Abstract
The association between apolipoprotein E (apoE) and amyloid-β peptide (Aβ) may significantly impact the function of both proteins, thus affecting the etiology of Alzheimer’s disease (AD). However, apoE/Aβ interactions remain fundamentally defined by the stringency of the detection method. Here we use size exclusion chromatography (SEC) as a non-stringent approach to the detection of apoE/Aβ interactions in solution, specifically apoE and both endogenous and exogenous Aβ from plasma, CSF and astrocyte conditioned media. By SEC analysis, Aβ association with plasma and CNS lipoproteins is apoE-dependent. While endogenous Aβ elutes to specific human plasma lipoproteins distinct from those containing apoE, it is the apoE-containing lipoproteins that absorb excess amounts of exogenous Aβ40. In human CSF, apoE, endogenous Aβ and phospholipid elute in an almost identical profile, as do apoE, exogenous Aβ and phospholipid from astrocyte conditioned media. Combining SEC fractionation with subsequent analysis for SDS-stable apoE/Aβ complex reveals that apoE-containing astrocyte lipoproteins exhibit the most robust interactions with Aβ. Thus, standardization of the methods for detecting apoE/Aβ complex is necessary to determine its functional significance in the neuropathology characteristic of AD. Importantly, a systematic understanding of the role of apoE-containing plasma and CNS lipoproteins in Aβ homeostasis could potentially contribute to identifying a plasma biomarker currently over-looked because it has multiple components.
1. Introduction
Two key proteins involved in Alzheimer’s disease (AD) are found circulating in both peripheral and CNS fluids associated with lipoprotein particles: apolipoprotein E (apoE) and amyloid-β peptide (Aβ). In humans, apoE is expressed as three naturally occurring common isoforms (apoE2, apoE3 and apoE4). ApoE modulates risk for AD, with ε2/2 decreasing risk 4-fold and ε4/4 increasing risk 12 fold [1-3]. ApoE expression is highest in the liver, followed by apoE expression in the brain. ApoE-containing plasma lipoproteins, synthesized primarily by the liver, do not cross the blood-brain barrier (BBB). ApoE is the major lipoprotein forming apolipoprotein produced in the brain (For review, [4]); secreted primarily by astrocytes as nascent apoE-containing discoidal particles [5, 6]. ApoE associates with lipoproteins to provide structural stability and serve as a ligand for receptor-mediated uptake of lipoproteins, facilitating cellular metabolism of cholesterol and lipids (for review, [7]).
Unlike apoE, the function of the association of Aβ with lipoproteins is less clear, although presumably the peptide associates with particles for its own stability and transport in plasma [8-15] or CSF [14, 16, 17]. The association of amphiphilic Aβ with lipoproteins would allow the peptide to remain soluble, either via an interaction with the lipids or apolipoprotein components of lipoproteins. Plasma lipoproteins in particular have been implicated in the transport of Aβ, including specific clearance from the brain [18]. Although the physiological consequence of the association of Aβ with lipoproteins remains unclear, it is interesting to note that in AD patients there is a decline in plasma lipoprotein-associated Aβ and an increase in free Aβ [19], consistent with disturbances in lipoprotein homeostasis that affect plasma Aβ levels in normolipidaemic AD patients [20].
ApoE/Aβ/lipoprotein interactions may be important for both clearance of the peptide and as a potential biomarker. Considerable work has focused on the role of apoE in the brain, including its association with Aβ. ApoE could serve as a chaperone, both in facilitating extracellular amyloid deposition and transporting soluble Aβ across the BBB to plasma [6, 21, 22]. As a concerted effort has been devoted to develop plasma biomarkers for AD, it is critical to understand the role of apoE-containing plasma and CNS lipoproteins in Aβ homeostasis during the development and progression of AD pathology. This knowledge could facilitate identification of a plasma biomarker currently over-looked because it has multiple components, apoE/Aβ/lipoproteins, with possibly distinct patterns of change that effect the overall complex.
A major unresolved issue in this field is the nature of the association between Aβ and apoE. This interaction is influenced by a number of parameters, with two particularly relevant to the data presented herein. First, apoE interactions with Aβ depend on the lipidation state of apoE, whether the apoE is purified [23-26], or associated with lipid-poor- [23, 26, 27], reconstituted “HDL”- [26], astrocyte- [28], CSF- [29] or plasma-lipoproteins [10, 24, 25]. Second, the nature and amount apoE/Aβ complex depends on the method of detecting the association between apoE and Aβ, whether in the presence of detergent or more “physiologic” buffers. Previous methods include, in an approximate order of descending stringency, gel-shift assay of SDS-PAGE [23-25, 28, 30], density gradient ultracentrifugation [10], non-denaturing gradient gel electrophoresis [28], co-immunoprecipitation (IP) [31] and solid-phase binding assays/ELISA [26, 29, 32-35]. Surprisingly, size exclusion chromatography (SEC)/gel-filtration, perhaps the most gentle method that can be used with physiological buffers, has not been used to directly study apoE interactions with Aβ. As this method has traditionally been used to separate the different classes of plasma lipoproteins by size, SEC has been used in the detailed characterization of the CNS-relevant lipoproteins found in CSF and secreted by astrocytes [17, 30, 36-38].
The present study examines the association of endogenous and exogenous Aβ with lipoproteins in solution, utilizing apoE-containing lipoproteins from both sides of the BBB, peripheral (plasma lipoproteins) and CNS-relevant (CSF and astrocyte-secreted lipoproteins). Using SEC by fast protein liquid chromatography (FPLC), apoE and Aβ elute with lipoproteins from plasma, CSF and astrocyte-conditioned media. The interaction of apoE and Aβ in specific fractions is further characterized with a non-reducing gel-shift assay to detect of the presence of SDS-stable apoE/Aβ complexes. By this measure, the most robust interaction is between Aβ and apoE-containing isolated rat astrocyte lipoproteins.
