Abstract
The neurotransmitter glutamate is the primary excitatory neurotransmitter in mammalian brain and is responsible for most corticocortical and corticofugal neurotransmission. Disturbances in glutamatergic function have been implicated in the pathophysiology of several neuropsychiatric disorders—including schizophrenia, drug abuse and addiction, autism, and depression—that were until recently poorly understood. Nevertheless, improvements in basic information regarding these disorders have yet to translate into Food and Drug Administration–approved treatments. Barriers to translation include the need not only for improved compounds but also for improved biomarkers sensitive to both structural and functional target engagement and for improved translational models. Overcoming these barriers will require unique collaborative arrangements between pharma, government, and academia. Here, we review a recent Institute of Medicine–sponsored meeting, highlighting advances in glutamatergic theories of neuropsychiatric illness as well as remaining barriers to treatment development.
INTRODUCTION
The 1990s was designated the decade of the brain to emphasize the great strides in neuroscience research that were being driven by pharmacology, molecular biology, neuroimaging, and other seminal technologies. More than a decade later, however, many of the major brain illnesses remain difficult or impossible to treat, including highly prevalent diseases such as schizophrenia, depression, substance abuse, Alzheimer’s disease, and neurodevelopmental disorders. The present paper reviews a meeting convened by the Institute of Medicine in June 2010 to bring together leading experts from academia, government, and pharma to address methods for harnessing the marked advances in brain research that have accrued over the past several decades for the development of new medications for major brain illnesses.
On the basis of the workshop presentations and discussions, a number of themes, opportunities, and suggestions for future efforts were identified. The statements, recommendations, and opinions summarized here are those of individual presenters and participants and should not be construed as reflecting consensus or endorsement by the workshop planning committee, the Institute of Medicine’s Forum on Neuroscience and Nervous System Disorders, or The National Academies. A full agenda of the meeting and summary of presentations is available online at http://www.iom.edu/Activities/Research/NeuroForum.aspx.
MODULATION OF GLUTAMATE NEUROTRANSMISSION: OVERVIEW
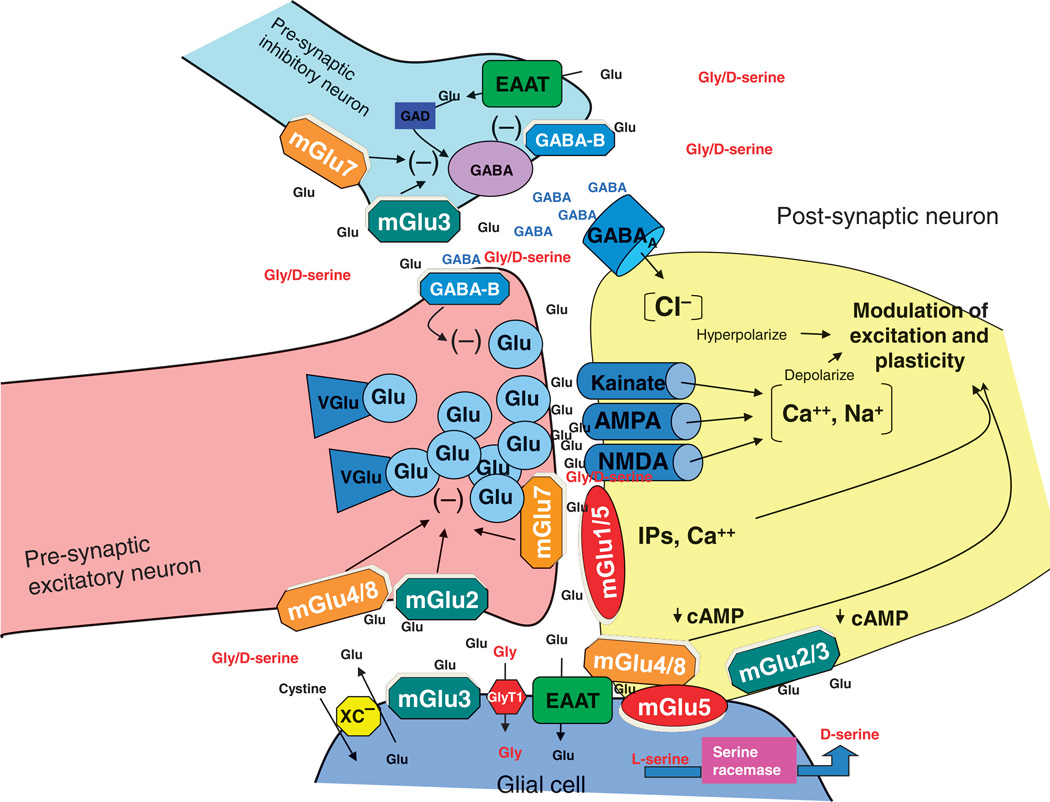
A primary focus of the meeting was an overview of the role of the neurotransmitter glutamate in normal brain function and identification of the levels at which intervention might take place. In mammals, the balance of excitation and inhibition in the central nervous system (CNS) is determined by synaptic inputs from the major inhibitory neurotransmitter γ-aminobutyric acid (GABA) and the major excitatory neurotransmitter glutamate. This ying-yang relationship between glutamate and GABA (Fig. 1) controls the major inputs and outputs of brain regions involved in essentially all physiological functions (1).
Fig. 1.
Schematic diagram of the glutamate synapse. Receptors most amenable to therapeutic manipulation include the AMPA and NMDA-type glutamate receptors; metabotropic type 2, 3, 5, and 7 receptors (mGluR2, mGluR3, mGluR5, and mGluR7); transporters for glutamate (GLT-1); glycine (GlyT1); and the cystine/glutamate antiporter (XC).
Glutamate mediates its effects at multiple ionotropic and metabotropic receptors. Ionotropic receptors are characterized by sensitivity to synthetic glutamate derivatives including N-methyl-d-aspartate (NMDA) and 2-amino-3-(5-methyl-3-oxo-1,2-oxazol-4-yl)propanoic acid (AMPA). Both NMDA (NMDAR) and AMPA (AMPAR) receptors are composed of multiple subunits surrounding a central pore, with receptor properties varying depending upon exact channel composition. In addition to the glutamate binding site, the NMDAR complex contains a binding site for the endogenous modulatory amino acids glycine and d-serine, as well as polyamine and redox-sensitive sites. NMDARs are uniquely voltage- and ligand-dependent, permitting them to function in a Hebbian fashion to integrate information across brain pathways (2).
Metabotropic glutamate receptors (mGluRs) are divided into groups on the basis of their second messenger coupling and ligand sensitivity. Group I receptors (mGluR1/5) predominantly potentiate both pre-synaptic glutamate release and postsynaptic NMDAR currents. In contrast, group II (mGluR2/3) receptors, in general, limit glutamate release, particularly during conditions of glutamate spillover from the synaptic cleft. Group III receptors (mGluR4/6/7/8) show more variable distribution but also generally inhibit glutamate function (3) (Fig. 1). Many of these receptors become rapidly down-regulated during stimulation. Thus, positive (PAM) and negative (NAM) allosteric modulators may be more effective in altering receptor activity thanmore traditional orthosteric agonists, antagonists, and mixed agonists/antagonists.
In addition to controlling excitation per se, glutamate neurotransmission, through its diverse downstream signaling mechanisms, represents a major system for controlling neuronal plasticity, learning, and memory (4, 5). However, not all glutamate receptors contribute equally to all behaviors. Thus, for example, NMDARs are involved in formation but not retention of memories, so that dissociation between such functions may be a hallmark of NMDAR-related disorders. In contrast, other types of glutamate receptors are involved in both formation and retention, and thus may lead to a different pattern of neuropsychological dysfunction.
Genetic contributions
An additional challenge to development of glutamate-based treatments is the complex genetics of most neuropsychiatric disorders. Heritability estimates for disorders such as schizophrenia, substance abuse, or autism are in the range of 50%, implying a strong genetic component but also implicating additional epigenetic or environmental factors. For some disorders, such as X-linked mental retardation or autism, informative pedigrees have recently been reported (6, 7). Most cases, however, are likely to be polygenic, with thousands of interacting genes of limited effect, each conferring small (5 to 25%) alteration in risk (8–10). It is also likely that in many instances, the contribution of genes to brain diseases is mediated by their involvement with brain development or though their modulation of responses to environmental factors (stress and drug exposure), limiting their use as targets for intervention once disease symptoms are already manifest.
Specific genetic contributions have been best characterized in the case of schizophrenia, where large-scale meta-analyses have been performed and significant convergence on glutamatergic pathways is observed (Table 1). Glutamate-related genes have also been identified in autism spectrum disorders (6, 7). Additional genes for these disorders undoubtedly remain to be identified. However, most genes implicated in neuropsychiatric illness may not be “druggable” because they are structural proteins (for example, dysbindin) or are not limited to CNS (for example, NRG1).
Table 1.
Glutamate- and nonglutamate-related genes implicated in schizophrenia: meta-analysis and genome-wide association studies (8, 153, 154).
| Glutamate-related genes | Dopamine-related genes | Other |
|---|---|---|
| NMDAR (GRIN2B) | Catechol-O-methyltransferase (COMT) | GABRB2 (GABA) |
| Serine racemase (SRR) | Dopamine type 1, 2, and 4 receptors (DRD1, DRD2, DRD4) | SLC6A4, TPH1 (serotonin) |
| d-Amino acid oxidase (DAO) | IL1BMHC (inflammatory) | |
| d-Amino acid oxidase activator (DAOA, G72) | MTHFR (folate) | |
| Dysbindin (DTNBP1) | HP (haptoblobin) | |
| Neuregulin (NRG1, ERBB4) | PLXNA2 (axon guidance) | |
| Neurogranin (NRGN) | TP53 (apoptosis) | |
| mGluR7 (GRIM7) | TCF4 (transcription) | |
| EAAT1 (SLCA13) | ZNF804A (unknown) |
Thus, although treatments need to compensate for underlying genetic disturbances, the best site of intervention may not be the proteins encoded by the risk genes themselves. Although there is always hope that genes will eventually be discovered that explain neuropsychiatric illness, a more immediate need is to develop improved technologies to exploit the knowledge that has accumulated over the past 25 years.
GLUTAMATE IN SPECIFIC DISORDERS
Along with advances in understanding of glutamate in normal brain function, the meeting highlighted recent advances in understanding the role of glutamatergic dysfunction in specific brain disorders, such as schizophrenia, drug abuse and addiction, depression, and autism. Although these disorders were considered the most promising for immediate translational research, other conditions such as Alzheimer’s disease, ischemia, amyotrophic lateral sclerosis, and pain syndromes were also discussed. In addition, the participants reviewed the basis for disease-specific molecular targets and provided a framework for prioritizing the translational tools necessary to test them in humans.
