibit modified surface properties and are therefore not suitable for such interaction experiments (9, 42, 68, 69, 88). To overcome this drawback and because of the biohazard risks associated with the manipulation of resistant parasites, some authors have proposed using surrogate microspheres, and these microspheres have been successfully employed for transport experiments. Typically, these substrates are fluorescent glass or latex beads that are designed or decorated to mimic the size and surface properties of the targeted parasites (48). These surrogates allow a relatively good prediction of the behavior of the parasites in waters and soils, including their removal by granular porous medium and their adhesion to strips bearing vegetation (24, 81, 82, 100, 101). Interestingly, divergent behaviors have been observed between the surrogate microspheres and the parasites they mimic under particular conditions of ionic strength and organic contamination close to natural field ones. This behavior could be linked to the existence of subtle differences in their respective surface chemistry (48, 82).
IMPLICATIONS FOR NATURAL RESOURCE MANAGEMENT
It has been clearly demonstrated that the monitoring of protozoan parasites in complex matrices as well as their removal and/or inactivation can be greatly impaired by their interactions with organic and inorganic contaminants (5, 31). For instance, these interactions critically affect the recovery rates of waterborne cysts and oocysts along the different steps of the process. Sampling surface waters may be problematic because the particle-attached parasites likely settle through gravity faster than free parasites in the water bed and thus may not be sampled (98, 99). More importantly, aggregation of parasites impacts their purification by immunomagnetic separation (IMS) techniques or flotation on dense solutions in cases of extensive organic contamination (15, 31, 66, 71, 113). Parasite-particle complexes exhibit a greater specific gravity and cannot be readily separated by flotation (55, 90, 98), whereas particles can also mask the antigenic sites at the parasite surface, thus hampering the antigen recognition by specific antibodies that are used for IMS or immunofluorescence techniques. The use of dispersant solutions, chelating agents, detergents, or biosurfactants is not always successful in preventing unwanted parasite-particle interactions during sample processing (54, 75). Furthermore, the surfaces of purified parasites may be still coated with divalent cations and organic substances (humic and fulvic acids) that may interfere with downstream applications such as PCR, which usually give useful information on the species, viability, and genetic type of the detected parasites (41, 58, 102).
Interestingly, interactions have a dual role in promoting or preventing parasite removal and/or inactivation, depending on treatment methods. Water industrials take advantage of these surface properties in order to remove the parasites from raw waters by using coagulant agents such as aluminum-based salts, iron-based salts, or organic polymers. Such agents enhance the aggregation of parasites with other particles, allowing the flocculation of the newly formed complexes and their removal following controlled settling (5). Another significant contribution of the particular surface properties relies on interactions that may occur between parasites and sand during their transport through granular porous media in water treatments, allowing parasite retention on sand filters (62, 110). In contrast, unwanted parasite-particle interactions are clearly detrimental when physical or chemical disinfectants are being used. UV has been shown to inactivate Cryptosporidium and Giardia at doses commonly applied by water industrials (>40 mJ/cm2) (51), while for Toxoplasma, complete and reliable inactivation has been reported in some studies but not others (33, 116). Parasites entrapped in soft mollusk tissues or attached to sediments may not be totally inactivated at these doses (10, 105). Also, the success of chemical-based inactivation processes may also be compromised for chlorine- and ozone-resistant microorganisms (5, 33, 117). The presence of organic and inorganic compounds associated to parasites in water may require the use of higher ozone doses to achieve parasite inactivation, which may lead to the formation of potentially harmful by-products such as bromates (115). In a similar way, Cryptosporidium oocysts and Giardia cysts entrapped in pipe wall biofilms survive the concentrations of free chlorine usually used in drinking water systems (2, 50). The capacity of the Toxoplasma oocyst to interact with biofilms has not been investigated so far. Such investigations would be of great interest, since the implication of biofilms cannot be ruled out for elucidating recurrent cases of waterborne toxoplasmosis in several areas (60).