2. Materials and Methods
2.1. Sources of apoE
Informed consent was obtained for experimentation with human subjects. All procedures were performed in compliance with the relevant Laws and Institutional guidelines and were approved by the institutional committee(s), from where the samples were obtained. 1) Human plasma from apoE genotyped (ε2/2, ε3/3, ε4/4) donors was kindly provided by Brad Hyman (Massachusetts General Hospital, Boston Mass). Donors were neurologically intact control subjects who were fasted. 2) Human CSF (ε3/3) was kindly provided by David Holtzman (Washington University, St Louis, Mo.). As previously described, the CSF was collected and concentrated 50-fold (Centriprep-10, Millipore, Bedford, MA) [30]. 3) Primary rat astrocyte conditioned media was generously provided by Linda Van Eldik (Northwestern University). Rat astrocyte conditioned media was prepared from neonatal (1-2 day old) Sprague-Dawley rats, concentrated 50-fold (Centriprep-10, Millipore, Bedford, MA) and prepared as previously described [30]. 4) Isolated rat astrocyte lipoproteins were prepared by pooling and concentrating FPLC fractions 30-45 of rat astrocyte conditioned media. 5) Rat apoE was purified as previously described [25]. Briefly, the lipoproteins were dialyzed against 0.01% EDTA, lyophilized, and delipidated in CHCl3:MeOH (2:1). Delipidated proteins were pelleted in MeOH and solubilized in 6M guanidine, 0.1M Tris, 0.01% EDTA (pH 7.4), and 1% 2-mercaptoethanol (bME). Proteins were fractionated on a Sephacryl S-300 (Pharmacia, Gaithersburg, MD) column equilibrated in 4M guanidine, 0.1M Tris, 0.01% EDTA (pH 7.4), and 0.1% bME. Fractions containing apoE were dialyzed into 5mM NH4HCO3, lyophilized, and resuspended in 0.1M NH4HCO3.
2.2. Amyloid-β peptide (Aβ)
Lyophilized powder of the 40 amino acid isoform of Aβ (Aβ40) peptide was initially solubilized at 5mM in 100% DMSO (Me2SO) and diluted prior to mixing with apoE, as described below.
2.3. Methods for separating lipoproteins
Some of the apparent disparity in the literature as it relates to interactions among apoE, Aβ and lipoproteins may be due to the differences in the methods for analyzing lipoproteins. For example, a common method is to isolate classes of lipoproteins. Lipoproteins have been isolated from normal and AD plasma by sequential ultracentrifugation, resulting in VLDL (d < 1.006) IDL/LDL (1.006 < d < 1.063) and HDL (1.063 < d < 1.21) particles [20, 25] and CSF particles further fractionated to densities of 1.063, 1.125, 1.21, and 1.25 [16]. While density centrifugation provides a gradient, rather than a single sample encompassing a wide range of densities, it is known to strip apolipoproteins from lipoprotein particles [30, 39]. Thus, characterization by size gradient (SEC) is perhaps the least disruptive method for both isolating lipoproteins and identifying apoE/Aβ interactions by co-elution.
2.4. Fast protein liquid chromatography (FPLC)
As previously described, size exclusion chromatography (SEC) by FPLC with tandem Superose-6 columns (Amersham Pharmacia Biotech) was used and samples eluted [0.02 M sodium phosphate, 0.05M NaCl, pH 7.4, 0.03% EDTA, and 0.02% sodium azide] at a flow rate of 0.4 mL/min. For plasma, 80 fractions (70 fractions for CSF and astrocyte media) of 400μl each were collected for subsequent analysis of protein and lipid content [30]. 1ml of human plasma was incubated with 0.5% DMSO (Me2SO) ± 25μM Aβ40 for 1 hour at room temperature and fractionated by FPLC. 1ml concentrated CSF was fractionated by FPLC. 1ml of concentrated astrocyte conditioned media was incubated with 0.5% DMSO ± 25μM Aβ40 for 1 hour at room temperature and fractionated by FPLC.
In human plasma, apoE concentrations are ~1.4mM[40-47] and plasma Aβ levels are between 0.1 and 5nM [48, 49] although highly variable across studies. Thus, the endogenous plasma apoE:Aβ molar ratio is likely between 1:7×10−5−1:3×10−3 Exogenous Aβ40 was added to the human plasma at 25mM, which equates to an apoE:Aβ ratio of between 1:5-1:16. In human CSF, apoE concentrations are ~0.2mM [50-52], while Abeta levels vary between 0.5 and 2nM [48], thus giving an apoE:Aβ ratio of 1:2.5-10×10−3 −1:1×10−2. For experiments using rat ACM the apoE:Aβ ratio was 1:36.
2.5. Immuno-blots and Western Blot
Antisera to human and rat apoE was prepared by immunizing rabbits with purified apoE isolated from human and rat plasma, respectively. Aβ was detected using monoclonal antibody 4G8 that recognizes amino acids 17-24 of Aβ (Senetec). As previously described, FPLC fractions were immuno-blotted directly onto Immobilon-P membrane using a vacuum manifold system, probed for human and rat apoE and Aβ, visualized by enhanced chemiluminescence (ECL) and quantified by densitometry using ImageQuant (Molecular Dynamics, Sunnyvale, CA). As previously described, SDS-stable apoE/Aβ complex formation was analyzed by non-reducing gel-shift assay (originally described in [23, 24]. Briefly, for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), selected FLPC fractions containing 2x Laemmli buffer (4% SDS) were boiled 5 minutes and electrophoresed on non-reducing 10-20% SDS-tricine gels. Gels were transferred to Immobilon-P (Millipore Corp., Bedford, MA) and probed with human or rat apoE anti-sera, or 4G8 antibody [25, 30, 36, 53].
2.6. Incubation of astrocyte lipoproteins with Aβ
As previously described, standard binding reaction conditions were used to identify SDS-stable apoE/Aβ complex using purified rat apoE or isolated apoE-containing rat astrocyte lipoproteins. Specifically, 25μg/ml (~700nM) apoE was incubated for 2 hours at room temperature with 5% DMSO ± 250μM Aβ40 [23-25, 54].
2.7. Lipid analysis
Aliquots of the FPLC fractions were analyzed for phospholipid (PL) content using an enzymatic assay (Wako, Richmond, VA) [30].
