Abstract
Autism spectrum disorders (ASD) represent a class of neurodevelopmental disorders characterized by impairments in social interaction, verbal and non-verbal communication, as well as restricted interests and repetitive behavior. This latter class of symptoms often includes features such as compulsive behaviors and resistance to change. The BTBR T+tf/J mouse strain has been used as an animal model to investigate the social communication and restricted interest features in ASD. Less is known about whether this mouse strain models cognitive flexibility deficits also observed in ASD. The present experiment investigated performance of BTBR T+tf/J and C57BL/6J on two different spatial reversal learning tests (100% accurate feedback and 80/20 probabilistic feedback), as well as marble burying and grooming behavior. BTBR T+tf/J and C57BL/6J mice exhibited similar performance on acquisition and reversal learning with 100% accurate feedback. BTBR T+tf/J mice were impaired in probabilistic reversal learning compared to that of C57BL/6J mice. BTBR T+tf/J mice also displayed increased stereotyped repetitive behaviors compared to that of C57BL/6J mice as shown by increased marble burying and grooming behavior. The present findings indicate that BTBR T+tf/J mice exhibit similar features related to “insistence on sameness” in ASD that include not only stereotyped repetitive behaviors, but also alterations in behavioral flexibility. Thus, BTBR T+tf/J mice can serve as a model to understand the neural mechanisms underlying alterations in behavioral flexibility, as well as to test potential treatments in alleviating these symptoms.
Keywords: BTBR T+ tf/J, Autism, Stereotypy, Reversal Learning, Mice, Memory
1. Introduction
Autism spectrum disorder (ASD) has a triad of symptoms that include deficits in social communication and interactions, impaired language development and presence of repetitive behaviors with restricted interests [10,54]. The diagnostic domain of restrictive and repetitive behaviors is commonly marked by motor stereotypies, e.g. rocking or hand flapping, and repetitively touching an object [25,28]. Furthermore, individuals with ASD display restricted interests for objects when there is an opportunity to freely explore them [28]. ASD subjects may also exhibit an insistence on sameness in cognitive tests that involve reversal learning or set-shifting [16,18,32]. These atypical behaviors are disruptive to daily functioning due to their interference of goal-directed behavior, interaction with environmental cues, and intermittently produce self-injurious actions [48]. To date, there is little known on the etiology underlying insistence on sameness behaviors. However, in recent years there has been increasing employment of animal models to understand the genetic and neurobiological changes that underlie particular symptom domains in ASD [38,42,62,70].
One mouse model that exhibits some of the behavioral symptoms observed in ASD is the BTBR T+ tf/J (BTBR) mouse [41,42,43,46,48]. This mouse strain exhibits certain social deficits comparable to that observed in ASD. For example, previous studies of social behavior have demonstrated that BTBR mice do not display a preference for a novel mouse over a novel object unlike other mouse strains, i.e. C57BL/6J mice [41,48,70]. Impairments are not limited to social interactions, because BTBR mice also exhibit restricted interests in exploration of olfactory stimuli [42] and display increased grooming compared to that of C57BL/6J mice [41,46,69]. Thus, BTBR mice exhibit social interaction deficits, restricted interests on exploratory tests and increased grooming behavior.
Although BTBR mice exhibit some behaviors comparable to that with insistence on sameness features in ASD, less is known about whether BTBR mice display another insistence on sameness feature – reversal learning deficits. This becomes important because, as described above, individuals with ASD can also show reversal learning or set-shifting deficits [16,18,32]. Past studies indicate that BTBR mice are not impaired on reversal learning compared to that of C57BL/6J mice [43,71]. These results would suggest that BTBR mice do not model this behavioral deficit observed in some ASD individuals.
To date, only reversal learning tests that provide 100% accurate feedback have been used with BTBR mice. In different clinical populations, reversal learning tests applying 100% accurate feedback are not always used, but instead probabilistic reinforcement paradigms are used to investigate cognitive flexibility [21,26,67]. This includes the study of cognitive flexibility in individuals with ASD [57]. In a probabilistic learning test, the ‘correct’ choice is reinforced on the majority of trials, e.g. 80%, and not reinforced on a minority of trials, e.g. 20%, in an unpredicted manner. Probabilistic learning tests are used, in part, because these tests are considered to be more ecologically relevant [14,64]. Further, probabilistic learning tests can have the advantage of determining whether a particular clinical population, mouse strain or drug manipulation is differentially affected by positive reinforcement or negative reinforcement when making a ‘correct’ choice. Thus, the use of a probabilistic reversal learning paradigm may be a preferable test to characterize behavioral flexibility performance in BTBR mice and use for translational studies related to ASD.
The objective of the present experiments was to characterize reversal learning performance in BTBR mice compared to that of C57BL/6J mice. C57BL/6J mice were used as a comparison group because this mouse is commonly used as a background strain in genetic studies and has been used in several experiments phenotyping the BTBR mouse [46,56,69]. Specifically, we examined acquisition and reversal learning performance in a spatial discrimination based on 100% accurate feedback and during a probabilistic learning procedure using a 80%/20% feedback contingency. In addition, to further characterize repetitive behaviors displayed in BTBR mice, both BTBR and C57BL/6J mice were tested for marble burying and grooming behavior.
2. Materials and methods
2.1. Animals
Male C57BL/6J and BTBR mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Mice were singly housed in plastic cages (28 cm wide × 17 cm long × 12 cm high) in humidity (30%) and temperature (22 ° C) controlled room with a 12-h light/dark cycle (lights on at 07:00 am). Ten to fourteen days after arrival behavioral testing procedures began. Animal care and use was in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and was approved by the Institutional Laboratory Animal Care and Use Committee at the University of Illinois at Chicago.
