Abstract
Drug dependence is a chronically relapsing disorder that places an enormous strain on healthcare systems. For treatments to have long-term clinical value, they must address the causes of relapse. Corticotropin-releasing factor (CRF), a neuropeptide central to the stress response, may be one key to solving the relapse cycle. CRF is hypothesized to mediate the elevated anxiety and negative emotional states experienced during the development of dependence. This review summarizes existing data on changes in the CRF system produced by drugs of abuse and the function of CRF receptors in regulating behavioural responses to drugs of abuse, with an emphasis on drug dependence. Drug-induced changes in neuronal excitability throughout the limbic system, as well as the reversal of these neuroadaptations by CRF receptor antagonists, are also addressed. CRF receptor antagonists, by reducing the motivational effects of drug withdrawal and protracted abstinence, are proposed to be novel therapeutic targets for drug abuse and addiction.
1. Introduction
Drug addiction is a chronically relapsing disorder in which cycles of compulsive drug taking are followed by periods of abstinence, resulting in withdrawal, characterized by heightened anxiety, irritability and negative affect.[1] Although stress can impact all stages of drug addiction,[2,3] relapse to drug taking is particularly sensitive to stress exposure because of heightened anxiety in the post-dependent state.[4] Therefore, delineation of the neuroadaptations underlying elevated stress responsiveness during abstinence in drug-dependent individuals is essential for the development of therapies to treat drug addiction. One such neuroadaptation involves the neuropeptide corticotropin-releasing factor (CRF), a molecule central to both stress and drug withdrawal responses. Polymorphisms in the genes that encode CRF receptors have been associated in humans with exacerbated stress responses and the propensity to develop drug addiction,[5-9] and the CRF system has significant potential as a target for medication development.
This review provides a brief overview of the role of CRF in hypothalamic stress responses, then focuses on existing behavioural data supporting a role for CRF in drug withdrawal, addressing not only acute but also protracted withdrawal, a behavioural model that may more appropriately replicate the relationship between drug taking and drug relapse periods in humans. Additionally, this article reviews electrophysiological data that demonstrate that CRF modulation of neuronal activity is a possible mechanism underlying drug dependence.
2. Corticotropin-Releasing Factor (CRF): The Central Component of the Stress Response
CRF is a 41-amino-acid peptide originally isolated from the hypothalamus[10] that acts via binding to two receptors: CRF1 and CRF2 .[11,12] The CRF receptors are 7-transmembrane G-protein-coupled receptors that principally function by interacting with the stimulatory G-protein (Gs), resulting in elevated adenylyl cyclase and cyclic adenosine monophosphate levels, although the receptors may also couple to other G-proteins.[13,14] Functional interactions between CRF and its receptors are antagonized by the CRF binding protein (CRF-BP), which sequesters CRF, thus reducing the quantity of CRF available for receptor binding.[15]
CRF was first characterized as the central activator of the endocrine stress response. Exposure to a stressor triggers the synthesis of CRF in the paraventricular nucleus of the hypothalamus. Subsequently, CRF is released via the median eminence into the portal blood to reach the pituitary gland. The peptide then activates CRF1 receptors on pituitary corticotrophs, thereby stimulating adrenocorticotropic hormone synthesis and release into the circulatory system, which subsequently elevates the production and secretion of cortisol (corticosterone in rodents) by the adrenal gland.[16,17] In addition to its function as an effector of the stress response, cortisol also provides negative feedback on hypothalamic-pituitary-adrenal (HPA) axis activity via binding to glucocorticoid receptors in the brain and pituitary,[18] including inhibition of hypothalamic CRF production.[19] As a primary component of the HPA axis, CRF plays a central role in the initiation, maintenance and adaptation of stress responses.
Furthermore, CRF from extrahypothalamic sources has been demonstrated to be key to the expression of behavioural responses to stressors.[20] CRF-immunoreactive perikarya can be found in various brain regions, with particularly strong expression in the extended amygdala (central nucleus of the amygdala [CeA] and medial amygdala [MeA], bed nucleus of the stria terminalis [BNST] and a transition area in the medial [shell] part of the nucleus accumbens [NAc]) and lateral septum,[21] all of which are activated by, and implicated in the expression of behavioural responses to, stressors.[22-24] CRF itself has been shown to be central to the involvement of these nuclei in behavioural stress responses, independent of HPA axis activation.[25] The distribution of the CRF-BP overlaps somewhat with that of CRF, with widespread expression in the cortex and high levels in the amygdala.[26] Interestingly, in the extended amygdala, terminals containing CRF-BP have been shown to colocalize with CRF-positive cell bodies,[26] suggesting that CRF-BP may directly regulate CRF function in these areas. CRF receptor distribution, determined by CRF binding assays, is even more widespread in the brain,[27] indicating a role for CRF and its receptors in regulating the development[28] and excitability[29-34] of many neuronal subpopulations.
The specific distribution of CRF1 and CRF2 receptors is minimally overlapping, with high-affinity CRF1 receptors showing more widespread distribution throughout the cortex and cerebellum. High levels of CRF1 receptors are found in the basolateral amygdala (BLA), MeA, medial septum and BNST, and moderate expression is found in the NAc and ventral tegmental area (VTA).[35,36] Interestingly, unlike other nuclei of the extended amygdala, very few CRF1 receptors can be found in the CeA despite the high content of CRF.[35,36] In contrast, extrahypothalamic forebrain CRF2 receptors are primarily confined to medial subdivisions of the extended amygdala (MeA and medial BNST), with the greatest expression in the lateral septum and ventromedial hypothalamus.[37] The high density of CRF and its receptors, as well as CRF-BP, in stress-responsive brain regions suggests the integral role of the peptide in the regulation of behavioural responses to stress. Not surprisingly, given the dissimilarity in receptor distribution, mice lacking either CRF1 or CRF2 receptors display differential alterations in stress responsiveness. CRF1 receptor null mutants show a blunting of anxiety-like behaviour, regardless of whether the deletion is constitutive[38] or restricted to the postnatal forebrain.[39] In contrast, mice lacking CRF2 receptors tend to exhibit elevated basal anxiety-like behaviour,[40,41] although this has not been observed in all CRF2 receptor null mutant mice.[42] However, even in the absence of changes in basal anxiety-like behaviour, deletion of CRF2 receptors resulted in impaired adaptation to prolonged stress exposure,[42,43] suggesting a deficient stress-coping system. This stress response system is both activated acutely by drugs of abuse and modulated by long-term drug exposure and withdrawal, suggesting a role for CRF systems in the development and maintenance of drug dependence and addiction.
3. Drugs of Abuse Acutely Upregulate CRF and Hypothalamic-Pituitary-Adrenal Axis Activity
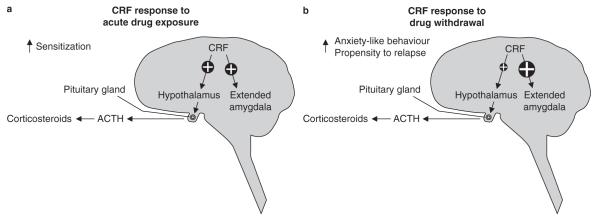
Most drugs with abuse potential, including opiates,[44] amphetamine,[45] cocaine,[46] nicotine,[47] marijuana (Δ9-tetrahydrocannabinol)[48] and alcohol (ethanol),[49] acutely activate the HPA axis via elevated hypothalamic production of CRF (figure 1a).[50,51] This acute HPA activation has been shown to participate in the development of drug-induced locomotor sensitization,[52] with antagonism of CRF1 receptors blocking behavioural sensitization to multiple drugs of abuse.[53,54] Likewise, deletion of CRF1, but not CRF2, receptors inhibits the development of behavioural sensitization to ethanol and blunts the HPA axis response to acute ethanol treatment.[55] Interestingly, blocking the production of the final HPA axis effector, corticosterone, by either adrenalectomy or pharmacological inhibition of its synthesis inhibits not only locomotor activation by cocaine but also the acquisition of cocaine self-administration,[56] suggesting that CRF-induced HPA activation may be involved in the onset of drug self-administration.
Fig. 1.
Time-dependent modulation of the corticotropin-releasing factor (CRF) response to exposure to drugs of abuse. (a) Acute exposure to drugs of abuse activates the hypothalamic-pituitary-adrenal (HPA) axis via increased hypothalamic synthesis and release of CRF, which is released into the portal blood from the median eminence. This triggers increased release of adrenocorticotropic hormone (ACTH) from the pituitary gland, which subsequently acts on the adrenal glands to elevate circulating corticosteroid levels. Acute drug exposure also increases CRF synthesis and release in the extended amygdala. (b) During drug withdrawal, activation of the HPA axis is attenuated compared with the drug-naive state, whereas CRF synthesis and release throughout the extended amygdala are greatly elevated. ↑ indicates increased.
4. Dysregulation of the CRF System during Withdrawal from Drugs of Abuse
While hypothalamic CRF may play a role in the acquisition of drug self-administration, the balance of data show that extrahypothalamic sources of CRF, particularly within the extended amygdala and other key limbic system structures, are integral to the development of negative reinforcement mechanisms associated with addiction.[57] That is, the primary involvement of CRF in the regulation of drug self-administration lies in the dysregulation of the CRF system following withdrawal of drug access in dependent individuals. Multiple lines of evidence have demonstrated that although acute drug exposure yields a transient elevation of CRF expression in multiple brain regions (figure 1a),[58,59] chronic exposure results in overactivation of the CRF system, which is central to the withdrawal and dependence phenotypes observed upon removal of drug access (figure 1b).[60-64] During the progression to dependence, drug exposure ceases to trigger elevated CRF expression,[58] resulting in a blunted HPA response.[65] However, when drug access is subsequently withdrawn, CRF release in the extended amygdala increases, accompanied by somatic and psychological withdrawal signs.[66-68] Particularly striking across multiple drugs of abuse is the elevation in CeA CRF at various withdrawal time points, which can be observed not only when assessing levels of messenger RNA (mRNA) expression[59,62,63] and protein content[60] but, importantly, also as an elevation of CRF released into the extracellular space.[64,66,69] Notably, a high level of CRF release at early withdrawal timepoints can yield paradoxically low CRF levels when protein content is determined at the intracellular level.[59,60,70,71] The synthesis of new CRF may lag behind the rate of release, thereby depleting the tissue content of CRF.