Schizophrenia
The development of glutamate-based models of schizophrenia can be dated to the late 1950s with the initial synthesis of phencyclidine (PCP) and ketamine. Although originally developed as general anesthetics, these compounds were found in early primate testing to produce a unique behavioral state in which animals were awake but seemingly disconnected from their environment. Subsequent testing in humans demonstrated that they induced schizophrenia-like symptoms and cognitive deficits (11, 12).
PCP and ketamine were subsequently shown to act by binding to a site (PCP receptor) located within the ion channel formed by the NMDAR complex, suggesting that NMDAR dysfunction may contribute to the pathophysiology of schizophrenia (13–15). NMDAR antagonists also reproduce specific neurophysiological deficits associated with schizophrenia, such as impaired generation of mismatch negativity (MMN), providing potential translational biomarkers for new treatment development (see Functional target engagement section).
Since the original studies implicating NMDAR in schizophrenia, significant postmortem data have accumulated showing alterations in NMDAR-related protein and gene expression (16, 17) and increased endogenous glutamate/NMDAR antagonists such as N-acetylaspartylglutamate (NAAG) (18) or kynurenic acid (19, 20). Reductions have also been reported in cerebrospinal fluid (CSF) levels of d-serine (21, 22), along with reduced expression of serine racemase (21), which synthesizes d-serine, and increased expression of d-amino acid oxidase (DAAO) (21, 23, 24), the enzyme responsible for d-serine degradation.
In rodents, serine racemase knockout induces a schizophrenia-like behavioral and neurohistological phenotype (25–27) that can be rescued by additional crossbreeding with DAAO knockouts (28). Finally, schizophrenia is associated with reduced glutathione, which modulates NMDAR via the redox site, and with genetic alteration of glutathione-related enzymes (29), potentially implicating oxidative stress as an environmental contributor to etiopathology. Over recent years, significant genetic data have also accumulated supporting glutamatergic, in addition to dopaminergic, theories of schizophrenia (Table 1). Although several etiological genes such as dysbindin or neuregulin make poor drug targets, others such as DAAO, especially in combination with d-serine, may be promising.
Most recently, anti-NMDAR antibodies have been linked to psychotic symptoms in paraneoplastic syndromes and autoimmune disorders such as systemic lupus erythematous (30), further implicating NMDAR in the pathophysiology of psychosis. Thus, treatments developed for NMDAR enhancement in schizophrenia may find applicability more broadly across a spectrum of neurological disorders.
Drug abuse and addiction
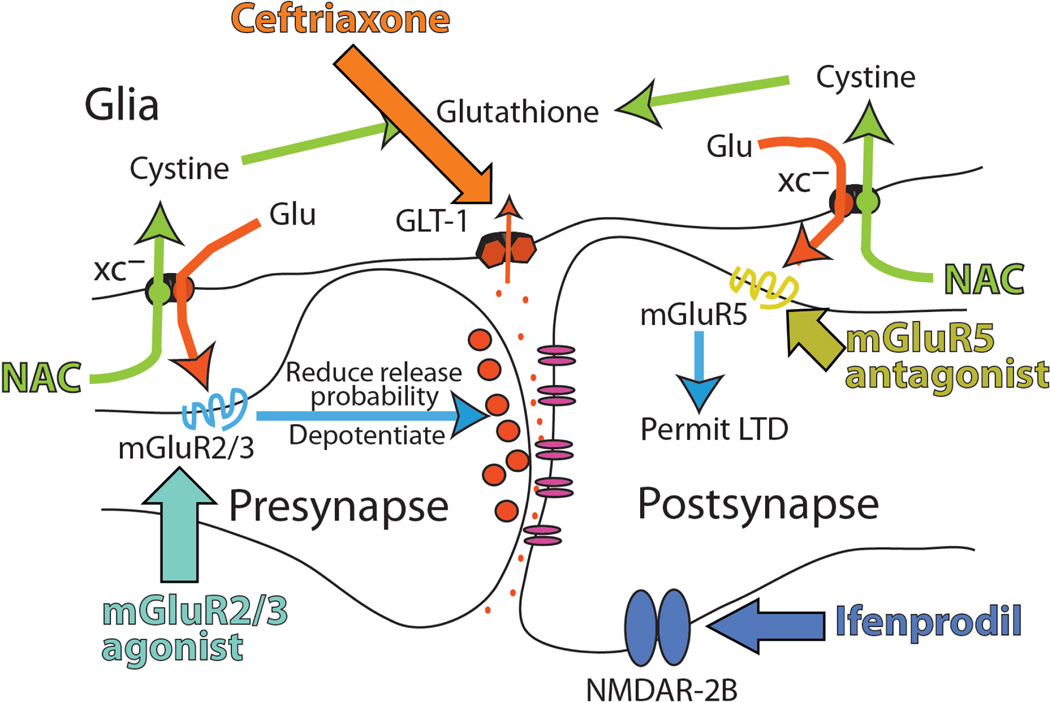
A second highlighted condition was drug abuse and addiction. Drug abuse is a major public health problem, with an estimated annual cost of about $500 billion (31). Neuroadaptations in glutamatergic transmission play a central role in addiction, so that medications that reverse these changes could be therapeutically beneficial (32) (Fig. 2).
Fig. 2.
Proposed glutamate-based treatment targets for drug abuse, including cystine/glutamate transporter (XC−) and the glutamate transporter (GLT-1). NAC, N-acetylcysteine; LTD, long-term depression. Updated from (32).
Drug-induced neuroadaptations in the context of abuse are embedded in circuitry regulating drug reward motivation and the vulnerability to relapse, and thereby strongly affect cardinal features of drug addiction, such as the reduced ability of addicts to experience natural reward, their enhanced sensitivity to the reinforcing value of drugs and drug cues, and the poor capacity of addicts to modify drug-seeking habits to avoid relapse and to exert restraint over their enhanced drive to take drugs (33).
Neuroimaging studies have documented reduced prefrontal and limbic activation in response to natural rewards and an enhanced reactivity to drug cues in addicted subjects (34). Similarly, preclinical studies have documented that an animal’s preference for drug over natural rewards increases with longer periods of drug exposure (35). This results in part from reduced frontal glutamatergic modulation of dopamine cell reactivity in response to natural rewards and an enhanced reactivity in response to drug cues.
Subcellular drug-induced changes have been identified in glutamate terminal fields in the striatum, particularly in nucleus accumbens, an area crucial to drug reward and conditioning. Perhaps most striking is the impairment in the ability of synapses that communicate between the prefrontal cortex and the accumbens to undergo synaptic plasticity (long-term potentiation and depression) (36–38). This is thought to limit the capacity of addicts to incorporate behavioral adaptations (including learning new conditioned associations) that can suppress or compete with drug seeking and taking (32, 36).
Reduced plasticity has been linked to molecular pathologies in the balance between nonsynaptic, glial, and synaptic glutamate transmission. For example, chronic cocaine, nicotine, and heroin use induces reductions in glial proteins (cystine-glutamate exchanger and glutamate transporters) responsible for regulating the levels of extracellular, nonsynaptic glutamate, and thereby glutamatergic tone at the mGluRs that modulate synaptic plasticity (39, 40).
Correspondingly, animal models of relapse have revealed that pharmacologically restoring the activity of either cystine-glutamate exchangers or glutamate transporters reduces cocaine or heroin seeking, marking these proteins and the regulation of synaptic plasticity as promising therapeutic targets (41–43). Similarly, enhancement of NMDAR function by the NMDAR partial agonist d-cycloserine facilitates extinction of cocaine-conditioned place preference and of naloxone-induced conditioned place aversion in morphine-dependent rats and retards subsequent reconditioning to ethanol and cocaine (44).
Autistic spectrum disorders
Autistic spectrum disorders are severe, highly prevalent neuropsychiatric disorders with unknown neurochemical pathologies. As with schizophrenia and drug abuse, no macrolevel structural brain disturbances have been described to explain the severe behavioral and neurocognitive impairments, suggesting instead that various forms of autism are related to functional impairment within distributed, potentially glutamatergic, brain circuits.
Autistic disorders are highly heritable, providing potentially important clues to underlying causes. Although substantial progress has been made in identifying specific etiological mechanisms, most of the genetic risk architecture remains unknown, and the genetic lesions that have been identified are highly heterogeneous. The prevailing view is that the syndrome of autism is a common manifestation of many, individually rare gene mutations (6). Understanding physiological processes affected by these mutations may establish a molecular logic for development of therapeutic interventions.
The leading hypothesis from this approach focuses on genes that couple mGluR1/5 to synaptic protein synthesis (45) and emerged as a result of the observation that mutations of key regulatory genes in this pathways cause several syndromic disorders with increased prevalence of autism. Markedly, fragile X syndrome (FXS) is caused by silencing of the FMR1 gene that encodes a repressor of mGluR-stimulated protein synthesis. Excessive mGluR-dependent protein synthesis has been shown to be pathogenic in FXS, and based on this discovery, NAMs of mGluR5 are now in human clinical trials.
If nonsyndromic autism is associated with genetic variation elsewhere in this complex regulatory pathway, one might expect a subset of patients to respond to treatments developed to treat FXS. But how will this subset of patients be identified? Clearly, there is a need for a predictive biomarker of treatment response in idiopathic autism. If it could be determined a priori by endophenotype or direct measurement that there is excessive cerebral protein synthesis, then a number of therapeutic strategies could be contemplated, including but not limited to NAMs of mGluR5.
The challenge of developing such a biomarker is further complicated by additional findings suggesting that the autistic phenotype can result from either too little or too much synaptic protein synthesis. If this model is correct, then a treatment with a beneficial response for some might be deleterious for others, and it will be important to determine whether mGluR-regulated biochemistry is up- or down-regulated.
TRANSLATIONAL APPROACHES AND BARRIERS TO TRANSLATION
Given the disconnection between the large increase in knowledge regarding glutamatergic systems and the slow pace of therapeutic development, a major focus of the meeting was the identification of barriers to translation. There is clearly a need for both improved compounds and improved preclinical models. A major focus, however, was on the need for improved biomarkers to exploit targets that have already been identified. A unique challenge in neuropsychiatric research is the fact that we are unable to ascertain actual drug concentrations within brain tissue and so cannot know whether the dose is appropriate relative to the affinity of the target. Thus, biomarkers are required to verify both structural target engagement (that doses of drug administered during clinical trials effectively occupy their intended target) and functional target engagement (that compounds, after binding, have the anticipated effects at the molecular, synaptic, and network targets).
Structural target engagement
Most currently available medications were developed before modern neuroimaging techniques. Dose selection, therefore, has been based primarily on the maximum tolerated dose (MTD), in which the dose was increased until CNS side effects were observed and the efficacy was then determined at this maximal dose. Only subsequently have receptor occupancy biomarkers been developed, and occupancy of the D2 dopamine receptor demonstrated for antipsychotics (46–48) and serotonin transporter occupancy shown for antidepressants (49, 50).