Modeling the fate and transport of parasitic protozoa may offer a valuable tool for risk assessment. Microbial pathogen modeling basically incorporates specific information about microbial dynamics in conjunction with soil composition, hydrological dynamics, climatic conditions, vegetation, and land management (35). It has been clearly demonstrated that mathematical models for bacteria are not suitable for predicting the fate and transport of parasitic protozoa (17, 28, 35). In particular, fecal indicators fail to reflect water contamination by Cryptosporidium and Giardia parasites because of the ability of oocysts and cysts to interact and aggregate reversibly with other particles, in contrast to other microorganisms (35, 49). Several specific models have been successfully used for estimating protozoan loads in watersheds in some particular contexts (17, 28, 35). To our knowledge, no such models exist for Toxoplasma oocysts on a large scale, mainly because there is too little information on their transport properties, survival, and prevalence in the environment (31, 60, 100). Modeling parasitic protozoa therefore carries with it several major challenges, among which are exact parasite surface characterization, the determination of inactivation rate, and improvements in the separation and molecular methods that are used to detect and characterize them in complex matrices. The first point is especially crucial for the study of the events that follow inactivation processes, in order to assess divergent aggregation and settling behaviors observed between viable and nonviable parasites.
CONCLUSIONS AND FUTURE PERSPECTIVES
The circulation of Giardia, Cryptosporidium, and Toxoplasma parasites in the different environmental sources leading to potential human infections strongly depends on how the parasites interact with their surrounding media, mainly other organic and inorganic particles. Hydrophobic, steric, and electrostatic attractive and/or repulsive forces created by the polymeric coverage of the parasite surface greatly enhance or hinder parasite adhesion following exposure to various environmental physicochemical factors. They contribute to parasite adhesion to natural organic matters, thus promoting their retention in soils or increasing their deposition kinetics in waterbeds. Consequently, such interactions make parasite fluxes hard to predict on a large scale, affecting the management of resources at risk of microbial pollution.
An accurate characterization of parasite-particle interactions clearly requires additional information on the topography and on the molecular composition of the outer surface of the cyst/oocyst wall. The combination of powerful microscopic and spectroscopic techniques, such as fluorescence microscopy, AFM, and/or Raman scattering microscopy, may give new insights into the biochemical nature and arrangement of surface macromolecules as well as the adhesive and mechanical properties of the robust wall (27, 86). To date, only the surface of the oocyst of Cryptosporidium parvum has been mapped in terms of force and mechanics, and most efforts should now be focused on the surfaces of other medically important Cryptosporidium species, Giardia cysts, and Toxoplasma oocysts and on the interaction forces that might result from the micro- and nanostructures of their coats.
ACKNOWLEDGMENTS
This work was supported by the French National Research Agency (grant ANR-09-ALIA-009) and Aix-Marseille University (Préciput 2011 program). P.-H.P. is supported by the ANR JCJC DissecTion program.
Footnotes
Published ahead of print 9 December 2011
REFERENCES
- 1. Abudalo RA, Ryan JN, Harvey RW, Metge DW, Landkamer L. 2010. Influence of organic matter on the transport of Cryptosporidium parvum oocysts in a ferric oxyhydroxide-coated quartz sand saturated porous medium. Water Res. 44:1104–1113 [DOI] [PubMed] [Google Scholar]
- 2. Angles ML, Chandy JP, Cox PT, Fisher IH, Warnecke MR. 2007. Implications of biofilm-associated waterborne Cryptosporidium oocysts for the water industry. Trends Parasitol. 23:352–356 [DOI] [PubMed] [Google Scholar]