Similar to the CeA, increased CRF release has also been observed in the lateral BNST during drug withdrawal,[67] suggesting an elevated activation of CRF signalling throughout the extended amygdala. This heightened CRF activity may be further augmented during abstinence by long-lasting changes in receptor expression levels. Contrasting changes in CRF1 and CRF2 receptor levels have been observed 3 weeks after cessation of ethanol exposure.[63] Specifically, following extended abstinence, CRF1 receptor levels in the BLA were elevated and CRF2 receptor levels decreased, whereas CRF1 receptor levels in the MeA were increased without changes in CRF2 receptor mRNA.[63] The effects may be specific to a given abused drug or withdrawal timepoint, because precipitated morphine withdrawal acutely reduced CRF1 receptor mRNA expression in the BLA and NAc.[72]
Data from CRF receptor knockout mice support a prominent role for CRF1 receptors in symptoms of drug withdrawal and dependence-induced elevations in drug intake. Deletion of the CRF1 receptor gene abolished dependence-induced elevations in the self-administration of ethanol[73] and opiate withdrawal-induced conditioned place aversion,[74] whereas deletion of the CRF2 receptor gene had a marginal effect on ethanol intake in nondependent mice under limited-access conditions.[75] The Marchigian Sardinian ethanol-preferring (msP) rat line carries a polymorphism in the promoter region of the gene encoding the CRF1 receptor, which is putatively responsible for the elevation in CRF1 receptor expression observed in multiple regions of the msP brain, particularly within the extended amygdala and other key limbic system structures.[76] msP rats display high basal alcohol intake, which can be reduced by antagonizing the CRF1 receptor.[76] These data suggest that altered expression of the CRF1 receptor may regulate excessive self-administration of ethanol (and perhaps other drugs of abuse).
The balance of gene expression studies suggests a more prominent role for CRF1 than CRF2 receptors in the motivational aspects of drug withdrawal and dependence. Data from CRF receptor knockout mice suggest the involvement of both receptors in the somatic withdrawal syndrome associated with drugs of abuse. CRF2 receptors may regulate the peripheral effects of opiate withdrawal, which were largely absent in CRF2 receptor knockouts.[77] Deletion of CRF1 receptors, which may cause a compensatory upregulation of CRF2 receptors, yielded heightened signs of somatic withdrawal from opiates.[78] Altogether, these data suggest that CRF receptors present attractive targets for the modulation of drug self-administration and somatic withdrawal syndromes in dependent populations.
5. CRF Receptor Antagonists as Potential Treatments for Drug Addiction
As discussed in section 4, CRF receptor antagonists, particularly those targeting CRF1 receptors, show promise for the development of treatments for drug abuse and addiction. Much effort has been placed on the development of high-affinity (low dissociation constant, Ki), blood-brain barrier-penetrating CRF1 receptor-selective antagonists with drug-like properties,[79,80] including good oral bioavailability, volume of distribution (moderate) and clearance rates (half-life suitable for once daily dosing, ~12–36 hours) that may be useful as medications for human patients. A subset of these antagonists and their basic pharmacological properties can be found in table I; for a comprehensive review of existing CRF1 receptor pharmacology, see Zorrilla and Koob.[93] Because of the upregulation of CRF and CRF1 receptors during drug withdrawal, many studies have explored the ability of CRF receptor antagonists to reduce withdrawal-induced elevations in anxiety and drug self-administration. Table II summarizes CRF receptor antagonist modulation of the behavioural and neuroendocrine effects of abused drugs, including the efficacy of the antagonists in inhibiting relapse to drug seeking.
Table I.
Pharmacological properties of selected corticotropin-releasing factor (CRF)1 receptor antagonists commonly used in animal models of drug addiction
| Compound | CRF1 receptor affinity (nmol/L)a |
Vd (L/kg)b | t½ (h)b | Commentsc | References |
|---|---|---|---|---|---|
| α-Helical CRF9-41 | 10.3 | NA | Also binds to CRF2 receptors and CRF binding protein; not blood-brain barrier penetrant |
81 | |
| D-Phe CRF12-41 | 15.5 | NA | Also binds to CRF2 receptors; not blood-brain barrier penetrant | 81 | |
| Antalarmin | 1.4 | 82 | |||
| CP-154,526 | 2.7 | 105 | 51 | 83,84 | |
| R121919 (NBI30775) |
3.5 | 16.7 | 1.7 | 85 | |
| NBI27914 | 2.3 | 86 | |||
| MJL-1-109-2 | 1.9 | 87 | |||
| CRA-1000 | 16–21 | Also binds to serotonin 5-HT1A and 5-HT4 receptors, L-type Ca2+ channel, site 2 Na2+ channel, platelet-activating factor, and serotonin reuptake channels weakly |
88,89 | ||
| LWH-63 (NIH-3) | 0.7 | 90 | |||
| MTIP | 0.22 | 1.7 | 3.9 | 91 | |
| MPZP | 4.9 | 92 |
CRF1 receptor affinity defined as Ki, the dissociation constant of the antagonist to the CRF1 receptor. Ki values determined at human or rat CRF1, as detailed in cited reference.
Vd and t½ determined in rats in vivo.
Unless otherwise noted, the antagonists are blood-brain barrier penetrant.
MPZP = N,N-bis(2-methoxyethyl)-3-(4-methoxy-2-methylphenyl)-2,5-dimethyl-pyrazolo[1,5-a] pyrimidin-7-amine; MTIP = 3-(4-chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine; NA = not applicable; t½ = compound half-life; Vd = volume of distribution of drug in plasma.
Table II.
Predicted therapeutic role of corticotropin-releasing factor (CRF) receptor antagonists in modulating processes related to drug addiction
| Drug | Acute HPA activation |
Behavioural sensitization |
Self-administration |
Withdrawal- induced place aversion |
Anxiety during withdrawal/ abstinence |
Relapse |
||
|---|---|---|---|---|---|---|---|---|
| maintenance | dependent/escalated drug taking |
stress-induced reinstatement |
drug- or cue-induced reinstatement |
|||||
| Cocaine | ↓ [51]a | ↓ [54]b | –/↓ [94-96]b,c | ↓ [94]b | ↓ [97]a | ↓ [98,99]b | –/↓ [98]a; [54,100]b | |
| Opiates | ↓ [101]b | _[102]b | ↓ [102,103]b | ↓ [104,105]b | ↓ [106]a; [99,107]b | –/↓ [106]a; [107]b | ||
| Ethanol | ↓ [108]a | ↓ [53,55]b | –/↓ [76,91,92,109-115]b,c | ↓ [116]a, [73,91,92,109,114-116]b |
↓ [116,117]a; [91,92,118]b |
↓ [119]a; [91,110,120]b,c |
–/↓ [119]a; [121]b | |
| Nicotine | ↓ [122]b | ↓ [123]a | _[64]b | ↓ [64]b | ↓ [124]a[64]b | ↓ [125,126]b | ||
| Δ9-tetrahydro- cannabinol |
↓ d | ↓ [127]a | ↓ e | |||||
| Palatable food | ↓ [128]b | ↓ [128]b | ↓ [129]b | _[129]b | ||||
Studies used CRF receptor subtype nonspecific antagonists.
Studies used CRF receptor-specific antagonists.
Systemic CRF receptor antagonism not yet tested with cannabinoid agonist exposure, but decreases in HPA activation and sensitization are predicted based on the ability of CRF receptor antagonists to inhibit similar effects following exposure to other drugs of abuse in conjunction with activation of the HPA axis by the cannabinoid receptor agonist anandamide[48] (although intracerebroventricular administration of D-Phe CRF12-41 does not significantly alter corticosterone levels after acute cannabinoid agonist administration).[127]
Systemic CRF receptor antagonism not yet tested with cannabinoid agonist exposure, but elevated anxiety-like behaviour and extracellular CRF observed in the extended amygdala during withdrawal from chronic cannabinoid agonist treatment.[69]
HPA = hypothalamic-pituitary-adrenal; ↓ indicates attenuation of HPA activation or behavioural measure following CRF receptor antagonist treatment; – indicates no effect of CRF receptor antagonist treatment on given behaviour.
5.1 CRF Receptor Subtype Nonspecific Antagonists
Prior to the discovery of the CRF receptor subtypes, several ligands that have affinity for both CRF receptor subtypes were developed as a means of competitively interfering with the function of endogenous CRF (table I). Among these (Met,[18] Lys,[23] Glu,[27,29,40] Ala,[32,41] Leu[33,36,38]) h/rCRF9–41 (α-helical CRF9-41),[130] a CRF receptor partial agonist,[93] and (D-Phe,[12] Nle,[21,38] CαMeLeu[37]) h/rCRF12-41 (D-Phe CRF12-41),[131] a full antagonist of the CRF receptor,[93] have been the most widely used, with similar results. Both ligands inhibited footshock-induced reinstatement of cocaine self-administration in rats, whether injected systemically[98] or locally infused into the BNST[132] or VTA.[133] Similar results were observed with stress-induced reinstatement of ethanol-seeking[119] and heroin-seeking[106,134] behaviour. Interestingly, α-helical CRF9-41 and D-Phe CRF12-41 were unable to antagonize drug- or cue-primed reinstatement, unless the cue was presented in conjunction with stress pre-exposure.[119] Nonetheless, the peptide ligands showed great efficacy in reducing ethanol withdrawal-induced elevations in both self-administration[116,135] and anxiety-like behaviour.[116,117]
For ethanol, the actions of CRF receptor antagonists in reducing both self-administration and anxiety-like behaviour in dependent rats have been localized to the CeA.[71,136] Similar effects have been found for nicotine, with either central or CeA-specific infusion of D-Phe CRF12-41 reducing withdrawal-associated elevations in brain reward stimulation thresholds,[124,137] an effect recently shown for ethanol withdrawal as well.[138] Likewise, CeA administration of α-helical CRF9-41 blocked conditioned place aversion to an environment paired with precipitated morphine withdrawal.[104]
These data demonstrate that the subtype-nonspecific peptide CRF receptor antagonists α-helical CRF9-41 and D-Phe CRF12-41 reduce both the heightened anxiety-like behaviour and elevated self-administration observed in drug dependence. However, to improve receptor specificity and efficacy of systemic administration, several nonpeptide antagonists have been developed that have CRF1 receptor specificity and show greater ability to penetrate the blood-brain barrier.
