Abstract
The purpose of the current study was to examine changes in dopamine D2 receptor (DA-D2R) expression within the basal ganglia of MPTP mice subjected to intensive treadmill exercise. Using Western immunoblotting analysis of synaptoneurosomes and in vivo positron emission tomography (PET) imaging employing the DA-D2R specific ligand [18F]fallypride, we found that high intensity treadmill exercise led to an increase in striatal DA-D2R expression that was most pronounced in MPTP compared to saline treated mice. Exercise-induced changes in the DA-D2R in the dopamine-depleted basal ganglia are consistent with the potential role of this receptor in modulating medium spiny neurons (MSNs) function and behavioral recovery. Importantly, findings from this study support the rationale for using PET imaging with [18F]fallypride to examine DA-D2R changes in individuals with Parkinson’s Disease (PD) undergoing high-intensity treadmill training.
Keywords: positron emission tomography, basal ganglia, neuroplasticity, treadmill exercise
Exercise improves motor performance in patients with Parkinson’s disease (PD).1–3 Animal models, such as the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse, provide a critical tool to investigate the molecular mechanisms of exercise-induced improvement in motor behavior.4–6 The dopamine D1 and D2 receptors (DA-D1R and DA-D2R) are the primary targets of dopamine on striatal medium spiny neurons (MSNs) and modulate physiological properties and cellular signaling. Specifically, the DA-D2R plays a major role in long-term depression (LTD), a form of synaptic plasticity that involves integration of glutamatergic and dopaminergic neurotransmission leading to the encoding of motor function in the dorsolateral striatum. Given the role of the DA-D2R in motor control, we sought to examine whether exercise enhanced improvement in motor function is due in part to an increase in striatal DA-D2R expression.
Positron emission tomography (PET)-imaging with DA-D2R radiotracers offers the ability to carry out longitudinal studies on the effect of exercise in humans. Previous studies with aerobic exercise have attempted to measure dopamine release in normal individuals7 and no change in the binding of [11C]raclopride was observed, leading the authors to suggest that little change in dopamine levels occurred. However, the effects of exercise on DA-D2R expression and synaptic activity has not been studied. The PET-imaging ligand [18F]fallypride is an excellent tool to examine this due to its high affinity and specificity for both DA-D2R and DA-D3R, and unlike [11C]raclopride, it is not readily displaced by baseline levels of endogenous dopamine.7–10 This was confirmed by reserpine pretreatment of animals (to deplete endogenous dopamine) that had no effect on [18F]fallypride binding,9,11 but significantly increased [11C]raclopride binding8 that was attributed to a change in the apparent binding affinity (Kd) rather than receptor number (Bmax).
As the binding potential (BP) of [18F]fallypride is resistant to changes due to depletion of dopamine, suggesting little effect on its Kd or Bmax at baseline or depleted state, we used [18F]fallypride to test our hypothesis that DA-D2R expression increases in the MPTP mouse model with intensive exercise.9,10,12,13 Furthermore, to support our PET imaging measures, we used the complementary technique of Western immunoblot analysis of synaptoneurosomal preparations to measure changes in DA-D2R protein expression at the level of synapse in the same animals. We report here the effects of exercise on DA-D2R expression and [18F]fallypride in groups of mice treated with either saline or MPTP.
METHODS
Animals, Treatment Groups, and MPTP Administration
Male C57BL/6 mice 8 weeks old (Charles River Laboratories, Wilmington, MA) were group-housed in a temperature-controlled room under 12 h light/12 h dark cycle. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals as approved by the USC IACUC. A total of 164 mice were used in four treatment groups: (1) saline (n = 42), (2) saline plus exercise (n = 55), (3) MPTP (n = 57), and (4) MPTP plus exercise (n = 42). For lesioning, mice received four intraperitoneal injections of 20 mg/kg MPTP (free-base; Sigma-Aldrich, St. Louis, MO) dissolved in 0.9% saline, at 2-h intervals or four intraperitoneal injections of 0.1 ml 0.9% NaCl as control. Lesioning was validated by HPLC analysis of striatal dopamine levels. At 10 days post-MPTP administration, there was 82.2% dopamine depletion in MPTP mice (48.0 ± 8.4 ng/mg of protein) compared with saline mice (269.5 ± 24.9 ng/mg of protein). At the end of the study, there was no significant difference in striatal dopamine levels between MPTP plus exercise mice (69.8 ± 11.7 ng/mg of protein) compared with MPTP (77.9 ± 12.0 ng/mg of protein). However, there was a significant increase of striatal dopamine in saline plus exercise mice (315.2 ± 9.0 ng/mg of protein) compared with saline (246.9 ± 19.8 ng/mg of protein) (F(3,16) = 7.78; P < 0.05).