- 3. Belli SI, Smith NC, Ferguson DJ. 2006. The coccidian oocyst: a tough nut to crack! Trends Parasitol. 22:416–423 [DOI] [PubMed] [Google Scholar]
- 4. Belli SI, Wallach MG, Luxford C, Davies MJ, Smith NC. 2003. Roles of tyrosine-rich precursor glycoproteins and dityrosine- and 3,4-dihydroxyphenylalanine-mediated protein cross-linking in development of the oocyst wall in the coccidian parasite Eimeria maxima. Eukaryot. Cell 2:456–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Betancourt WQ, Rose JB. 2004. Drinking water treatment processes for removal of Cryptosporidium and Giardia. Vet. Parasitol. 126:219–234 [DOI] [PubMed] [Google Scholar]
- 6. Brush CF, Walter MF, Anguish LJ, Ghiorse WC. 1998. Influence of pretreatment and experimental conditions on electrophoretic mobility and hydrophobicity of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 64:4439–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Butkus MA, Bays JT, Labare MP. 2003. Influence of surface characteristics on the stability of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 69:3819–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Byrd TL, Walz JY. 2005. Interaction force profiles between Cryptosporidium parvum oocysts and silica surfaces. Environ. Sci. Technol. 39:9574–9582 [DOI] [PubMed] [Google Scholar]
- 9. Byrd TL, Walz JY. 2007. Investigation of the interaction force between Cryptosporidium parvum oocysts and solid surfaces. Langmuir 23:7475–7483 [DOI] [PubMed] [Google Scholar]
- 10. Cantwell RE, Hofmann R. 2011. Ultraviolet absorption properties of suspended particulate matter in untreated surface waters. Water Res. 45:1322–1328 [DOI] [PubMed] [Google Scholar]
- 11. Chalmers RM, Davies AP. 2010. Minireview: clinical cryptosporidiosis. Exp. Parasitol. 124:138–146 [DOI] [PubMed] [Google Scholar]
- 12. Chatterjee A, et al. 2010. Evidence for mucin-like glycoproteins that tether sporozoites of Cryptosporidium parvum to the inner surface of the oocyst wall. Eukaryot. Cell 9:84–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chatterjee A, et al. 2010. Giardia cyst wall protein 1 is a lectin that binds to curled fibrils of the GalNAc homopolymer. PLoS Pathog. 6:e1001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chávez-MunguíA C, Cedillo-Rivera R, Martínez-Palomo A. 2004. The ultrastructure of the cyst wall of Giardia lamblia. J. Eukaryot. Microbiol. 51:220–226 [DOI] [PubMed] [Google Scholar]
- 15. Chesnot T, Schwartzbrod J. 2004. Quantitative and qualitative comparison of density-based purification methods for detection of Cryptosporidium oocysts in turbid environmental matrices. J. Microbiol. Methods 58:375–386 [DOI] [PubMed] [Google Scholar]
- 16. Chiu PW, Huang YC, Pan YJ, Wang CH, Sun CH. 2010. A novel family of cyst proteins with epidermal growth factor repeats in Giardia lamblia. PLoS Negl. Trop. Dis. 2010 4:e677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Coffey R, Cummins E, Flaherty VO, Cormican M. 2010. Analysis of the soil and water assessment tool (SWAT) to model Cryptosporidium in surface water sources. Biosystems Eng. 106:303–314 [Google Scholar]
- 18. Considine RF, Dixon DR, Drummond CJ. 2000. Laterally-resolved force microscopy of biological microspheres—oocysts of Cryptosporidium parvum. Langmuir 16:1323–1330 [Google Scholar]
- 19. Considine RF, Dixon DR, Drummond CJ. 2002. Oocysts of Cryptosporidium parvum and model sand surfaces in aqueous solutions: an atomic force microscope (AFM) study. Water Res. 36:3421–3428 [DOI] [PubMed] [Google Scholar]
- 20. Considine RF, Drummond CJ, Dixon DR. 2001. Force of interaction between a biocolloid and an inorganic oxide: complexity of surface deformation, roughness, and brushlike behavior. Langmuir 17:6325–6335 [Google Scholar]
- 21. Dai X, Boll J. 2003. Evaluation of attachment of Cryptosporidium parvum and Giardia lamblia to soil particles. J. Environ. Qual. 32:296–304 [DOI] [PubMed] [Google Scholar]
- 22. Dai X, Boll J. 2006. Settling velocity of Cryptosporidium parvum and Giardia lamblia. Water Res. 40:1321–1325 [DOI] [PubMed] [Google Scholar]