5.2 Specific CRF1 Receptor Antagonism
A significant advance in CRF1 receptor pharmacology occurred with the discovery of blood-brain barrier-penetrating CRF1 receptor antagonists, including CP-154,526[139] and antalarmin.[82] These compounds were the first major CRF1 receptor antagonists with therapeutic potential for CNS disorders, such as drug addiction. Importantly, antalarmin was shown to be capable of inhibiting anxiety-like behaviour, resulting from intracerebroventricular CRF treatment, in the elevated plus maze,[140] indicating not only brain penetrance, but also efficacy following systemic delivery in blocking the central effects of CRF. Like the subtype nonselective peptide antagonists, the small-molecule antalarmin reduced withdrawal-associated elevations in ethanol self-administration in both rats[109] and mice,[73] as well as ethanol intake in ethanol-preferring msP rats.[76]
Similarly, the ability of antalarmin to reduce cocaine self-administration was evident selectively in rats given extended (6-hour) daily access to cocaine (‘long access’ [LgA]), a schedule with which self-administration escalates across days, but not in rats given brief (1-hour) daily access (‘short access’ [ShA]), in which intake does not escalate.[94] In addition to reducing elevated drug self-administration in dependent animals, antalarmin also inhibited stress-primed reinstatement of ethanol self-administration[110] as well as stress-induced elevations in ethanol self-administration[110] and palatable food intake.[129] These effects may be due to the ability of antalarmin to reduce the negative emotional state of withdrawal, as the compound reduced the development of conditioned avoidance of a location previously paired with acute morphine withdrawal.[105] These data demonstrate that antalarmin specifically reduces drug withdrawal effects and self-administration in dependent, but not nondependent, individuals.
Similar to antalarmin, CP-154,526 effectively antagonized stress-induced reinstatement of drug seeking for cocaine,[99] heroin[99] and ethanol,[120] as well as cue- and drug-primed methamphetamine reinstatement.[141] It also inhibited stress-induced reinstatement of conditioned place preference to morphine[142,143] and cocaine.[144] Interestingly, the compound may have efficacy in reducing maintenance self-administration responding,[95,111-113] although many experiments that showed effects on maintenance responding were performed at some point post-stress, such as following a history of forced swim testing or extinction training. CP-154,526 also inhibited the reduction in social interaction observed after restraint stress in rats with a history of multiple cycles of ethanol withdrawal.[145] Important for efficacy in human drug treatment, this anxiolytic-like effect was observed whether the CRF1 receptor antagonist was administered prior to the restraint stress or during each of the withdrawal periods, suggesting that the use of the antagonist to alleviate the stress of withdrawal may have a lasting ability to blunt the heightened stress sensitivity in post-dependent individuals.
Wills and colleagues[146] suggested that stress pre-exposure may be integral to the ability of CP-154,526 to reduce self-administration in non-dependent rats. However, recent data have shown that CP-154,526 reduced ethanol intake in the absence of stress in nondependent mice under-going a limited-access two-bottle choice paradigm.[111,113] CP-154,526 has also been used to inhibit locomotor sensitization to multiple drugs of abuse, demonstrating the involvement of CRF activation of CRF1 receptors in the expression,[54] or acquisition and expression,[53] of sensitization to cocaine and ethanol, respectively. Importantly, following systemic administration, this antagonist blocked the central generation of anxiety-like responses without altering peripheral HPA axis activity,[147] a key feature for efficacy in human treatment because blunting of all stress responses would be disadvantageous.
The search for optimal, drug-like CRF1 receptor antagonists has spurred the development and testing of many additional antagonists in recent years. Similar to CP-154,526, CRA-1000 reduced ethanol withdrawal-induced anxiety.[148,149] R121919 (also known as NBI30775),[150] at doses that do not alter normal HPA function, has been shown to reduce anxiety-like behaviour in rats with high basal anxiety, but not in rats without high basal anxiety.[151] Low doses of R121919 have similarly been demonstrated to reduce anxiety-like behaviour in mice[152] and depression in humans[153,154] without significantly modulating basal HPA activity. Similar to antalarmin, R121919 also reduced LgA but not ShA cocaine self-administration,[94] as well as LgA, but not ShA, heroin self-administration.[102] The antagonist also reduced both binge eating of palatable food and the anxiety-like behaviour precipitated by the removal of access to that food.[128]
Both R121919 and MJL-1-109-2 dose-dependently decreased ethanol self-administration during withdrawal, with no effect in nondependent rats,[109] as did LWH-63 when administered to dependent Sardinian alcohol-preferring rats.[114] None of these three CRF1 receptor antagonists reduced homecage drinking when ethanol was available continuously, although they paradoxically slightly increased ethanol intake under limited homecage access conditions in nondependent rats.[114]
The newer heterocyclic CRF1 receptor antagonists MPZP[92] and MTIP[91] also reduced dependence-induced ethanol self-administration. Surprisingly, MTIP also reduced excessive drinking in nondependent msP rats. This reduction in nondependent ethanol intake was likely attributable to genetic differences between msP rats and other strains, rather than via a novel action of MTIP compared with other CRF1 receptor antagonists, as the msP line shows a high incidence of a CRF1 receptor promoter polymorphism that yields elevated CRF1 receptor expression.[91] The striking similarity in the ability of the various CRF1 receptor-selective antagonists to inhibit withdrawal-associated elevated anxiety-like responses and drug self-administration confirms the integral role of CRF activity at the CRF1 receptor in regulating the negative effects of drug withdrawal.
5.3 Targeting the CRF2 Receptor
Unlike CRF1 receptors, fewer pharmacological tools have been developed to specifically modulate the CRF2 receptor, perhaps because of the perception that CRF1 receptors regulate the majority of central CRF effects. Indeed, several studies have shown an inability of CRF2 receptor antagonists to modulate withdrawal-induced behavioural adaptations.[142,148] However, the functionality of the CRF2 receptor in modulating drug self-administration and reinstatement has begun to emerge. Activation of the CRF2 receptor in the CeA by urocortin 3 (Ucn3) reduced ethanol self-administration in dependent rats while increasing self-administration in nondependent rats.[155] The effects of Ucn3 on dependent ethanol self-administration may stem from the ability of Ucn3 to inhibit anxiety-like behaviour during acute ethanol withdrawal.[156] Under a two-bottle choice limited-access paradigm, central infusions of Ucn3 reduced ethanol intake similarly to CRF1 receptor antagonists,[113,157] suggesting that either inhibition of CRF1 receptors or activation of CRF2 receptors can decrease the propensity to consume ethanol even in non-dependent individuals.
Unlike ethanol self-administration, footshock-induced reinstatement of cocaine seeking can be reduced by blockade, rather than activation, of the CRF2 receptor. Using the preferential CRF2 receptor antagonist antisauvagine-30 in rats, reinstatement of self-administration of cocaine following footshock stress was blocked.[158] The involvement of CRF2 receptors in the modulation of stress-induced relapse likely operates through a different circuitry than for CRF1 receptor regulation of drug self-administration. Whereas CRF1 receptor antagonists may exert their greatest effects in the extended amygdala, CRF2 receptor antagonists inhibited stress-induced reinstatement in rats via activity in the VTA[158] and reduced withdrawal-induced anxiety-like behaviour in rats via the dorsal raphe nucleus.[159] Together, these data demonstrate that developing pharmacological tools for more selective targeting of CRF2 receptors warrants increased attention. The currently available compounds lack utility as treatment options for drug abuse because they must be centrally administered and display much lower CRF receptor subtype specificity than CRF1 receptor antagonists (e.g. antisauvagine-30, although roughly 100-fold more selective for CRF2 than CRF1 receptors, is not CRF2 receptor specific[160]).
6. Role of CRF in Drug Relapse Following Extended Periods of Abstinence
To date, much of the preclinical research into the role of CRF in regulating drug dependence has focused heavily on acute withdrawal paradigms. These studies are very pertinent to human drug addiction in the early stages of ceasing drug taking (i.e. the ability to gain sobriety), but it is uncertain how they translate to the more common human situation, in which relapse to drug taking occurs following extended periods of abstinence. In contrast to the acute withdrawal window, which is characterized by both somatic withdrawal symptoms and negative affect, the protracted abstinence period is distinguished by heightened anxiety-like behaviour and drug craving.[161-163] For example, in rats, withdrawal of chronic ethanol access via either liquid diet[164] or ethanol vapour[165] yielded elevated anxiety-like behaviour and brain reward thresholds within the first 24 hours,[164,165] followed by a return to normal baseline but heightened stress-induced anxiety-like behaviour at 2 weeks post-withdrawal[164,165] and a resurgence of increased baseline anxiety-like behaviour after 6 weeks of abstinence.[165] These data demonstrate that abstinence is not a static condition but rather one in which neuroadaptations continue over a prolonged period of time.
One study in rats suggested that this difference between acute and extended withdrawal may not diminish the clinical relevance of the preclinical findings. Administration of CRF receptor antagonists during multiple ethanol withdrawal periods had a long-lasting ability to decrease stress-induced anxiety-like behaviour during protracted abstinence periods.[145] Nevertheless, several additional studies have begun to address the role of the CRF system in long-term abstinence, all of which suggest continued CRF receptor antagonist efficacy throughout the abstinence period. Elevated anxiety-like behaviour and ethanol self-administration observed in dependent rats after 4 weeks of abstinence were reversed by D-Phe CRF12-41 antagonism of CRF receptors.[116] Similarly, following 6 weeks of abstinence, post-dependent rats showed increased sensitivity to the effects of restraint stress on anxiety-like behaviour, an effect that was blocked by D-Phe CRF12-41.[166] These data suggest that the increased CRF-like immunoreactivity observed in the amygdala 6 weeks after withdrawal of chronic access to ethanol or cocaine[60] may regulate the heightened anxiety-like behaviour observed during protracted withdrawal. More recently, Heilig and colleagues[63] found elevated CRF1 receptor mRNA expression in the BLA and MeA in post-dependent rats following 3 weeks of abstinence from ethanol vapour. These rats also showed elevated drinking following stress exposure compared with rats without a history of dependence, as well as elevated stress sensitivity that was ameliorated by the CRF1 receptor antagonist MTIP.[63] Although similar data are lacking for other drugs of abuse, the sensitivity to CRF1 receptor antagonist blockade of stress-induced drug reinstatement has been shown in multiple paradigms that involve prolonged extinction training,[99,142,158] suggesting persistent CRF system sensitivity over long periods of abstinence, regardless of the abused substance.
