Abstract
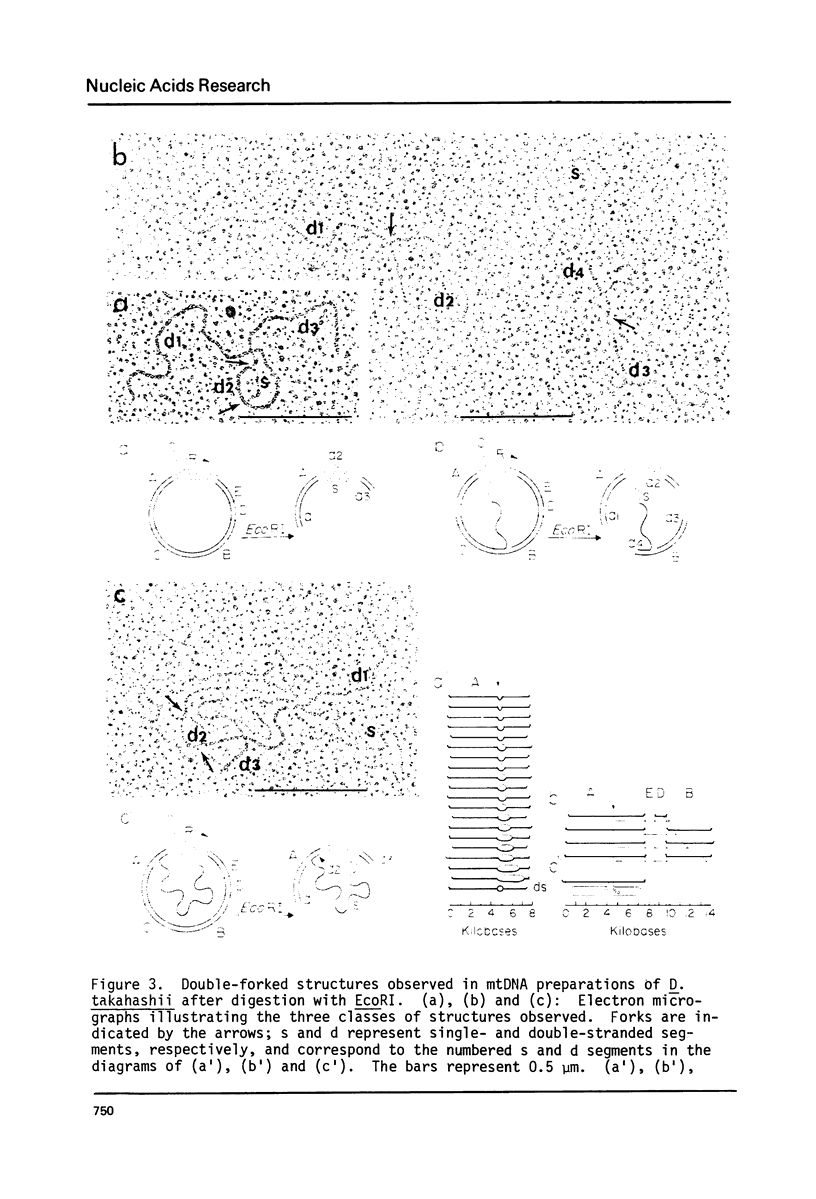
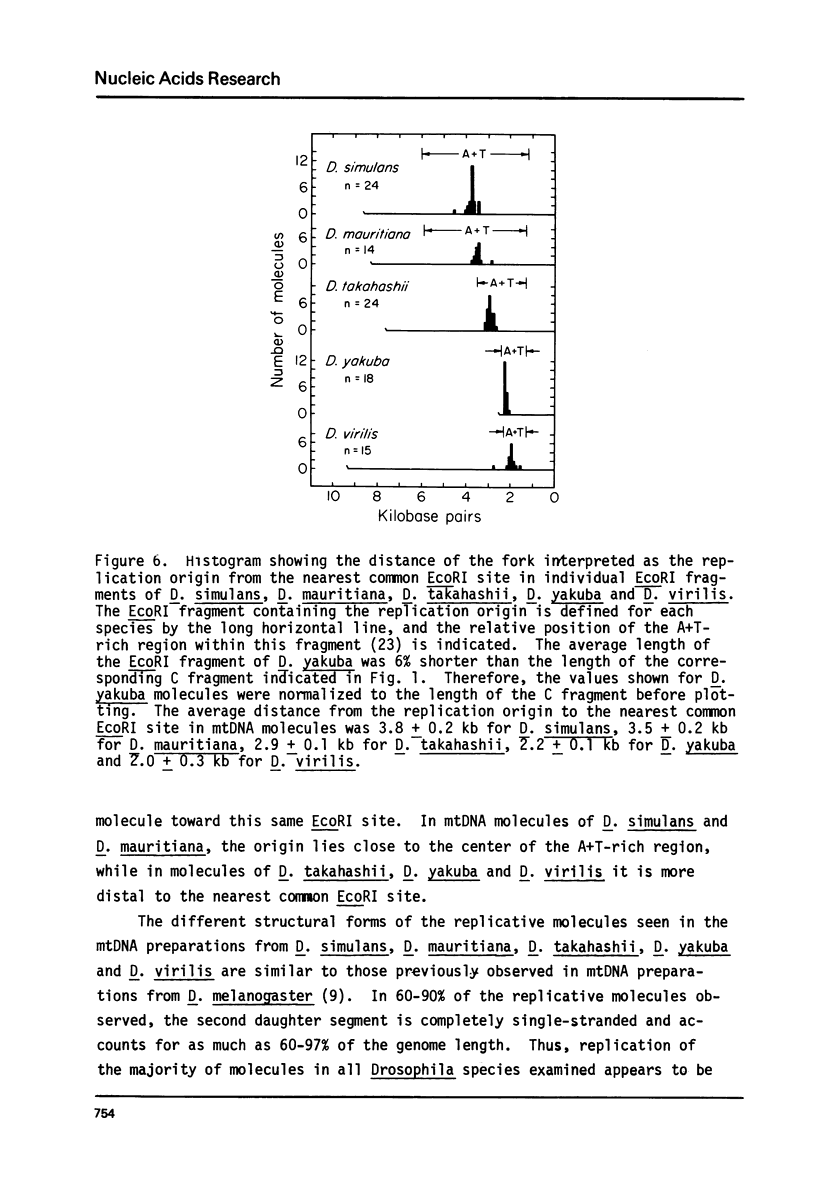
Mitochondrial DNA (mtDNA) obtained from ovaries of Drosophila simulans, D. mauritiana, D. takahashii, D. yakuba and D. virilis was examined by electron microscopy. From a consideration of the structural properties of replicative intermediates, it was concluded that in mtDNA molecules of each species, synthesis on one strand can be up to 97% complete before synthesis on the complementary strand is initiated. MtDNA molecules of each species contain a single A+T-rich region which shows species-specific size variation from 1.0 kb (D. virilis) to 4.8 kb (D. simulans), and maps at the same position in all molecules relative to three common EcoRI sites. The structural properties of complex forms, interpreted as having originated from replicative intermediates, and produced by either partial denaturation or EcoRI digestion, are consistent with the hypothesis that replication is initiated within the A+T-rich region and proceeds unidirectionally around the molecule towards the nearest common EcoRI site. The replication origin is located near the center of the A+T-rich region in D. simulans and D. mauritiana, but lies closer to that end of the A+T-rich region which is distal to the nearest common EcoRI site in D. takahashii, D. yakuba and D. virilis.
Full text
PDF


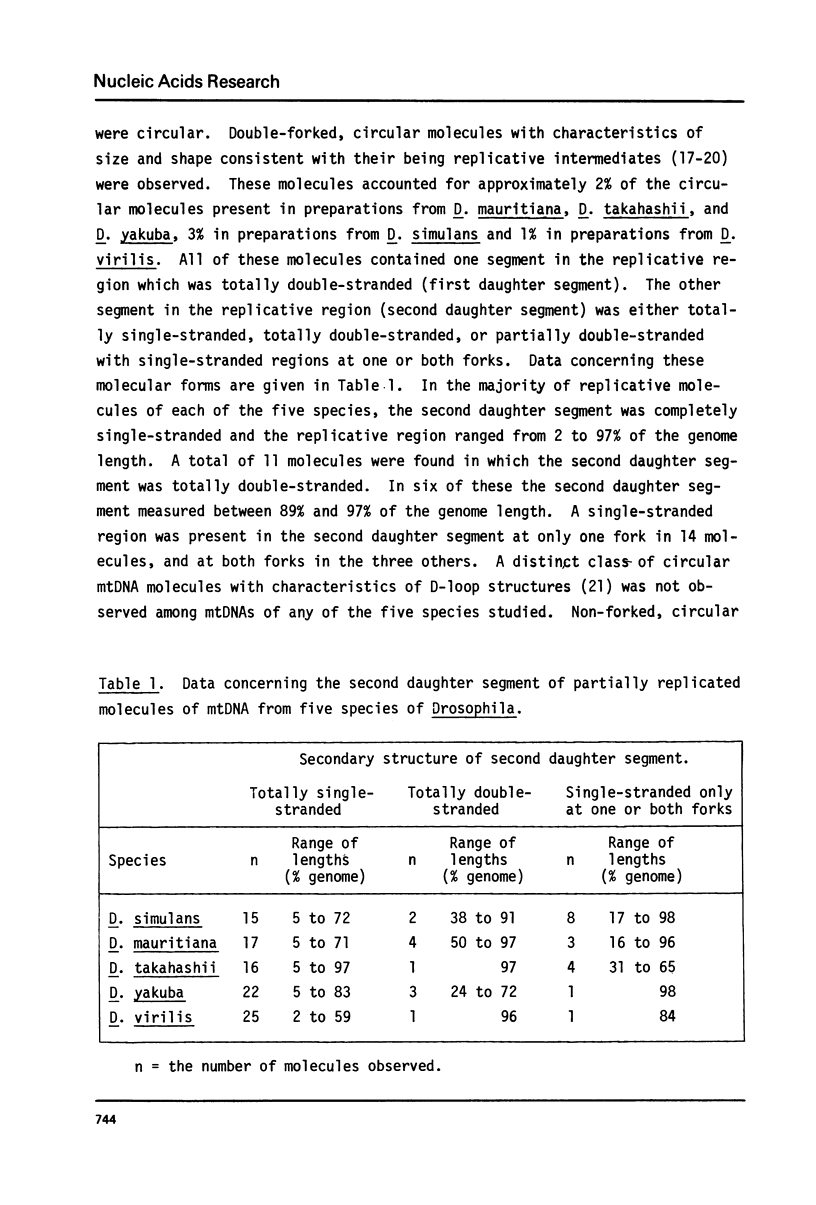









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Crews S. T., Nishiguchi J., Ojala D. K., Posakony J. W. Nucleotide sequence of a fragment of HeLa-cell mitochondrial DNA containing the precisely localized origin of replication. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):179–192. doi: 10.1101/sqb.1979.043.01.024. [DOI] [PubMed] [Google Scholar]
- Battey J., Clayton D. A. The transcription map of mouse mitochondrial DNA. Cell. 1978 May;14(1):143–156. doi: 10.1016/0092-8674(78)90309-4. [DOI] [PubMed] [Google Scholar]