- 23. Dai X, Boll J, Hayes ME, Aston DE. 2004. Adhesion of Cryptosporidium parvum and Giardia lamblia to solid surfaces: the role of surface charge and hydrophobicity. Colloids Surf. B 34:259–263 [DOI] [PubMed] [Google Scholar]
- 24. Dai X, Hozalski RM. 2003. Evaluation of microspheres as surrogates for Cryptosporidium parvum oocysts in filtration experiments. Environ. Sci. Technol. 37:1037–1042 [DOI] [PubMed] [Google Scholar]
- 25. Davids BJ, et al. 2006. A new family of giardial cysteine-rich non-VSP protein genes and a novel cyst protein. PLoS One 1:e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Derjaguin BV, Landau LD. 1941. Theory of the stability of strongly charged lyophobic sols and of the adhesion of strongly charged particles in solutions of electrolytes. Acta Physicochim. URSS 14:733–762 [Google Scholar]
- 27. de Souza W, Rocha GM. 2011. Atomic force microscopy: a tool to analyze the structural organization of pathogenic protozoa. Trends Parasitol. 27:160–167 [DOI] [PubMed] [Google Scholar]
- 28. Dorner SM, Huck PM, Slawson RM. 2004. Estimating potential environmental loadings of Cryptosporidium spp. and Campylobacter spp. from livestock in the Grand River watershed, Ontario, Canada. Environ. Sci. Technol. 38:3370–3380 [DOI] [PubMed] [Google Scholar]
- 29. Dorny P, Praet N, Deckers N, Gabriel S. 2009. Emerging food-borne parasites. Vet. Parasitol. 163:196–206 [DOI] [PubMed] [Google Scholar]
- 30. Drozd C, Schwartzbrod J. 1996. Hydrophobic and electrostatic cell surface properties of Cryptosporidium parvum. Appl. Environ. Microbiol. 62:1227–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dumètre A, Dardé ML. 2003. How to detect Toxoplasma gondii oocysts in environmental samples? FEMS Microbiol. Rev. 27:651–661 [DOI] [PubMed] [Google Scholar]
- 32. Dumètre A, Dardé ML. 2004. Purification of Toxoplasma gondii oocysts by cesium chloride gradient. J. Microbiol. Methods 56:427–430 [DOI] [PubMed] [Google Scholar]
- 33. Dumètre A, et al. 2008. Effects of ozone and ultraviolet radiation treatments on the infectivity of Toxoplasma gondii oocysts. Vet. Parasitol. 153:209–213 [DOI] [PubMed] [Google Scholar]
- 34. Fayer R, Dubey JP, Lindsay DS. 2004. Zoonotic protozoa: from land to sea. Trends Parasitol. 20:531–536 [DOI] [PubMed] [Google Scholar]
- 35. Ferguson C, de Roda Husman AM, Altavilla N, Deere D, Ashbolt N. 2003. Fate and transport of surface water pathogens in watersheds. Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 33:299–361 [Google Scholar]
- 36. Ferguson DJ, Birch-Andersen A, Siim JC, Hutchison WM. 1979. Ultrastructural studies on the sporulation of oocysts of Toxoplasma gondii. III. Formation of the sporozoites within the sporocysts. Acta Pathol. Microbiol. Scand. B 87:253–260 [DOI] [PubMed] [Google Scholar]
- 37. Ferguson DJ, Brecht S, Soldati D. 2000. The microneme protein MIC4, or an MIC4-like protein, is expressed within the macrogamete and associated with oocyst wall formation in Toxoplasma gondii. Int. J. Parasitol. 30:1203–1209 [DOI] [PubMed] [Google Scholar]
- 38. Fierro FA, et al. 2008. BCR/ABL expression of myeloid progenitors increases beta 1-integrin mediated adhesion to stromal cells. J. Mol. Biol. 377:1082–1093 [DOI] [PubMed] [Google Scholar]
- 39. Forslund A, et al. 2011. Leaching of Cryptosporidium parvum oocysts, Escherichia coli, and a Salmonella enterica serovar Typhimurium bacteriophage through intact soil cores following surface application and injection of slurry. Appl. Environ. Microbiol. 77:8129–8138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Franz CM, Puech PH. 2008. Atomic force microscopy: a versatile tool for studying cell morphology, adhesion and mechanics source. Cell. Mol. Bioeng. 1:289–300 [Google Scholar]
- 41. Frostegård A, et al. 1999. Quantification of bias related to the extraction of DNA directly from soils. Appl. Environ. Microbiol. 65:5409–5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gao X, Chorover J. 2009. In-situ monitoring of Cryptosporidium parvum oocyst surface adhesion using ATR-FTIR spectroscopy. Colloids Surf. B Biointerfaces 71:169–176 [DOI] [PubMed] [Google Scholar]