2.2 Apparatus
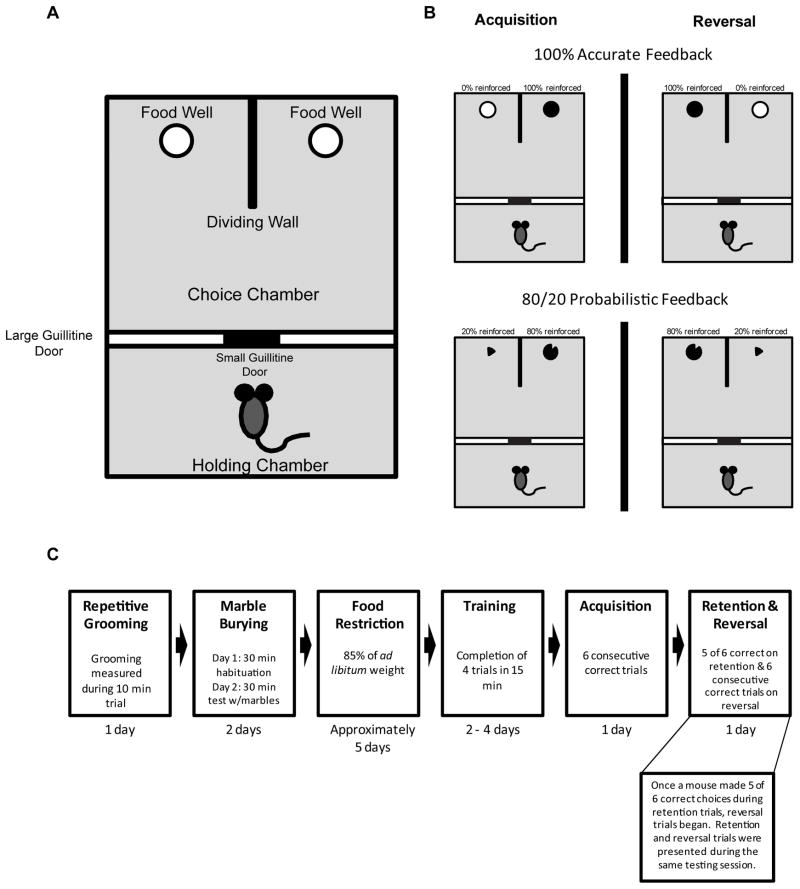
Spatial discrimination training and testing was conducted in a black acrylic rectangular-shaped maze (76 cm long × 50 cm wide × 30 cm high). The maze was divided in half by a plastic guillotine door (52 cm high × 49 cm wide) that extended the width of the maze (see Figure 1A). One half served as the start area and the other half served as the choice area. To ensure that mice started each trial from the same central area and not biased toward one side, a small plastic door (10 cm high × 5 cm wide) was located at the bottom, center of the guillotine door. A hook on the smaller door, located on the choice area side, allowed for easy opening and closing of the start door. In the choice area, an acrylic piece (30 cm long × 16 cm high) extended out from the back wall that divided the choice area into two equally-sized and distinct spatial locations. Each choice location had distinct visual cues attached to the back and side walls, including two-dimensional black and white shapes approximately 8 cm by 12 cm in size. Different three-dimensional objects (e.g. toy space ship or toy alien) were also mounted to the back wall of each spatial location approximately 15 cm above the floor. In each choice location a food well was centered and placed 3 cm away from the back wall. The current studies utilized a rectangular maze with high walls instead of a standard T-maze due to preliminary work with BTBR mice, in which mice escaped the maze area. Furthermore, the current apparatus was used because, unlike the T-maze and water maze tests, the rectangular maze minimized experimenter handling of a mouse and likely reduced any stress induced by handling.
Figure 1.
Acquisition and reversal learning of a spatial discrimination. A) Diagram of spatial reversal learning apparatus. B) In one study, C57BL/6J and BTBR mice were tested on acquisition and reversal learning of a spatial discrimination with 100% accurate feedback (top). In another study, C57BL/6J and BTBR mice were tested on acquisition and reversal learning of a spatial discrimination, but a 80/20 probabilistic reinforcement schedule was used (bottom). C) Order of behavioral testing completed by C57BL/6J and BTBR mice.
2.3. Spatial Discrimination Training
The training procedure was identical for the deterministic and probabilistic spatial discrimination test. One week prior to training, mice were food restricted to 85% of their ad lib feeding weight. After being stabilized at 85% of their ad lib body weight, mice were trained for 2 – 4 days before acquisition testing. Behavioral procedures were modified from past studies by Ragozzino and colleagues [11,51]. At the beginning of training, mice were first placed in the start area. The start door was opened allowing a mouse to freely navigate in the choice area and consume a ½ piece of Fruity Pebbles cereal (Post Foods, St. Louis, MO) from each food well (1.5 cm wide × 1.25 cm deep). Once the cereal pieces were consumed from both compartments, the guillotine door was immediately raised to allow a mouse to return to the start area. If a mouse did not navigate back to the start area within 5 sec after consuming both cereal pieces, the experimenter gently nudged the mouse back toward the start area. By the last session of training all mice returned to the start area within 3 – 5 sec after completing a trial without having to be handled by the experimenter. After returning to the start area the guillotine door was lowered and a mouse was restricted from entering the choice area. Subsequently, the food wells were re-baited and the start door opened to begin a new trial. This procedure continued until 15 min had elapsed. Once a mouse completed 4 – 6 trials in 15 min for 2 consecutive days, acquisition testing began the following day.
2.4 Acquisition and Reversal Learning Testing of a Spatial Discrimination (100% Accurate Feedback)
In this experiment there were 8 BTBR and 8 C57BL/6J mice tested. In the acquisition test, a mouse was required to always enter the same spatial location in order to retrieve a cereal reinforcement. Previously, we ran probes trials in the rectangular maze to determine whether rodents were using spatial cues or egocentric response cues to guide their choice. Based on probe trials in which the choice area and the start area were switched, as well as probe trials in which cues were switched between the two choice locations, the findings indicate that rodents were using visuospatial cues to guide their choice pattern (Minneti & Ragozzino, unpublished observations). At the beginning of testing a mouse was placed in the start area. The start door was opened and a mouse could choose to enter one of two spatial locations. Only one of the two foodwells was baited with a ½ piece of cereal in each trial. If a mouse chose the correct location, it could eat the cereal piece, the guillotine door was raised and subsequently lowered after a mouse returned to the start area. If a mouse chose the incorrect spatial location, it was allowed to navigate to the unbaited foodwell in that spatial location. Following navigation to the unbaited foodwell, the baited foodwell was temporarily removed from the maze and the guillotine door was raised allowing a mouse to return to the start area. For incorrect choices, the baited foodwell was temporarily removed to prevent a mouse from quickly navigating over to the correct spatial location and obtain a cereal reinforcer after making an incorrect choice. Between trials, the choice area was cleaned with 2% ammonium chloride solution to minimize use of odor cues, as used in previous experiments [51,53]. Acquisition criterion was achieved when a mouse completed 6 consecutive correct trials. This is the same learning criterion used in similar discrimination and reversal learning tests [58,59,60].
Reversal learning was conducted the day after acquisition testing. Just prior to the reversal learning test, each mouse received a retention test. This was conducted to determine whether there was a difference in retaining the initially learned spatial discrimination between mouse strains. In addition, the retention test was conducted to have mice began at a similar level of the originally learned discrimination just prior to reversal learning. In the retention test, a mouse was reinforced for choosing the spatial location that was correct in acquisition. Criterion was achieved when a mouse successfully chose the correct spatial location on 5 out of 6 trials, similar to the learning criterion in the acquisition phase. Immediately after achieving retention criterion, the reversal learning session began. All aspects of the reversal learning test were similar to those in the acquisition phase, except that the opposite spatial location was reinforced as in acquisition. The criterion for the reversal learning test was 6 consecutive correct trials.
As in previous experiments, [2,12,23,24,40], an analysis of errors in reversal learning was conducted to determine whether there was any difference between mouse strains in the ability to initially inhibit the previously relevant choice pattern (perseverative errors) and/or the ability to maintain the new choice pattern after being initially selected (regressive errors). Perseverative errors were counted when a mouse continued to choose the previously correct spatial location in reversal learning. If a mouse chose the previously correct spatial location on the first trial of reversal learning this was not counted as a perseverative error, but served as initial negative feedback that this choice was no longer correct. If a mouse continued to choose the previously correct spatial location on subsequent trials these were counted as perseverative errors until a mouse chose the new correct spatial location the first time. After selecting the correct spatial location for the first time, all subsequent entries into the previously reinforced spatial location were scored as regressive errors. For example, a mouse might have initially learned to choose spatial location A and then during reversal learning had to learn to choose spatial location B. The following represents an example of the spatial location chosen on consecutive trials during reversal learning: A, A, A, B, A, B, A, A, B, B, B, B, B, B. In this example, a mouse would have two perseverative errors (bold) and three regressive errors (italics).