Treadmill Exercise
Exercise started 5 days after lesioning. Mice from the two exercise groups (saline plus exercise and MPTP plus exercise) were trained to run on a 100-cm motorized treadmill (Exer 6M, Columbus Instruments, OH) at incremental speeds for 6 weeks (5 days/week) to reach duration of 60 min/day and speed of 18–20 m/min.5,6
Magnetic Resonance Imaging
A three-dimensional volumetric T1-weighted magnetic resonance (MR) image of the mouse brain was obtained with a 7-T micro-MRI system (Bruker Biospin, Billerica, MA). Parameters of image acquisition were: TE = 46.1 ms, TR = 6292.5 ms, 0.4-mm slice thickness, 0.45-mm interslice thickness, 128 × 128 × 128 matrix size.
Radiochemistry
Synthesis of [18F]fallypride was performed as previously described through nucleophilic substitution reaction of the tosyl precursor with [18F] using a custom-made radiochemistry apparatus.12 Purification was achieved by reverse-phase HPLC on a C8(2) Phenomenex Luna column using acetonitrile and sodium phosphate buffer as mobile phase (55:45). UV absorbance was measured at 254 nm and AUFS 0.05. Radioactive peak (retention time 17 min) corresponding to [18F]fallypride, was collected and solvent removed on a rotary evaporator. The final product was tested for pyrogenicity, sterility, pH, and removal of organic solvents by gas chromatography. Specific activity and radiochemical purity was assessed with a Waters HPLC system using a C8(2) Phenomenex Luna analytical. Specific activity was in the range of 3,000–12,000 Ci/mmol.
PET Measurements and Image Analysis
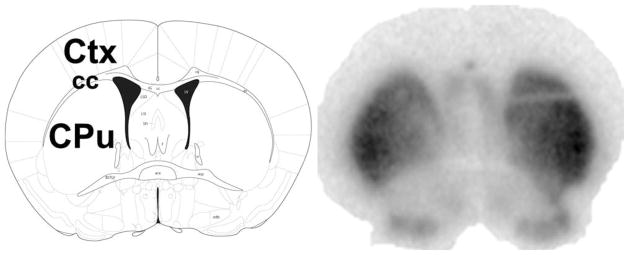
Twenty mice were used for PET imaging (n = 6 saline; n = 3 saline plus exercise; n = 5 MPTP; and n = 6 MPTP plus exercise). Scans were acquired with a Concorde microPET R4 scanner (CTI Concorde Microsystems, Knoxville, TN) with a 60-min list mode acquisition protocol after 20-min transmission scan for attenuation correction with a 68Ge source. [18F]fallypride (10.92–11.28 MBq) was injected via the tail vein (single bolus) at the start of the emission scan. Mice were anesthetized with 2% isofluorane and 98% oxygen. The dynamic list mode data were sorted to sinograms with 26 frames (6 × 20 sec, 4 × 40 sec, 6 × 1 min, and 10 × 5 min) and reconstructed by two iterations of OSEM (ordered subsets expectation maximization) followed by 18 iterations of the MAP (maximum a posteriori) reconstruction algorithm.14 Reconstructed images were cropped to contain the head and linearly interpolated in the Z-direction to produce a 128 × 128 × 63 image with isotropic 0.4 × 0.4 × 0.4 mm3 voxels. High-resolution binding potential (BP) images of the striatum were computed from the reconstructed dynamic images using a multilinear tissue reference model15 and Logan plots16 with high activity in the striatum and very low activity in the cerebellum (reference region). Anatomical regions of interest (striatum and cerebellum) were manually defined in both hemispheres in PET images coregistered with MRI using Rview (version 8.21Beta).17 Quantification of specific binding of [18F]fallypride in the mouse striatum was performed using the BP value that provides a measure of the ratio of specific/nonspecific binding at equilibrium.18,19 To demonstrate binding specificity in the striatum, four mice were harvested 60 min after ligand injection, brains quickly frozen in liquid nitrogen, sectioned at 30-μm thickness, and sections apposed to a phospho-imager (Typhoon 9200, GE Healthcare Inc., Piscataway, NJ) (Fig. 1). Studies have shown that [18F]fallypride binds specifically to the DA-D2R, and as very little DA-D3R is in the striatum, binding indicates DA-D2R occupancy.9,10,12,13
FIG. 1.
[18F]Fallypride shows high biding specificity to the mouse striatum. The left panel shows an anatomical rendering of the coronal section at approximate level bregma 0.20. The right panel shows a representative autoradiograph with intensive labeling corresponding to the striatum.