- 43. Gao X, Chorover J. 2011. Amphiphile disruption of pathogen attachment at the hematite (α-Fe2O3)-water interface. Langmuir 27:5936–5943 [DOI] [PubMed] [Google Scholar]
- 44. Gottig N, et al. 2006. Active and passive mechanisms drive secretory granule biogenesis during differentiation of the intestinal parasite Giardia lamblia. J. Biol. Chem. 281:18156–18166 [DOI] [PubMed] [Google Scholar]
- 45. Gupta V, et al. 2009. Riverbank filtration: comparison of pilot scale transport with theory. Environ. Sci. Technol. 43:669–676 [DOI] [PubMed] [Google Scholar]
- 46. Hampoelz B, et al. 2011. Microtubule-induced nuclear envelope fluctuations control chromatin dynamics in Drosophila embryos. Development 138:3377–3386 [DOI] [PubMed] [Google Scholar]
- 47. Harris JR, Petry F. 1999. Cryptosporidium parvum structural components of the oocyst wall. J. Parasitol. 85:839–849 [PubMed] [Google Scholar]
- 48. Harvey RW, Metge D, Sheets R, Jasperse J. 2011. Fluorescent microspheres as surrogates in evaluating the efficacy of riverbank filtration for removing Cryptosporidium parvum oocysts and other pathogens, p 81–96. In Chittaranjan R, Shamrukh M. (ed), Riverbank filtration for water security in desert countries. Springer, Amsterdam, Netherlands [Google Scholar]
- 49. Hawkins PR, Swanson P, Warnecke M, Shanker SR, Nicholson C. 2000. Understanding the fate of Cryptosporidium and Giardia in storage reservoirs: a legacy of Sydney's water contamination incident. Aqua 49:289–306 [Google Scholar]
- 50. Helmi K, et al. 2008. Interactions of Cryptosporidium parvum, Giardia lamblia, vaccinal poliovirus type 1, and bacteriophages phiX174 and MS2 with a drinking water biofilm and a wastewater biofilm. Appl. Environ. Microbiol. 74:2079–2088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hijnen WA, Beerendonk EF, Medema GJ. 2006. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: a review. Water Res. 40:3–22 [DOI] [PubMed] [Google Scholar]
- 52. Hijnen WA, Brouwer-Hanzens AJ, Charles KJ, Medema GJ. 2005. Transport of MS2 phage, Escherichia coli, Clostridium perfringens, Cryptosporidium parvum, and Giardia intestinalis in a gravel and a sandy soil. Environ. Sci. Technol. 39:7860–7868 [DOI] [PubMed] [Google Scholar]
- 53. Hsu BM, Huang C. 2002. Influence of ionic strength and pH on hydrophobicity and zeta potential of Giardia and Cryptosporidium. Colloids Surf. A 201:201–206 [Google Scholar]
- 54. Inoue M, et al. 2003. A new filter-eluting solution that facilitates improved recovery of Cryptosporidium oocysts from water. J. Microbiol. Methods 55:679–686 [DOI] [PubMed] [Google Scholar]
- 55. Inoue M, et al. 2006. Changes of physical and biochemical properties of Cryptosporidium oocysts with various storage conditions. Water Res. 40:881–886 [DOI] [PubMed] [Google Scholar]
- 56. Janjaroen D, Liu Y, Kuhlenschmidt MS, Kuhlenschmidt TB, Nguyen TH. 2010. Role of divalent cations on deposition of Cryptosporidium parvum oocysts on natural organic matter surfaces. Environ. Sci. Technol. 44:4519–4524 [DOI] [PubMed] [Google Scholar]
- 57. Jenkins MB, et al. 2010. Significance of wall structure, macromolecular composition, and surface polymers to the survival and transport of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 76:1926–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Jiang J, Alderisio KA, Singh A, Xiao L. 2005. Development of procedures for direct extraction of Cryptosporidium DNA from water concentrates and for relief of PCR inhibitors. Appl. Environ. Microbiol. 71:1135–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jones JL, et al. 2009. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 49:878–884 [DOI] [PubMed] [Google Scholar]
- 60. Jones JL, Dubey JP. 2010. Waterborne toxoplasmosis—recent developments. Exp. Parasitol. 124:10–25 [DOI] [PubMed] [Google Scholar]
- 61. Karanis P, Kourenti C, Smith H. 2007. Waterborne transmission of protozoan parasites: a worldwide review of outbreaks and lessons learnt. J. Water Health 5:1–38 [DOI] [PubMed] [Google Scholar]