3. Results
3.1. Endogenous and exogenous Aβ elute with specific human plasma lipoproteins
Human plasma from apoE genotyped donors (ε2/2, ε3/3, ε4/4) was fractionated by SEC using FPLC to determine whether the isoforms of human apoE affect endogenous Aβ association with plasma lipoproteins. Endogenous Aβ clearly has an elution profile that indicates a specific preference for certain classes of lipoproteins in the plasma, although this profile does not match the apoE profile. Indeed, 95% of the endogenous Aβ elutes in lipoprotein-containing fractions (Figure 1D). The elution profile for endogenous Aβ from ε2/2, ε3/3, ε4/4 human plasma presents the general profile of a peak at IDL and a broad HDL peak centering on HDL-2, with minor variations among the apoE isoforms (Figure 1-A, B and C). For example, with apoE4, there is also a small Aβ peak at VLDL. The general elution profile for apoE includes a peak at VLDL that decreases across the IDL/LDL range and then peaks at HDL-1 (large HDL) (Figure 1-A, B and C), although there are isoform-specific variations. ApoE2 has a broad profile from VLDL to HDL-1 with no prominent peaks. ApoE3 has a large, distinct peak at VLDL, a shoulder at IDL and a peak at HDL-1. ApoE4 has a peak at VLDL with a broad shoulder to IDL and a peak at HDL-1. There was also a peak of endogenous Aβ that eluted in fractions consistent with small apoA1-containing HDL lipoproteins. Furthermore, we have previously demonstrated that apoA1 forms a complex with Aβ using isolated plasma HDL from human, rat and rabbit [25].
Figure 1. Endogenous and exogenous Aβ elute with specific human plasma lipoproteins.
Elution profiles of apoE and Aβ in human plasma from ε2/2 , ε3/3, ε4/4 without (A, B, C) or with (E, F, G) the addition of exogenous Aβ40. Graphs are an average of n = 3 separate experiments. Graph of the average proportion of endogenous Aβ (D) or exogenous Aβ (H) that elute with plasma lipoproteins or as free protein. (Note: the characteristic distribution of human plasma lipoproteins are given on graphs below the units of the X-axis: VLDL, IDL/LDL, HDL, and free protein).
With the addition of 25μM Aβ40 to the plasma, both the Aβ and apoE elution profiles are similar for the 3 apoE isoforms, although the elution profile for exogenous Aβ was not as well-defined as the elution profile of endogenous Aβ (Figure 1-E, F and G). These results indicate that apoE-containing lipoproteins absorb the majority of Aβ when the plasma is challenged by incubation with a bolus of Aβ40 (~70%; Figure 1-H), consistent with previous reports that both endogenous and exogenous Aβ can associate with specific plasma lipoproteins [9, 10, 12]. In contrast, Biere and co-workers used density gradient ultracentrifugation to demonstrate that 89% of exogenous I125-Aβ eluted in the free protein fractions, primarily bound to albumin [10]. In the current study, the use of density gradient ultracentrifugation also resulted in >90% of the endogenous Aβ eluting as free protein (data not shown), a pattern consistent with previous studies showing the loss of actual apolipoproteins from CSF and astrocyte conditioned media [30]. Specifically, the majority of the apolipoproteins from CSF and astrocyte conditioned media eluted as free protein with density gradient ultracentrifugation, while these apolipoproteins were lipoprotein-associated when fractionated by SEC [30]. An additional consideration is the use of I125-Aβ vs. unlabeled Aβ as we have observed that radioiodinated-Aβ interferes with interactions between apoE and Aβ (M.J. LaDu and G. Bu, unpublished results). Thus, there are several explanations for the partitioning of Aβ with free protein and possible association with albumin.
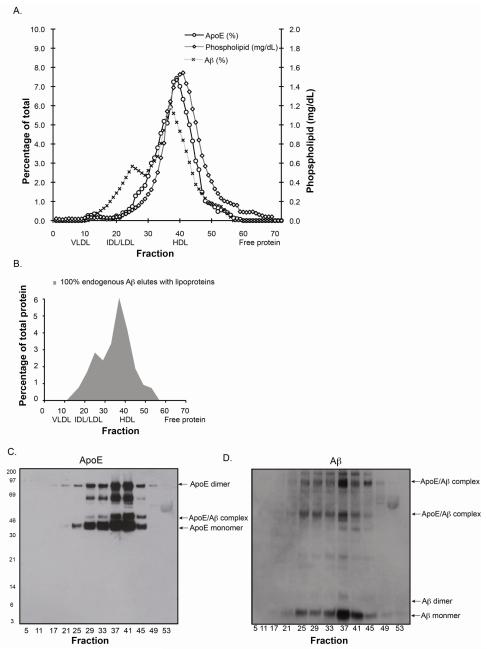
3.2. Endogenous Aβ exists mainly in apoE containing HDL-like particles in CSF
The primary lipoprotein present in human CSF is the size of HDL-2 with apoE as the primary apolipoprotein (for review, [55, 56]). The lipid composition of human CSF lipoproteins is not significantly different among the 3 apoE genotypes [17]. Further, Aβ was primarly associated with CSF lipoproteins [17]. Here, human CSF from an ε3/ε3 carrier was used to further characterize the nature of the interaction between apoE and endogenous Aβ. ApoE, Aβ and phospholipid eluted in a single peak primarily between fractions 35-43 (Figure 2-A), with 100% of the endogenous Aβ associated with this apoE-containing lipoprotein (Figure 2-B). Western blot analysis of SDS-PAGE of selected FLPC fractions confirmed the co-localization of apoE and Aβ to the same fractions (Figure 2-C and D). Importantly, a portion of the Aβ was associated with apoE via an SDS-stable complex, as demonstrated by an Aβ band at ~45 and ~97 kDa, consistent with apoE monomer/Aβ and apoE dimer/Aβ complexes (Figure 2-D)(for example, [25]). In addition, a significant portion of the Aβ is monomer and dimer by Western blot, suggesting that Aβ forms a non-SDS-stable complex either with apoE or directly with a lipid component of CSF-lipoproteins.
Figure 2. Endogenous Aβ co-elutes with CSF lipoproteins.
Elution profiles of apoE, Aβ and phospholipid (PL) in human CSF (ε3/3) (A). Graph of the average proportion of endogenous Aβ that elutes with apoE-containing CSF lipoproteins or as free protein (B). Representative Western blots of SDS-PAGE of selected FPLC fractions probed for Aβ (C) or apoE (D).