Altogether, these data indicate the potential for success in using CRF receptor antagonists to combat the proximal causes of relapse even following long periods of sobriety. This perseverance of CRF sensitivity throughout the abstinence period also suggests that common changes in neuroplasticity within the CRF systems of the extended amygdala and other limbic regions may underlie withdrawal-induced elevations in drug intake.
7. Elevated CRF Signalling Alters Plasticity Throughout the Extended Amygdala and Mesocorticolimbic System during Drug Withdrawal
The chronically relapsing nature of drug addiction suggests that long-lasting neuroadaptations govern the persistence of drug-seeking and drug-taking behaviour, for example, via changes in the strength of the synaptic connections within neurocircuits that subserve the response to drugs of abuse.[167] Because withdrawal from multiple drugs of abuse results in elevated levels of CRF and CRF1 receptors in the extended amygdala, in particular within the amygdala[60,62,63,66,168] and BNST,[67] these nuclei present likely loci for drug-induced synaptic modifications that may underlie elevated anxiety-like behaviour and drug self-administration in dependent individuals. Acute ethanol treatment of brain slices increased inhibitory GABA signalling in the CeA via activation of CRF1 receptors, and this effect could be blocked by deletion of the CRF1 receptor gene or by D-Phe CRF12-41,[169] antalarmin, LWH-63 and R121919 treatment,[170] but not by the CRF2 receptor antagonist astressin2-B.[169] However, deletion of the CRF2 receptor gene augmented ethanol-induced inhibitory postsynaptic currents (IPSCs).[169] Interestingly, the development of ethanol dependence in vivo did not preclude the ability of acute ethanol treatment to increase GABA IPSCs in brain slices of the CeA collected during early withdrawal.[170] However, unlike nondependent rats, in which CRF1 receptor antagonists inhibited only the potentiation of CeA IPSCs by ethanol, co-application of antalarmin, LWH-63 or R121919 not only blocked ethanol-induced IPSCs but also reduced baseline inhibitory firing in the absence of ethanol.[170] Thus, CeA neurons maintain responsiveness to ethanol during withdrawal, and elevated extracellular CRF observed in vivo at this timepoint increases the baseline firing rate of CeA GABA-ergic neurons.
This dampening of baseline inhibitory tone by CRF1 receptor antagonists may also occur, albeit at a much lesser level, in nondependent animals, providing a putative explanation for the reduction of ethanol intake by CRF1 receptor antagonists in nondependent animals, similar to observations in several recent studies using limited access paradigms that aimed to replicate binge drinking.[111,113] These data suggest that the reduction in baseline IPSCs may, in fact, have functional consequences for the regulation of ethanol intake in nondependent individuals under certain access/intake conditions.
Whereas ethanol studies have focused on the effects of dependence on CRF modulation of inhibitory signalling in the CeA, cocaine withdrawal elevated excitatory synaptic firing, with effects appearing 2 weeks, but not 1 day, after withdrawal from drug treatment.[168,171,172] This potentiated response, observed at synapses from BLA projections to the CeA, involved not only greater activation in response to CRF treatment,[171] but also elevated long-term potentiation following high-frequency stimulation, an effect blocked by the CRF1 receptor antagonist NBI27914[168,172] and reduced in magnitude by the CRF receptor antagonist astressin2-B.[172] These data demonstrate that although multiple drugs of abuse alter plasticity within the CeA, the specific CRF synapses modified by drug withdrawal may not be uniform across different drugs.
Unlike the CeA, withdrawal-induced synaptic changes in the BNST appear to act via a common pathway, regardless of the abused substance. Similar to the CeA, CRF treatment increased GABAergic neuron firing via a CRF1 receptor-dependent mechanism, as CRF modulation of inhibitory currents was blocked by NBI27914, but not by the CRF2 receptor antagonist antisauvagine-30.[173] However, unlike the divergent mechanisms observed for ethanol and cocaine withdrawal in the CeA, withdrawal from chronic intermittent ethanol vapour, LgA cocaine self-administration and LgA heroin self-administration all reduced the fidelity of the intrinsic excitability of BNST juxtacapsular neurons, thus disrupting the long-term potentiation of these neurons.[174] This shift away from the excitation threshold occurred via a CRF1 receptor-dependent mechanism, as R121919 treatment normalized the intrinsic excitability to levels comparable to nondependent controls.[174] These data demonstrate that withdrawal-induced upregulation of the CRF/CRF1 receptor system in the CeA and BNST alters the excitability, and thus intrinsic responsiveness, of the extended amygdala to subsequent neural signals. The blockade of CRF1 receptors returns these brain regions to activity levels similar to normal controls.
Unlike the extended amygdala, in which drug withdrawal alters synaptic efficacy mainly via a CRF1 receptor-dependent mechanism, behavioural results suggest a prominent role for CRF2 receptors in the VTA as mediators of drug seeking, as well as glutamate and dopamine release during stress-induced drug reinstatement.[158] At the synaptic level, application of CRF potentiated the response of VTA glutamate N-methyl-D-aspartate (NMDA) receptors to stimulation, an effect that was further heightened by chronic cocaine exposure.[175] This enhancement of CRF regulation of VTA excitability following chronic cocaine exposure was reduced by CRF1 receptor antagonism but was completely blocked by CRF2 receptor antagonism.[175] These results align with the existing data for stress-induced drug reinstatement, supporting a model of CRF regulation of VTA neuronal activity in which elevated CRF, caused by stress exposure, activates CRF2 receptors to enhance dopamine and glutamate release, resulting in reinstatement of drug seeking. Within the same population of neurons, chronic cocaine exposure unmasked an additional ability of CRF to enhance excitatory responses via the glutamate α-amino-3-hydroxy-5-methyl-4-iso-xazolepropionic acid (AMPA) receptor, which was insensitive to CRF in drug-naive animals.[175] Unlike the NMDA effect, however, potentiation of AMPA signalling by CRF in cocaine-experienced animals was insensitive to CRF2 receptor antagonism and instead was blocked by inhibition of the CRF1 receptor.[175] Thus, although CRF2 receptors play a predominant role in CRF regulation of VTA neuronal activity, chronic drug exposure may produce an additional mechanism for elevated excitation via a CRF1 receptor-dependent pathway. Together with the in vivo studies discussed previously, these data suggest that inhibition of CRF activity presents a useful pharmacological target for normalizing maladaptive changes in neuronal activity that may underlie the persistence of drug addiction.
8. Conclusion
As a chronically relapsing condition, drug abuse and addiction cannot be successfully treated without addressing the underlying cause of relapse. CRF is a key modulator of the anxiety observed in both acute and protracted abstinence from multiple drugs of abuse and thus presents an ideal target for medication development. The success of multiple CRF receptor antagonists in animal models of drug dependence and the variety of compounds now available provides hope that a clinically effective CRF receptor antagonist for the treatment of drug addiction may be on the horizon.
Acknowledgements
The authors would like to thank Michael Arends for editorial assistance in the preparation of this manuscript and Janet Hightower for help with figure preparation. Financial support was received from the Pearson Center for Alcoholism and Addiction Research and National Institutes of Health grant DK26741 from the National Institute of Diabetes and Digestive and Kidney Diseases, AA06420, AA08459 and AA018914 from the National Institute on Alcohol Abuse and Alcoholism, and DA04043, DA04398 and DA023957 from the National Institute on Drug Abuse. G.F. Koob and E.P. Zorrilla are inventors on a provisional patent filed for CRF1 antagonists (US provisional patent number 60/902,479).
Footnotes
M.L. Logrip has no conflicts of interest to disclose. This is publication number 20696 from The Scripps Research Institute.