Whereas the MTD concept is arguably appropriate for some classes of compounds, this approach is inadequate for most glutamatergic targets. For example, many glutamate receptors become down-regulated at full occupancy, requiring partial activation for effective stimulation. In such cases, doses above a critical range may lead to a deceptive lack of efficacy. For some targets, such as mGluR5 (51) or GlyT1 (glycine transporter 1) (52, 53), positron emission tomography (PET) ligands are available. Moreover, in a recent GlyT1 inhibitor trial, dosing designed to produce midrange occupancy on the basis of PET occupancy led to preliminary but encouraging evidence of beneficial effect (53), consistent with animal findings (54, 55). In contrast, studies with this inhibitor at the MTD produced negative results, highlighting the importance of monitoring occupancy in glutamatergic trials.
For most of the glutamatergic targets, no validated PET ligands have been developed, and development of further ligands is complicated by high costs, the need for specialized chemistry expertise in fast synthesis (because PET isotopes are short-lived), and access to imaging resources. High costs may be difficult to justify for isolated single drug development program. However, once developed, such ligands would be useful to academic, government, and pharma users.
In response to this specific problem, a work group has been convened through the National Institutes of Health to generate specific proposals for consortia that would share resources across academia, government, and industry to foster ligand development while permitting pharma to maintain proprietary development candidates (see Models for collaborative drug development section). Such consortia may be critical to overcoming a practical, but key, barrier to translational medicine approaches for neuropharmaceutical development.
PET ligands may also be crucial for patient stratification. For example, in autism, it has been suggested that the autistic phenotype can result from either too little or too much synaptic protein synthesis (45). If this is correct, then a treatment with a beneficial response for some might be deleterious for others, complicating treatment development. Thus, knowledge of pretreatment target density may be critical for patient selection and therapeutic design.
Functional target engagement
In addition to demonstrating structural target engagement, a critical step in drug development is demonstration that binding of a drug within the CNS produces the expected functional engagement of the target and the expected effect on network-level neural processing. As with structural target engagement, demonstration of functional target engagement is critical for selecting doses that produce engagement that can be related to behavioral changes in animal models and therapeutic effects in humans. Moreover, functional biomarkers may prove essential for identifying disease subpopulations for an informative trial with a specific class of drugs.
Several promising candidate biomarkers that can be implemented in both preclinical and clinical phases of drug development have been explored over recent years. In particular, electrophysiological measures in rodents, primates, and humans are sensitive to NMDAR antagonists such as ketamine or PCP. However, ideal parameters for use in preclinical and clinical development programs have not been established. A recurrent limitation at present is that academic development of biomarkers is difficult to accomplish because academic researchers frequently do not have access to high-affinity tool compounds to assist in biomarker development. Conversely, without biomarkers to guide compound development, it is difficult for industry to develop and prioritize new compounds. As with structural measures, development of biomarkers within a precompetitive space with compounds contributed by pharma would facilitate progress for all compounds while permitting companies to retain proprietary rights.
Electrophysiological biomarkers
This class of biomarkers is based on well-characterized alterations in brain electrical activity during disease states and the relationship of these signals to underlying glutamatergic function. In academic research, such measures are used to evaluate pathophysiological mechanisms preclinically and some have been used to verify effects of NMDAR antagonists. The participants at the meeting discussed which of these have the sensitivity and specificity needed for hypothesis testing in drug development and could become standards for the field. Academic researchers have focused auditory responses when exploring glutamatergic function. To date, only brain electroencephalography measures have been subject to sufficient standardization to be applied, albeit narrowly, to the development of drugs (especially GABAergic) that inhibit brain activity.
Mismatch negativity
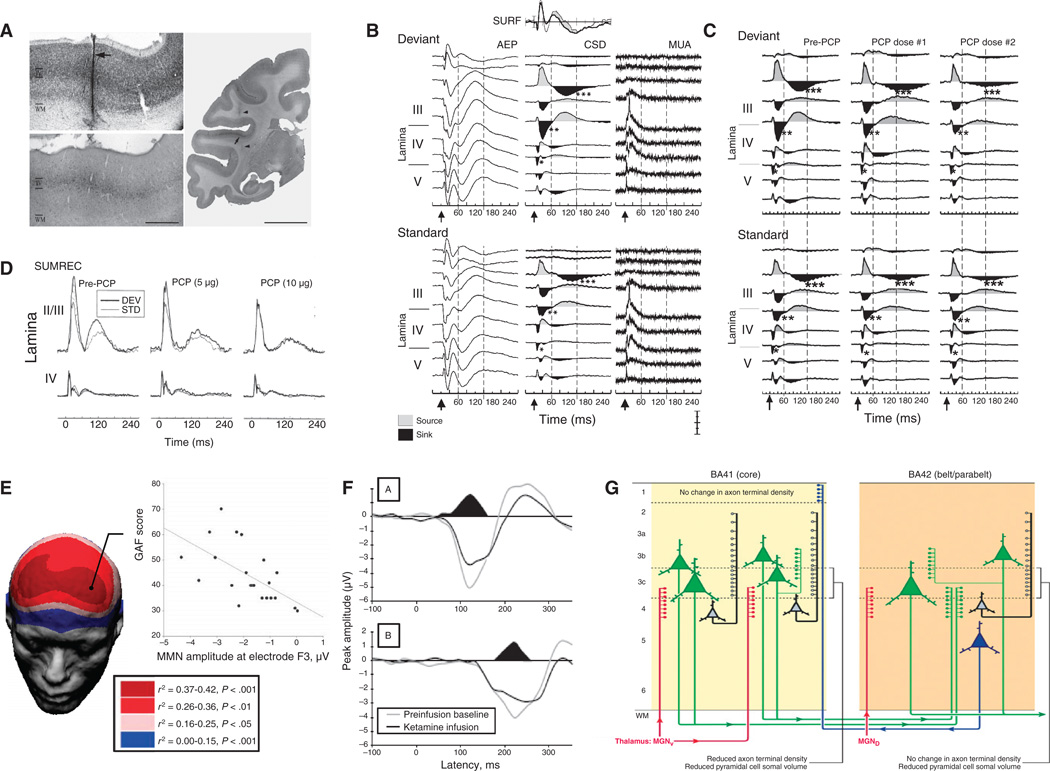
MMN is an auditory event-related potential component elicited by deviant stimuli in an auditory “oddball” paradigm, in which a series of repetitive tones is interrupted infrequently by a physically distinct oddball stimulus. MMN measures functioning of the auditory sensory memory system (56). Because MMN is elicited by unattended as well as attended stimuli, this paradigm can be used across clinical populations relatively free from confounds relating to motivation or task engagement. Furthermore, it is generated in the auditory cortex, a brain region that is relatively conserved across rodents, monkeys, and humans (57) (Fig. 3).
Fig. 3.
An example biomarker for glutamate-based drug development. (A) MMN is an event-related potential generated in primary auditory cortex. (B to D) Auditory-evoked field potentials (AEP), current source density (CSD), and multiunit activity (MUA) analyses show MMN-related activity in superficial layers of auditory cortex (B) and dose-dependent inhibition by local administration of the NMDAR antagonist PCP (C), leading to a reduction in local summed (SUMREC) current flow (D) (65). (E) In schizophrenia, reductions in MMN generation correlate with overall function, as reflected in the global assessment of function (GAF) score (60). (F) Deficits similar to those observed in schizophrenia are induced in volunteers by acute administration of ketamine (69). (G) Histological investigation of auditory cortex shows reduced glutamatergic pyramidal cell volume, consistent with underlying glutamatergic dysfunction (155). (A) to (D) is reprinted from (65). (E) is reprinted with permission from (60). (F) is reprinted with permission from (69). (G) is reprinted by permission from Macmillan Publishers Ltd. (155), copyright (2009).
Deficits in MMN generation are reliably observed in schizophrenia (58, 59) and are related to overall level of function (60), but are not observed in other psychotic disorders or depression (61). Abnormalities of MMN generation are reported in autism (62, 63) and Alzheimer’s disease (64), although patterns of deficit differ from those of schizophrenia. Schizophrenia-like deficits in MMN generation are observed in monkeys (65), humans (66–69), and rodents (70, 71) after treatment with NMDAR antagonists. These results point to the possibility of using MMN-like responses in rodents as a tool to interrogate the NMDAR system (72) and to evaluate compounds that increase or facilitate NMDAR function.
Auditory N100
Auditory N100 (N1) is a somewhat simpler auditory response that reflects response to individual stimuli independent of deviance from previous stimuli. N1 declines exponentially in amplitude as stimulation interval decreases below 9 s, suggesting that its generation is also modulated by short-term sensory memory traces. Schizophrenia patients show deficits in N1 generation that can be reproduced in monkeys (73) by acute NMDAR antagonist administration. In mice, N1 shows similar characteristics as in humans (74, 75), suggesting that it may serve as a simple tool for translational biomarker approaches. In autism, increases in N1 latency are observed both in patients and in mice prenatally exposed to valproic acid (76).
Auditory 40-Hz steady-state response
Auditory function may also be probed with rapidly presented stimuli, which elicit steady-state (as opposed to transient) responses that can be analyzed as a function of spectral frequency over time. The auditory system shows a peak resonance at 40 Hz (that is, within the γ range), a response that is impaired in schizophrenic, bipolar, and autistic patients (77, 78), and is hypothesized to reflect impaired synchrony within local glutamate/GABA circuits in auditory cortex (79). As with other passive auditory measures, the auditory 40-Hz steady-state response may be ideal for cross-species translation, although ideal frequencies for stimulation in animals remain unknown.
Neuroimaging-based approaches
Brain function may also be assessed with functional brain measures such as functional magnetic resonance imaging (fMRI) that can be applied to a variety of tasks to probe specific activation patterns in neural circuits as well as during resting conditions to map functional networks. Several initiatives are under way to permit standardization of fMRI approaches across platforms (such as the Biomedical Informatics Research Network). An advantage of fMRI is that most cognitive tasks currently used to assess dysfunction in neuropsychiatric conditions can be adapted to fMRI conditions. In general, however, fMRI cannot be readily obtained in either awake rodents or primates, limiting their use for translational research from animals to humans. Nonetheless, for establishing drug effects on glutamate function in humans, fMRI may prove to have broader application than evoked auditory electrophysiological approaches because of its potential to explore the dynamic interactions across multiple brain regions during specific stimulation protocols and at rest.
The use of fMRI to assess resting functional connectivity is arguably the most promising new application of the blood oxygen level–dependent (BOLD) signal to identify brain functional networks and has already been shown to be highly reproducible between and within subjects (80) and to be sensitive to disruption by diseases (81). Its reproducibility as well as its sensitivity to neuropathology makes it a promising tool to serve as a translational biomarker in brain diseases. Resting-state fMRI (rsfMRI) has been investigated to date in Alzheimer’s disease (82, 83), schizophrenia (84), depression (81), and addiction (85). In addition, rsfMRI measures can be obtained in primates and rodents (86), facilitating its use for translational research. Other measures, such as diffusion tensor imaging (87–89), may provide a structural correlate that could be applicable in human, primate, and rodent species (90).