- 62. Kim HN, Walker SL, Bradford SA. 2010. Coupled factors influencing the transport and retention of Cryptosporidium parvum oocysts in saturated porous media. Water Res. 44:1213–1223 [DOI] [PubMed] [Google Scholar]
- 63. King BJ, Monis PT. 2007. Critical processes affecting Cryptosporidium oocyst survival in the environment. Parasitology 134:309–323 [DOI] [PubMed] [Google Scholar]
- 64. Konrad C, Spycher C, Hehl AB. 2010. Selective condensation drives partitioning and sequential secretion of cyst wall proteins in differentiating Giardia lamblia. PLoS Pathog. 6:e1000835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Krieg M, et al. 2008. Tensile forces govern germ-layer organization in zebrafish. Nature Cell Biol. 10:429–436 [DOI] [PubMed] [Google Scholar]
- 66. Kuczynska E, Shelton DR. 1999. Method for detection and enumeration of Cryptosporidium parvum oocysts in feces, manures, and soils. Appl. Environ. Microbiol. 65:2820–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kuznar ZA, Elimelech M. 2004. Adhesion kinetics of viable Cryptosporidium parvum oocysts to quartz surfaces. Environ. Sci. Technol. 38:6839–6845 [DOI] [PubMed] [Google Scholar]
- 68. Kuznar ZA, Elimelech M. 2005. Role of surface proteins in the deposition kinetics of Cryptosporidium parvum oocysts. Langmuir 21:710–716 [DOI] [PubMed] [Google Scholar]
- 69. Kuznar ZA, Elimelech M. 2006. Cryptosporidium oocyst surface macromolecules significantly hinder oocyst attachment. Environ. Sci. Technol. 40:1837–1842 [DOI] [PubMed] [Google Scholar]
- 70. Lauwaet T, Davids BJ, Reiner DS, Gillin FD. 2007. Encystation of Giardia lamblia: a model for other parasites. Curr. Opin. Microbiol. 10:554–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lélu M, et al. 2011. Development of a sensitive method for Toxoplasma gondii oocyst extraction in soil. Vet. Parasitol. 183:59–67 [DOI] [PubMed] [Google Scholar]
- 72. Liang Y, Hilal N, Langston P, Starov V. 2007. Interaction forces between colloidal particles in liquid: theory and experiment. Adv. Colloid Interface Sci. 134–135:151–166 [DOI] [PubMed] [Google Scholar]
- 73. Lindquist HD, et al. 2003. Autofluorescence of Toxoplasma gondii and related coccidian oocysts. J. Parasitol. 89:865–867 [DOI] [PubMed] [Google Scholar]
- 74. Liu Y, Kuhlenschmidt MS, Kuhlenschmidt TB, Nguyen TH. 2010. Composition and conformation of Cryptosporidium parvum oocyst wall surface macromolecules and their effect on adhesion kinetics of oocysts on quartz surface. Biomacromolecules 11:2109–2115 [DOI] [PubMed] [Google Scholar]
- 75. Macarisin D, Bauchan G, Fayer R. 2010. Spinacia oleracea L. leaf stomata harboring Cryptosporidium parvum oocysts: a potential threat to food safety. Appl. Environ. Microbiol. 76:555–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mai K, et al. 2009. Oocyst wall formation and composition in coccidian parasites. Mem. Inst. Oswaldo Cruz 104:281–289 [DOI] [PubMed] [Google Scholar]
- 77. Maubon D, Ajzenberg D, Brenier-Pinchart MP, Dardé ML, Pelloux H. 2008. What are the respective host and parasite contributions to toxoplasmosis? Trends Parasitol. 24:299–303 [DOI] [PubMed] [Google Scholar]
- 78. Medema GJ, Schets FM, Teunis PF, Havelaar AH. 1998. Sedimentation of free and attached Cryptosporidium oocysts and Giardia cysts in water. Appl. Environ. Microbiol. 64:4460–4466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Metge DW, et al. 2011. Effects of sediment-associated extractable metals, degree of sediment grain sorting, and dissolved organic carbon upon Cryptosporidium parvum removal and transport within riverbank filtration sediments, Sonoma County, California. Environ. Sci. Technol. 45:5587–5595 [DOI] [PubMed] [Google Scholar]
- 80. Milosavljevic N, et al. 2010. Nongenomic effects of cisplatin: acute inhibition of mechanosensitive transporters and channels without actin remodeling. Cancer Res. 70:7514–7522 [DOI] [PubMed] [Google Scholar]