References
- 1.Koob GF. A role for brain stress systems in addiction. Neuron. 2008 Jul 10;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci. 2008 Oct;1141:105–30. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goeders NE. The impact of stress on addiction. Eur Neuropsychopharmacol. 2003 Dec;13(6):435–41. doi: 10.1016/j.euroneuro.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Weiss F, Ciccocioppo R, Parsons LH, et al. Compulsive drug-seeking behavior and relapse: neuroadaptation, stress, and conditioning factors. Ann N Y Acad Sci. 2001 Jun;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 5.De Luca V, Tharmalingam S, Kennedy JL. Association study between the corticotropin-releasing hormone receptor 2 gene and suicidality in bipolar disorder. Eur Psychiatry. 2007 Jul;22(5):282–7. doi: 10.1016/j.eurpsy.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Enoch MA, Shen PH, Ducci F, et al. Common genetic origins for EEG, alcoholism and anxiety: the role of CRH-BP. PLoS One. 2008;3(10):e3620. doi: 10.1371/journal.pone.0003620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke TK, Schumann G. Gene-environment interactions resulting in risk alcohol drinking behaviour are mediated by CRF and CRF1. Pharmacol Biochem Behav. 2009 Sep;93(3):230–6. doi: 10.1016/j.pbb.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Blomeyer D, Treutlein J, Esser G, et al. Interaction between CRHR1 gene and stressful life events predicts adolescent heavy alcohol use. Biol Psychiatry. 2008 Jan 15;63(2):146–51. doi: 10.1016/j.biopsych.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 9.Treutlein J, Kissling C, Frank J, et al. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiatry. 2006 Jun;11(6):594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- 10.Spiess J, Rivier J, Rivier C, et al. Primary structure of corticotropin-releasing factor from ovine hypothalamus. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6517–21. doi: 10.1073/pnas.78.10.6517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CP, Pearse RV, 2nd, O’Connell S, et al. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993 Dec;11(6):1187–95. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- 12.Lovenberg TW, Liaw CW, Grigoriadis DE, et al. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci U S A. 1995 Jan 31;92(3):836–40. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grammatopoulos DK, Randeva HS, Levine MA, et al. Rat cerebral cortex corticotropin-releasing hormone receptors: evidence for receptor coupling to multiple G-proteins. J Neurochem. 2001 Jan;76(2):509–19. doi: 10.1046/j.1471-4159.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- 14.Blank T, Nijholt I, Grammatopoulos DK, et al. Corticotropin-releasing factor receptors couple to multiple G-proteins to activate diverse intracellular signaling pathways in mouse hippocampus: role in neuronal excitability and associative learning. J Neurosci. 2003 Jan 15;23(2):700–7. doi: 10.1523/JNEUROSCI.23-02-00700.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linton EA, Behan DP, Saphier PW, et al. Corticotropin-releasing hormone (CRH)-binding protein: reduction in the adrenocorticotropin-releasing activity of placental but not hypothalamic CRH. J Clin Endocrinol Metab. 1990 Jun;70(6):1574–80. doi: 10.1210/jcem-70-6-1574. [DOI] [PubMed] [Google Scholar]
- 16.Guillemin R, Dear WE, Nichols B, Jr, et al. ACTH releasing activity in vivo of a CRF preparation and lysine vasopressin. Proc Soc Exp Biol Med. 1959 May;101(1):107–11. doi: 10.3181/00379727-101-24848. [DOI] [PubMed] [Google Scholar]
- 17.Hiroshige T, Ogura C, Itoh S. ACTH release by purified hypothalamic CRF preparation as assayed by intrapituitary microinjection. Endocrinol Jpn. 1968 Sep;15(3):379–82. doi: 10.1507/endocrj1954.15.379. [DOI] [PubMed] [Google Scholar]
- 18.McEwen BS. Influences of adrenocortical hormones on pituitary and brain function. Monogr Endocrinol. 1979;12:467–92. doi: 10.1007/978-3-642-81265-1_25. [DOI] [PubMed] [Google Scholar]
- 19.Kretz O, Reichardt HM, Schutz G, et al. Corticotropin-releasing hormone expression is the major target for glucocorticoid feedback-control at the hypothalamic level. Brain Res. 1999 Feb 13;818(2):488–91. doi: 10.1016/s0006-8993(98)01277-3. [DOI] [PubMed] [Google Scholar]
- 20.Heinrichs SC, Menzaghi F, Merlo Pich E, et al. The role of CRF in behavioral aspects of stress. Ann N Y Acad Sci. 1995 Dec 29;771:92–104. doi: 10.1111/j.1749-6632.1995.tb44673.x. [DOI] [PubMed] [Google Scholar]
- 21.Pilcher WH, Joseph SA. Co-localization of CRF-ir perikarya and ACTH-ir fibers in rat brain. Brain Res. 1984 May 7;299(1):91–102. doi: 10.1016/0006-8993(84)90791-1. [DOI] [PubMed] [Google Scholar]
- 22.Melia KR, Sananes CB, Davis M. Lesions of the central nucleus of the amygdala block the excitatory effects of septal ablation on the acoustic startle reflex. Physiol Behav. 1992 Jan;51(1):175–80. doi: 10.1016/0031-9384(92)90220-v. [DOI] [PubMed] [Google Scholar]
- 23.Day HE, Nebel S, Sasse S, et al. Inhibition of the central extended amygdala by loud noise and restraint stress. Eur J Neurosci. 2005 Jan;21(2):441–54. doi: 10.1111/j.1460-9568.2005.03865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammack SE, Richey KJ, Watkins LR, et al. Chemical lesion of the bed nucleus of the stria terminalis blocks the behavioral consequences of uncontrollable stress. Behav Neurosci. 2004 Apr;118(2):443–8. doi: 10.1037/0735-7044.118.2.443. [DOI] [PubMed] [Google Scholar]
- 25.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999 Nov 1;46(9):1167–80. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 26.Potter E, Behan DP, Linton EA, et al. The central distribution of a corticotropin-releasing factor (CRF)-binding protein predicts multiple sites and modes of interaction with CRF. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4192–6. doi: 10.1073/pnas.89.9.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Souza EB, Battaglia G. Corticotropin-releasing hormone (CRH) receptors in brain. Adv Exp Med Biol. 1988;245:123–36. doi: 10.1007/978-1-4899-2064-5_9. [DOI] [PubMed] [Google Scholar]
- 28.Swinny JD, Kalicharan D, Blaauw EH, et al. Corticotropin-releasing factor receptor types 1 and 2 are differentially expressed in pre- and post-synaptic elements in the post-natal developing rat cerebellum. Eur J Neurosci. 2003 Aug;18(3):549–62. doi: 10.1046/j.1460-9568.2003.02776.x. [DOI] [PubMed] [Google Scholar]
- 29.Schierloh A, Deussing J, Wurst W, et al. Corticotropin-releasing factor (CRF) receptor type 1-dependent modulation of synaptic plasticity. Neurosci Lett. 2007 Apr 6;416(1):82–6. doi: 10.1016/j.neulet.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 30.Fu Y, Neugebauer V. Differential mechanisms of CRF1 and CRF2 receptor functions in the amygdala in pain-related synaptic facilitation and behavior. J Neurosci. 2008 Apr 9;28(15):3861–76. doi: 10.1523/JNEUROSCI.0227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miyata M, Okada D, Hashimoto K, et al. Corticotropin-releasing factor plays a permissive role in cerebellar long-term depression. Neuron. 1999 Apr;22(4):763–75. doi: 10.1016/s0896-6273(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 32.Rainnie DG, Bergeron R, Sajdyk TJ, et al. Corticotrophin releasing factor-induced synaptic plasticity in the amygdala translates stress into emotional disorders. J Neurosci. 2004 Apr 7;24(14):3471–9. doi: 10.1523/JNEUROSCI.5740-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmolesky MT, De Ruiter MM, De Zeeuw CI, et al. The neuropeptide corticotropin-releasing factor regulates excitatory transmission and plasticity at the climbing fibre-Purkinje cell synapse. Eur J Neurosci. 2007 Mar;25(5):1460–6. doi: 10.1111/j.1460-9568.2007.05409.x. [DOI] [PubMed] [Google Scholar]
- 34.Gallagher JP, Orozco-Cabal LF, Liu J, et al. Synaptic physiology of central CRH system. Eur J Pharmacol. 2008 Apr 7;583(2-3):215–25. doi: 10.1016/j.ejphar.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Pett K, Viau V, Bittencourt JC, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000 Dec 11;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 36.Potter E, Sutton S, Donaldson C, et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8777–81. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995 Oct;15(10):6340–50. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timpl P, Spanagel R, Sillaber I, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998 Jun;19(2):162–6. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 39.Muller MB, Zimmermann S, Sillaber I, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003 Oct;6(10):1100–7. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 40.Bale TL, Contarino A, Smith GW, et al. Mice deficient for corticotropin-releasing hormone receptor-2 display anxiety-like behaviour and are hypersensitive to stress. Nat Genet. 2000 Apr;24(4):410–4. doi: 10.1038/74263. [DOI] [PubMed] [Google Scholar]
- 41.Kishimoto T, Radulovic J, Radulovic M, et al. Deletion of crhr2 reveals an anxiolytic role for corticotropin-releasing hormone receptor-2. Nat Genet. 2000 Apr;24(4):415–9. doi: 10.1038/74271. [DOI] [PubMed] [Google Scholar]
- 42.Coste SC, Kesterson RA, Heldwein KA, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat Genet. 2000 Apr;24(4):403–9. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- 43.Coste SC, Heard AD, Phillips TJ, et al. Corticotropin-releasing factor receptor type 2-deficient mice display impaired coping behaviors during stress. Genes Brain Behav. 2006 Mar;5(2):131–8. doi: 10.1111/j.1601-183X.2005.00142.x. [DOI] [PubMed] [Google Scholar]
- 44.Buckingham JC. Secretion of corticotrophin and its hypothalamic releasing factor in response to morphine and opioid peptides. Neuroendocrinology. 1982;35(2):111–6. doi: 10.1159/000123364. [DOI] [PubMed] [Google Scholar]
- 45.Swerdlow NR, Koob GF, Cador M, et al. Pituitary-adrenal axis responses to acute amphetamine in the rat. Pharmacol Biochem Behav. 1993 Jul;45(3):629–37. doi: 10.1016/0091-3057(93)90518-x. [DOI] [PubMed] [Google Scholar]
- 46.Calogero AE, Gallucci WT, Kling MA, et al. Cocaine stimulates rat hypothalamic corticotropin-releasing hormone secretion in vitro. Brain Res. 1989 Dec 25;505(1):7–11. doi: 10.1016/0006-8993(89)90109-1. [DOI] [PubMed] [Google Scholar]