Tissue Collection for HPLC and Protein Analysis
At the end of the study, brains were quickly removed and dorsal striatum dissected fresh corresponding to anatomical regions from bregma 1.2 to 0.6 with the corpus callosum as dorsal border, the lateral aspect of the corpus callosum as lateral border, and above the anterior commissure as the ventral border.20
HPLC Analysis of Dopamine and Its Metabolites
Dopamine levels in striatal homogenates (n = 4 per group) were determined by HPLC with electrochemical detection.6 The system consisted of an ESA auto-sampler (ESA, Chelmsford, MA) equipped with a 150 × 3.2 mm reverse-phase C-18 column (3μm diameter) and a CoulArray 5600A (ESA, Chelmsford, MA), equipped with a four-channel analytical cell with potentials set at −75, 50, 220, and 350 mV.
Western Immunoblot Analysis
Exercise effect on synaptic expression of DA-D1R and DA-D2R was analyzed in synaptoneurosome preparations made fresh from eight pooled dorsolateral striatum.21 This procedure was performed on three sets of mice for a total of 24 mice per experimental group (n = 3 preps per group). Relative expression of proteins for DA-D1R (~50 kDa), DA-D2R (~50 kDa), tyrosine hydroxylase (58 kDa), dopamine transporter (68 kDa), and α-tubulin (50 kDa) (as loading control) were analyzed by Western immunoblot22 using commercially available primary antibodies (rabbit polyclonal and mouse monoclonal antibodies, Millipore, Temecula, CA). Protein bands were visualized by affinity purified goat anti-rabbit or anti-mouse secondary antibodies conjugated to IRDye680 or IRDye800 (Rockland, Gilbertsville, PA). Fluorescent signal was detected by scanning the filter in a LI-COR Odyssey near infrared imaging platform and quantified using Odyssey 2.1 software (LI-COR Biotechnology, Lincoln, NE). The results are shown as relative expression levels compared with the saline group (set to 100%).
Statistical Analysis
Differences between groups in BP of [18F]fallypride, DA-D1R, and DA-D2R protein levels were analyzed using two-way analysis of variance (ANOVA) with treatment as between subject factor (saline vs. MPTP), and exercise as within subject factor (no exercise vs. exercise). For maximal treadmill speed test, time was used as between subject factor (week 1, 2, etc.) and treatment was used as within subject factor (saline vs. MPTP). The Bonferroni post hoc test was used to correct for multiple comparisons when assessing significance of interest. Significance level was set to P < 0.05. To explore the practical significance of group differences, an estimate of the magnitude of the differences between groups was calculated using the effect size (ES) (ES = MeanGroup 1 – MeanGroup 2/SDpooled). The ES reflects the impact of treatment within a population of interest and is reported according to established criteria as small (<0.41), medium (0.41–0.70), or large (>0.70).23 Analysis was performed using Prism5 for Windows (GraphPad, San Diego, CA).
RESULTS
High-Intensity Treadmill Exercise Improved Motor Behavior in MPTP-Lesioned Mice
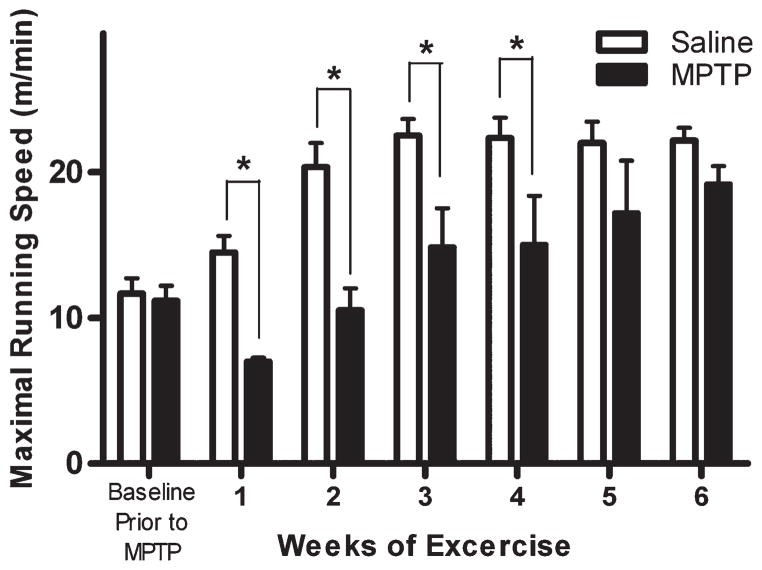
Prior to MPTP-lesioning and start of exercise, average baseline velocities of all mice in two exercise groups were similar (saline plus exercise: 11.7 ± 1.1 m/min, and MPTP plus exercise: 11.2 ± 1.1 m/min). Daily exercise for 6 weeks improved maximal treadmill velocities in both exercise groups with the saline plus exercise mice displaying significantly greater maximal velocity compared to the MPTP plus exercise mice in weeks 1 through 4 (Fig. 2). However, MPTP plus exercise mice had similar maximal treadmill speeds as saline plus exercise mice in week 5 (MPTP plus exercise: 17.2 ± 3.6 m/min and saline plus exercise: 22.0 ± 1.5 m/min) and week 6 (19.2 ± 1.2 m/min and 22.2 ± 0.9 m/min, respectively). As previously reported, MPTP-lesioned mice that did not undergo treadmill training displayed no spontaneous recovery of motor behavior with their maximal velocity of 7.0 ± 0.3 m/min at the end of the 6-week exercise period.5
FIG. 2.