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: topology of circular daughter molecules and dynamics of catenated oligomer formation. J Mol Biol. 1976 Jan 5;100(1):85–92. doi: 10.1016/s0022-2836(76)80036-8. [DOI] [PubMed] [Google Scholar]
- Bultmann H., Laird C. D. Mitochondrial DNA from Drosophila melanogaster. Biochim Biophys Acta. 1973 Mar 19;299(2):196–209. doi: 10.1016/0005-2787(73)90342-0. [DOI] [PubMed] [Google Scholar]
- Bultmann H., Zakour R. A., Sosland M. A. Evolution of Drosophila mitochondrial DNAs. Comparison of denaturation maps. Biochim Biophys Acta. 1976 Nov 12;454(1):21–44. doi: 10.1016/0005-2787(76)90351-8. [DOI] [PubMed] [Google Scholar]
- Buzzo K., Fouts D. L., Wolstenholme D. R. EcoRI cleavage site variants of mitochondrial DNA molecules from rats. Proc Natl Acad Sci U S A. 1978 Feb;75(2):909–913. doi: 10.1073/pnas.75.2.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauron C. M., Wolstenholme D. R. Structural heterogeneity of mitochondrial DNA molecules within the genus Drosophila. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3623–3627. doi: 10.1073/pnas.73.10.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. M., Cummings D. J. Mitochondrial DNA replication in Paramecium aurelia. Cross-linking of the initiation end. J Mol Biol. 1977 Jan 15;109(2):327–344. doi: 10.1016/s0022-2836(77)80037-5. [DOI] [PubMed] [Google Scholar]
- Goddard J. M., Wolstenholme D. R. Origin and direction of replication in mitochondrial DNA molecules from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3886–3890. doi: 10.1073/pnas.75.8.3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring E. S., Peacock W. J. Intramolecular heterogeneity of mitochondrial DNA of Drosophila melanogaster. J Cell Biol. 1977 May;73(2):279–286. doi: 10.1083/jcb.73.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Robberson D. L., Vinograd J. A novel closed-circular mitochondrial DNA with properties of a replicating intermediate. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2252–2257. doi: 10.1073/pnas.68.9.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu H., Vinograd J. Unidirectionality of replication in mouse mitochondrial DNA. Nat New Biol. 1973 Jan 24;241(108):103–105. doi: 10.1038/newbio241103a0. [DOI] [PubMed] [Google Scholar]