Imaging of biochemistry
Other imaging-based procedures may also be developed for assessment of effects of glutamatergic compounds. Magnetic resonance spectroscopy (MRS) can be used to measure N-acetyl aspartate (NAA) levels, which reflect the integrity of glutamatergic neurons, as well as glutamate/glutamine turnover (91, 92). Abnormalities of these measures have been reported in schizophrenia (93), as have glutamate/glutamine ratios in CSF (94).
Finally, because binding of NMDAR channel blockers such as PCP or ketamine is use-dependent, radioligand-based approaches [PET and SPECT (single-photon emission computed tomography)] may also be used to image glutamatergic function. To date, use-dependent ligands such as CNS-1261 have been tested (91), but the resolution of such agents is not yet sufficient for widespread translational use.
Models for collaborative drug development
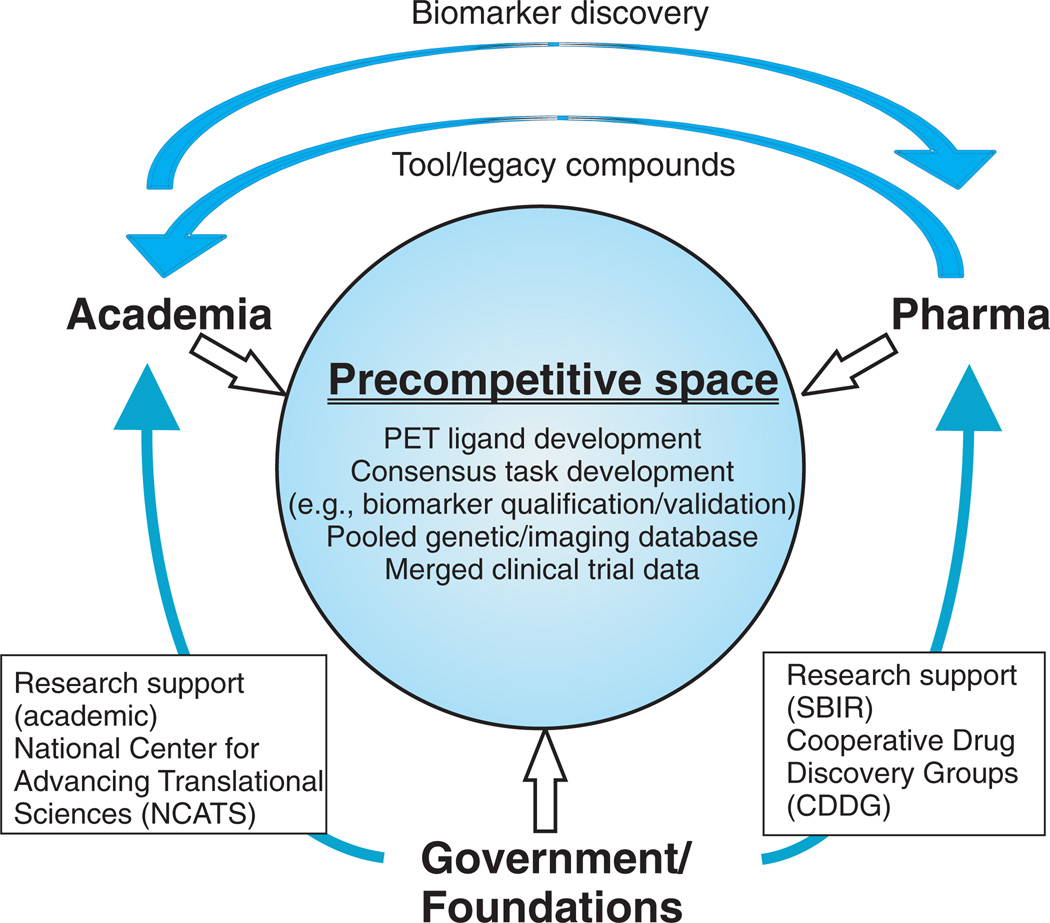
Given the high cost of biomarker development, current collaborative models require that much or all of the work be conducted in the precompetitive space—with pharma and academia providing expertise; pharma providing compounds; and government, foundations, and pharma providing funding or in-kind contributions (Fig. 4). Technology developed in the precompetitive space would be available to all stakeholders, with companies maintaining proprietary rights only for specific development candidates.
Fig. 4.
Schematic model of interactive drug development among academia, government, and pharma, designed to overcome barriers to translational drug development. In this model, biomarkers are developed in a precompetitive space, with pharma providing nonproprietary compounds, expertise, and financial support, which is matched by government and foundations. In addition, baseline genetic and consensus behavioral and biomarker data from clinical trials are pooled to permit comparison among disease states and genotype/phenotype investigation.
In the United States, small-scale support for ligand development is currently available through Small Business Innovation Research funding mechanisms. Such models, however, specifically limit collaborations to small entities that may or may not have sufficient resources or access to high-affinity ligands to successfully complete the task. Other examples of collaboration include the Alzheimer Disease Neuroimaging Initiative (ADNI) in which funding from the National Institute of Aging, industry, and advocacy groups has been combined to develop biomarkers of disease state and progression that can be applied to drug development.
The original 5-year ADNI effort has been expanded and extended to a second phase in which multiple brain imaging and blood and CSF measures are being refined and standardized for use in clinical trials that follow subjects with predementia. A similar consortium of government and foundations was recently developed for Parkinson’s disease [Parkinson’s Progression Markers Initiative (PPMI)] with a goal of achieving synergy with the Alzheimer’s effort because structural and functional imaging methodologies as well as proteomics and/or metabolomics of blood and CSF require the same performance characteristics to be useful in drug development independent of diagnosis.
An alternative model for collaboration is the European Innovative Medicines Initiative (IMI), which is a joint initiative between the European Union and pharma. Under this initiative, academic consortia compete for funding for research defined by a consortium of companies, with funding coming from pooled resources of government and industry. For instance, the challenges in drug development for schizophrenia and depression are being addressed under the project entitled “Novel Methods leading to New Medicines in Depression and Schizophrenia” (NEWMEDS), which contains 10 work packages covering system-based animal models and translational and patient stratification biomarkers.
In IMI, government and industry contribute equal funding, with the latter’s contribution coming mostly from“in-kind” support rather than funds committed to a central source. No such program is presently in place in the United States. In general, a 1:1 funding match between government/academia and industry would produce a program attractive enough to align industry while still providing sufficient value added to government to justify the added complexity that such collaborations necessarily entail.
PROOF-OF-CONCEPT TRIALS, SYNTHESIS, AND FUTURE DIRECTIONS
To date, no Food and Drug Administration–approved compounds are available that take advantage of the glutamatergic basis of neuropsychiatric illness. Nevertheless, glutamatergic hypotheses have led to positive phase 2 trials in schizophrenia and anxiety disorders, and clinical trials are under way to evaluate group 1 mGluRs in the treatment of FXS (7). In addition, the recent fortuitous discovery of antidepressant effects of the NMDAR antagonist ketamine has opened a new window on basic neural mechanisms of depression and may initiate a new wave of treatment development.
NMDAR potentiation in schizophrenia
Given the ability of NMDAR antagonists to induce schizophrenia-like psychotic effects, one straightforward hypothesis is that compounds that stimulate NMDAR should be therapeutically beneficial in schizophrenia. Initial preclinical (95) and clinical (96) studies targeted the glycine modulatory site of the NMDAR with the natural compounds glycine and d-serine. High doses of these compounds were required because of their extensive peripheral metabolism/elimination, poor CNS penetrance, and dilution into the general glycine/d-serine pool in brain. Thus, target engagement remains unverified. Despite these limitations, several small-scale studies showed positive results, although negative findings have also been obtained. Varied results have also been obtained with d-cycloserine, a mixed glycine-site agonist/antagonist with a resultant U-shaped dose-response curve (97, 98).
A second-generation approach to NMDAR stimulation has been the use of glycine transport (GlyT1) inhibitors. GlyT1 transporters are colocalized in the CNS with NMDAR and serve to maintain low, sub-saturating glycine concentrations within the protected space of the synaptic cleft (99). Initial behavioral studies were performed with the glycine derivative glycyldodecylamide (GDA) (100, 101) and physiological studies with NFPS (ALX-5407) (102). Subsequent studies have shown the effectiveness of high-affinity glycine transport inhibitors in a range of preclinical models relevant to schizophrenia, including PCP-induced hyperactivity and dopaminergic dysregulation (99).
Significant clinical results have been obtained with sarcosine (N-methylglycine), a naturally occurring glycine transport inhibitor (98), and, most recently, with the high-affinity glycine transport inhibitor R1678 (103). As described above (see Structural target engagement section), success of this approach may depend on dosing designed to achieve midrange target occupancy to prevent receptor down-regulation (54, 55). Another approach to modulate the glycine site has been the use of d-serine combined with a DAAO inhibitor, which acts either peripherally or centrally to prevent d-serine degradation (104). However, this approach has yet to be evaluated in clinical studies.
mGluR2/3 in anxiety
Although reduced NMDAR function appears to occur in patients with schizophrenia, increased glutamate signaling may contribute to anxiety. Thus, negative modulation of glutamate signaling via mGluR is a promising approach to treatment of anxiety [see (105, 106) for reviews]. This approach aims to suppress excitatory glutamate neurotransmission in key areas of the brain associated with fear and anxiety, such as the amygdala, hippocampus, and thalamus (1).
The first in vivo tool for mGluR2/3 stimulation was the orthosteric agonist LY354740. LY354740 blocks veratridine-induced glutamate release in vitro, penetrates the CNS at receptor relevant concentrations in vivo (107), and shows activity in preclinical rodent models (105). Genetic deletion of either mGluR2 or mGluR3 abolishes the anxiolytic actions of LY354740 in rodents, suggesting the need for blockade of both mGluR2 and mGluR3 receptors for a robust effect (108).