Exercise improves motor behavior in the MPTP mouse. The maximum running speed of saline (n = 12) and MPTP (n = 12) mice on the motorized treadmill was tested at the end of each week. The baseline treadmill velocities were measured prior to MPTP lesioning. By the end of the running period, there was no difference in velocity between saline and MPTP mice. Results are shown as mean ± SEM. Data were analyzed by two-way ANOVA with repeated measures; the symbol “*” represents significance level P < 0.05. Significant differences in maximal treadmill velocity were seen at weeks 1 through 4.
High-Intensity Treadmill Exercise Increased Striatal DA-D2R but not DA-D1R Protein
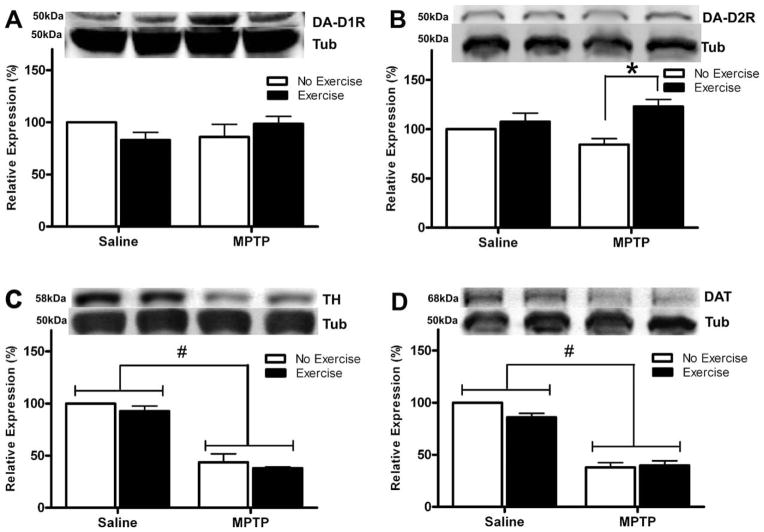
High-intensity treadmill exercise differentially affected DA-D2R and DA-D1R levels in synaptoneurosomal preparations from the dorsal striatum as shown by Western blot analysis (Fig. 3). MPTP plus exercise mice had 48.8% increase in striatal DA-D2R compared with MPTP mice (Fig. 3B), and significant interaction between exercise and MPTP lesioning on DA-D2R protein level (F(1,8) = 6.0; P < 0.05). Conversely, there was no exercise effect on DA-D1R protein levels between the groups (Fig. 3A; F(1,8) = 0.1, P = 0.78). MPTP lesioning alone did not significantly alter either DA-D2R (F(1,8) = 0.0; P = 0.88) or DA-D1R expression (F(1,8) = 0.0; P = 0.92). In addition, two different protein markers of midbrain dopaminergic fibers integrity, tyrosine hydroxylase (TH; Fig. 3C) and dopamine transporter (DAT; Fig. 3D), showed that MPTP significantly decreased striatal TH protein (F(1,8) = 757.3; P < 0.05) and DAT expression (F(1,8) = 218.0; P < 0.05).
FIG. 3.
Exercise selectively up-regulates DA-D2R but not DA-D1R striatal protein. Panel (A) shows Western immunoblot analysis of synaptoneurosome preparations from the dorsal striatum for DA-D1R protein. There was no statistically significant difference between treatment groups. Panel (B) shows Western immunoblot analysis of synaptoneurosome preparations from the dorsal striatum for DA-D2R protein. MPTP plus exercise mice showed elevated DA-D2R protein compared to MPTP mice. (C) Analysis of tyrosine-hydroxylase (TH) protein and (D) dopamine transporter (DAT) protein (markers of midbrain dopaminergic terminals) in synaptoneurosome preparations from four different groups. MPTP treatment significantly decreased levels of TH and DAT proteins in dorsal striatum. These data were generated in three separate experiments, each consisting from pooled tissue (n = 8 brains/group). Results are shown as mean ± SEM. The symbol “#” represents significant MPTP effect (P < 0.05), and the symbol “*” represents significant effect of exercise in MPTP groups as shown by two-way ANOVA with Bonferroni post hoc correction (P < 0.05).