2.5. Acquisition and Reversal Learning Testing of a Spatial Discrimination (80/20 Probabilistic Learning)
The goal of Experiment 2 was to determine whether spatial discrimination performance differed between BTBR and C57BL/6J mice using a probabilistic learning procedure. A separate group of BTBR mice (n = 10) and C57BL/6J mice (n = 11) were used from those in Experiment 1. Food restriction and training were the same as described in section 2.3. A similar test procedure was used as in the 100% accurate feedback spatial discrimination except that in acquisition one choice location was designated as the ‘correct’ spatial location and contained a ½ piece of cereal on 80% of trials. On the other 20% of trials, the ‘incorrect’ location was baited with a ½ piece of cereal. This 80/20 probabilistic learning procedure was used in the acquisition, retention and reversal learning phases of testing. The first two trials of each test phase always contained a food reinforcement in the ‘correct’ arm. On any individual trial, only one choice location was baited. Learning criterion was achieved when a mouse chose the “correct” location on 6 consecutive trials.
The day after acquisition testing, each mouse received a retention test as described above. The retention criterion involved choosing the “correct” location on 5 out of 6 consecutive trials. Subsequently, each mouse received the reversal learning test. In reversal learning, the previously ‘incorrect’ location on acquisition was now the ‘correct’ location during reversal learning. The first two trials of the test always contained a food reinforcement in the ‘correct’ arm. Criterion was obtained when a mouse chose the new “correct” location for 6 consecutive trials.
As described in section 2.4, perseverative and regressive errors were calculated in the reversal learning session. In addition, an analysis was conducted to determine if BTBR mice compared to C57BL/6J mice had differential sensitivity to reinforcement or no reinforcement on ‘correct’ trials as carried out in a past study [3]. A similar analysis was also conducted for ‘incorrect’ trials. To determine this, trials were analyzed based on the outcome (reinforcement or no reinforcement) of each previous trial and shown as a ratio. For correct trials, win-stay ratios were determined by the number of times a subject received reinforcement in the ‘correct’ compartment and then returned to the same compartment as that chosen on the previous trial, divided by the total number of reinforced trials for only the ‘correct’ trials. The lose-shift ratio was determined by the number of times a subject reversed its choice after receiving no reinforcement in the ‘correct’ spatial location on the previous trial, divided by the total non-reinforced trials for ‘correct’ trials. For ‘incorrect’ trials, win-stay ratios were determined by the number of times a subject received reinforcement in the ‘incorrect’ spatial location and then returned to the same spatial location as that chosen on the previous trial, divided by the total number of reinforced trials for ‘incorrect’ trials. The lose-shift ratio was determined by the number of times a subject reversed its choice after receiving no reinforcement in the ‘incorrect’ spatial location on the previous trial, divided by the total non-reinforced trials a rat chose the ‘incorrect’ spatial location.
2.6 Repetitive Grooming Behavior
With the exception of three C57BL/6J and two BTBR mice, all subjects tested in probabilistic reversal learning were also tested for repetitive self-grooming and marble burying described below (see Figure 1C). The procedure used to measure spontaneous grooming behavior was modified from McFarlane et al. [41]. Mice were individually placed in a clear plastic cage (28 cm wide × 17 cm long × 12 cm high) for 20 min. The plastic cage was placed in a room separate from the room in which mice were housed. Subjects were allowed to freely explore the cage for the entirety of the test. The first 10 min served as a habituation period. During the second 10 min of testing a trained observer recorded cumulative time spent grooming all body regions. The trained observer sat approximately 1.6 m from the test cage. After each mouse was tested, the cage was thoroughly cleaned with a 2% ammonium chloride solution.
2.7 Marble Burying
The day after the self-grooming test, mice were habituated to the plastic container used for the marble burying test. The marble burying test procedure was modified from Njung’e and Handley [44], as well as Thomas et al. [63]. The day after the grooming test, mice were individually placed in a plastic container (46 cm long by 24 cm wide by 21 cm deep) with 3 cm of clean woodchip bedding (Northeastern Products, NY). The plastic container was placed in a room used for behavioral testing. Mice were allowed to freely explore a container for 30 min undisturbed. This served to habituate mice to the chamber. Twenty-four hours later, 20 glass marbles (1.5 cm in diameter) were arranged in 5 rows of 4. The marbles were placed on top of 3 cm of clean woodchip bedding. A template was used to ensure that there was a consistent positioning of marbles. Each mouse was placed in the testing container and a wire lid was placed on top. Mice were allowed to explore the container and marbles for 30 min. After 30 min, each mouse was removed from the testing container and returned to their home cage. Marbles were considered buried if ≥ 2/3 of the surface area was covered in woodchip bedding. Latency to begin burying and number of buried marbles were recorded. Between testing, marbles were thoroughly cleaned and new bedding was used for each mouse.
2.7. Statistical Analysis
Separate unpaired t-tests were conducted to compare strain differences on trials to criterion for the acquisition phase, retention phase and reversal learning phase in the spatial discrimination tests. For the reversal learning results, separate unpaired t-tests were conducted to determine if there was a strain difference for perseverative and regressive errors, as well as win-stay and lose-shift probabilities. A Benajmini-Hochberg correction was applied when there was significance with multiple outcome measures in reversal learning [4,5]. An unpaired t-test was used to determine differences between strains in cumulative time grooming. For the marble burying test, unpaired t-tests were conducted on total marbles buried and latency to begin burying for both BTBR and C57BL/6J mice. The statistical significance level was set at p < 0.05.
3. Results
3.1. Acquisition and Reversal Learning Testing of a Spatial Discrimination (100% Accurate Feedback)
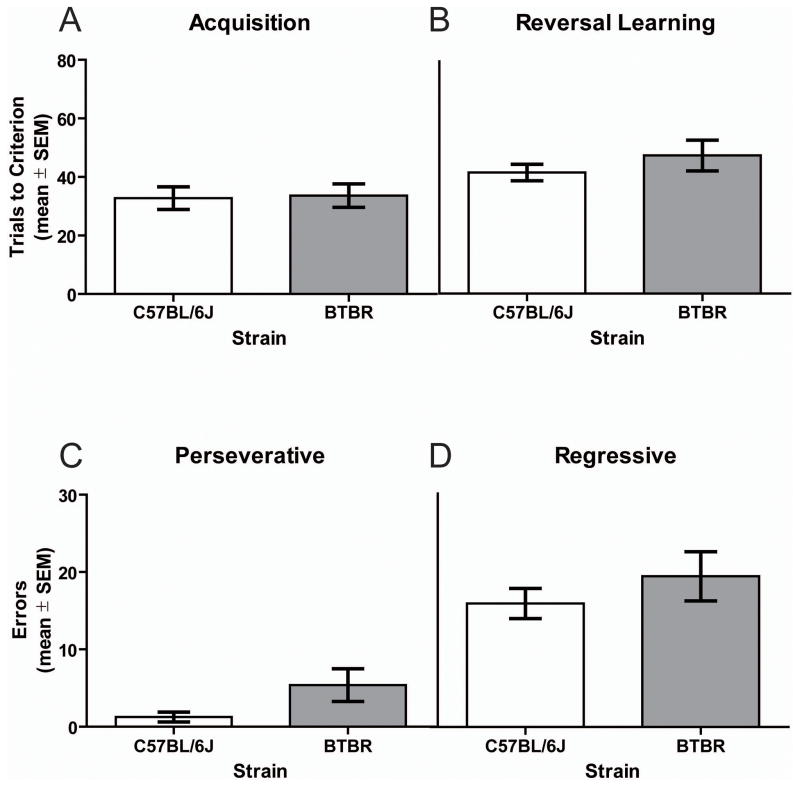
As shown in Figure 2, the BTBR and C57BL/6J mice required a comparable number of trials to reach criterion in acquisition of the spatial discrimination, t(14) = 0.16, ns. Retention performance of the spatial discrimination was not significantly different in BTBR (mean = 9.5 ± 2.1) and C57BL/6J (mean = 6.4 ± 0.3) mice, t(14) = 1.49, ns. As observed in acquisition, both mouse strains achieved reversal learning criterion at a similar rate, t(14) = 0.98, ns.