- 47.Buckingham JC, Hodges JR. Hypothalamic receptors influencing the secretion of corticotrophin releasing hormone in the rat. J Physiol. 1979 May;290(2):421–31. doi: 10.1113/jphysiol.1979.sp012780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weidenfeld J, Feldman S, Mechoulam R. Effect of the brain constituent anandamide, a cannabinoid receptor agonist, on the hypothalamo-pituitary-adrenal axis in the rat. Neuroendocrinology. 1994 Feb;59(2):110–2. doi: 10.1159/000126646. [DOI] [PubMed] [Google Scholar]
- 49.Rivier C, Bruhn T, Vale W. Effect of ethanol on the hypothalamic-pituitary-adrenal axis in the rat: role of corticotropin-releasing factor (CRF) J Pharmacol Exp Ther. 1984 Apr;229(1):127–31. [PubMed] [Google Scholar]
- 50.Rivier CL, Grigoriadis DE, Rivier JE. Role of corticotropin-releasing factor receptors type 1 and 2 in modulating the rat adrenocorticotropin response to stressors. Endocrinology. 2003 Jun;144(6):2396–403. doi: 10.1210/en.2002-0117. [DOI] [PubMed] [Google Scholar]
- 51.Sarnyai Z, Biro E, Penke B, et al. The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Res. 1992 Aug 28;589(1):154–6. doi: 10.1016/0006-8993(92)91176-f. [DOI] [PubMed] [Google Scholar]
- 52.Cole BJ, Cador M, Stinus L, et al. Critical role of the hypothalamic pituitary adrenal axis in amphetamine-induced sensitization of behavior. Life Sci. 1990;47(19):1715–20. doi: 10.1016/0024-3205(90)90344-q. [DOI] [PubMed] [Google Scholar]
- 53.Fee JR, Sparta DR, Picker MJ, et al. Corticotropin releasing factor-1 receptor antagonist, CP-154,526, blocks the expression of ethanol-induced behavioral sensitization in DBA/2J mice. Neuroscience. 2007 Nov 30;150(1):14–21. doi: 10.1016/j.neuroscience.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Przegalinski E, Filip M, Frankowska M, et al. Effects of CP 154,526, a CRF1 receptor antagonist, on behavioral responses to cocaine in rats. Neuropeptides. 2005 Oct;39(5):525–33. doi: 10.1016/j.npep.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Pastor R, McKinnon CS, Scibelli AC, et al. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci U S A. 2008 Jul 1;105(26):9070–5. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Goeders NE, Guerin GF. Effects of surgical and pharmacological adrenalectomy on the initiation and maintenance of intravenous cocaine self-administration in rats. Brain Res. 1996 May 25;722(1-2):145–52. doi: 10.1016/0006-8993(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 57.Koob GF. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 2010 Feb 16;1314:3–14. doi: 10.1016/j.brainres.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou Y, Spangler R, LaForge KS, et al. Corticotropin-releasing factor and type 1 corticotropin-releasing factor receptor messenger RNAs in rat brain and pituitary during ‘binge’-pattern cocaine administration and chronic withdrawal. J Pharmacol Exp Ther. 1996 Oct;279(1):351–8. [PubMed] [Google Scholar]
- 59.Maj M, Turchan J, Smialowska M, et al. Morphine and cocaine influence on CRF biosynthesis in the rat central nucleus of amygdala. Neuropeptides. 2003 Apr;37(2):105–10. doi: 10.1016/s0143-4179(03)00021-0. [DOI] [PubMed] [Google Scholar]
- 60.Zorrilla EP, Valdez GR, Weiss F. Changes in levels of regional CRF-like-immunoreactivity and plasma corticosterone during protracted drug withdrawal in dependent rats. Psychopharmacology (Berl) 2001 Dec;158(4):374–81. doi: 10.1007/s002130100773. [DOI] [PubMed] [Google Scholar]
- 61.Zhou Y, Spangler R, Ho A, et al. Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic ‘binge’ cocaine. Brain Res Mol Brain Res. 2003 May 26;114(1):73–9. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]
- 62.Caberlotto L, Rimondini R, Hansson A, et al. Corticotropin-releasing hormone (CRH) mRNA expression in rat central amygdala in cannabinoid tolerance and withdrawal: evidence for an allostatic shift? Neuropsychopharmacology. 2004 Jan;29(1):15–22. doi: 10.1038/sj.npp.1300296. [DOI] [PubMed] [Google Scholar]
- 63.Sommer WH, Rimondini R, Hansson AC, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008 Jan 15;63(2):139–45. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 64.George O, Ghozland S, Azar MR, et al. CRF-CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci U S A. 2007 Oct 23;104(43):17198–203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007 Aug;164(8):1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richter RM, Weiss F. In vivo CRF release in rat amygdala is increased during cocaine withdrawal in self-administering rats. Synapse. 1999 Jun 15;32(4):254–61. doi: 10.1002/(SICI)1098-2396(19990615)32:4<254::AID-SYN2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 67.Olive MF, Koenig HN, Nannini MA, et al. Elevated extracellular CRF levels in the bed nucleus of the stria terminalis during ethanol withdrawal and reduction by subsequent ethanol intake. Pharmacol Biochem Behav. 2002 May;72(1-2):213–20. doi: 10.1016/s0091-3057(01)00748-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merlo Pich E, Lorang M, Yeganeh M, et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J Neurosci. 1995 Aug;15(8):5439–47. doi: 10.1523/JNEUROSCI.15-08-05439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodriguez de Fonseca F, Carrera MR, Navarro M, et al. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science. 1997 Jun 27;276(5321):2050–4. doi: 10.1126/science.276.5321.2050. [DOI] [PubMed] [Google Scholar]
- 70.Sarnyai Z, Biro E, Gardi J, et al. Brain corticotropin-releasing factor mediates ‘anxiety-like’ behavior induced by cocaine withdrawal in rats. Brain Res. 1995 Mar 27;675(1-2):89–97. doi: 10.1016/0006-8993(95)00043-p. [DOI] [PubMed] [Google Scholar]
- 71.Funk CK, O’Dell LE, Crawford EF, et al. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci. 2006 Nov 1;26(44):11324–32. doi: 10.1523/JNEUROSCI.3096-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iredale PA, Alvaro JD, Lee Y, et al. Role of corticotropin-releasing factor receptor-1 in opiate withdrawal. J Neurochem. 2000 Jan;74(1):199–208. doi: 10.1046/j.1471-4159.2000.0740199.x. [DOI] [PubMed] [Google Scholar]
- 73.Chu K, Koob GF, Cole M, et al. Dependence-induced increases in ethanol self-administration in mice are blocked by the CRF1 receptor antagonist antalarmin and by CRF1 receptor knockout. Pharmacol Biochem Behav. 2007 Apr;86(4):813–21. doi: 10.1016/j.pbb.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Contarino A, Papaleo F. The corticotropin-releasing factor receptor-1 pathway mediates the negative affective states of opiate withdrawal. Proc Natl Acad Sci U S A. 2005 Dec 20;102(51):18649–54. doi: 10.1073/pnas.0506999102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharpe AL, Coste SC, Burkhart-Kasch S, et al. Mice deficient in corticotropin-releasing factor receptor type 2 exhibit normal ethanol-associated behaviors. Alcohol Clin Exp Res. 2005 Sep;29(9):1601–9. doi: 10.1097/01.alc.0000179371.46716.5e. [DOI] [PubMed] [Google Scholar]
- 76.Hansson AC, Cippitelli A, Sommer WH, et al. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc Natl Acad Sci U S A. 2006 Oct 10;103(41):15236–41. doi: 10.1073/pnas.0604419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Papaleo F, Ghozland S, Ingallinesi M, et al. Disruption of the CRF(2) receptor pathway decreases the somatic expression of opiate withdrawal. Neuropsychopharmacology. 2008 Nov;33(12):2878–87. doi: 10.1038/npp.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papaleo F, Kitchener P, Contarino A. Disruption of the CRF/CRF1 receptor stress system exacerbates the somatic signs of opiate withdrawal. Neuron. 2007 Feb 15;53(4):577–89. doi: 10.1016/j.neuron.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 79.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000 Jul-Aug;44(1):235–49. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 80.Zhao H. Lead optimization in the nondrug-like space. Drug Discov Today. 2011 Feb;16(3-4):158–63. doi: 10.1016/j.drudis.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 81.Curtis AL, Grigoriadis DE, Page ME, et al. Pharmacological comparison of two corticotropin-releasing factor antagonists: in vivo and in vitro studies. J Pharmacol Exp Ther. 1994 Jan;268(1):359–65. [PubMed] [Google Scholar]
- 82.Webster EL, Lewis DB, Torpy DJ, et al. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996 Dec;137(12):5747–50. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- 83.Keller C, Bruelisauer A, Lemaire M, et al. Brain pharmacokinetics of a nonpeptidic corticotropin-releasing factor receptor antagonist. Drug Metab Dispos. 2002 Feb;30(2):173–6. doi: 10.1124/dmd.30.2.173. [DOI] [PubMed] [Google Scholar]
- 84.Lundkvist J, Chai Z, Teheranian R, et al. A non peptidic corticotropin releasing factor receptor antagonist attenuates fever and exhibits anxiolytic-like activity. Eur J Pharmacol. 1996 Aug 8;309(2):195–200. doi: 10.1016/0014-2999(96)00337-8. [DOI] [PubMed] [Google Scholar]
- 85.Chen C, Wilcoxen KM, Huang CQ, et al. Design of 2,5-dimethyl-3-(6-dimethyl-4-methylpyridin-3-yl)-7-dipropylaminopyrazolo[1 ,5-a]pyrimidine (NBI 30775/R121919) and structure: activity relationships of a series of potent and orally active corticotropin-releasing factor receptor antagonists. J Med Chem. 2004 Sep 9;47(19):4787–98. doi: 10.1021/jm040058e. [DOI] [PubMed] [Google Scholar]
- 86.Chen C, Dagnino R, Jr, De Souza EB, et al. Design and synthesis of a series of non-peptide high-affinity human corticotropin-releasing factor1 receptor antagonists. J Med Chem. 1996 Oct 25;39(22):4358–60. doi: 10.1021/jm960149e. [DOI] [PubMed] [Google Scholar]
- 87.Jagoda E, Contoreggi C, Lee MJ, et al. Autoradiographic visualization of corticotropin releasing hormone type 1 receptors with a nonpeptide ligand: synthesis of [(76)Br]MJL-1-109-2. J Med Chem. 2003 Aug 14;46(17):3559–62. doi: 10.1021/jm034077k. [DOI] [PubMed] [Google Scholar]
- 88.McCarthy JR, Heinrichs SC, Grigoriadis DE. Recent advances with the CRF1 receptor: design of small molecule inhibitors, receptor subtypes and clinical indications. Curr Pharm Des. 1999 May;5(5):289–315. [PubMed] [Google Scholar]
- 89.Okuyama S, Chaki S, Kawashima N, et al. Receptor binding, behavioral, and electrophysiological profiles of nonpeptide corticotropin-releasing factor subtype 1 receptor antagonists CRA1000 and CRA1001. J Pharmacol Exp Ther. 1999 May;289(2):926–35. [PubMed] [Google Scholar]