High-Intensity Treadmill Exercise Increased Striatal [18F]Fallypride Binding Potential (BP)
While Western immunoblotting analysis of receptor protein expression measured total antibody epitopes (both surface and internal cellular stores), in vivo PET-imaging with the high-affinity DA-D2R-specific radioligand [18F]fallypride can delineate the effects of exercise on availability of DA-D2R to bind ligand (Fig. 4). Statistical analysis revealed that there was a significant effect of exercise (F(1,16) = 12.3; P < 0.05) as well as MPTP lesion (F(1,16) = 160.3; P < 0.05) with no significant interaction between MPTP and exercise (F(1,16) = 3.5; P = 0.07) on [18F]fallypride BP. The Bonferroni post hoc analysis showed significant difference in BP values between MPTP and MPTP plus exercise mice (t = 1.1, Df = 1, 16; P < 0.01), and no significant difference between saline and saline plus exercise mice (t = 4.1, Df = 1, 16; P > 0.05). Specifically, MPTP plus exercise mice had a 73.1% increase in [18F]fallypride BP compared to MPTP mice (average BP values for MPTP plus exercise: 7.1 ± 0.7; average BP values for MPTP mice: 4.1 ± 0.3) (Fig. 4B). In addition, saline plus exercise mice had an 8.2% increase in [18F]fallypride BP (13.2 ± 1.0) compared to saline mice (12.2 ± 0.3). Consistent with these findings “effect size” calculations revealed a larger exercise effect between MPTP groups (ES = 2.61) than that observed between the saline groups (ES = 0.94).
FIG. 4.
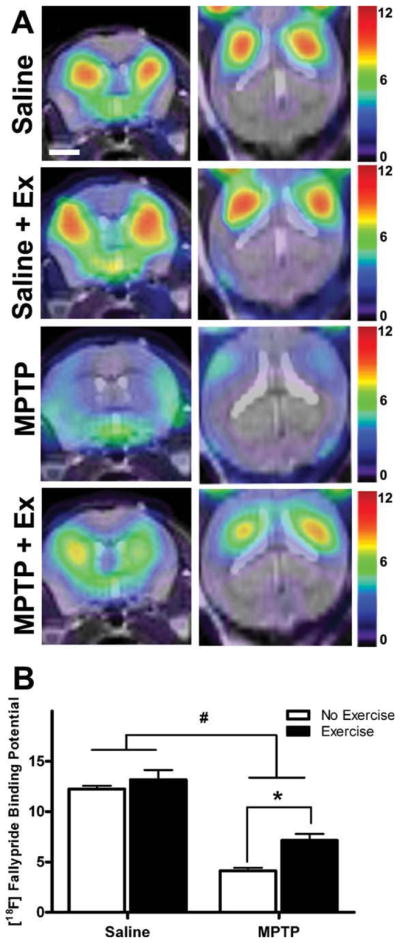
Exercise selectively increases [18F]fallypride binding potential (BP) in the striatum of MPTP mice. Panel (A) shows [18F]fallypride BP representative images in the coronal orientation (left side) and horizontal orientation (right side). The scale bar to the right shows relative BP intensity with high in red and low in blue. Panel (B) graphically depicts the BP data showing that MPTP reduced [18F]fallypride BP, while exercise increased [18F]fallypride BP in both saline and MPTP mice. The numbers of mice used for each group were saline (n = 6), saline plus exercise (n = 3), MPTP (n = 5), and MPTP plus exercise mice (n = 6). Results are shown as mean ± SEM. The symbol “#” represents significance level of MPTP effect (P < 0.05), and “*” represents the effect of exercise in MPTP groups as shown by two-way ANOVA with Bonferroni post hoc correction (P < 0.01).