In generalized anxiety disorder (GAD) patients, LY354740 is administered in its prodrug form (LY544344) to increase oral bioavailability. In an initial double-blind study, LY544344 treatment led to reductions in HAMA-A scores at well-tolerated doses (109). However, this study was terminated before all patients were enrolled because risk of seizures became apparent in chronic toxicology studies in animals.
mGluR2 PAMs also show positive effects in animal models of anxiety (105, 110). Overall, these early studies suggest that activation of mGluR3 and/or mGluR2 receptors has promise in treating anxiety disorders. Newer agents that can be given orally in humans may be needed to understand their clinical potential across the range of anxiety-related disorders, including GAD, and also posttraumatic stress disorders, obsessive compulsive disorder, and possibly phobias. In addition, markers of target engagement are required to better evaluate optimal occupancy levels for clinical response. To date, no anxiety-related biomarkers have been developed that would aid in clinical development.
mGluR2/3 in schizophrenia
In addition to anxiety disorders, mGluR2/3 agonists may also be effective for hyperglutamatergic states associated with schizophrenia. In rodents, NMDAR blockade produces a rebound hyperglutamatergia that may itself be pathological. Blockade of hyperglutamatergia by stimulation of mGluR2/3 receptors, therefore, may be therapeutically beneficial. In rats, the mGluR2/3 agonist LY354740 inhibited the behavioral and neurochemical actions of PCP, suggesting that it may have antipsychotic activity (111, 112). Similar effects were subsequently observed with improved mGluR2/3 ligands such as LY379268 and LY404039 (113). In addition to blocking PCP-induced effects, the mGluR2/3 agonists also block the hyperlocomotion in rodents induced by amphetamine, also supporting their antipsychotic efficacy (114). Unlike antipsychotics, the effects of mGluR2/3 agonists are prevented by pretreatment with mGluR2/3 antagonists (114) or knockout of the mGluR2/3 receptor (115), indicating that the mGluR2/3 receptor mediates the effect.
At the cellular level, mGluR2/3 agonists block NMDAR antagonist–induced neuronal degeneration and the direct or indirect release of multiple neurotransmitters in vivo, including glutamate, dopamine, serotonin, and norepinephrine (116–118). The ability of mGluR2/3 agonists to reverse behaviors induced by the different psychostimulants linked to psychosis in humans (PCP, amphetamine, and serotonin 2A agonists), which are all mechanistically distinct, is an interesting feature of this pharmacological class. These results likely reflect the multifaceted role of glutamate in the direct and indirect modulation of many neurotransmitter systems in the brain (1, 119) and support the view that mGluR2/3 agonists act on nonglutamate systems downstream from their effects on negative modulation of glutamate release (1, 120).
Human studies also support mGluR2/3 agonist treatment approaches. In an initial human study, LY354740 alleviated ketamine-induced deficits in working memory, although not other effects of ketamine (121). These studies, however, were likely limited by the low oral bioavailability and potency of LY354740. Subsequent human studies, therefore, were performed with the compound LY2140023, which is an oral dipeptide prodrug that delivers receptor-active levels of LY404039 when administered orally in humans (115).
In an initial phase 2 clinical study (115), LY2140023, along with olanzapine, was more effective than placebo at weeks 1, 2, 3, and 4 of treatment by the Positive and Negative Syndrome Scale (PANSS) total, positive, and negative scales. The compound was also well tolerated, and thus, this may be a promising approach for treating schizophrenia. Unfortunately, a follow-up study (clinicaltrials.gov NCT00520923) failed to show efficacy for either LY2140023 or olanzapine relative to placebo.
Because of the lack of biomarkers for structural target engagement in these studies, even a clear clinical result would leave open the question of whether a specific target hypothesis had been adequately tested. In clinical studies, LY2140023 dose was ultimately selected on the basis of steady-state concentrations of the compound in CSF on the assumption that this was a reasonable proxy for free drug concentration at mGluR2/3 receptors in human brain. Such an assumption, however, may or may not be true. Furthermore, doses required for efficacy in animal schizophrenia models are much higher than the ones showing efficacy in animal anxiety models, suggesting unexplained mediators between occupancy and efficacy.
At present, the convergence of preclinical and clinical findings has spawned interest in this approach, including the use of mGluR2 PAMs, which, like full agonists, are effective in animal PCP models of psychosis (110, 122). Nevertheless, ideal target occupancy levels remain unknown for both anxiety disorders and schizophrenia, limiting dose selection strategies for clinical study.
NMDAR antagonists in depression
Mood disorders [major depressive disorder (MDD) and bipolar disorder] are common, chronic, disabling mental disorders affecting the lives of millions worldwide. A large number of individuals fail to respond or tolerate existing therapeutics. Current treatments for depression act by increasing the synaptic availability of serotonin and norepinephrine. They often take weeks to months to achieve response and remission of disease, which usually results in substantial morbidity and disruption in personal, family, professional, and social life, as well as risk for suicidal behavior. Despite the well-recognized limitations of existing antidepressants, there has been little progress in developing more effective or rapidly acting agents since the first antidepressant was introduced for clinical use more than 50 years ago.
The potential role of glutamatergic neurotransmission in depression was first highlighted nearly 20 years ago (123, 124) on the basis of the efficacy of NMDAR antagonists in different preclinical models of depression (125, 126). Effects were observed for a variety of NMDAR antagonists including ketamine (127–131), dizocilpine (MK-801), CGP 37849, and CP-101,606 (132–136). However, the ability of these model systems to predict efficacy in humans remained relatively untested until recently.
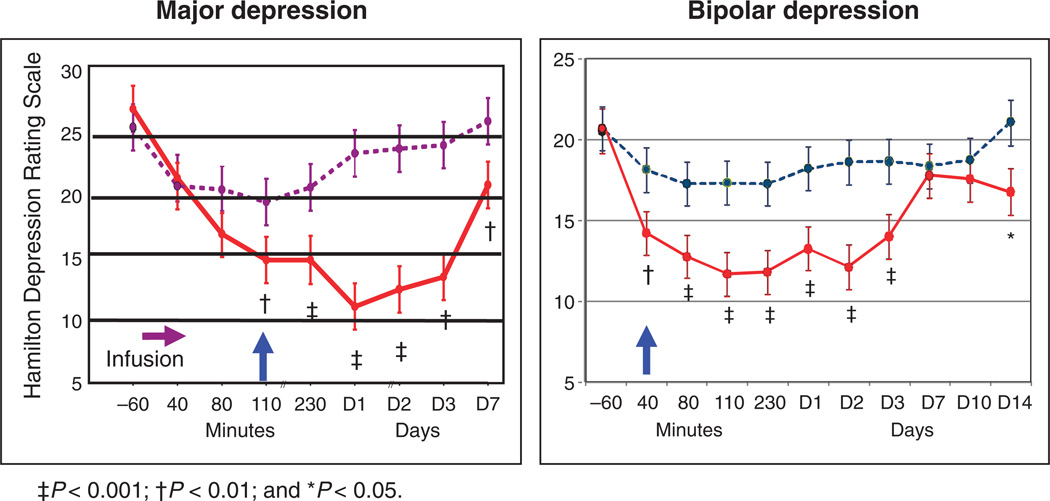
The impetus for clinical research was the serendipitous discovery that ketamine had significant antidepressant effects within 72 hours in seven patients with treatment-resistant MDD participating in ketamine-challenge research (137). Since this initial report, there have been two double-blind, placebo-controlled studies in treatment-resistant major depression: one in MDD and the other in bipolar depression (Fig. 5).
Fig. 5.
(A and B) Effects of the NMDAR antagonist ketamine in unipolar (A) (138) and bipolar (B) depression, showing persistent effect. Adapted from (138) and (140).
In the first study, a single intravenous infusion of ketamine (0.5 mg/kg over 40 min) resulted in a rapid (within 110 min) and relatively sustained (1 to 2 weeks) antidepressant effect (138). The antidepressant effects persisted well beyond the 2- to 3-hour half-life of ketamine and its metabolite norketamine. Ketamine was superior to placebo on the 21-item Hamilton Depression Rating Scale scores from 110 min through 7 days after the single infusion. In categorical analyses, about 70% of patients were found to have responded to ketamine (50% improvement) at 24 hours after infusion, and 35% maintained a sustained response after 1 week of the infusion. It is of note that comparable remission rates with monoaminergic-based antidepressants takes about 10 weeks in patients with nontreatment-resistant major depression (139). Similar effects have subsequently been observed in bipolar depression (140), suggesting generalizability across depressive disorders (Fig. 5).
The antidepressant response to ketamine in treatment-resistant major depression has since been reported in several other, albeit uncontrolled, studies (141, 142). Ketamine has also been found to have significant antisuicidal effects as early as 40 min after infusion (140, 143). Although controlled studies are necessary to confirm ketamine’s rapid antisuicidal properties, the public health implications are potentially enormous.
Ongoing studies are investigating alternatives to ketamine that may be easier to administer and may be associated with reduced psychotomimetic effect. For example, a recent, double-blind, randomized, placebo-controlled clinical trial evaluating the NMDAR-2B subunit–selective antagonist CP-101,606 found that this agent induced significant and relatively rapid antidepressant effects (by day 5) in patients with treatment-resistant MDD, but also showed evidence of psychotomimetic properties (144). As a result, the CP-101,606 research program was discontinued. Additional clinical studies with subunit NMDAR-2A and NMDAR-2B antagonists, however, including AZD6765 (AstraZeneca Pharmaceuticals; ongoing, NCT00986479) and EVT 101 (Evotec Neurosciences; terminated, NCT01128452) are under way or completed.
Although biomarkers have not yet been developed to guide dosing, several variables have been reported to predict initial antidepressant response to ketamine including family history of alcohol dependence (142) and increased pretreatment rostral anterior cingulate cortex activity (145). More recently, anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task was found to predict antidepressant response to ketamine (146). These variables may serve as markers of glutamatergic dysfunction within key brain circuits linked to depression.
On a cellular level, ketamine’s antidepressant effect may reflect increased glutamatergic input through AMPAR relative to NMDAR. In support, one preclinical study showed that ketamine’s antidepressant-like effects in the forced swim test were selectively abolished by using an AMPAR antagonist (NBQX) before ketamine infusion (129). In contrast to ketamine, traditional antidepressants induce delayed effects via intracellular signaling changes (124).
Further extension of this preclinical work suggests that the mammalian target of rapamycin (mTOR)–dependent synapse formation underlies the rapid antidepressant properties of ketamine. In a series of elegant studies by the Duman lab, it was reported that ketamine rapidly activates mTOR pathway, leading to increased synaptic signaling proteins and increased number and function of new spine synapses in the prefrontal cortex of rats (147). However, the current lack of tools to evaluate downstream consequences of NMDAR blockade in humans at both structural and functional levels limits the degree to which mechanistic studies in rodents can be translated into improved antidepressant treatments in patients.
Other conditions
Given the postulated role of FMR1 promoter methylation and resultant mGluR5 up-regulation in FXS, mGluR5 compounds represent a promising treatment for this and related conditions (148, 149). A recent proof-of-concept trial in adult patients with FXS supports this approach, with beneficial results observed only in patients with full FMR1 promoter methylation (150). If mGluR5 target engagement biomarkers become available, future studies assessing both mGluR5 levels in fragile X–related disorders and their engagement by mGluR5 antagonists will yield definitive tests of this approach.
NMDAR-mediated plasticity may also play a key role in neurogenic pain development at both central and peripheral levels. Indeed, ketamine was initially described as an anesthetic and analgesic and remains in use for combination anesthesia. Ketamine continues to be used experimentally for chronic pain, although its use remains limited by psychotomimetic effects. Recently, peripheral NMDARs have been implicated in craniofacial pain (151), although peripheral NMDAR blockade has yet to be proven effective against pain (152).