3.3. Aβ associates with apoE-containing rat astrocyte lipoproteins
To extend the characterization of Aβ interactions with apoE-containing CNS lipoproteins, rat astrocyte conditioned media with or without the addition of exogenous Aβ was fractionated by SEC. Initially, SDS-stable apoE/Aβ complex formation was analyzed in the SEC fractions that contained both apoE and exogenous Aβ. In addition, in the absence of exogenous Aβ SEC fractions containing isolated apoE-containing astrocyte lipoproteins were pooled and incubated with Aβ for comparison to purified rat apoE.
SEC fractionation of conditioned media from primary rat astrocyte cultures and analysis for apoE and phospholipid revealed a broad peak between fractions 35-45 (Figure 3-A) [30, 36], suggesting particles similar in size to CSF but more heterogeneous (Figure 2-A) [17, 30]. Incubation of this conditioned media with 25μM Aβ40 prior to SEC resulted in Aβ eluting primarily in fractions 35-45, with the excess found in the free protein fractions (Figure 3-B), as observed with human plasma incubated with exogenous Aβ40 (Figure 1-E, F, G and H). In addition, Aβ also eluted in fractions 3-8, consistent with large particles or, more likely, large Aβ aggregates as these fractions contain minimal amounts of apoE. SEC fractionation of serum free media + 25μM Aβ40 resulted in Aβ eluting entirely in the free protein fractions, suggesting that a component in the conditioned media facilitated the aggregation of the peptide (data not shown). Thus, the distribution of exogenous Aβ in SEC of astrocyte conditioned media results in Aβ eluting in very early fractions (8%), with the apoE/phospholipid peak (53%) and as free protein (39%) (Figure 3-C).
Figure 3. Exogenous Aβ co-elutes with astrocyte lipoproteins and forms SDS-stable complex with apoE in isolated rat astrocyte lipoproteins.
Elution profiles for rat apoE and PL in conditioned media from primary rat astrocyte cultures without (A) or with the addition of exogenous Aβ40 (B). Graphs are an average of n = 2 separate experiments. Graph of the average proportion of exogenous Aβ that elutes with apoE-containing astrocyte lipoproteins or as free protein (C). Representative Western blots of SDS-PAGE of selected FPLC fractions probed for Aβ (D) or apoE (E). Western blots of purified rat apoE and isolated rat astrocyte lipoproteins incubated with Aβ40 and probed for apoE (F, right) or Aβ (F, left).
Western blot analysis of SDS-PAGE of selected fractions again confirmed the co-localization of apoE and Aβ to the same fractions (Figure 3-D and E), where >50% of the Aβ appears to be in an SDS-stable complex with apoE, although Aβ monomer and dimer bands are clearly visible between 3-14kDa (Figure 3-E). These results are in apparent conflict with Morikawa and coworkers who incubated conditioned media from immortalized astrocytes from GFAP-apoE transgenic mice [57] with Aβ and observed very little complex under denaturing conditions but were able to detect apoE/Aβ complex by nondenaturing gradient gel electrophoresis [28]. While the precise reason for these differences is not entirely clear, the immortalized cell lines described in this initial paper did not become a widely used reagent as the apoE production varied with both isoform and passage number (M.J. LaDu, unpublished observations). Finally, in the absence of exogenous Aβ, SEC fractions 35-45 from rat astrocyte conditioned media were pooled and concentrated. Standard binding reaction conditions were used to analyze SDS-stable apoE/Aβ complex formation between Aβ40 (250μM) and isolated apoE-containing rat astrocyte lipoproteins or purified rat apoE (700nM) (Figure 3-F) [23-25, 54]. While a complex at 45kDa (Aβ) is visible with the pure rat apoE, the vast majority of the Aβ runs as a smear between 3-14kDa. However, the majority of the Aβ incubated with the astrocyte lipoproteins appears as 45 and ~97kDA bands, consistent with apoE monomer/Aβ and apoE dimer/Aβ complex [25]. Although it is possible that the higher molecular weight Aβ species correspond to Aβ aggregates, the demonstration of these bands with both α-apoE and α-Aβ antibodies indicates that most if not all of this immunodetection corresponds to an ApoE/Aβ complex. The majority of the Aβ incubated with rat astrocyte lipoproteins forms an SDS-stable complex with apoE.
4. Discussion
Aging is a common risk factor for both AD and cardiovascular diseases (CVD). Plasma lipoprotein dysfunction/dysregulation is largely responsible for CVD as people age. The greatest genetic factor modulating AD risk is apoE, a component of plasma lipoproteins. As a concerted effort has been devoted to develop plasma biomarkers for AD, it is critical to understand the association of apoE and Aβ with plasma lipoproteins. This is possible only via a systematic understanding of the role of peripheral and apoE-containing CNS lipoproteins in Aβ homeostasis during the development and progression of AD pathology. This knowledge could potentially contribute to identifying a plasma biomarker currently over-looked because it has multiple components, including a specific lipid profile, apoE conformation and assembly of Aβ [58, 59].
One of the primary challenges remains identifying apoE/Aβ interactions, as the method of detection continues to define what constitutes an interaction. This study focused on 2 components of this interaction: 1) the source of the apoE-containing lipoproteins, specifically human plasma, human CSF and rat astrocyte conditioned media; and 2) the method of detection, specifically a combination of co-elution from SEC fractionation (mild) and subsequent analysis of apoE/Aβ containing fractions for SDS-stable complex via gel-shift assay (stringent). The elution of apoE and Aβ from human plasma revealed that while the profile of endogenous Aβ was specific and consistent among plasma from the 3 apoE genotypes, the profile did not align with the apoE profile. However, when an excess of 25μM Aβ40 was incubated with plasma, the elution profiles for apoE and Aβ were nearly identical, demonstrating that apoE-containing lipoproteins have the capacity to absorb a large excess of Aβ as the normal concentration of Aβ in plasma is below 10nM [48, 49, 60]. In human CSF, the elution profiles for apoE and endogenous Aβ were virtually identical, while exogenous Aβ incubated with rat astrocyte conditioned media eluted primarily with apoE, with the excess Aβ in free protein fractions similar to the pattern of exogenous Aβ in plasma. Thus, under mild conditions using SEC, the profiles of Aβ and apoE-containing CNS lipoproteins are virtually identical, specifically for lipoproteins from CSF and astrocyte conditioned media.