DISCUSSION
This study demonstrates that high-intensity treadmill exercise leads to an increase in [18F]fallypride BP (DA-D2R availability) in the striatum of MPTP treated mice. Conversely, there was no significant change in total striatal dopamine levels between MPTP plus exercise compared with MPTP no exercise mice. [18F]fallypride is a highly selective DA-D2/D3R antagonist whose BP reflects an in vivo measure of available receptors (Bmax)/binding affinity (Kd). As DA-D2Rs are the predominant dopamine receptor subtype within dorsal striatum, an exercise-induced increase in [18F]fallypride BP represents an increase in DA-D2R number and is supported by an increase in protein expression using Western immunoblotting and our previous studies showing an increase in striatal DA-D2R mRNA transcript expression using in situ hybridization histochemistry.5 This interpretation of BP elevation is further supported by the fact that displacement of [18F]fallypride by dopamine is not likely to occur in MPTP mice as dopamine levels remain low.24 Hence, changes in apparent binding affinity (Kd) are negligible and are unlikely to effect BP. The enhanced effect of exercise in MPTP mice may reflect an attempt of the injured brain to optimize dopaminergic neurotransmission through increased receptor number while dopamine levels remain depleted. Increased responsiveness of MPTP mice to exercise reveals a greater potential of the injured versus the intact brain to undergo neuroplasticity, which may not be essential when striatal circuitry is intact. The fact that dopamine levels do not change significantly with exercise in MPTP mice suggests that compensatory changes in DA-D2R are critical for exercise related improved motor performance.
Using PET imaging, we observed a decrease in DA-D2R BP after MPTP lesioning relative to saline-treated mice. This was in contrast to Western immunoblotting in which no change in DA-D2R protein expression was observed. The DA-D2R exists in a dynamic equilibrium between surface and intracellular compartments, with the latter not generally available to binding to PET radioligands. In the dopamine-depleted state, compensatory mechanisms may lead to changes in the intracellular pool for DA-D2R, which may be unavailable for [18F]fallypride binding but yet available for detection in Western immunoblotting.
Unlike our findings, a compensatory increase in the DA-D2R has been reported in individuals with PD and after administration of MPTP in nonhuman primates, or 6-OHDA in rats.25 In the literature, the loss of DA-D2Rs is reportedly due to the degeneration of dopaminergic neurons, whereas the increase in DA-D2Rs results from increased expression on remaining dopaminergic terminals and/or increased synthesis within striatopallidal neurons or cholinergic interneurons. This discrepancy between our PET study, and those of the literature, may be due to differences in the severity of the lesion between studies.11 Specifically, the loss of a greater number of presynaptic DA-D2Rs through MPTP-induced cell loss may be sufficient to offset any postsynaptic compensatory changes induced by the lesion alone. Alternatively, our inability to observe an increase in DA-D2R BP and expression levels in MPTP (non-exercise) mice may be due to a modest recovery of dopamine levels at the end of the study (82% dopamine depletion at 10 days versus 68% depletion at 42 days postlesion). However, this is unlikely as the MPTP plus exercise mice, which also displayed a small recovery of dopamine (not significantly different from the MPTP no exercise mice) had increase of DA-D2R BP.
The majority of DA-D1Rs and D2Rs are expressed on dendritic spines of MSNs with additional receptors expressed on cholinergic interneurons and terminals of glutamatergic and dopaminergic neurons originating from the cortex (or thalamus) and substantia nigra pars compacta, respectively.26 A major role of dopamine is to modulate corticostriatal or thalamostriatal glutamatergic neurotransmission at the MSN. Glutamatergic neurotransmission is enhanced through DA-D1Rs and diminished through DA-D2Rs.27–29 Under conditions of dopamine depletion, spines and synaptic connections are selectively lost on DA-D2R containing MSNs of the indirect pathway.30 This loss is accompanied with a hyperexcitability state within the MSNs due to increased glutamatergic corticostriatal neurotransmission.31–33 In animal models of PD, this increased glutamatergic drive correlates with parkinsonian-like motor behavior.34 Attenuation of this hyperexcitable state through the application of dopamine or its agonists leads to reversal of parkinsonian motor deficits.35,36 In light of these reports and our findings, we hypothesize that the benefits of high-intensity exercise are to enhance dopaminergic signaling through increased DA-D2R expression in the indirect pathway (but not the DA-D1R direct pathway) and to improve motor function through suppression of glutamatergic excitability.
The primary conclusion of our study is that exercise in the form of intensive treadmill running facilitates neuroplasticity through increased expression of striatal DA-D2Rs, a process most evident in the injured brain. Based on our findings, a noninvasive PET-imaging approach with [18F]fallypride can be used to investigate whether intensive treadmill exercise also leads to changes in the DA-D2R in individuals with PD. Our study highlights the value of preclinical research in animal models of dopamine depletion and the importance of translational research for providing both rationale and insight toward understanding imaging and exercise studies in individuals with PD.