Figure 2.
Acquisition and reversal learning of a spatial discrimination (100% accurate feedback). A) Mean (± SEM) trials to criterion in acquisition. BTBR (n = 8) and C57BL/6J (n = 8) mice performance did not differ on trials to criterion. B) Mean (± SEM) trials to criterion on reversal learning. BTBR and C57BL/6J mice did not differ on trials to criterion. C) Mean (± SEM) perseverative errors committed during reversal learning. BTBR and C57BL/6J mice did not differ on perseverative errors. D) Mean (± SEM) regressive errors during reversal learning. BTBR and C57BL/6J mice committed a similar number of regressive errors.
An analysis of the errors committed during reversal learning indicated that the two strains did not differ in initially inhibiting the previously correct choice or maintaining the new correct choice after being initially selected (see Figures 2C and 2D). Specifically, the difference in perseverative errors between the mouse strains was not significant, t(14) = 1.87, ns. Similarly, C57BL/6J and BTBR mice committed a comparable number of regressive errors during reversal learning, t(14) = 0.95, ns.
3.2. Acquisition and Reversal Learning Testing of a Spatial Discrimination (80/20 Probabilistic Learning)
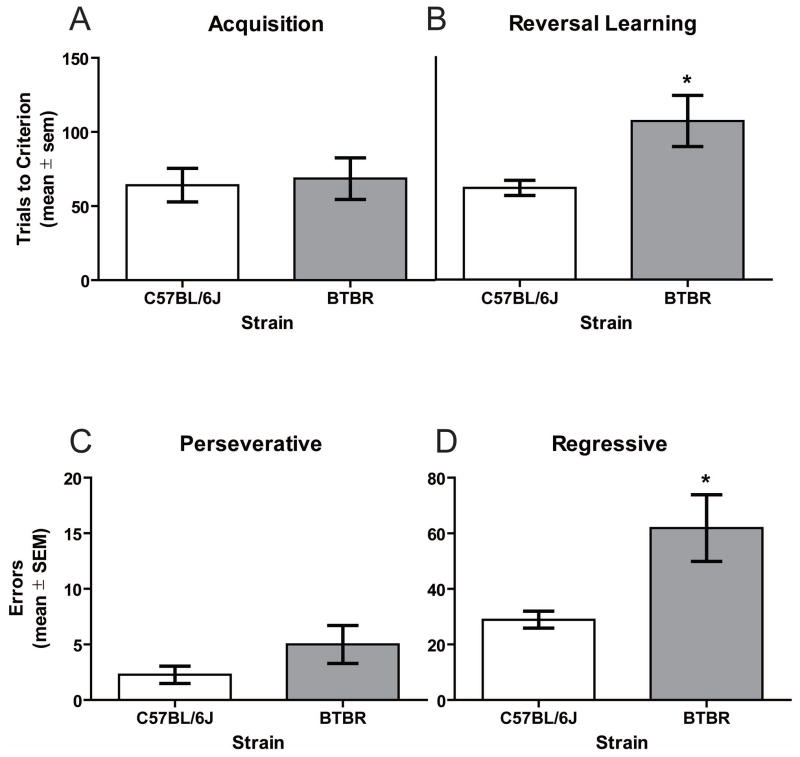
As shown in Figure 3, BTBR and C57BL/6J mice required a comparable number of trials to criterion for acquisition when applying probabilistic reinforcement, t(19) = 0.25, ns. In the retention test, BTBR mice (mean = 13.7 ± 5.6) and C57BL/6J mice (mean = 8.5 ± 1.1) achieved retention criterion at a rate that was not significantly different, t(19) = 0.97, ns. In contrast, BTBR mice required significantly more trials to reach reversal learning criterion compared to C57BL/6J mice, t(19) = 2.62, p < .05.
Figure 3.
Acquisition and reversal learning of a spatial discrimination (80/20 probabilistic learning procedure). A) Mean (± SEM) trials to criterion on acquisition. BTBR (n = 10) and C57BL/6J (n = 11) mice performance did not differ on trials to criterion. B) Mean (± SEM) trials to criterion on reversal learning. BTBR mice required more trials to reach criterion compared to that of C57BL/6J mice. C) Mean (± SEM) perseverative errors during reversal learning. C57BL/6J and BTBR mice did not differ on the number of perseverative errors. D) Mean (± SEM) regressive errors during reversal learning. BTBR mice committed more regressive errors compared to that of C57BL/6J mice during reversal learning; * p < .05 vs. C57BL/6J.
An analysis of the error pattern during probabilistic reversal learning showed that the difference in the number of perseverative errors was not significantly different between BTBR and C57BL/6J mice, t(19) = 1.50, ns. However, BTBR mice did commit significantly more regressive errors than that of C57BL/6J mice, t(19) = 2.79, p = .012 (see Figure 3D).
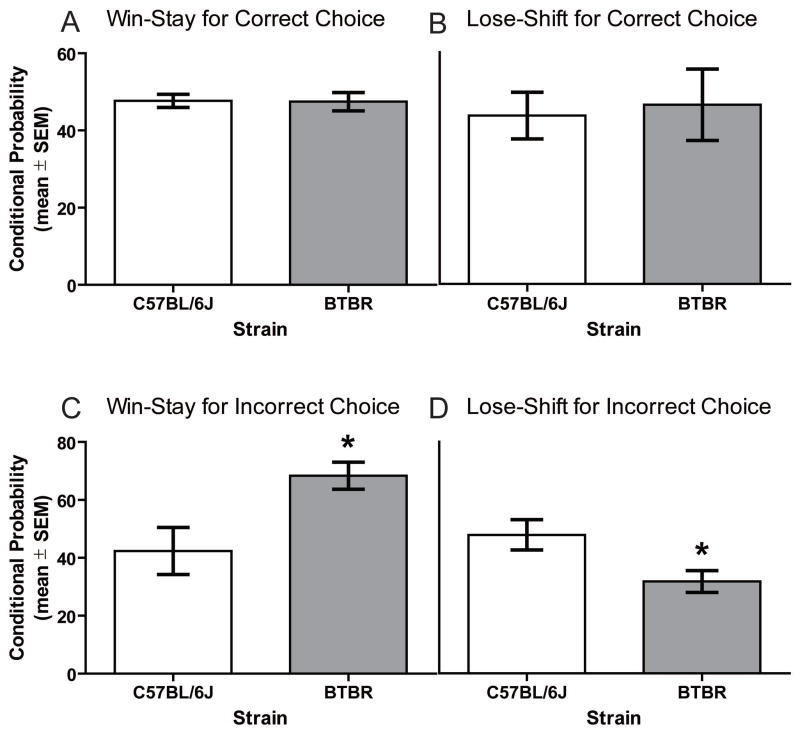
To determine whether the mouse strains differed in responsiveness after receiving a reinforcement or no reinforcement for ‘correct’ and ‘incorrect’ trials, win-stay and lose-shift probabilities were analyzed (see Figure 4). The mean number of trials in which reinforcement was received on a ‘correct’ trial was 31.8 ± 5.3 for the BTBR group and 23.9 ± 2.2 for the C57BL/6J group. The difference in reinforcements for ‘correct’ trials between the strains was not significantly, t(19) = 0.14, ns. The probability for win-stay and lose-shift was approximately 50% in both mouse strains. The difference in win-stay performance was not significant between the mouse strains, t(19) = 0.08, ns. The mean number of trials in which no reinforcement was received on a ‘correct’ trial was 8.1 ± 2.1 for the BTBR group and 5.7 ± 0.6 for the C57BL/6J group. This difference between the strains was not significant, t(19) = 0.4, ns. The difference in lose-shift probabilities between the groups was not significant, t(19) = 0.26, ns.