Aβ is both an amphipathic and lipophilic peptide that interacts with several types of lipids, including phospholipids and gangliosides [61-63], and vesicles formed from human brain lipids [64]. Given past reports of other hydrophobic molecules such as serum amyloid A “remodeling” plasma and CSF lipoproteins [65], the addition of exogenous Aβ could modify lipoprotein size. Surprisingly, interaction with exogenous Aβ did not induce drastic changes to the overall size of the Aβ/apoE-containing lipoprotein particles, as indicated by the basically unmodified apoE elution profiles in the presence and absence of excess exogenous Aβ in plasma and astrocyte conditioned media. However, although the specific biological functions of SDS stable apoE/Aβ complex remain unclear, the apparent gradient of complex formation using purified apoE (low), apoE-containing CSF lipoproteins (medium), and apoE-containing astrocyte lipoproteins (high) suggests that: 1) the conformation of lipoprotein-associated apoE can facilitate its interaction with Aβ and 2) lipids are critical to this process although their precise identity and role are not known at present.
While the exact physiological consequence of the association of Aβ with lipoproteins, specifically apoE-containing lipoproteins, is not entirely clear, evidence suggests that it maintains the solubility of the Aβ peptide and may facilitate Aβ clearance and transport across the BBB [21, 22, 66]. Currently Aβ and apoE are measured in total plasma protein preparations, which may not be sensitive to detect subtle changes in protein levels. In this current study, the interactions between Aβ and apoE can be detected with specific lipoprotein assemblies, which may reveal differences in early stage AD. Further work is needed to assess whether interactions between lipoproteins and Aβ could serve as useful biomarkers for pre-clinical diagnosis of AD.
5. Conclusion
In the AD field, a major focus is on identification of a plasma biomarker. An understanding of apoE/Aβ/lipoprotein interactions may facilitate the identification of a constellation of markers that meet this need. By focusing on plasma, CSF and astrocyte lipoproteins, we have demonstrated that not only do apoE and Aβ co-elute with CSF and astrocyte lipoproteins, apoE-containing human plasma lipoproteins can absorb a bolus of excess Aβ. Combining SEC fractionation with subsequent analysis for SDS-stable complex reveals that apoE-containing astrocyte lipoproteins exhibit the most robust interactions with Aβ. Further work is needed to assess whether changing patterns in these interactions can serve as useful biomarkers for the pre-clinical diagnosis of AD.
Highlights.
SEC is a non-stringent approach to the detection of apoE/Aβ interactions in solution.
By SEC analysis, Aβ association with CNS and plasma lipoproteins is apoE-dependent.
ApoE-containing human plasma lipoproteins absorb excess amounts of exogenous Aβ40.
Aβ and apoE-containing astrocyte lipoproteins exhibit the most robust interaction.
Aβ/lipoprotein interactions are important for clearance and as a potential biomarker.
Acknowledgements
The authors thank Brad Hyman (Mass. General) for apoE genotyped human plasma, David Holtzman (Washington University) for human CSF and Linda Van Eldik (University of Kentucky) for the rat astrocyte conditioned media. This work was supported by grants from the Alzheimer’s Association ZEN-08-899000 (MJL), NIH/NIA PO1AG021184 (MJL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- [2].Corder EH, Saunder SM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr., Rimmler JB, Locke PA, Conneally PM, Schmader KE, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nature Genetics. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- [3].Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet. 2007;39:17–23. doi: 10.1038/ng1934. [DOI] [PubMed] [Google Scholar]
- [4].Elliott DA, Weickert CS, Garner B. Apolipoproteins in the brain: implications for neurological and psychiatric disorders. Clin Lipidol. 2010;51:555–573. doi: 10.2217/CLP.10.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yu C, Youmans KL, Ladu MJ. Proposed mechanism for lipoprotein remodelling in the brain. Biochim Biophys Acta. 2010;1801:819–823. doi: 10.1016/j.bbalip.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hauser PS, Narayanaswami V, Ryan RO. Apolipoprotein E: from lipid transport to neurobiology. Prog Lipid Res. 2011;50:62–74. doi: 10.1016/j.plipres.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Seubert P, Vigo-Pelfrey C, Esch F, Lee M, Dovey H, Davis D, Sinha S, Schiossmacher M, Whaley J, Swindlehurst C, McCormack R, Wolfert R, Selkoe D, Lieberburg I, Schenk D. Isolation and quantification of soluble Alzheimer’s [beta]-peptide from biological fluids. Nature. 1992;359:325–327. doi: 10.1038/359325a0. [DOI] [PubMed] [Google Scholar]
- [9].Koudinov A, Matsubara E, Frangione B, Ghiso J. The soluble form of Alzheimer’s amyloid beta protein is complexed to high density lipoprotein 3 and very high density lipoprotein in normal human plasma. Biochem Biophys Res Commun. 1994;205:1164–1171. doi: 10.1006/bbrc.1994.2788. [DOI] [PubMed] [Google Scholar]