SUMMARY
To date, all available classes of medication for neuropsychiatric disease have been discovered fortuitously, and theories of disease have been reverse-engineered to explain the findings. Attempts to translate basic scientific advances, including new genetic findings, into effective therapies, therefore, remain a daunting task. To be effective, a highly disciplined approach to drug development is required with target engagement and proof-of-mechanism biomarkers applied at key stages to ensure that both positive and negative clinical results are readily interpretable. Such approaches require ongoing collaboration between basic and clinical scientists and, ideally, ongoing collaboration among industry, academia, and pharma (Fig. 4). Consortia have been developed for conditions such as Alzheimer’s disease, depression, and Parkinson’s disease and should be considered for other CNS disorders such as schizophrenia, autism, substance abuse, and depression. Such approaches will promise to end the current half-century (or more) drought in new mechanistic drug development for neuropsychiatric disorders.
Acknowledgments
Funding: Manuscript preparation funded in part by grants R37MH49334, R01DA03383, and P50MH086385 to D.C.J. Supported by the Intramural Research Program at the National Institute of Mental Health. M.F.B. acknowledges support from NIH, Howard Hughes Medical Institute, FRAXA, and Simons Foundation Autism Research Initiative.
Footnotes
Competing interests: D.C.J. holds intellectual property rights for use of NMDA modulators in treatment of mental disorders including schizophrenia and depression. He holds equity interest in Glytech Inc. He has served as a paid consultant to Schering-Plough, Takeda, NPS, Solvay, Sepracor, AstraZeneca, Pfizer, Cypress, Merck, Sunovion Eli Lilly, and BMS. He has received research grants from Pfizer, Roche, and Jazz and serves on the advisory board for Glytech and Promentis Pharmaceuticals. P.W.K. is listed as co-inventor on a patent application for the combined use of mGluR5-negative allosteric modulators and N-acetylcysteine in the treatment of addiction. P.W.K. has financial interest in Promentis Pharmaceuticals Inc., which is developing compounds to modulate the cystine-glutamate exchanger for treating schizophrenia. C.Z. is listed as a co-inventor on a patent application for the use of ketamine in major depression. C.Z. has assigned his rights in the patent to the U.S. government but will share a percentage of any royalties that may be received by the government. M.F.B. holds equity in, and is a paid consultant for, Seaside Therapeutics Inc. W.Z.P. is a paid consultant to AgeneBio, Amgen, AstraZeneca, BMS, InVivo, Orasi, Pfizer, and Theravance. Hoffmann–La Roche holds the patents for the compound RG1678 referred to in the paper. The other authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 2.Cotman CW, Monaghan DT, Ganong AH. Excitatory amino acid neurotransmission: NMDA receptors and Hebb-type synaptic plasticity. Annu. Rev. Neurosci. 1988;11:61–80. doi: 10.1146/annurev.ne.11.030188.000425. [DOI] [PubMed] [Google Scholar]
- 3.Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol. Psychiatry. 2004;9:984–997. 979. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- 4.Anwyl R. Metabotropic glutamate receptors: Electrophysiological properties and role in plasticity. Brain Res. Brain Res. Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi S. Metabotropic glutamate receptors: Synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 6.Betancur C. Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Res. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 7.Krueger DD, Bear MF. Toward fulfilling the promise of molecular medicine in fragile X syndrome. Annu. Rev. Med. 2011;62:411–429. doi: 10.1146/annurev-med-061109-134644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.International Schizophrenia Consortium. Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, Sklar P. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, Naiman D, Liu QR. Molecular genetics of addiction and related heritable phenotypes: Genome-wide association approaches identify “connectivity constellation” and drug target genes with pleiotropic effects. Ann. N. Y. Acad. Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miles JH. Autism spectrum disorders—A genetics review. Genet. Med. 2011;13:278–294. doi: 10.1097/GIM.0b013e3181ff67ba. [DOI] [PubMed] [Google Scholar]
- 11.Luby ED, Gottlieb JS, Cohen BD, Rosenbaum G, Domino EF. Model psychoses and schizophrenia. Am. J. Psychiatry. 1962;119:61–67. doi: 10.1176/ajp.119.1.61. [DOI] [PubMed] [Google Scholar]
- 12.Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuro-endocrine responses. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 13.Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- 14.Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv. Rev. Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- 15.Javitt DC. Glutamate and schizophrenia: Phencyclidine, N-methyl-d-aspartate receptors, and dopamine–glutamate interactions. Int. Rev. Neurobiol. 2007;78:69–108. doi: 10.1016/S0074-7742(06)78003-5. [DOI] [PubMed] [Google Scholar]
- 16.Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr. Opin. Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 17.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol. Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- 18.Tsai SJ. Central N-acetyl aspartylglutamate deficit: A possible pathogenesis of schizophrenia. Med. Sci. Monit. 2005;11:HY39–HY45. [PubMed] [Google Scholar]
- 19.Wonodi I, Schwarcz R. Cortical kynurenine pathway metabolism: A novel target for cognitive enhancement in schizophrenia. Schizophr. Bull. 2010;36:211–218. doi: 10.1093/schbul/sbq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G, Samuelsson M, Erhardt S. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr. Bull. 2010 doi: 10.1093/schbul/sbq086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bendikov I, Nadri C, Amar S, Panizzutti R, De Miranda J, Wolosker H, Agam G. A CSF and postmortem brain study of d-serine metabolic parameters in schizophrenia. Schizophr. Res. 2007;90:41–51. doi: 10.1016/j.schres.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindström LH, Iyo M. Reduced d-serine to total serine ratio in the cerebrospinal fluid of drug naive schizophrenic patients. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:767–769. doi: 10.1016/j.pnpbp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 23.Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain d-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr. Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Verrall L, Burnet PW, Betts JF, Harrison PJ. The neurobiology of D-amino acid oxidase and its involvement in schizophrenia. Mol. Psychiatry. 2010;15:122–137. doi: 10.1038/mp.2009.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labrie V, Fukumura R, Rastogi A, Fick LJ, Wang W, Boutros PC, Kennedy JL, Semeralul MO, Lee FH, Baker GB, Belsham DD, Barger SW, Gondo Y, Wong AH, Roder JC. Serine racemase is associated with schizophrenia susceptibility in humans and in a mouse model. Hum. Mol. Genet. 2009;18:3227–3243. doi: 10.1093/hmg/ddp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basu AC, Tsai GE, Ma CL, Ehmsen JT, Mustafa AK, Han L, Jiang ZI, Benneyworth MA, Froimowitz MP, Lange N, Snyder SH, Bergeron R, Coyle JT. Targeted disruption of serine racemase affects glutamatergic neurotransmission and behavior. Mol. Psychiatry. 2009;14:719–727. doi: 10.1038/mp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeVito LM, Balu DT, Kanter BR, Lykken C, Basu AC, Coyle JT, Eichenbaum H. Serine racemase deletion disrupts memory for order and alters cortical dendritic morphology. Genes Brain Behav. 2011;10:210–222. doi: 10.1111/j.1601-183X.2010.00656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labrie V, Wang W, Barger SW, Baker GB, Roder JC. Genetic loss of D-amino acid oxidase activity reverses schizophrenia-like phenotypes in mice. Genes Brain Behav. 2010;9:11–25. doi: 10.1111/j.1601-183X.2009.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr. Opin. Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Kayser MS, Dalmau J. The emerging link between autoimmune disorders and neuropsychiatric disease. J. Neuropsychiatry Clin. Neurosci. 2011;23:90–97. doi: 10.1176/appi.neuropsych.23.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Office of National Drug Control Policy. Washington, DC: Executive Office of the President, Office of National Drug Control Policy; 2004. The Economic Costs of Drug Abuse in the United States, 1992–2002. http://www.ncjrs.gov/ondcppubs/publications/pdf/economic_costs.pdf. [Google Scholar]
- 32.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 33.Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 36.Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science. 2010;328:1709–1712. doi: 10.1126/science.1187801. [DOI] [PubMed] [Google Scholar]
- 37.Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A. Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat. Neurosci. 2006;9:868–869. doi: 10.1038/nn1713. [DOI] [PubMed] [Google Scholar]
- 38.Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-acetylcysteine reverses cocaine-induced metaplasticity. Nat. Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol. Psychiatry. 2009;65:841–845. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madayag A, Kau KS, Lobner D, Mantsch JR, Wisniewski S, Baker DA. Drug-induced plasticity contributing to heightened relapse susceptibility: Neurochemical changes and augmented reinstatement in high-intake rats. J. Neurosci. 2010;30:210–217. doi: 10.1523/JNEUROSCI.1342-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou W, Kalivas PW. N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol. Psychiatry. 2008;63:338–340. doi: 10.1016/j.biopsych.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sari Y, Smith KD, Ali PK, Rebec GV. Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J. Neurosci. 2009;29:9239–9243. doi: 10.1523/JNEUROSCI.1746-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol. Psychiatry. 2010;67:81–84. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelleher RJ, III, Bear MF. The autistic neuron: Troubled translation? Cell. 2008;135:401–406. doi: 10.1016/j.cell.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 46.Nordström AL, Farde L, Wiesel FA, Forslund K, Pauli S, Halldin C, Uppfeldt G. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: A double-blind PET study of schizophrenic patients. Biol. Psychiatry. 1993;33:227–235. doi: 10.1016/0006-3223(93)90288-o. [DOI] [PubMed] [Google Scholar]