Figure 4.
Win-stay and lose shift probabilities for correct and incorrect choices during probabilistic reversal learning. A) Mean (± SEM) win-stay probability for correct choices. BTBR (n = 10) mice had a similar win-stay probability for the correct choice compared to that of C57BL/6J (n = 11) mice. B) Mean (± SEM) lose-shift probability for correct choices. BTBR and C57BL/6J mice did not differ in lose-shift probability for correct choices. C) Mean (± SEM) win-stay probability for incorrect choices. BTBR mice had a significantly greater probability of staying with the incorrect choice following obtainment of a reinforcement on an incorrect trial compared to that of C57BL/6J mice, * p < .05 vs. C57BL/6J. D) Mean (± SEM) lose-shift probability for incorrect choices. BTBR mice were significantly less likely to shift to the correct location following no reinforcement on an incorrect choice compared to that of C57BL/6J mice, * p < .05 vs. C57BL/6J.
The analysis of win-stay and lose-shift probabilities for ‘incorrect’ trials revealed a different pattern of findings than for ‘correct’ trials. First, the mean number of trials in which a reinforcement was received on a ‘incorrect’ trial was 14.3 ± 2.3 for BTBR mice and 7.1 ± 1.0 for C57BL/6J mice, t(19) = 3.0, p < .05. BTBR mice exhibited a significantly greater probability of returning to the ‘incorrect’ location after being reinforced in this location (win-stay), t(19) = 2.70, p = .014. The mean number of trials in which no reinforcement was received on a ‘incorrect’ trial was 53.7 ± 10.2 for BTBR mice and 25.2 ± 2.6 for C57BL/6J mice, t(19) = 2.80, p < .05 BTBR mice were significantly less likely to shift to the ‘correct’ location following no reinforcement on an ‘incorrect’ choice compared to that of C57BL/6J mice (lose-shift), t(19) = 2.45, p = .024.
Because multiple comparisons of the choice patterns in probabilistic reversal learning were significant and to control for a false discovery rate, a Benjamini-Hochberg correction was conducted on regressive errors, win-stay probabilities and lose-shift probabilities for incorrect choices. The analysis revealed there was still a significant effect for regressive errors (p < 0.017), win-stay probabilities for incorrect choices (p < 0.03) and lose-shift probabilities for incorrect choices (p < 0.05).
3.3. Repetitive Grooming
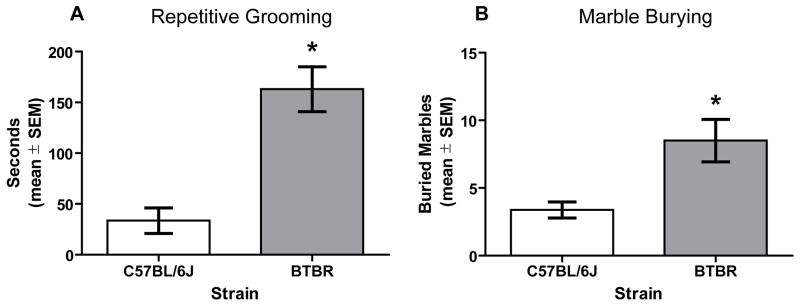
The results for repetitive grooming are shown in Figure 5A. During the 10 min test, BTBR mice spent over 180 sec grooming, while C57BL/6J mice spent approximately 30 sec grooming. The difference in time grooming between the mouse strains was significant, t(14) = 5.09, p < .05.
Figure 5.
Comparison of marble burying and repetitive grooming in BTBR (n = 8) and C57BL/6J (n = 8) mice. A) Mean (± SEM) seconds spent grooming during 10 min test session. BTBR mice spent more time grooming compared to C57BL/6J mice. B) Mean (± SEM) marbles buried during the 30 min testing session. BTBR mice buried more marbles compared to C57BL/6J mice. * p < .05 vs. C57BL/6J.
3.4. Marble Burying
BTBR mice buried significantly more marbles compared to that of C57BL/6J mice, t(14) = 3.05, p < .05 (Figure 5B). In contrast, the latency to initiate burying a marble was comparable between the BTBR group (mean = 86.8 ± 7.8 sec) and C57BL/6J group (mean = 91.4 ± 9.9 sec), t(14) = 0.37, ns.
4. Discussion
The present experiments investigated spatial reversal learning in BTBR and C57BL/6J mice using two different reinforcement contingencies. In the spatial discrimination test with 100% accurate feedback, BTBR mice performed similarly to C57BL/6J mice in the acquisition, retention, and reversal learning phases. This is comparable to previous studies showing that BTBR mice are not impaired on reversal learning tests with 100% accurate feedback compared to that of other inbred strains of mice, i.e. C57BL/6J [43]. Further, BTBR and C57BL/6J mice had comparable performance on the acquisition of a spatial discrimination when using a probabilistic reinforcement procedure. However, BTBR mice were impaired on probabilistic reversal learning compared to that of C57BL/6J mice. Thus, when conditions involved an unpredicted and occasional lack of reinforcement for a ‘correct’ choice along with an occasional reinforcement on ‘incorrect’ trials, BTBR mice did not reverse choice patterns as quickly as C57BL/6J mice.
In the probabilistic reversal learning test, BTBR mice showed a selective increase in regressive errors. Thus, BTBR mice could initially inhibit the previously relevant choice pattern similar to that of C57BL/6J mice, but after initially choosing the new correct spatial location, BTBR mice more often reverted back to choosing the previously correct spatial location. Therefore, BTBR mice were not able to maintain the new choice pattern as efficiently as C57BL/6J mice in probabilistic reversal learning. This behavioral pattern has been reported in ASD individuals who show deficits in maintaining a set in various cognitive flexibility tests [16,33]. Therefore, BTBR mice may not only show alterations in cognitive flexibility similar to that found in individuals with ASD, but similar processes, e.g. maintenance of a strategy, may be affected that underlie the cognitive flexibility impairments in both individuals with ASD and BTBR mice.
Several experiments in rodents have examined the neural circuitry that supports the process for initially shifting away from a previous choice pattern and the process for maintaining or reliably executing a choice pattern after initially selecting it [12,15,24,40,64]. In particular, we previously found that the dorsomedial striatum and parafascicular thalamic nucleus, two brain regions that are interconnected [35], are important for maintaining a choice pattern when conditions require a change in strategies [11,50]. Furthermore, orbitofrontal cortex lesions or inactivation often lead to perserverative responding in reversal learning [15,34]. However, under certain conditions, i.e. increased level of difficulty, the orbitofrontal cortex is important for maintaining a newly selected response pattern during reversal learning [34]. The orbitofrontal cortex projects to the dorsomedial striatum [7] and receives input from intralaminar nuclei that include the parafascicular thalamic nucleus [8]. Therefore, these brain areas may represent a frontal-basal ganglia-thalamic circuit that enables maintenance of a response pattern when conditions require behavioral flexibility. This raises the possibility that the increase in regressive errors observed in BTBR mice compared to that of C57BL/6J mice, may reflect altered functioning in this neural circuit that reduces behavioral flexibility. If this is the case, this altered neural network would be comparable to anatomical and functional imaging findings in ASD that indicate alterations in frontal cortex – basal ganglia – thalamic circuitry [8,14,22,61].