- 90.Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004 Jul;13(7):799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- 91.Gehlert DR, Cippitelli A, Thorsell A, et al. 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethyl-imidazo[1,2-b]pyridazine: a novel brain-penetrant, orally available corticotropin-releasing factor receptor 1 antagonist with efficacy in animal models of alcoholism. J Neurosci. 2007 Mar 7;27(10):2718–26. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Richardson HN, Zhao Y, Fekete EM, et al. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol Biochem Behav. 2008 Feb;88(4):497–510. doi: 10.1016/j.pbb.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zorrilla EP, Koob GF. Progress in corticotropin-releasing factor-1 antagonist development. Drug Discov Today. 2010 May;15(9-10):371–83. doi: 10.1016/j.drudis.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Specio SE, Wee S, O’Dell LE, et al. CRF(1) receptor antagonists attenuate escalated cocaine self-administration in rats. Psychopharmacology (Berl) 2008 Feb;196(3):473–82. doi: 10.1007/s00213-007-0983-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Goeders NE, Guerin GF. Effects of the CRH receptor antagonist CP-154,526 on intravenous cocaine self-administration in rats. Neuropsychopharmacology. 2000 Nov;23(5):577–86. doi: 10.1016/S0893-133X(00)00148-2. [DOI] [PubMed] [Google Scholar]
- 96.Mello NK, Negus SS, Rice KC, et al. Effects of the CRF1 antagonist antalarmin on cocaine self-administration and discrimination in rhesus monkeys. Pharmacol Biochem Behav. 2006 Dec;85(4):744–51. doi: 10.1016/j.pbb.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 97.Basso AM, Spina M, Rivier J, et al. Corticotropin-releasing factor antagonist attenuates the ‘anxiogenic-like’ effect in the defensive burying paradigm but not in the elevated plus-maze following chronic cocaine in rats. Psychopharmacology (Berl) 1999 Jul;145(1):21–30. doi: 10.1007/s002130051028. [DOI] [PubMed] [Google Scholar]
- 98.Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998 Jul 15;18(14):5529–36. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Shaham Y, Erb S, Leung S, et al. CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berl) 1998 May;137(2):184–90. doi: 10.1007/s002130050608. [DOI] [PubMed] [Google Scholar]
- 100.Goeders NE, Clampitt DM. Potential role for the hypothalamo-pituitary-adrenal axis in the conditioned reinforcer-induced reinstatement of extinguished cocaine seeking in rats. Psychopharmacology (Berl) 2002 May;161(3):222–32. doi: 10.1007/s00213-002-1007-4. [DOI] [PubMed] [Google Scholar]
- 101.Skelton KH, Oren D, Gutman DA, et al. The CRF1 receptor antagonist, R121919, attenuates the severity of precipitated morphine withdrawal. Eur J Pharmacol. 2007 Sep 24;571(1):17–24. doi: 10.1016/j.ejphar.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 102.Greenwell TN, Funk CK, Cottone P, et al. Corticotropin-releasing factor-1 receptor antagonists decrease heroin self-administration in long- but not short-access rats. Addict Biol. 2009 Apr;14(2):130–43. doi: 10.1111/j.1369-1600.2008.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Negus SS, Rice KC. Mechanisms of withdrawal-associated increases in heroin self-administration: pharmacologic modulation of heroin vs food choice in heroin-dependent rhesus monkeys. Neuropsychopharmacology. 2009 Mar;34(4):899–911. doi: 10.1038/npp.2008.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heinrichs SC, Menzaghi F, Schulteis G, et al. Suppression of corticotropin-releasing factor in the amygdala attenuates aversive consequences of morphine withdrawal. Behav Pharmacol. 1995 Jan;6(1):74–80. [PubMed] [Google Scholar]
- 105.Stinus L, Cador M, Zorrilla EP, et al. Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology. 2005 Jan;30(1):90–8. doi: 10.1038/sj.npp.1300487. [DOI] [PubMed] [Google Scholar]
- 106.Shaham Y, Funk D, Erb S, et al. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997 Apr 1;17(7):2605–14. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang J, Fang Q, Liu Z, et al. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology (Berl) 2006 Mar;185(1):19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- 108.Rivier C, Rivier J, Lee S. Importance of pituitary and brain receptors for corticotrophin-releasing factor in modulating alcohol-induced ACTH secretion in the rat. Brain Res. 1996 May 20;721(1-2):83–90. doi: 10.1016/0006-8993(96)00164-3. [DOI] [PubMed] [Google Scholar]
- 109.Funk CK, Zorrilla EP, Lee MJ, et al. Corticotropin-releasing factor 1 antagonists selectively reduce ethanol self-administration in ethanol-dependent rats. Biol Psychiatry. 2007 Jan 1;61(1):78–86. doi: 10.1016/j.biopsych.2006.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Marinelli PW, Funk D, Juzytsch W, et al. The CRF1 receptor antagonist antalarmin attenuates yohimbine-induced increases in operant alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2007 Dec;195(3):345–55. doi: 10.1007/s00213-007-0905-x. [DOI] [PubMed] [Google Scholar]
- 111.Sparta DR, Sparrow AM, Lowery EG, et al. Blockade of the corticotropin releasing factor type 1 receptor attenuates elevated ethanol drinking associated with drinking in the dark procedures. Alcohol Clin Exp Res. 2008 Feb;32(2):259–65. doi: 10.1111/j.1530-0277.2007.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lowery EG, Sparrow AM, Breese GR, et al. The CRF-1 receptor antagonist, CP-154,526, attenuates stress-induced increases in ethanol consumption by BALB/cJ mice. Alcohol Clin Exp Res. 2008 Feb;32(2):240–8. doi: 10.1111/j.1530-0277.2007.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lowery EG, Spanos M, Navarro M, et al. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010 May;35(6):1241–52. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sabino V, Cottone P, Koob GF, et al. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2006 Dec;189(2):175–86. doi: 10.1007/s00213-006-0546-5. [DOI] [PubMed] [Google Scholar]
- 115.Gilpin NW, Richardson HN, Koob GF. Effects of CRF1-receptor and opioid-receptor antagonists on dependence-induced increases in alcohol drinking by alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2008 Sep;32(9):1535–42. doi: 10.1111/j.1530-0277.2008.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Valdez GR, Roberts AJ, Chan K, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002 Oct;26(10):1494–501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 117.Baldwin HA, Rassnick S, Rivier J, et al. CRF antagonist reverses the ‘anxiogenic’ response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103(2):227–32. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- 118.Wills TA, Knapp DJ, Overstreet DH, et al. Sensitization, duration, and pharmacological blockade of anxiety-like behavior following repeated ethanol withdrawal in adolescent and adult rats. Alcohol Clin Exp Res. 2009 Mar;33(3):455–63. doi: 10.1111/j.1530-0277.2008.00856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J Neurosci. 2002 Sep 15;22(18):7856–61. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Le AD, Harding S, Juzytsch W, et al. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000 Jun;150(3):317–24. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- 121.Sparta DR, Ferraro FM, 3rd, Fee JR, et al. The alcohol deprivation effect in C57BL/6J mice is observed using operant self-administration procedures and is modulated by CRF-1 receptor signaling. Alcohol Clin Exp Res. 2009 Jan;33(1):31–42. doi: 10.1111/j.1530-0277.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Okada S, Shimizu T, Yokotani K. Extrahypothalamic corticotropin-releasing hormone mediates (−)-nicotine-induced elevation of plasma corticosterone in rats. Eur J Pharmacol. 2003 Jul 25;473(2-3):217–23. doi: 10.1016/s0014-2999(03)01966-6. [DOI] [PubMed] [Google Scholar]
- 123.Tucci S, Cheeta S, Seth P, et al. Corticotropin releasing factor antagonist, alpha-helical CRF(9-41), reverses nicotine-induced conditioned, but not unconditioned, anxiety. Psychopharmacology (Berl) 2003 May;167(3):251–6. doi: 10.1007/s00213-003-1403-4. [DOI] [PubMed] [Google Scholar]
- 124.Marcinkiewcz CA, Prado MM, Isaac SK, et al. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology. 2009 Jun;34(7):1743–52. doi: 10.1038/npp.2008.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zislis G, Desai TV, Prado M, et al. Effects of the CRF receptor antagonist D-Phe CRF(12-41) and the alpha2-adrenergic receptor agonist clonidine on stress-induced reinstatement of nicotine-seeking behavior in rats. Neuropharmacology. 2007 Dec;53(8):958–66. doi: 10.1016/j.neuropharm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009 Jul 15;66(2):110–7. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodriguez de Fonseca F, Rubio P, Menzaghi F, et al. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J Pharmacol Exp Ther. 1996 Jan;276(1):56–64. [PubMed] [Google Scholar]
- 128.Cottone P, Sabino V, Roberto M, et al. CRF system recruitment mediates dark side of compulsive eating. Proc Natl Acad Sci U S A. 2009 Nov 24;106(47):20016–20. doi: 10.1073/pnas.0908789106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ghitza UE, Gray SM, Epstein DH, et al. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF1 receptors. Neuropsychopharmacology. 2006 Oct;31(10):2188–96. doi: 10.1038/sj.npp.1300964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rivier J, Rivier C, Vale W. Synthetic competitive antagonists of corticotropin-releasing factor: effect on ACTH secretion in the rat. Science. 1984 May 25;224(4651):889–91. doi: 10.1126/science.6326264. [DOI] [PubMed] [Google Scholar]
- 131.Menzaghi F, Howard RL, Heinrichs SC, et al. Characterization of a novel and potent corticotropin-releasing factor antagonist in rats. J Pharmacol Exp Ther. 1994 May;269(2):564–72. [PubMed] [Google Scholar]
- 132.Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999 Oct 15;19(20):RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang B, Shaham Y, Zitzman D, et al. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005 Jun 1;25(22):5389–96. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shalev U, Finnie PS, Quinn T, et al. A role for corticotropin-releasing factor, but not corticosterone, in acute food-deprivation-induced reinstatement of heroin seeking in rats. Psychopharmacology (Berl) 2006 Aug;187(3):376–84. doi: 10.1007/s00213-006-0427-y. [DOI] [PubMed] [Google Scholar]