- 47.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D2 occupancy, clinical response, and side effects: A double-blind PET study of first-episode schizophrenia. Am. J. Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 48.Howes OD, Egerton A, Allan V, McGuire P, Stokes P, Kapur S. Mechanisms underlying psychosis and antipsychotic treatment response in schizophrenia: Insights from PET and SPECT imaging. Curr. Pharm. Des. 2009;15:2550–2559. doi: 10.2174/138161209788957528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Talbot PS, Bradley S, Clarke CP, Babalola KO, Philipp AW, Brown G, McMahon AW, Matthews JC. Brain serotonin transporter occupancy by oral sibutramine dosed to steady state: A PET study using 11C-DASB in healthy humans. Neuropsychopharmacology. 2010;35:741–751. doi: 10.1038/npp.2009.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moresco RM, Matarrese M, Fazio F. PET and SPET molecular imaging: Focus on serotonin system. Curr. Top. Med. Chem. 2006;6:2027–2034. doi: 10.2174/156802606778522140. [DOI] [PubMed] [Google Scholar]
- 51.Burger C, Deschwanden A, Ametamey S, Johayem A, Mancosu B, Wyss M, Hasler G, Buck A. Evaluation of a bolus/infusion protocol for 11C-ABP688, a PET tracer for mGluR5. Nucl. Med. Biol. 2010;37:845–851. doi: 10.1016/j.nucmedbio.2010.04.107. [DOI] [PubMed] [Google Scholar]
- 52.Passchier J, Gentile G, Porter R, Herdon H, Salinas C, Jakobsen S, Audrain H, Laruelle M, Gunn RN. Identification and evaluation of [11C]GSK931145 as a novel ligand for imaging the type 1 glycine transporter with positron emission tomography. Synapse. 2010;64:542–549. doi: 10.1002/syn.20760. [DOI] [PubMed] [Google Scholar]
- 53.Umbricht D, Martin-Facklam M, Pizzagalli E, Youssef E, Yoo K, Doerflinger E, Bausch A, Arrowsmith R, Alberati D, Santarelli L. Glycine transporter type 1 (GLYT1) inhibition RG1678: Results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Schiz. Bull. 2011;37 Suppl. 1:324. [Google Scholar]
- 54.Alberati D, Borroni E, Moreau J, Hainzl D, Pinnard E, Wettstein JG. Partial occupancy of the glycine transporter type 1 in rat by RG1678 leads to efficacy in mouse models relevant to schizophrenia. Schiz. Bull. 2011;37 Suppl. 1:286. [Google Scholar]
- 55.Borroni E, Wong DF, Wallace TL, Zhou Y, Kumar A, Pinard E, Alberati D. Partial occupancy of the glycine transporter type 1 in monkey by RG1678 leads to efficacy in a model of prefrontal cortical function. Schiz. Bull. 2011;37 Suppl. 1:296–297. [Google Scholar]
- 56.Näätänen R. The mismatch negativity: A powerful tool for cognitive neuroscience. Ear Hear. 1995;16:6–18. [PubMed] [Google Scholar]
- 57.Javitt DC. Intracortical mechanisms of mismatch negativity dysfunction in schizophrenia. Audiol. Neurootol. 2000;5:207–215. doi: 10.1159/000013882. [DOI] [PubMed] [Google Scholar]
- 58.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: Defining the pattern. Arch. Gen. Psychiatry. 2000;57:1131–1137. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 59.Umbricht D, Krljes S. Mismatch negativity in schizophrenia: A meta-analysis. Schizophr. Res. 2005;76:1–23. doi: 10.1016/j.schres.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 60.Light GA, Braff DL. Mismatch negativity deficits are associated with poor functioning in schizophrenia patients. Arch. Gen. Psychiatry. 2005;62:127–136. doi: 10.1001/archpsyc.62.2.127. [DOI] [PubMed] [Google Scholar]
- 61.Umbricht D, Koller R, Schmid L, Skrabo A, Grübel C, Huber T, Stassen H. How specific are deficits in mismatch negativity generation to schizophrenia? Biol. Psychiatry. 2003;53:1120–1131. doi: 10.1016/s0006-3223(02)01642-6. [DOI] [PubMed] [Google Scholar]
- 62.Gomot M, Blanc R, Clery H, Roux S, Barthelemy C, Bruneau N. Candidate electrophysiological endophenotypes of hyper-reactivity to change in autism. J. Autism Dev. Disord. 2011;41:705–714. doi: 10.1007/s10803-010-1091-y. [DOI] [PubMed] [Google Scholar]
- 63.Dunn MA, Gomes H, Gravel J. Mismatch negativity in children with autism and typical development. J. Autism Dev. Disord. 2008;38:52–71. doi: 10.1007/s10803-007-0359-3. [DOI] [PubMed] [Google Scholar]
- 64.Pekkonen E. Mismatch negativity in aging and in Alzheimer’s and Parkinson’s diseases. Audiol. Neurootol. 2000;5:216–224. doi: 10.1159/000013883. [DOI] [PubMed] [Google Scholar]
- 65.Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-d-aspartate receptors in auditory sensory memory and mismatch negativity generation: Implications for schizophrenia. Proc. Natl. Acad. Sci. U.S.A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN. The effects of a sub-anaesthetic dose of ketamine on human selective attention. Neuropsychopharmacology. 2000;22:293–302. doi: 10.1016/S0893-133X(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 67.Heekeren K, Daumann J, Neukirch A, Stock C, Kawohl W, Norra C, Waberski TD, Gouzoulis-Mayfrank E. Mismatch negativity generation in the human 5HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology. 2008;199:77–88. doi: 10.1007/s00213-008-1129-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kreitschmann-Andermahr I, Rosburg T, Demme U, Gaser E, Nowak H, Sauer H. Effect of ketamine on the neuromagnetic mismatch field in healthy humans. Brain Res. Cogn. Brain Res. 2001;12:109–116. doi: 10.1016/s0926-6410(01)00043-x. [DOI] [PubMed] [Google Scholar]
- 69.Umbricht D, Schmid L, Koller R, Vollenweider FX, Hell D, Javitt DC. Ketamine-induced deficits in auditory and visual context-dependent processing in healthy volunteers: Implications for models of cognitive deficits in schizophrenia. Arch. Gen. Psychiatry. 2000;57:1139–1147. doi: 10.1001/archpsyc.57.12.1139. [DOI] [PubMed] [Google Scholar]
- 70.Ehrlichman RS, Maxwell CR, Majumdar S, Siegel SJ. Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. J. Cogn. Neurosci. 2008;20:1403–1414. doi: 10.1162/jocn.2008.20097. [DOI] [PubMed] [Google Scholar]
- 71.Tikhonravov D, Neuvonen T, Pertovaara A, Savioja K, Ruusuvirta T, Näätänen R, Carlson S. Effects of an NMDA-receptor antagonist MK-801 on an MMN-like response recorded in anesthetized rats. Brain Res. 2008;1203:97–102. doi: 10.1016/j.brainres.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 72.Umbricht D, Vyssotki D, Latanov A, Nitsch R, Lipp H. Deviance-related electrophysiological activity in mice: Is there mismatch negativity in mice? Clin. Neurophysiol. 2005;116:353–363. doi: 10.1016/j.clinph.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 73.Javitt DC, Jayachandra M, Lindsley RW, Specht CM, Schroeder CE. Schizophrenia-like deficits in auditory P1 and N1 refractoriness induced by the psychomimetic agent phencyclidine (PCP) Clin. Neurophysiol. 2000;111:833–836. doi: 10.1016/s1388-2457(99)00313-2. [DOI] [PubMed] [Google Scholar]
- 74.Maxwell CR, Ehrlichman RS, Liang Y, Trief D, Kanes SJ, Karp J, Siegel SJ. Ketamine produces lasting disruptions in encoding of sensory stimuli. J. Pharmacol. Exp. Ther. 2006;316:315–324. doi: 10.1124/jpet.105.091199. [DOI] [PubMed] [Google Scholar]
- 75.Umbricht D, Vyssotky D, Latanov A, Nitsch R, Brambilla R, D’Adamo P, Lipp HP. Midlatency auditory event-related potentials in mice: Comparison to midlatency auditory ERPs in humans. Brain Res. 2004;1019:189–200. doi: 10.1016/j.brainres.2004.05.097. [DOI] [PubMed] [Google Scholar]
- 76.Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating γ oscillations and delayed auditory responses as translational biomarkers of autism. Biol. Psychiatry. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Donnell BF, Vohs JL, Hetrick WP, Carroll CA, Shekhar A. Auditory event-related potential abnormalities in bipolar disorder and schizophrenia. Int. J. Psychophysiol. 2004;53:45–55. doi: 10.1016/j.ijpsycho.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state γ responses. Biol. Psychiatry. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gonzalez-Burgos G, Lewis DA. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008;34:944–961. doi: 10.1093/schbul/sbn070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tomasi D, Volkow ND. Functional connectivity density mapping. Proc. Natl. Acad. Sci. U.S.A. 2010;107:9885–9890. doi: 10.1073/pnas.1001414107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Horn DI, Yu C, Steiner J, Buchmann J, Kaufmann J, Osoba A, Eckert U, Zierhut KC, Schiltz K, He H, Biswal B, Bogerts B, Walter M. Glutamatergic and resting-state functional connectivity correlates of severity in major depression—The role of pregenual anterior cingulate cortex and anterior insula. Front. Syst. Neurosci. 2010;4:33. doi: 10.3389/fnsys.2010.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang HY, Wang SJ, Liu B, Ma ZL, Yang M, Zhang ZJ, Teng GJ. Resting brain connectivity: Changes during the progress of Alzheimer disease. Radiology. 2010;256:598–606. doi: 10.1148/radiol.10091701. [DOI] [PubMed] [Google Scholar]
- 83.Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry. 2010;67:584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D’Angelo D, Mauro CJ, Milham MP. Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophr. Res. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol. Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Magnuson M, Majeed W, Keilholz SD. Functional connectivity in blood oxygenation level-dependent and cerebral blood volume-weighted resting state functional magnetic resonance imaging in the rat brain. J. Magn. Reson. Imaging. 2010;32:584–592. doi: 10.1002/jmri.22295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lim KO, Helpern JA. Neuropsychiatric applications of DTI—A review. NMR Biomed. 2002;15:587–593. doi: 10.1002/nbm.789. [DOI] [PubMed] [Google Scholar]
- 88.Karlsgodt KH, Niendam TA, Bearden CE, Cannon TD. White matter integrity and prediction of social and role functioning in subjects at ultra-high risk for psychosis. Biol. Psychiatry. 2009;66:562–569. doi: 10.1016/j.biopsych.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ingalhalikar M, Parker D, Bloy L, Roberts TP, Verma R. Diffusion based abnormality markers of pathology: Toward learned diagnostic prediction of ASD. Neuroimage. 2011;57:918–927. doi: 10.1016/j.neuroimage.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guilfoyle DN, Gerum S, Hrabe J. Murine diffusion imaging using snapshot interleaved EPI acquisition at 7T. J. Neurosci. Methods. 2011;199:10–14. doi: 10.1016/j.jneumeth.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stone JM. Imaging the glutamate system in humans: Relevance to drug discovery for schizophrenia. Curr. Pharm. Des. 2009;15:2594–2602. doi: 10.2174/138161209788957438. [DOI] [PubMed] [Google Scholar]