In addition to examination of perseverative and regressive errors in reversal learning, an analysis was also carried out on trials following a reward (win-stay) or no reward (lose-shift) for both ‘correct’ and ‘incorrect’ trials. Performance did not differ between the mouse strains for either measure on ‘correct’ trials. However, when BTBR mice chose the ‘incorrect’ spatial location and received a reward, they showed a greater preference for subsequently visiting that ‘incorrect’ spatial location. Thus, BTBR mice showed a greater sensitivity to reward on a choice that was previously correct leading these mice to revert back to the previously correct choice pattern. Moreover, BTBR mice had a decreased lose-shift probability for ‘incorrect’ trials compared to that of C57BL/6J mice. This pattern also indicates that BTBR mice exhibited a greater preference for the previously ‘correct’ choice pattern even though it was not reinforced on the majority of trials. Taken together, the findings suggest that BTBR mice were not differentially sensitive to reinforcement or no reinforcement, but more generally showed a greater preference for the previously ‘correct’ choice during probabilistic reversal learning.
BTBR mice may have exhibited a reversal learning deficit with the probabilistic reinforcement procedure not because of the decrease in accurate feedback, but more generally because the probabilistic learning test had an increased level of difficulty compared to reversal learning with 100% accurate feedback. Thus, simply increasing the level of difficulty may lead to behavioral flexibility deficits in BTBR mice, as opposed to a specific alteration in the reinforcement probabilities.
BTBR mice not only exhibited alterations in probabilistic reversal learning, but also displayed increased repetitive behaviors. Consistent with previous studies, BTBR mice demonstrated an increase in marble burying and grooming compared to C57BL/6J mice [30,41,46,55,56,69]. Marble burying has been traditionally administered as a test of anxiety; however, studies have shown that marble burying is indicative of repetitive behavior or motor stereotypy [63]. Because mice were exposed to the chamber 24 h before testing without the presence of marbles, the addition of marbles may have led to increased anxiety and therefore an increase in marble burying. However, Thomas and colleagues [63] demonstrated that the amount of marbles buried in this test is not correlated with anxiety scores found in the light-dark exploration test or with pre-exposure to the marbles. Furthermore, BTBR mice do not exhibit increased anxiety when tested in the elevated-plus maze, a test that is commonly used to measure anxiety in rodents [6,43,56]. Thus, the increase in marble burying observed in BTBR mice likely represents a form of increased motor stereotypy as opposed to an increase in anxiety.
The difference in total marbles buried between the two mouse strains may be explained by the relatively low number of marbles buried by C57BL/6J mice compared to previous studies [29,39,63,65]. In previous studies, C57BL/6J mice tend to bury 8 – 12 marbles during a 30 min session, about two times as many marbles as observed here. Unlike these studies, the current experiment pre-exposed mice to the testing apparatus for 30 min undisturbed, 24 hr prior to testing. This pre-exposure may explain the number of marbles buried in C57BL/6J mice in the present experiment compared to past studies. In addition, other studies used a burying criterion of 1/2 buried to be counted while in the present study, a marble had to be at least 2/3 buried for it to be counted. Thus, the differences in the amount a marble had to be buried may also explain, in certain cases, the differences on this test for C57BL/6J mice. Despite the lower number of marbles buried by C57BL/6J mice compared to that of past studies, we observed that BTBR mice buried a comparable number of marbles as reported in a recent study by Gould et al. [30]. Thus, using the present experimental protocol for marble-burying behavior, BTBR mice displayed significantly greater marble burying compared to that of C57BL/6J mice.
The present results showed that BTBR mice also had increased grooming behavior compared to C57BL/6J mice as demonstrated previously [55]. Different treatments have been examined to determine whether grooming behavior in BTBR mice could be attenuated and thus be effective in altering repetitive behaviors in ASD. For example, treatment with the atypical antipsychotic, risperidone decreases repetitive grooming in the BTBR mouse [55]. However, grooming was only reduced at doses that that had sedative effects. There has also been interest in the use of selective serotonin reuptake inhibitors to treat certain repetitive behaviors in ASD [31]. Although the effects of a SSRI on grooming behavior in BTBR mice are unknown, SSRI treatment has been shown to reduce marble burying in BTBR mice [19,30]. Furthermore, SSRI treatment decreases repetitive grooming behavior in SAPAP3 knock-out mice, a strain that exhibits high rates of compulsive grooming [68]. However, to date, unknown is whether SSRI treatment only affects repetitive motor behaviors, but also alleviates other repetitive behaviors or restricted interests in BTBR mice.
Because BTBR mice exhibit motor stereotypies, as well as behavioral flexibility impairments compared to C57BL/6J mice, the BTBR mouse strain may serve as a useful animal model of autism in understanding the neural mechanisms underlying repetitive behaviors and for testing potential pharmacological treatments to alleviate these behaviors. One biochemical alteration consistently observed in ASD is elevated serotonin in blood platelets [1,36]. This elevated serotonin may result from altered serotonin transporter function [12,17]. Related to repetitive behaviors, the long allele form of 5-HTTLPR (serotonin-transporter-linked polymorphic region) is associated with higher serotonin transporter activity [27,37] and appears to be over-transmitted in some ASD patients which is also associated with increased aggression and repetitive behaviors in the disorder [12,20]. This has been the rationale for treating repetitive behaviors in ASD with a SSRI [31,45,49]. At present, unknown is whether a similar treatment would also be effective in reducing the reversal learning alterations observed in BTBR mice. Thus, future studies can determine the extent to which SSRI treatment may reduce ‘insistence on sameness’ behaviors in BTBR mice.
4.1. Conclusions
The present experiments demonstrate for the first time that BTBR mice exhibit reversal learning impairments compared to that of C57BL/6J mice, but only when a probabilistic learning procedure is employed. The reversal learning deficit in BTBR mice results from an impairment in maintaining a new choice pattern after being initially selected. Taken together with the alterations in repetitive motor behaviors, the findings indicate that BTBR mice show several repetitive behaviors that make BTBR mice a useful model to study the mechanisms underlying these behavioral features and test potential treatments to alleviate these deficits in ASD.
Acknowledgments
This research was supported by NIH grant P50 HD055751 (JAS, MER).