Acknowledgments
This work was supported by a grant from the USC CTSI Full Pilot Grant Program, and generous funds from Parkinson’s Disease Foundation, Team Parkinson (Los Angeles), the Parkinson Alliance, the Whittier Parkinson’s Disease Education Group, NINDS RO1 NS44327-1, NIA (AG 21937) and U.S. Army NETRP W81XWH-04-1-0444. M.G.V. is a recipient of USC Neuroscience Graduate Program Merit Fellowship. We wish to thank Ryan Park and Dr. Peter Conti from the USC Small Animal Imaging Core for assistance with micro-PET imaging and Dr. Rex Moats from the Small Animal Imaging Research Core at the Saban Research Institute for assistance with the mouse MRI. We would like to thank Yi-Hsuan (Lilian) Lai for help with treadmill exercise, and Avery Abernathy for his expertise in HPLC analysis. We are thankful to the Friends of the USC Parkinson’s Disease Research Group including George and MaryLou Boone, Walter and Susan Doniger, and Roberto Gonzales for their generous support.
Footnotes
Potential conflict of interest: Nothing to report.
Note added in proof: This article was published online on 19 October 2010. An error was subsequently identified. This notice is included in the online and print versions to indicate that both have been corrected.
Financial Disclosures: USC Neuroscience Graduate Program Merit Fellowship (MV), NINDS RO1 NS44327-1 (MV, CW, JW, MJ and GP), USC CTSI Full Pilot Grant Program (QL, AN, MJ, GP).
Author Roles: All authors were instrumental in generating this manuscript. Research Project Conception: GP, BF, MJ, RL, JW. Execution of Project: MV, QL, AN, CW, MJ, GP. Data Collection, Processing, Statistical Analysis: MV, QL, BF, AN, RL, MJ, GP. Manuscript Preparation: MV, QL, BF, RL, JW, MJ, GP.
References
- 1.Bergen JL, Toole T, Elliott RGr, Wallace B, Robinson K, Maitland CG. Aerobic exercise intervention improves aerobic capacity and movement initiation in Parkinson’s disease patients. NeuroRehabilitation. 2002;17:16–168. [PubMed] [Google Scholar]
- 2.Comella CL, Stebbins GT, Brown-Toms N, Goetz CG. Physical therapy and Parkinson’s disease: a controlled clinical trial. Neurology. 1994;44(3 Part 1):376–378. doi: 10.1212/wnl.44.3_part_1.376. [DOI] [PubMed] [Google Scholar]
- 3.Schenkman M, Hall D, Kumar R, Kohrt WM. Endurance exercise training to improve economy of movement of people with Parkinson disease: three case reports. Phys Ther. 2008;88:63–76. doi: 10.2522/ptj.20060351. [DOI] [PubMed] [Google Scholar]
- 4.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci. 2009;10:1–14. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher BE, Petzinger GM, Nixon K, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77:378–390. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 6.Petzinger GM, Walsh JP, Akopian G, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27:5291–5300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang GJ, Volkow ND, Fowler JS, et al. PET studies of the effects of aerobic exercise on human striatal dopamine release. J Nucl Med. 2000;41:1352–1356. [PubMed] [Google Scholar]
- 8.Ginovart N, Farde L, Halldin C, Swahn CG. Effect of reserpine-induced depletion of synaptic dopamine on [11C]raclopride binding to D2-dopamine receptors in the monkey brain. Synapse. 1997;25:321–325. doi: 10.1002/(SICI)1098-2396(199704)25:4<321::AID-SYN2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee J, Christian BT, Narayanan TK, Shi B, Mantil J. Evaluation of dopamine D-2 receptor occupancy by clozapine, risperidone, and haloperidol in vivo in the rodent and nonhuman primate brain using 18F-fallypride. Neuropsychopharmacology. 2001;25:476–488. doi: 10.1016/S0893-133X(01)00251-2. [DOI] [PubMed] [Google Scholar]
- 10.Honer M, Bruhlmeier M, Missimer J, Schubiger AP, Ametamey SM. Dynamic imaging of striatal D2 receptors in mice using quad-HIDAC PET. J Nucl Med. 2004;45:464–470. [PubMed] [Google Scholar]
- 11.Falardeau P, Bedard PJ, Di Paolo T. Relation between brain do-pamine loss and D2 dopamine receptor density in MPTP monkeys. Neurosci Lett. 1988;86:225–229. doi: 10.1016/0304-3940(88)90575-7. [DOI] [PubMed] [Google Scholar]
- 12.Mukherjee J, Yang ZY, Brown T, et al. Preliminary assessment of extrastriatal dopamine D-2 receptor binding in the rodent and nonhuman primate brains using the high affinity radioligand, 18F-fallypride. Nucl Med Biol. 1999;26:519–527. doi: 10.1016/s0969-8051(99)00012-8. [DOI] [PubMed] [Google Scholar]