- [10].Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem. 1996;271:32916–32922. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- [11].Scharnagl H, Tisljar U, Winkler K, Huttinger M, Nauck MA, Gross W, Wieland H, Ohm TG, Marz W. The betaA4 amyloid peptide complexes to and enhances the uptake of beta-very low density lipoproteins by the low density lipoprotein receptor-related protein and heparan sulfate proteoglycans pathway. Lab Invest. 1999;79:1271–1286. [PubMed] [Google Scholar]
- [12].Koudinov AR, Berezov TT, Kumar A, Koudinova NV. Alzheimer’s amyloid beta interaction with normal human plasma high density lipoprotein: association with apolipoprotein and lipids. Clin Chim Acta. 1998;270:75–84. doi: 10.1016/s0009-8981(97)00207-6. [DOI] [PubMed] [Google Scholar]
- [13].Permanne B, Perez C, Soto C, Frangione B, Wisniewski T. Detection of apolipoprotein E/dimeric soluble amyloid ß complexes in Alzheimer’s disease brain supernatants. Biochem. Biophys. Res. Commun. 1997;240:715–720. doi: 10.1006/bbrc.1997.7727. [DOI] [PubMed] [Google Scholar]
- [14].Ghiso J, Matsubara E, Koudinov A, Choi-Miura NH, Tomita M, Wisniewski T, Frangione B. The cerebrospinal-fluid form of Alzheimer’s amyloid beta is complexed to SP-40,40 (apolipoprotein J), an inhibitor of the complement membrane-attack complex. Biochem. J. 1993;293:27–30. doi: 10.1042/bj2930027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Koudinov AR, Berezov TT, Koudinova NV. The levels of soluble amyloid beta in different high density lipoprotein subfractions distinguish Alzheimer’s and normal aging cerebrospinal fluid: implication for brain cholesterol pathology? Neurosci Lett. 2001;314:115–118. doi: 10.1016/s0304-3940(01)02263-7. [DOI] [PubMed] [Google Scholar]
- [16].Koudinov AR, Koudinova NV, Kumar A, Beavis RC, Ghiso J. Biochemical characterization of Alzheimer’s soluble amyloid beta protein in human cerebrospinal fluid: association with high density lipoproteins. Biochem Biophys Res Commun. 1996;223:592–597. doi: 10.1006/bbrc.1996.0940. [DOI] [PubMed] [Google Scholar]
- [17].Fagan AM, Younkin LH, Morris JC, Fryer JD, Cole TG, Younkin SG, Holtzman DM. Differences in the Abeta40/Abeta42 ratio associated with cerebrospinal fluid lipoproteins as a function of apolipoprotein E genotype. Ann Neurol. 2000;48:201–210. [PubMed] [Google Scholar]
- [18].Sagare A, Deane R, Bell RD, Johnson B, Hamm K, Pendu R, Marky A, Lenting PJ, Wu Z, Zarcone T, Goate A, Mayo K, Perlmutter D, Coma M, Zhong Z, Zlokovic BV. Clearance of amyloid-beta by circulating lipoprotein receptors. Nat Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Matsubara E, Sekijima Y, Tokuda T, Urakami K, Amari M, Shizuka-Ikeda M, Tomidokoro Y, Ikeda M, Kawarabayashi T, Harigaya Y, Ikeda S, Murakami T, Abe K, Otomo E, Hirai S, Frangione B, Ghiso J, Shoji M. Soluble Abeta homeostasis in AD and DS: impairment of anti-amyloidogenic protection by lipoproteins. Neurobiol Aging. 2004;25:833–841. doi: 10.1016/j.neurobiolaging.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [20].Mamo JC, Jian L, James AP, Flicker L, Esselmann H, Wiltfang J, Mamo JCL. Plasma lipoprotein beta-amyloid in subjects with Alzheimer’s disease or mild cognitive impairment. Annals of Clinical Biochemistry. 2008;45:395–403. doi: 10.1258/acb.2008.007214. [DOI] [PubMed] [Google Scholar]
- [21].Koudinov AR, Koudinova NV, Berezov TT, Ivanov YD. HDL phospholipid: a natural inhibitor of Alzheimer’s amyloid beta-fibrillogenesis? Clin Chem Lab Med. 1999;37:993–994. doi: 10.1515/CCLM.1999.148. [DOI] [PubMed] [Google Scholar]
- [22].Cole GM, Beech W, Frautschy SA, Sigel J, Glasgow C, Ard MD. Lipoprotein effects on Abeta accumulation and degradation by microglia in vitro. J Neurosci Res. 1999;57:504–520. [PubMed] [Google Scholar]
- [23].LaDu MJ, Falduto MT, Manelli AM, Reardon CA, Getz GS, Frail DE. Isoform-specific binding of apolipoprotein E to beta-amyloid. J Biol Chem. 1994;269:23403–23406. [PubMed] [Google Scholar]
- [24].LaDu MJ, Pederson TM, Frail DE, Reardon CA, Getz GS, Falduto MT. Purification of apolipoprotein E attenuates isoform-specific binding to beta-amyloid. J Biol Chem. 1995;270:9039–9042. doi: 10.1074/jbc.270.16.9039. [DOI] [PubMed] [Google Scholar]
- [25].LaDu MJ, Lukens JR, Reardon CA, Getz GS. Association of human, rat, and rabbit apolipoprotein E with beta-amyloid. J Neurosci Res. 1997;49:9–18. [PubMed] [Google Scholar]
- [26].Tokuda T, Calero M, Matsubara E, Vidal R, Kumar A, Permanne B, Zlokovic B, Smith JD, Ladu MJ, Rostagno A, Frangione B, Ghiso J. Lipidation of apolipoprotein E influences its isoform-specific interaction with Alzheimer’s amyloid beta peptides. The Biochemical journal. 2000;348(Pt 2):359–365. [PMC free article] [PubMed] [Google Scholar]
- [27].Bentley NM, Ladu MJ, Rajan C, Getz GS, Reardon CA. Apolipoprotein E structural requirements for the formation of SDS-stable complexes with beta-amyloid-(1-40): the role of salt bridges. Biochem J. 2002;366:273–279. doi: 10.1042/BJ20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Morikawa M, Fryer JD, Sullivan PM, Christopher EA, Wahrle SE, DeMattos RB, O’Dell MA, Fagan AM, Lashuel HA, Walz T, Asai K, Holtzman DM. Production and characterization of astrocyte-derived human apolipoprotein E isoforms from immortalized astrocytes and their interactions with amyloid-beta. Neurobiol Dis. 2005;19:66–76. doi: 10.1016/j.nbd.2004.11.005. [DOI] [PubMed] [Google Scholar]