References
- 1.Anderson GM, Freedman DX, Cohen DJ, Volkmar FR, Hoder L, McPhedran P, Minderaa RB, Hansen CR, Young G. Whole blood serotonin in autistic and normal subjects. J Child Psychol Psychiatry. 1987;26:885–900. doi: 10.1111/j.1469-7610.1987.tb00677.x. [DOI] [PubMed] [Google Scholar]
- 2.Baker PM, Thompson JL, Sweeney JA, Ragozzino ME. Differential effects of 5-HT(2A) and 5-HT(2C) receptor blockade on strategy-switching. Behav Brain Res. 2011;219:123–31. doi: 10.1016/j.bbr.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, Dalley JW, Robbins TW. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J Roy Statistical Society. 1995;57:289–300. [Google Scholar]
- 5.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
- 6.Benno R, Smirnova Y, Vera S, Liggett A, Schanz N. Exaggerated responses to stress in the BTBR T+tf/J mouse: An unusual behavioral phenotype. Behav Brain Res. 2009;197:462–65. doi: 10.1016/j.bbr.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 7.Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–47. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- 8.Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- 9.Bernardi S, Anagnostou E, Shen J, Kolevzon A, Buxbaum JD, Hollander E, Hof PR, Fan J. In vivo 1H-magnetic resonance spectroscopy study of the attentional networks in autism. Brain Res. 2011;22:198–205. doi: 10.1016/j.brainres.2010.12.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bodfish JW, Symons FJ, Parker DE, Lewis MH. Varieties of repetitive behavior in autism: comparisons to mental retardation. J Autism Dev Disord. 2000;30:237–43. doi: 10.1023/a:1005596502855. [DOI] [PubMed] [Google Scholar]
- 11.Brown HD, Baker PM, Ragozzino ME. The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats. J Neurosci. 2010;30:14390–8. doi: 10.1523/JNEUROSCI.2167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brune CW, Kim S, Salt J, Leventhal BL, Lord C, Cook EH. 5-HTTLPR Genotype-specific phenotype in children and adolescents with autism. Am J Psychiatry. 2006;163:2148–56. doi: 10.1176/ajp.2006.163.12.2148. [DOI] [PubMed] [Google Scholar]
- 13.Budhani S, Marsh AA, Pine DS, Blair RJ. Neural correlates of response reversal: considering acquisition. Neuroimage. 2007;34:1754–65. doi: 10.1016/j.neuroimage.2006.08.060. [DOI] [PubMed] [Google Scholar]
- 14.Cheung C, Yu K, Fung G, Leung M, Wong C, Li Q, Sham P, Chua S, McAlonan G. Autistic disorders and schizophrenia: related or remote? An anatomical likelihood estimation. PLoS One. 5:12233. doi: 10.1371/journal.pone.0012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–80. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coldren JT, Halloran C. Spatial reversal as a measure of executive functioning in children with autism. J Genet Psychol. 2003;164:29–41. doi: 10.1080/00221320309597501. [DOI] [PubMed] [Google Scholar]
- 17.Cook EH, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL. Evidence of linkage between the serotonin transporter and autistic disorder. Mol Psychiatry. 1997;2:247–50. doi: 10.1038/sj.mp.4000266. [DOI] [PubMed] [Google Scholar]
- 18.Corbett BA, Constantine LJ, Hendren R, Rocke D, Ozonoff S. Examining executive functioning in children with autism spectrum disorder, attention deficit hyperactivity disorder and typical development. Psychiatry Res. 2009;166:210–22. doi: 10.1016/j.psychres.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crowley JJ, Blendy JA, Lucki I. Strain-dependent antidepressant-like effects of citalopram in the mouse tail suspension test. Psychopharmacology (Berl) 2005;183:257 –264. doi: 10.1007/s00213-005-0166-5. [DOI] [PubMed] [Google Scholar]
- 20.Devlin B, Cook EH, Jr, Coon H, Dawson G, Grigorenko EL, McMahon W, Minshew N, Pauls D, Smith M, Spence MA, Rodier PM, Stodgell C, Schellenberg GD. Autism and the serotonin transporter: the long and short of it. Mol Psychiatry. 2005;10:1110–6. doi: 10.1038/sj.mp.4001724. [DOI] [PubMed] [Google Scholar]
- 21.Dickstein DP, Finger EC, Brotman MA, Rich BA, Pine DS, Blair JR, Leibenluft E. Impaired probabilistic reversal learning in youths with mood and anxiety disorders. Psychol Med. 2010;40:1089–100. doi: 10.1017/S0033291709991462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DiMartino A, Ross K, Uddin LQ, Sklar AB, Castellanos FX, Milham MP. Functional brain correlates of social and nonsocial processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biol Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Floresco SB, Ghods-Sharifi S, Vexelman C, Magyar O. Dissociable roles for the nucleus accumbens core and shell in regulating set shifting. J Neurosci. 2006;26:2449–57. doi: 10.1523/JNEUROSCI.4431-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Floresco SB, Magyar O, Ghods-Sharifi S, Vexelman C, Tse MTL. Multiple dopamine receptor subtypes in the medial prefrontal cortex of the rat regulate set-shifting. Neuropsychopharmacology. 2006;31:297–309. doi: 10.1038/sj.npp.1300825. [DOI] [PubMed] [Google Scholar]
- 25.Fombonne E. Epidemiology of Pervasive Developmental Disorders. Pediatri Res. 2009;65:591–98. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- 26.Freyer T, Kloppel S, Tuscher O, Kordon A, Zurowski B, Kuelz AK, Speck O, Glauche V, Voderholzer U. Frontostriatal activation in patients with obsessive-compulsive disorder before and after cognitive behavioral therapy. Psychol Med. 2011;41:207–216. doi: 10.1017/S0033291710000309. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg BD, Tolliver TJ, Huang S, Qian L, Bengel D, Murphy DL. Genetic variation in the serotonin transporter promoter region affects serotonin uptake in human blood platelets. Am J Med Genet. 1999;88:83–7. [PubMed] [Google Scholar]
- 28.Goldman S, Wang C, Salgado MW, Greene PE, Kim M, Rapin I. Motor stereotypies in children with autism and other developmental disorders. Dev Med Child Neurol. 2009;51:30–8. doi: 10.1111/j.1469-8749.2008.03178.x. [DOI] [PubMed] [Google Scholar]
- 29.Gomes FV, Casarotto PC, Resstel LB, Guimarães FS. Facilitation of CB1 receptor-mediated neurotransmission decreases marble burying behavior in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2011;30:434–8. doi: 10.1016/j.pnpbp.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 30.Gould GG, Hensler JG, Burke TF, Benno RH. Density and function of central serotonin (5HT) transporters, 5-HT1A and 5-HT2A receptors, and effects of their targeting on BTBR T+tf/J mouse social behavior. J Neurochem. 2011;116:291–303. doi: 10.1111/j.1471-4159.2010.07104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollander E, Phillips A, Chaplin W, Zagursky K, Novotny S, Wasserman S, Iyengar R. A placebo controlled crossover trial of liquid fluoxetine on repetitive behaviors in childhood and adolescent autism. Neuropsychopharmacology. 2005;30:582–9. doi: 10.1038/sj.npp.1300627. [DOI] [PubMed] [Google Scholar]
- 32.Hughes C, Russell J, Robbins TW. Evidence for executive dysfunction in autism. Psychol Med. 1994;32:477–92. doi: 10.1016/0028-3932(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 33.Kaland N, Smith L, Mortensen EL. Brief report: cognitive flexibility and focused attention in children and adolescents with Asperger syndrome or high-functioning autism as measured on the computerized version of the Wisconsin Card Sorting Test. J Autism Dev Disord. 2008;31:1161–5. doi: 10.1007/s10803-007-0474-1. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Ragozzino ME. The involvement of the orbitofrontal cortex in learning under changing task contingencies. Neurobiol Learn Mem. 2005;83:125–33. doi: 10.1016/j.nlm.2004.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lapper SR, Bolam JP. Input from the frontal cortex and the parafascicular nucleus to cholinergic interneurons in the dorsal striatum of the rat. Neuroscience. 1992;51:533–45. doi: 10.1016/0306-4522(92)90293-b. [DOI] [PubMed] [Google Scholar]