- 13.Christian BT, Narayanan TK, Shi B, Mukherjee J. Quantitation of striatal and extrastriatal D-2 dopamine receptors using PET imaging of [(18)F]fallypride in nonhuman primates. Synapse. 2000;38:71–79. doi: 10.1002/1098-2396(200010)38:1<71::AID-SYN8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Qi J, Leahy RM, Cherry SR, Chatziioannou A, Farquhar TH. High-resuulution 3D Bayesian image reconstruction using micro-PET small-animal scanner. Phys Med Biol. 1998;43:1001–1013. doi: 10.1088/0031-9155/43/4/027. [DOI] [PubMed] [Google Scholar]
- 15.Ichise M, Toyama H, Innis RB, Carson RE. Strategies to improve neuroreceptor parameter estimation by linear regression analysis. J Cereb Blood Flow Metab. 2002;22:1271–1281. doi: 10.1097/01.WCB.0000038000.34930.4E. [DOI] [PubMed] [Google Scholar]
- 16.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 17.Studholme C, Hill DL, Hawkes DJ. Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med Phys. 1997;24:25–35. doi: 10.1118/1.598130. [DOI] [PubMed] [Google Scholar]
- 18.Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- 19.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4(3 Part 1):153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. 2. New York: Academic Press; 2001. [Google Scholar]
- 21.Johnson MW, Chotiner JK, Watson JB. Isolation and characterization of synaptoneurosomes from single rat hippocampal slices. J Neurosci Methods. 1997;77:151–156. doi: 10.1016/s0165-0270(97)00120-9. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Thomas JR, Salazar W, Landers DM. What is missing in p <05? Effect size. Res Q Exerc Sport. 1991;62:344–348. doi: 10.1080/02701367.1991.10608733. [DOI] [PubMed] [Google Scholar]
- 24.Cropley VL, Innis RB, Nathan PJ, et al. Small effect of dopamine release and no effect of dopamine depletion on [(18)F]fallypride binding in healthy humans. Synapse. 2008;62:399–408. doi: 10.1002/syn.20506. [DOI] [PubMed] [Google Scholar]
- 25.Hurley MJ, Jenner P. What has been learnt from study of dopamine receptors in Parkinson’s disease? Pharmacol Ther. 2006;111:715–728. doi: 10.1016/j.pharmthera.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 26.Smith Y, Villalba R. Striatal and extrastriatal dopamine in the basal ganglia: An overview of its anatomical organization in normal and Parkinsonian brains. Mov Disord. 2008;23(Suppl 3):S534–S547. doi: 10.1002/mds.22027. [DOI] [PubMed] [Google Scholar]
- 27.Cepeda C, Buchwald NA, Levine MS. Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proc Natl Acad Sci USA. 1993;90:9576–9580. doi: 10.1073/pnas.90.20.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine MS, Altemus KL, Cepeda C, et al. Modulatory actions of dopamine on NMDA receptor-mediated responses are reduced in D1A-deficient mutant mice. J Neurosci. 1996;16:5870–5882. doi: 10.1523/JNEUROSCI.16-18-05870.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Umemiya M, Raymond LA. Dopaminergic modulation of excitatory postsynaptic currents in rat neostriatal neurons. J Neurophysiol. 1997;78:1248–1255. doi: 10.1152/jn.1997.78.3.1248. [DOI] [PubMed] [Google Scholar]
- 30.Day M, Wang Z, Ding J, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 31.VanLeeuwen JE, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci Res. 2010;88:650–668. doi: 10.1002/jnr.22216. [DOI] [PubMed] [Google Scholar]
- 32.Hernandez-Echeagaray E, Starling AJ, Cepeda C, Levine MS. Modulation of AMPA currents by D2 dopamine receptors in striatal medium-sized spiny neurons: are dendrites necessary? Eur J Neurosci. 2004;19:2455–2463. doi: 10.1111/j.0953-816X.2004.03344.x. [DOI] [PubMed] [Google Scholar]
- 33.Surmeier DJ, Ding J, Day M, Wang Z, Shen W. D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons. Trends Neurosci. 2007;30:228–235. doi: 10.1016/j.tins.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Calabresi P, Mercuri NB, Sancesario G, Bernardi G. Electrophysiology of dopamine-denervated striatal neurons. Implications for Parkinson’s disease. Brain. 1993;116 (Part 2):433–452. [PubMed] [Google Scholar]
- 35.Ballion B, Frenois F, Zold CL, Chetrit J, Murer MG, Gonon F. D2 receptor stimulation, but not D1, restores striatal equilibrium in a rat model of Parkinsonism. Neurobiol Dis. 2009;35:376–384. doi: 10.1016/j.nbd.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev. 1997;21:519–523. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]