- 81. Mohanram A, et al. 2010. Comparison of transport and attachment behaviors of Cryptosporidium parvum oocysts and oocyst-sized microspheres being advected through three minerologically different granular porous media. Water Res. 44:5334–5344 [DOI] [PubMed] [Google Scholar]
- 82. Mohanram A, et al. 2011. Effect of dissolved organic carbon on the transport and attachment behaviors of Cryptosporidium parvum oocysts and carboxylate-modified microspheres advected through temperate humic and tropical volcanic agricultural soil. Environ. Sci. Technol. [Epub ahead of print]. doi:10.1021/es2003342 [DOI] [PubMed]
- 83. Moitinho MLR, Bertoli M, Guedes TA, Ferreira CS. 1999. Influence of refrigeration and formalin on the floatability of Giardia duodenalis cysts. Mem. Inst. Oswaldo Cruz 94:571–574 [DOI] [PubMed] [Google Scholar]
- 84. Mons C, Dumètre A, Gosselin S, Galliot C, Moulin L. 2009. Monitoring of Cryptosporidium and Giardia river contamination in Paris area. Water Res. 43:211–217 [DOI] [PubMed] [Google Scholar]
- 85. Montoya JG, Liesenfeld O. 2004. Toxoplasmosis. Lancet 363:1965–1976 [DOI] [PubMed] [Google Scholar]
- 86. Murugkar S, Evans CL, Xie XS, Anis H. 2009. Chemically specific imaging of Cryptosporidium oocysts using coherent anti-Stokes Raman scattering (CARS) microscopy. J. Microsc. 233:244–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nanduri JS, Williams S, Aji T, Flanigan TP. 1999. Characterization of an immunogenic glycocalyx on the surfaces of Cryptosporidium parvum oocysts and sporozoites. Infect. Immun. 67:2022–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ongerth JE, Pecoraro JP. 1996. Electrophoretic mobility of Cryptosporidium oocysts and Giardia cysts. J. Environ. Eng. ASCE 122:228 [Google Scholar]
- 89. Petry F. 2004. Structural analysis of Cryptosporidium parvum. Microsc. Microanal. 10:586–601 [DOI] [PubMed] [Google Scholar]
- 90. Poitras C, Fatisson J, Tufenkji N. 2009. Real-time microgravimetric quantification of Cryptosporidium parvum in the presence of potential interferents. Water Res. 43:2631–2638 [DOI] [PubMed] [Google Scholar]
- 91. Possenti A, et al. 2010. Molecular characterisation of a novel family of cysteine-rich proteins of Toxoplasma gondii and ultrastructural evidence of oocyst wall localisation. Int. J. Parasitol. 40:1639–1649 [DOI] [PubMed] [Google Scholar]
- 92. Puech PH, et al. 2011. Force measurements of TCR/pMHC recognition at T cell surface. PLoS One 6:e22344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Puech PH, Poole K, Knebel D, Muller DJ. 2006. A new technical approach to quantify cell-cell adhesion forces by AFM. Ultramicroscopy 106:637–644 [DOI] [PubMed] [Google Scholar]
- 94. Puech PH, et al. 2005. Measuring cell adhesion forces of primary gastrulating cells from zebrafish using atomic force microscopy. J. Cell Sci. 118:4199–4206 [DOI] [PubMed] [Google Scholar]
- 95. Robertson LJ. 2007. The potential for marine bivalve shellfish to act as transmission vehicles for outbreaks of protozoan infections in humans: a review. Int. J. Food Microbiol. 120:201–216 [DOI] [PubMed] [Google Scholar]
- 96. Rose JB, Sliko TR. 1999. Giardia, Cryptosporidium, and Cyclospora and their impact on foods: a review. J. Food Prot. 62:1059–1070 [DOI] [PubMed] [Google Scholar]
- 97. Santamaría J, et al. 2011. Transport of Cryptosporidium parvum oocysts in sandy soil: impact of length scale. J. Environ. Monit. 13:3481–3484 [DOI] [PubMed] [Google Scholar]
- 98. Searcy KE, Packman AI, Atwill ER, Harter T. 2005. Association of Cryptosporidium parvum with suspended particles: impact on oocyst sedimentation. Appl. Environ. Microbiol. 71:1072–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Searcy KE, Packman AI, Atwill ER, Harter T. 2006. Deposition of Cryptosporidium oocysts in streambeds. Appl. Environ. Microbiol. 72:1810–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shapiro K, et al. 2010. Effect of estuarine wetland degradation on transport of Toxoplasma gondii surrogates from land to sea. Appl. Environ. Microbiol. 76:6821–6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Shapiro K, et al. 2009. Surface properties of Toxoplasma gondii oocysts and surrogate microspheres. Appl. Environ. Microbiol. 75:1185–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Sluter SD, Tzipori S, Widmer G. 1997. Parameters affecting polymerase chain reaction detection of waterborne Cryptosporidium parvum oocysts. Appl. Microbiol. Biotechnol. 48:325–330 [DOI] [PubMed] [Google Scholar]