- Kirschner R. H., Wolstenholme D. R., Gross N. J. Replicating molecules of circular mitochondrial DNA. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1466–1472. doi: 10.1073/pnas.60.4.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klukas C. K., Dawid I. B. Characterization and mapping of mitochondrial ribosomal RNA and mitochondrial DNA in Drosophila melanogaster. Cell. 1976 Dec;9(4 Pt 1):615–625. doi: 10.1016/0092-8674(76)90044-1. [DOI] [PubMed] [Google Scholar]
- Kroon A. M., Pepe G., Bakker H., Holtrop M., Bollen J. E., Van Bruggen E. F., Cantatore P., Terpstra P., Saccone C. The restriction fragment map of rat-liver mitochondrial DNA: a reconsideration. Biochim Biophys Acta. 1977 Sep 20;478(2):128–145. doi: 10.1016/0005-2787(77)90177-0. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davis R. W., Davidson N. A physical study by electron microscopy of the terminally reptitious, circularly permuted DNA from the coliphage particles of Escherichia coli 15. J Mol Biol. 1970 Feb 28;48(1):1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojala D., Attardi G. Precise localization of the origin of replication in a physical map of HeLa cell mitochondrial DNA and isolation of a small fragment that contains it. J Mol Biol. 1978 Jul 5;122(3):301–319. doi: 10.1016/0022-2836(78)90192-4. [DOI] [PubMed] [Google Scholar]
- Peacock W. J., Brutlag D., Goldring E., Appels R., Hinton C. W., Lindsley D. L. The organization of highly repeated DNA sequences in Drosophila melanogaster chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:405–416. doi: 10.1101/sqb.1974.038.01.043. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Friedman S., Gall J. G., Gehring W. Isolation and characterization of mitochondrial DNA from Drosophila melanogaster. J Cell Biol. 1973 Feb;56(2):580–589. doi: 10.1083/jcb.56.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J. L., Dawid I. B. Mapping of mitochondrial DNA in Xenopus laevis and X. borealis: the positions of ribosomal genes and D-loops. J Mol Biol. 1978 Feb 15;119(1):133–146. doi: 10.1016/0022-2836(78)90273-5. [DOI] [PubMed] [Google Scholar]