- 36.Leboyer M, Philippe A, Bouvard M, Guilloud-Bataille M, Bondoux D, Tabuteau F, Feingold J, Mouren-Simeoni M, Launay J. Whole blood serotonin and plasma beta-endorphin in autistic probands and their first degree relatives. Biol Psychiatry. 1999;15:158–63. doi: 10.1016/s0006-3223(97)00532-5. [DOI] [PubMed] [Google Scholar]
- 37.Lesch K, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- 38.Lewis MH, Tanimura Y, Lee LW, Bodfish LW. Animal models of restricted repetitive behavior in autism. Behav Brain Res. 2007;176:66–74. doi: 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Line SJ, Barkus C, Coyle C, Jennings KA, Deacon RM, Lesch KP, Sharp T, Bannerman DM. Opposing alterations in anxiety and species-typical behaviours in serotonin transporter overexpressor and knockout mice. Eur Neuropsychopharmacol. 2011;21:108–16. doi: 10.1016/j.euroneuro.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McCool MF, Patel S, Talati R, Ragozzino ME. Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem. 2008;89:114–24. doi: 10.1016/j.nlm.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McFarlane HG, Kusek GK, Yang M, Phoenix JL, Bolivar VJ, Crawley JN. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes Brain Behav. 2008;7:152–63. doi: 10.1111/j.1601-183X.2007.00330.x. [DOI] [PubMed] [Google Scholar]
- 42.Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawely JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav Brain Res. 2008;191:118–29. doi: 10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Njung’e K, Handley SL. Evaluation of marble-burying behavior as a model of anxiety. Pharmacol Biochem Behav. 1991;38:63–7. doi: 10.1016/0091-3057(91)90590-x. [DOI] [PubMed] [Google Scholar]
- 45.Owley T, Brune CW, Salt JD, Walton L, Guter S, Ayuyao BA, Gibbons RD, Leventhal BL, Cook EH. A pharmacogenetic study of escitalopram in autism spectrum disorders. Austim Res. 2010;3:1–7. doi: 10.1002/aur.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pearson B, Pobbe R, Defensor E, Oasay L, Bolivar V, Blanchard D, Blanchard R. Motor and cognitive stereotypies in the BTBR T+tf/J mouse model of autism. Genes Brain Behav. 2011;10:228–35. doi: 10.1111/j.1601-183X.2010.00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pierce K, Courchesne E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol Psychiatry. 2001;49:655–64. doi: 10.1016/s0006-3223(00)01008-8. [DOI] [PubMed] [Google Scholar]
- 48.Pobbe RL, Defensor EB, Pearson BL, Bolivar VJ, Blanchard DC, Blanchard RJ. General and social anxiety in the BTBR T+ tf/J mouse strain. Behav Brain Res. 2011;216:446–51. doi: 10.1016/j.bbr.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Posey DJ, Erickson CA, Stigler KA, McDougle CJ. The use of selective serotonin reuptake inhibitors in autism and related disorders. J Child Adolesc Psychopharmacol. 2006;1:181–6. doi: 10.1089/cap.2006.16.181. [DOI] [PubMed] [Google Scholar]
- 50.Ragozzino ME, Choi D. Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem. 2004;11:70–7. doi: 10.1101/lm.65404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci. 2003;117:1054–65. doi: 10.1037/0735-7044.117.5.1054. [DOI] [PubMed] [Google Scholar]
- 52.Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem. 2009;91:13–22. doi: 10.1016/j.nlm.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116:105–15. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rapin I, Tuchman RF. What is new in autism? Curr Opin Neurol. 2008;21:143–9. doi: 10.1097/WCO.0b013e3282f49579. [DOI] [PubMed] [Google Scholar]
- 55.Silverman JL, Tolu SS, Barkan CL, Crawley JN. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology. 2010;35:976–89. doi: 10.1038/npp.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Silverman JL, Yang M, Turner SM, Katz AM, Bell DB, Koenig JI, Crawley JN. Low stress reactivity and neuroendocrine factors in the BTBR T+tf/J mouse model of autism. Neuroscience. 2010;171:1197–208. doi: 10.1016/j.neuroscience.2010.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solomon M, Smith AC, Frank MJ, Ly S, Carter CS. Probabilistic reinforcement learning in adults with autism spectrum disorders. Autism Res. 2011;4:109–20. doi: 10.1002/aur.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tait DS, Brown VJ. Difficulty overcoming learned non-reward during reversal learning in rats with ibotenic acid lesions of orbital prefrontal cortex. Ann N Y Acad Sci. 2007;1121:407–20. doi: 10.1196/annals.1401.010. [DOI] [PubMed] [Google Scholar]
- 59.Tait DS, Brown VJ. Lesions of the basal forebrain impair reversal learning but not shifting of attentional set in rats. Behav Brain Res. 2008;187:100–8. doi: 10.1016/j.bbr.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 60.Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–24. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- 61.Takarae Y, Minshew NJ, Luna B, Sweeney JA. Atypical involvement of frontostriatal systems during sensorimotor control in autism. Psychiatry Res. 2007;156:117–27. doi: 10.1016/j.pscychresns.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanimura Y, Yang MC, Lewis MH. Procedural learning and cognitive flexibility in a mouse model of restricted, repetitive behaviour. Behav Brain Res. 2008;189:250–6. doi: 10.1016/j.bbr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 63.Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology (Berl) 2009;204:361–73. doi: 10.1007/s00213-009-1466-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsuchida A, Doll BB, Fellows LK. Beyond reversal: a critical role for human orbitofrontal cortex in flexible learning from probabilistic feedback. J Neurosci. 2010;30:16868–75. doi: 10.1523/JNEUROSCI.1958-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tzavos A, Jih J, Ragozzino ME. Differential effects of M1 muscarinic receptor blockade and nicotinic receptor blockade in the dorsalmedial striatum on response reversal learning. Behav Brain Res. 2004;154:245–53. doi: 10.1016/j.bbr.2004.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Veeraragavan S, Bui N, Perkins JR, Yuva-Paylor LA, Carpenter RL, Paylor R. Modulation of behavioral phenotypes by a muscarinic M1 antagonist in a mouse model of fragile X syndrome. Psychopharmacology (Berl) 2011;217:143–51. doi: 10.1007/s00213-011-2276-6. [DOI] [PubMed] [Google Scholar]
- 67.Weiler JA, Bellebaum C, Brune M, Juckel G, Daum I. Impairment of probabilistic reward-based learning in schizophrenia. Neuropsychology. 2009;23:571–80. doi: 10.1037/a0016166. [DOI] [PubMed] [Google Scholar]
- 68.Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G. Cortico-striatal synaptic defects and OCD-like behaviours in Sapap3-mutant mice. Nature. 2007;448:894–900. doi: 10.1038/nature06104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang M, Perry K, Weber MD, Katz AM, Crawley JN. Social peers rescue autism-relevant sociability deficits in adolescent mice. Autism Res. 2011;3:1–11. doi: 10.1002/aur.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yang M, Scattoni ML, Zhodzishsky V, Chen T, Caldwell H, Young WS, McFarlane HG, Crawley JN. Social approach behaviors are similar on conventional versus reverse lighting cycles, and in replications across cohorts, in BTBR T+ tf/J, C57BL/6J, and vasopressin receptor 1B mutant mice. Front Behav Neurosci. 2007;1:1. doi: 10.3389/neuro.08/001.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zagreda L, Goodman J, Druin DP, McDonald D, Diamond A. Cognitive deficits in a genetic mouse model of the most common biochemical cause of human mental retardation. J Neurosci. 1999;19:6175–82. doi: 10.1523/JNEUROSCI.19-14-06175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]