- 103. Spano F, Puri C, Ranucci L, Putignani L, Crisanti A. 1997. Cloning of the entire COWP gene of Cryptosporidium parvum and ultrastructural localization of the protein during sexual parasite development. Parasitology 114:427–437 [DOI] [PubMed] [Google Scholar]
- 104. Speer CA, Clark S, Dubey JP. 1998. Ultrastructure of the oocysts, sporocysts, and sporozoites of Toxoplasma gondii. J. Parasitol. 84:505–512 [PubMed] [Google Scholar]
- 105. Sunnotel O, et al. 2007. Effectiveness of standard UV depuration at inactivating Cryptosporidium parvum recovered from spiked Pacific oysters (Crassostrea gigas). Appl. Environ. Microbiol. 73:5083–5087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Tate KW, Pereira MD, Atwill ER. 2004. Efficacy of vegetated buffer strips for retaining Cryptosporidium parvum. J. Environ. Qual. 33:2243–2251 [DOI] [PubMed] [Google Scholar]
- 107. Taubenberger A, et al. 2007. Revealing early steps of α2β1 integrin-mediated adhesion to collagen type I by using single-cell force spectroscopy. Mol. Biol. Cell 18:1634–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Templeton TJ, et al. 2004. The Cryptosporidium oocyst wall protein is a member of a multigene family and has a homolog in Toxoplasma. Infect. Immun. 72:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Thompson RC. 2004. The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Vet. Parasitol. 126:15–35 [DOI] [PubMed] [Google Scholar]
- 110. Tufenkji N, Nixon DR, Considine R, Drummond CJ. 2006. Multi-scale Cryptosporidium/sand interactions in water treatments. Water Res. 40:3315–3331 [DOI] [PubMed] [Google Scholar]
- 111. Ubbink J, Schär-Zammaretti P. 2005. Probing bacterial interactions: integrated approaches combining atomic force microscopy, electron microscopy and biophysical techniques. Micron 36:293–320 [DOI] [PubMed] [Google Scholar]
- 112. Ulrich F, et al. 2005. Wnt11 functions in gastrulation by controlling cell cohesion through Rab5c and E-cadherin. Dev. Cell 9:555–564 [DOI] [PubMed] [Google Scholar]
- 113. U.S. EPA 2005. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. EPA-815-R-05-002. U.S. Environmental Protection Agency, Washington, DC [Google Scholar]
- 114. Vitte J, Benoliel AM, Pierres A, Bongrand P. 2004. Is there a predictable relationship between surface physical-chemical properties and cell behaviour at the interface? Eur. Cell. Mater. 7:52–63 [DOI] [PubMed] [Google Scholar]
- 115. von Gunten U. 2003. Ozonation of drinking water: part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 37:1469–1487 [DOI] [PubMed] [Google Scholar]
- 116. Wainwright KE, et al. 2007. Physical inactivation of Toxoplasma gondii oocysts in water. Appl. Environ. Microbiol. 73:5663–5666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wainwright KE, et al. 2007. Chemical inactivation of Toxoplasma gondii oocysts in water. J. Parasitol. 93:925–931 [DOI] [PubMed] [Google Scholar]
- 118. Webb HK, Truong VK, Hasan J, Crawford RJ, Ivanova EP. 2011. Physico-mechanical characterisation of cells using atomic force microscopy—current research and methodologies. J. Microbiol. Methods 86:131–139 [DOI] [PubMed] [Google Scholar]
- 119. Wright CJ, Shah MK, Powell LC, Armstrong I. 2010. Application of AFM from microbial cell to biofilm. Scanning 32:134–149 [DOI] [PubMed] [Google Scholar]
- 120. Young PL, Komisar SJ. 2005. Impacts of viability and purification on the specific gravity of Cryptosporidium oocysts. Water Res. 39:3349–3359 [DOI] [PubMed] [Google Scholar]
- 121. Young PL, Komisar SJ. 2005. Settling behavior of unpurified Cryptosporidium oocysts in laboratory settling columns. Environ. Sci. Technol. 39:2636–2644 [DOI] [PubMed] [Google Scholar]