- [29].Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].LaDu MJ, Gilligan SM, Lukens JR, Cabana VG, Reardon CA, Van Eldik LJ, Holtzman DM. Nascent astrocyte particles differ from lipoproteins in CSF. J Neurochem. 1998;70:2070–2081. doi: 10.1046/j.1471-4159.1998.70052070.x. [DOI] [PubMed] [Google Scholar]
- [31].Russo C, Angelini G, Dapino D, Piccini A, Piombo G, Schettini G, Chen S, Teller JK, Zaccheo D, Gambetti P, Tabaton M. Opposite roles of apolipoprotein E in normal brains and in Alzheimer’s disease. Proc. Natl. Acad. Sci. USA. 1998;95:15598–15602. doi: 10.1073/pnas.95.26.15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Golabek AA, Soto C, Vogel T, Wisniewski T. The interaction between apolipoprotein E and Alzheimer’s amyloid ß-peptide is dependent on ß-peptide conformation. J. Biol. Chem. 1996;271:10602–10606. doi: 10.1074/jbc.271.18.10602. [DOI] [PubMed] [Google Scholar]
- [33].Pillot T, Goethals M, Vanloo B, Lins L, Brasseur R, Vandekerckhove J, Rosseneu M. Specific modulation of the fusogenic properties of the Alzheimer beta-amyloid peptide by apolipoprotein E isoforms. Euro. J. Biochem. 1997;243:650–659. doi: 10.1111/j.1432-1033.1997.00650.x. [DOI] [PubMed] [Google Scholar]
- [34].Pillot T, Goethals M, Najib J, Labeur C, Lins L, Chambaz J, Brasseur R, Vandekerckhove J, Rosseneu M. Beta-amyloid peptide interacts specifically with the carboxy-terminal domain of human apolipoprotein E: relevance to Alzheimer’s disease. J Neurochem. 1999;72:230–237. doi: 10.1046/j.1471-4159.1999.0720230.x. [DOI] [PubMed] [Google Scholar]
- [35].Yamauchi K, Tozuka M, Hidaka H, Nakabayashi T, Sugano M, Kondo Y, Nakagawara A, Katsuyama T. Effect of apolipoprotein AII on the interaction of apolipoprotein E with beta-amyloid: some apo(E-AII) complexes inhibit the internalization of beta-amyloid in cultures of neuroblastoma cells. J Neurosci Res. 2000;62:608–614. doi: 10.1002/1097-4547(20001115)62:4<608::AID-JNR16>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [36].Fagan AM, Holtzman DM, Munson G, Mathur T, Schneider D, Chang LK, Getz GS, Reardon CA, Lukens J, Shah JA, LaDu MJ. Unique lipoproteins secreted by primary astrocytes from wild type, apoE (-/-), and human apoE transgenic mice. J Biol Chem. 1999;274:30001–30007. doi: 10.1074/jbc.274.42.30001. [DOI] [PubMed] [Google Scholar]
- [37].DeMattos RB, Brendza RP, Heuser JE, Kierson M, Cirrito JR, Fryer J, Sullivan PM, Fagan AM, Han X, Holtzman DM. Purification and characterization of astrocyte-secreted apolipoprotein E and J-containing lipoproteins from wild-type and human apoE transgenic mice. Neurochem Int. 2001;39:415–425. doi: 10.1016/s0197-0186(01)00049-3. [DOI] [PubMed] [Google Scholar]
- [38].Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, Beisiegel U. Characterization of four lipoprotein classes in human cerebrospinal fluid. J Lipid Res. 2001;42:1143–1151. [PubMed] [Google Scholar]
- [39].Castro GR, Fielding CJ. Evidence for the distribution of apolipoprotein E between lipoprotein classes in human normocholesterolemic plasma and for the origin of unassociated apolipoprotein E (Lp-E) J. Lipid Res. 1984;25:58–67. [PubMed] [Google Scholar]
- [40].Corbo RM, Scacchi R. Apolipoprotein E (APOE) allele distribution in the world. Is APOE*4 a ‘thrifty’ allele? Ann Hum Genet. 1999;63:301–310. doi: 10.1046/j.1469-1809.1999.6340301.x. [DOI] [PubMed] [Google Scholar]
- [41].Folin M, Baiguera S, Conconi MT, Di Liddo R, De Carlo E, Parnigotto PP, Nussdorfer GG. Apolipoprotein E as vascular risk factor in neurodegenerative dementia. Int J Mol Med. 2004;14:609–613. [PubMed] [Google Scholar]
- [42].Mooijaart SP, van Vliet P, van Heemst D, Rensen PC, Berbee JF, Jolles J, de Craen AJ, Westendorp RG. Plasma levels of apolipoprotein E and cognitive function in old age. Ann N Y Acad Sci. 2007;1100:148–161. doi: 10.1196/annals.1395.013. [DOI] [PubMed] [Google Scholar]
- [43].Panza F, Solfrizzi V, Colacicco AM, Basile AM, D’Introno A, Capurso C, Sabba M, Capurso S, Capurso A. Apolipoprotein E (APOE) polymorphism influences serum APOE levels in Alzheimer’s disease patients and centenarians. Neuroreport. 2003;14:605–608. doi: 10.1097/00001756-200303240-00016. [DOI] [PubMed] [Google Scholar]
- [44].Siest G, Bertrand P, Qin B, Herbeth B, Serot JM, Masana L, Ribalta J, Passmore AP, Evans A, Ferrari M, Franceschi M, Shepherd J, Cuchel M, Beisiegel U, Zuchowsky K, Rukavina AS, Sertic J, Stojanov M, Kostic V, Mitrevski A, Petrova V, Sass C, Merched A, Salonen JT, Tiret L, Visvikis S, ApoEurope group Apolipoprotein E polymorphism and serum concentration in Alzheimer’s disease in nine European centres: the ApoEurope study. Clin Chem Lab Med. 2000;38:721–730. doi: 10.1515/CCLM.2000.102. [DOI] [PubMed] [Google Scholar]
- [45].Slooter AJ, Tang MX, van Duijn CM, Stern Y, Ott A, Bell K, Breteler MM, Van Broeckhoven C, Tatemichi TK, Tycko B, Hofman A, Mayeux R, A population-based investigation Apolipoprotein E epsilon4 and the risk of dementia with stroke. Jama. 1997;277:818–821. doi: 10.1001/jama.277.10.818. [DOI] [PubMed] [Google Scholar]
- [46].van Vliet P, Mooijaart SP, de Craen AJ, Rensen PC, van Heemst D, Westendorp RG. Plasma levels of apolipoprotein E and risk of stroke in old age. Ann N Y Acad Sci. 2007;1100:140–147. doi: 10.1196/annals.1395.012. [DOI] [PubMed] [Google Scholar]