- 135.Finn DA, Snelling C, Fretwell AM, et al. Increased drinking during withdrawal from intermittent ethanol exposure is blocked by the CRF receptor antagonist D-Phe-CRF(12-41) Alcohol Clin Exp Res. 2007 Jun;31(6):939–49. doi: 10.1111/j.1530-0277.2007.00379.x. [DOI] [PubMed] [Google Scholar]
- 136.Rassnick S, Heinrichs SC, Britton KT, et al. Microinjection of a corticotropin-releasing factor antagonist into the central nucleus of the amygdala reverses anxiogenic-like effects of ethanol withdrawal. Brain Res. 1993 Mar 5;605(1):25–32. doi: 10.1016/0006-8993(93)91352-s. [DOI] [PubMed] [Google Scholar]
- 137.Bruijnzeel AW, Zislis G, Wilson C, et al. Antagonism of CRF receptors prevents the deficit in brain reward function associated with precipitated nicotine withdrawal in rats. Neuropsychopharmacology. 2007 Apr;32(4):955–63. doi: 10.1038/sj.npp.1301192. [DOI] [PubMed] [Google Scholar]
- 138.Bruijnzeel AW, Small E, Pasek TM, et al. Corticotropin-releasing factor mediates the dysphoria-like state associated with alcohol withdrawal in rats. Behav Brain Res. 2010 Jul 11;210(2):288–91. doi: 10.1016/j.bbr.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schulz DW, Mansbach RS, Sprouse J, et al. CP-154,526: a potent and selective nonpeptide antagonist of corticotropin releasing factor receptors. Proc Natl Acad Sci U S A. 1996 Sep 17;93(19):10477–82. doi: 10.1073/pnas.93.19.10477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zorrilla EP, Valdez GR, Nozulak J, et al. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002 Oct 18;952(2):188–99. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]
- 141.Moffett MC, Goeders NE. CP-154,526, a CRF type-1 receptor antagonist, attenuates the cue-and methamphetamine-induced reinstatement of extinguished methamphetamine-seeking behavior in rats. Psychopharmacology (Berl) 2007 Feb;190(2):171–80. doi: 10.1007/s00213-006-0625-7. [DOI] [PubMed] [Google Scholar]
- 142.Lu L, Ceng X, Huang M. Corticotropin-releasing factor receptor type I mediates stress-induced relapse to opiate dependence in rats. Neuroreport. 2000 Aug 3;11(11):2373–8. doi: 10.1097/00001756-200008030-00008. [DOI] [PubMed] [Google Scholar]
- 143.Lu L, Liu D, Ceng X, et al. Differential roles of corticotropin-releasing factor receptor subtypes 1 and 2 in opiate withdrawal and in relapse to opiate dependence. Eur J Neurosci. 2000 Dec;12(12):4398–404. [PubMed] [Google Scholar]
- 144.Lu L, Liu D, Ceng X. Corticotropin-releasing factor receptor type 1 mediates stress-induced relapse to cocaine-conditioned place preference in rats. Eur J Pharmacol. 2001 Mar;415(2-3):203–8. doi: 10.1016/s0014-2999(01)00840-8. [DOI] [PubMed] [Google Scholar]
- 145.Breese GR, Overstreet DH, Knapp DJ, et al. Prior multiple ethanol withdrawals enhance stress-induced anxiety-like behavior: inhibition by CRF1- and benzodiazepine-receptor antagonists and a 5-HT1a-receptor agonist. Neuropsychopharmacology. 2005 Sep;30(9):1662–9. doi: 10.1038/sj.npp.1300706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wills TA, Knapp DJ, Overstreet DH, et al. Interactions of stress and CRF in ethanol-withdrawal induced anxiety in adolescent and adult rats. Alcohol Clin Exp Res. 2010 Sep 1;34(9):1603–12. doi: 10.1111/j.1530-0277.2010.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Arborelius L, Skelton KH, Thrivikraman KV, et al. Chronic administration of the selective corticotropin-releasing factor 1 receptor antagonist CP-154,526: behavioral, endocrine and neurochemical effects in the rat. J Pharmacol Exp Ther. 2000 Aug;294(2):588–97. [PubMed] [Google Scholar]
- 148.Overstreet DH, Knapp DJ, Breese GR. Modulation of multiple ethanol withdrawal-induced anxiety-like behavior by CRF and CRF1 receptors. Pharmacol Biochem Behav. 2004 Feb;77(2):405–13. doi: 10.1016/j.pbb.2003.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Knapp DJ, Overstreet DH, Moy SS, et al. SB242084, flumazenil, and CRA1000 block ethanol withdrawal-induced anxiety in rats. Alcohol. 2004 Feb;32(2):101–11. doi: 10.1016/j.alcohol.2003.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heinrichs SC, De Souza EB, Schulteis G, et al. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002 Aug;27(2):194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- 151.Keck ME, Welt T, Wigger A, et al. The anxiolytic effect of the CRH(1) receptor antagonist R121919 depends on innate emotionality in rats. Eur J Neurosci. 2001 Jan;13(2):373–80. doi: 10.1046/j.0953-816x.2000.01383.x. [DOI] [PubMed] [Google Scholar]
- 152.Oshima A, Flachskamm C, Reul JM, et al. Altered serotonergic neurotransmission but normal hypothalamic-pituitary-adrenocortical axis activity in mice chronically treated with the corticotropin-releasing hormone receptor type 1 antagonist NBI 30775. Neuropsychopharmacology. 2003 Dec;28(12):2148–59. doi: 10.1038/sj.npp.1300267. [DOI] [PubMed] [Google Scholar]
- 153.Kunzel HE, Zobel AW, Nickel T, et al. Treatment of depression with the CRH-1-receptor antagonist R121919: endocrine changes and side effects. J Psychiatr Res. 2003 Nov-Dec;37(6):525–33. doi: 10.1016/s0022-3956(03)00070-0. [DOI] [PubMed] [Google Scholar]
- 154.Zobel AW, Nickel T, Kunzel HE, et al. Effects of the high-affinity corticotropin-releasing hormone receptor 1 antagonist R121919 in major depression: the first 20 patients treated. J Psychiatr Res. 2000 May-Jun;34(3):171–81. doi: 10.1016/s0022-3956(00)00016-9. [DOI] [PubMed] [Google Scholar]
- 155.Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007 Jun 25;1155:172–8. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Valdez GR, Sabino V, Koob GF. Increased anxiety-like behavior and ethanol self-administration in dependent rats: reversal via corticotropin-releasing factor-2 receptor activation. Alcohol Clin Exp Res. 2004 Jun;28(6):865–72. doi: 10.1097/01.alc.0000128222.29875.40. [DOI] [PubMed] [Google Scholar]
- 157.Sharpe AL, Phillips TJ. Central urocortin 3 administration decreases limited-access ethanol intake in nondependent mice. Behav Pharmacol. 2009 Jul;20(4):346–51. doi: 10.1097/FBP.0b013e32832f01ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wang B, You ZB, Rice KC, et al. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007 Aug;193(2):283–94. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- 159.Vuong SM, Oliver HA, Scholl JL, et al. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav Brain Res. 2010 Mar 17;208(1):278–81. doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ruhmann A, Bonk I, Lin CR, et al. Structural requirements for peptidic antagonists of the corticotropin-releasing factor receptor (CRFR): development of CRFR2beta-selective antisauvagine-30. Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15264–9. doi: 10.1073/pnas.95.26.15264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bruijnzeel AW, Gold MS. The role of corticotropin-releasing factor-like peptides in cannabis, nicotine, and alcohol dependence. Brain Res Brain Res Rev. 2005 Nov;49(3):505–28. doi: 10.1016/j.brainresrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 162.Heilig M, Egli M, Crabbe JC, et al. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010 Apr;15(2):169–84. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bossert JM, Ghitza UE, Lu L, et al. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005 Dec 5;526(1-3):36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 164.Rylkova D, Shah HP, Small E, et al. Deficit in brain reward function and acute and protracted anxiety-like behavior after discontinuation of a chronic alcohol liquid diet in rats. Psychopharmacology (Berl) 2009 Apr;203(3):629–40. doi: 10.1007/s00213-008-1409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhao Y, Weiss F, Zorrilla EP. Remission and resurgence of anxiety-like behavior across protracted withdrawal stages in ethanol-dependent rats. Alcohol Clin Exp Res. 2007 Sep;31(9):1505–15. doi: 10.1111/j.1530-0277.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 166.Valdez GR, Zorrilla EP, Roberts AJ, et al. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003 Feb;29(2):55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- 167.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007 Nov;8(11):844–58. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 168.Fu Y, Pollandt S, Liu J, et al. Long-term potentiation (LTP) in the central amygdala (CeA) is enhanced after prolonged withdrawal from chronic cocaine and requires CRF1 receptors. J Neurophysiol. 2007 Jan;97(1):937–41. doi: 10.1152/jn.00349.2006. [DOI] [PubMed] [Google Scholar]
- 169.Nie Z, Schweitzer P, Roberts AJ, et al. Ethanol augments GABAergic transmission in the central amygdala via CRF1 receptors. Science. 2004 Mar 5;303(5663):1512–4. doi: 10.1126/science.1092550. [DOI] [PubMed] [Google Scholar]
- 170.Roberto M, Cruz MT, Gilpin NW, et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol Psychiatry. 2010 May 1;67(9):831–9. doi: 10.1016/j.biopsych.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pollandt S, Liu J, Orozco-Cabal L, et al. Cocaine withdrawal enhances long-term potentiation induced by corticotropin-releasing factor at central amygdala glutamatergic synapses via CRF, NMDA receptors and PKA. Eur J Neurosci. 2006 Sep;24(6):1733–43. doi: 10.1111/j.1460-9568.2006.05049.x. [DOI] [PubMed] [Google Scholar]
- 172.Krishnan B, Centeno M, Pollandt S, et al. Dopamine receptor mechanisms mediate corticotropinreleasing factor-induced long-term potentiation in the rat amygdala following cocaine withdrawal. Eur J Neurosci. 2010;31:1027–42. doi: 10.1111/j.1460-9568.2010.07148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kash TL, Winder DG. Neuropeptide Y and corticotropin-releasing factor bi-directionally modulate inhibitory synaptic transmission in the bed nucleus of the stria terminalis. Neuropharmacology. 2006 Oct;51(5):1013–22. doi: 10.1016/j.neuropharm.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 174.Francesconi W, Berton F, Repunte-Canonigo V, et al. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009 Apr 29;29(17):5389–401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009 May 20;29(20):6535–44. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]