- Robberson D. L., Kasamatsu H., Vinograd J. Replication of mitochondrial DNA. Circular replicative intermediates in mouse L cells. Proc Natl Acad Sci U S A. 1972 Mar;69(3):737–741. doi: 10.1073/pnas.69.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein J. L., Brutlag D., Clayton D. A. The mitochondrial DNA of Drosophila melanogaster exists in two distinct and stable superhelical forms. Cell. 1977 Oct;12(2):471–482. doi: 10.1016/0092-8674(77)90123-4. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upholt W. B., Dawid I. B. Mapping of mitochondrial DNA of individual sheep and goats: rapid evolution in the D loop region. Cell. 1977 Jul;11(3):571–583. doi: 10.1016/0092-8674(77)90075-7. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Fauron C. M. A partial map of the circular mitochondrial genome of Drosophila melanogaster. Location of EcoRI-sensitive sites and the adenine-thymine-rich region. J Cell Biol. 1976 Nov;71(2):434–448. doi: 10.1083/jcb.71.2.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme D. R., Koike K., Cochran-Fouts P. Replication of mitochondrial DNA: replicative forms of molecules from rat tissues and evidence for discontinuous replication. Cold Spring Harb Symp Quant Biol. 1974;38:267–280. doi: 10.1101/sqb.1974.038.01.030. [DOI] [PubMed] [Google Scholar]
- Wolstenholme D. R., Koike K., Cochran-Fouts P. Single strand-containing replicating molecules of circular mitochondrial DNA. J Cell Biol. 1973 Jan;56(1):230–245. doi: 10.1083/jcb.56.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstonholme D. R., Kirschner R. G., Gross N. J. Heart denaturation studies of rat liver mitrochondrial DNA. A denaturation map and changes in molecular configurations. J Cell Biol. 1972 May;53(2):393–406. doi: 10.1083/jcb.53.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]