- [47].van Vliet P, Oleksik AM, Mooijaart SP, de Craen AJ, Westendorp RG. APOE genotype modulates the effect of serum calcium levels on cognitive function in old age. Neurology. 2009;72:821–828. doi: 10.1212/01.wnl.0000343852.10018.24. [DOI] [PubMed] [Google Scholar]
- [48].Lichtlen P, Mohajeri MH. Antibody-based approaches in Alzheimer’s research: safety, pharmacokinetics, metabolism, and analytical tools. J Neurochem. 2008;104:859–874. doi: 10.1111/j.1471-4159.2007.05064.x. [DOI] [PubMed] [Google Scholar]
- [49].Yaffe K, Weston A, Graff-Radford NR, Satterfield S, Simonsick EM, Younkin SG, Younkin LH, Kuller L, Ayonayon HN, Ding J, Harris TB. Association of plasma beta-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305:261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Carlsson J, Armstrong VW, Reiber H, Felgenhauer K, Seidel D. Clinical relevance of the quantification of apolipoprotein E in cerebrospinal fluid. Clin. Chim. Acta. 1991;196:167–176. doi: 10.1016/0009-8981(91)90070-s. [DOI] [PubMed] [Google Scholar]
- [51].Hesse C, Larsson H, Fredman P, Minthon L, Andreasen N, Davidsson P, Blennow K. Measurement of apolipoprotein E (apoE) in cerebrospinal fluid. Neurochem Res. 2000;25:511–517. doi: 10.1023/a:1007516210548. [DOI] [PubMed] [Google Scholar]
- [52].Wahrle SE, Shah AR, Fagan AM, Smemo S, Kauwe JS, Grupe A, Hinrichs A, Mayo K, Jiang H, Thal LJ, Goate AM, Holtzman DM. Apolipoprotein E levels in cerebrospinal fluid and the effects of ABCA1 polymorphisms. Mol Neurodegener. 2007;2:7. doi: 10.1186/1750-1326-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].LaDu MJ, Stine WB, Jr., Narita M, Getz GS, Reardon CA, Bu G. Self-Assembly of HEK Cell-Secreted ApoE Particles Resembles ApoE Enrichment of Lipoproteins as a Ligand for the LDL Receptor-Related Protein. Biochemistry. 2006;45:381–390. doi: 10.1021/bi051765s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Manelli AM, Stine WB, Van Eldik LJ, LaDu MJ. ApoE and Abeta1-42 interactions: effects of isoform and conformation on structure and function. J Mol Neurosci. 2004;23:235–246. doi: 10.1385/JMN:23:3:235. [DOI] [PubMed] [Google Scholar]
- [55].Ladu MJ, Reardon C, Van Eldik L, Fagan AM, Bu G, Holtzman D, Getz GS. Lipoproteins in the central nervous system. Ann N Y Acad Sci. 2000;903:167–175. doi: 10.1111/j.1749-6632.2000.tb06365.x. [DOI] [PubMed] [Google Scholar]
- [56].Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer’s disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Sun Y, Wu S, Bu G, Onifade MK, Patel SN, LaDu MJ, Fagan AM, Holtzman DM. Glial fibrillary acidic protein-apolipoprotein E (apoE) transgenic mice: astrocyte-specific expression and differing biological effects of astrocyte-secreted apoE3 and apoE4 lipoproteins. J Neurosci. 1998;18:3261–3272. doi: 10.1523/JNEUROSCI.18-09-03261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nat Rev Neurol. 2010;6:131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- [59].Lui JK, Laws SM, Li QX, Villemagne VL, Ames D, Brown B, Bush AI, De Ruyck K, Dromey J, Ellis KA, Faux NG, Foster J, Fowler C, Gupta V, Hudson P, Laughton K, Masters CL, Pertile K, Rembach A, Rimajova M, Rodrigues M, Rowe CC, Rumble R, Szoeke C, Taddei K, Taddei T, Trounson B, Ward V, Martins RN, R A. Group, Plasma amyloid-beta as a biomarker in Alzheimer’s disease: the AIBL study of aging. J Alzheimers Dis. 2010;20:1233–1242. doi: 10.3233/JAD-2010-090249. [DOI] [PubMed] [Google Scholar]
- [60].Graff-Radford NR, Crook JE, Lucas J, Boeve BF, Knopman DS, Ivnik RJ, Smith GE, Younkin LH, Petersen RC, Younkin SG. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- [61].Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid beta-protein (A beta): a possible form of preamyloid in Alzheimer’s disease. Nature medicine. 1995;1:1062–1066. doi: 10.1038/nm1095-1062. [DOI] [PubMed] [Google Scholar]
- [62].McLaurin J, Chakrabartty A. Membrane disruption by Alzheimer beta-amyloid peptides mediated through specific binding to either phospholipids or gangliosides. Implications for neurotoxicity. J Biol Chem. 1996;271:26482–26489. doi: 10.1074/jbc.271.43.26482. [DOI] [PubMed] [Google Scholar]
- [63].Hayashi H, Kimura N, Yamaguchi H, Hasegawa K, Yokoseki T, Shibata M, Yamamoto N, Michikawa M, Yoshikawa Y, Terao K, Matsuzaki K, Lemere CA, Selkoe DJ, Naiki H, Yanagisawa K. A seed for Alzheimer amyloid in the brain. J Neurosci. 2004;24:4894–4902. doi: 10.1523/JNEUROSCI.0861-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Waschuk SA, Elton EA, Darabie AA, Fraser PE, McLaurin JA. Cellular membrane composition defines A beta-lipid interactions. J Biol Chem. 2001;276:33561–33568. doi: 10.1074/jbc.M103598200. [DOI] [PubMed] [Google Scholar]
- [65].Cai L, de Beer MC, de Beer FC, van der Westhuyzen DR. Serum amyloid A is a ligand for scavenger receptor SR-BI and inhibits HDL binding and selective lipid uptake. J Biol Chem. 2004 doi: 10.1074/jbc.M411555200. [DOI] [PubMed] [Google Scholar]
- [66].Mulder M, Terwel D. Possible link between lipid metabolism and cerebral amyloid angiopathy in Alzheimer’s disease: A role for high-density lipoproteins? Haemostasis. 1998;28:174–194. doi: 10.1159/000022429. [DOI] [PubMed] [Google Scholar]