- 92.Henry ME, Lauriat TL, Shanahan M, Renshaw PF, Jensen JE. Accuracy and stability of measuring GABA, glutamate, and glutamine by proton magnetic resonance spectroscopy: A phantom study at 4 Tesla. J. Magn. Reson. 2011;208:210–218. doi: 10.1016/j.jmr.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bustillo JR, Rowland LM, Mullins P, Jung R, Chen H, Qualls C, Hammond R, Brooks WM, Lauriello J. 1H-MRS at 4 Tesla in minimally treated early schizophrenia. Mol. Psychiatry. 2010;15:629–636. doi: 10.1038/mp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindström LH, Iyo M. Elevated glutamine/glutamate ratio in cerebrospinal fluid of first episode and drug naive schizophrenic patients. BMC Psychiatry. 2005;5:6. doi: 10.1186/1471-244X-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toth E, Lajtha A. Antagonism of phencyclidine-induced hyperactivity by glycine in mice. Neurochem. Res. 1986;11:393–400. doi: 10.1007/BF00965013. [DOI] [PubMed] [Google Scholar]
- 96.Waziri R. Glycine therapy of schizophrenia. Biol. Psychiatry. 1988;23:210–211. doi: 10.1016/0006-3223(88)90093-5. [DOI] [PubMed] [Google Scholar]
- 97.Kantrowitz JT, Javitt DC. N-methyl-d-aspartate (NMDA) receptor dysfunction or dysregulation: The final common pathway on the road to schizophrenia? Brain Res. Bull. 2010;83:108–121. doi: 10.1016/j.brainresbull.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsai GE, Lin PY. Strategies to enhance N-methyl-d-aspartate receptor-mediated neurotransmission in schizophrenia, a critical review and meta-analysis. Curr. Pharm. Des. 2010;16:522–537. doi: 10.2174/138161210790361452. [DOI] [PubMed] [Google Scholar]
- 99.Javitt DC. Glycine transport inhibitors for the treatment of schizophrenia: Symptom and disease modification. Curr. Opin. Drug Discov. Devel. 2009;12:468–478. [PubMed] [Google Scholar]
- 100.Javitt DC, Frusciante M. Glycyldodecylamide, a phencyclidine behavioral antagonist, blocks cortical glycine uptake: Implications for schizophrenia and substance abuse. Psychopharmacology. 1997;129:96–98. doi: 10.1007/s002130050168. [DOI] [PubMed] [Google Scholar]
- 101.Javitt DC, Balla A, Sershen H, Lajtha A. A.E. Bennett Research Award. Reversal of phencyclidine-induced effects by glycine and glycine transport inhibitors. Biol. Psychiatry. 1999;45:668–679. doi: 10.1016/s0006-3223(98)00237-6. [DOI] [PubMed] [Google Scholar]
- 102.Bergeron R, Meyer TM, Coyle JT, Greene RW. Modulation of N-methyl-d-aspartate receptor function by glycine transport. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15730–15734. doi: 10.1073/pnas.95.26.15730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Umbricht D, Yoo K, Youssef E, Dorflinger E, Martin-Facklam M, Bausch A, Arrowsmith R, Alberati D, Marder SR, Santarelli L. Glycine transporter type 1 (GLYT1) inhibitor RG1678: Positive results of the proof-of-concept study for the treatment of negative symptoms in schizophrenia. Neuropsychopharmacology. 2010;35:S320–S321. [Google Scholar]
- 104.Hashimoto K, Fujita Y, Horio M, Kunitachi S, Iyo M, Ferraris D, Tsukamoto T. Co-administration of a D-amino acid oxidase inhibitor potentiates the efficacy of D-serine in attenuating prepulse inhibition deficits after administration of dizocilpine. Biol. Psychiatry. 2009;65:1103–1106. doi: 10.1016/j.biopsych.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 105.Swanson CJ, Bures M, Johnson MP, Linden AM, Monn JA, Schoepp DD. Metabotropic glutamate receptors as novel targets for anxiety and stress disorders. Nat. Rev. Drug Discov. 2005;4:131–144. doi: 10.1038/nrd1630. [DOI] [PubMed] [Google Scholar]
- 106.Linden AM, Schoepp DD. Metabotropic glutamate receptor targets for neuropsychiatric disorders. Drug Discov. Today Ther. Strateg. 2006;3:507–517. [Google Scholar]
- 107.Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci. Lett. 1997;229:161–164. doi: 10.1016/s0304-3940(97)00442-4. [DOI] [PubMed] [Google Scholar]
- 108.Linden AM, Shannon H, Baez M, Yu JL, Koester A, Schoepp DD. Anxiolytic-like activity of the mGLU2/3 receptor agonist LY354740 in the elevated plus maze test is disrupted in metabotropic glutamate receptor 2 and 3 knock-out mice. Psychopharmacology. 2005;179:284–291. doi: 10.1007/s00213-004-2098-x. [DOI] [PubMed] [Google Scholar]
- 109.Dunayevich E, Erickson J, Levine L, Landbloom R, Schoepp DD, Tollefson GD. Efficacy and tolerability of an mGlu2/3 agonist in the treatment of generalized anxiety disorder. Neuropsychopharmacology. 2008;33:1603–1610. doi: 10.1038/sj.npp.1301531. [DOI] [PubMed] [Google Scholar]
- 110.Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD. Metabotropic glutamate 2 receptor potentiators: Receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s) Psychopharmacology. 2005;179:271–283. doi: 10.1007/s00213-004-2099-9. [DOI] [PubMed] [Google Scholar]
- 111.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 112.Krystal JH, Belger A, D’Souza DC, Anand A, Charney DS, Aghajanian GK, Moghaddam B. Therapeutic implications of the hyperglutamatergic effects of NMDA antagonists. Neuropsychopharmacology. 1999;21:S143–S157. [Google Scholar]
- 113.Schoepp DD, Wright RA, Levine LR, Gaydos B, Potter WZ. LY354740, an mGlu2/3 receptor agonist as a novel approach to treat anxiety/stress. Stress. 2003;6:189–197. doi: 10.1080/1025389031000146773. [DOI] [PubMed] [Google Scholar]
- 114.Cartmell J, Monn JA, Schoepp DD. The metabotropic glutamate 2/3 receptor agonists LY354740 and LY379268 selectively attenuate phencyclidine versus d-amphetamine motor behaviors in rats. J. Pharmacol. Exp. Ther. 1999;291:161–170. [PubMed] [Google Scholar]
- 115.Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD. Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: A randomized phase 2 clinical trial. Nat. Med. 2007;13:1102–1107. doi: 10.1038/nm1632. [DOI] [PubMed] [Google Scholar]
- 116.Cartmell J, Salhoff CR, Perry KW, Monn JA, Schoepp DD. Dopamine and 5-HT turnover are increased by the mGlu2/3 receptor agonist LY379268 in rat medial prefrontal cortex, nucleus accumbens and striatum. Brain Res. 2000;887:378–384. doi: 10.1016/s0006-8993(00)03067-5. [DOI] [PubMed] [Google Scholar]
- 117.Swanson CJ, Schoepp DD. A role for noradrenergic transmission in the actions of phencyclidine and the antipsychotic and antistress effects of mGlu2/3 receptor agonists. Ann. N. Y. Acad. Sci. 2003;1003:309–317. doi: 10.1196/annals.1300.019. [DOI] [PubMed] [Google Scholar]
- 118.Marek GJ, Wright RA, Schoepp DD. 5-Hydroxytryptamine2A (5-HT2A) receptor regulation in rat prefrontal cortex: Interaction of a phenethylamine hallucinogen and the metabotropic glutamate2/3 receptor agonist LY354740. Neurosci. Lett. 2006;403:256–260. doi: 10.1016/j.neulet.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 119.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 120.Schoepp DD, Marek GJ. Preclinical pharmacology of mGlu2/3 receptor agonists: Novel agents for schizophrenia? Curr. Drug Targets CNS Neurol. Disord. 2002;1:215–225. doi: 10.2174/1568007024606177. [DOI] [PubMed] [Google Scholar]
- 121.Krystal JH, Abi-Saab W, Perry E, D’Souza DC, Liu N, Gueorguieva R, McDougall L, Hunsberger T, Belger A, Levine L, Breier A. Preliminary evidence of attenuation of the disruptive effects of the NMDA glutamate receptor antagonist, ketamine, on working memory by pretreatment with the group II metabotropic glutamate receptor agonist, LY354740, in healthy human subjects. Psychopharmacology. 2005;179:303–309. doi: 10.1007/s00213-004-1982-8. [DOI] [PubMed] [Google Scholar]
- 122.Conn PJ, Tamminga C, Schoepp DD, Lindsey C. Schizophrenia: Moving beyond monoamine antagonists. Mol. Interv. 2008;8:99–107. doi: 10.1124/mi.8.2.7. [DOI] [PubMed] [Google Scholar]
- 123.Skolnick P, Popik P, Trullas R. Glutamate-based antidepressants: 20 years. Trends Pharmacol. Sci. 2009;30:563–569. doi: 10.1016/j.tips.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 124.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Machado-Vieira R, Salvadore G, Ibrahim LA, Diaz-Granados N, Zarate CA., Jr Targeting glutamatergic signaling for the development of novel therapeutics for mood disorders. Curr. Pharm. Des. 2009;15:1595–1611. doi: 10.2174/138161209788168010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zarate CA, Quiroz J, Payne J, Manji HK. Modulators of the glutamatergic system: Implications for the development of improved therapeutics in mood disorders. Psychopharmacol. Bull. 2002;36:35–83. [PubMed] [Google Scholar]
- 127.Aguado L, San Antonio A, Pérez L, del Valle R, Gómez J. Effects of the NMDA receptor antagonist ketamine on flavor memory: Conditioned aversion, latent inhibition, and habituation of neophobia. Behav. Neural Biol. 1994;61:271–281. doi: 10.1016/s0163-1047(05)80010-x. [DOI] [PubMed] [Google Scholar]
- 128.Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, Quevedo J. Acute administration of ketamine induces antidepressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 129.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: Role of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol. Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 130.Mickley GA, Schaldach MA, Snyder KJ, Balogh SA, Len T, Neimanis K, Goulis P, Hug J, Sauchak K, Remmers-Roeber DR, Walker C, Yamamoto BK. Ketamine blocks a conditioned taste aversion (CTA) in neonatal rats. Physiol. Behav. 1998;64:381–390. doi: 10.1016/s0031-9384(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 131.Silvestre JS, Nadal R, Pallarés M, Ferré N. Acute effects of ketamine in the holeboard, the elevated-plus maze, and the social interaction test in Wistar rats. Depress. Anxiety. 1997;5:29–33. [PubMed] [Google Scholar]
- 132.Meloni D, Gambarana C, De Montis MG, Dal Prá P, Taddei I, Tagliamonte A. Dizocilpine antagonizes the effect of chronic imipramine on learned helplessness in rats. Pharmacol. Biochem. Behav. 1993;46:423–426. doi: 10.1016/0091-3057(93)90374-3. [DOI] [PubMed] [Google Scholar]
- 133.Padovan CM, Guimarães FS. Antidepressant-like effects of NMDA-receptor antagonist injected into the dorsal hippocampus of rats. Pharmacol. Biochem. Behav. 2004;77:15–19. doi: 10.1016/j.pbb.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 134.Papp M, Moryl E. New evidence for the antidepressant activity of MK-801, a noncompetitive antagonist of NMDA receptors. Pol. J. Pharmacol. 1993;45:549–553. [PubMed] [Google Scholar]
- 135.Skolnick P, Miller R, Young A, Boje K, Trullas R. Chronic treatment with 1-aminocyclopropanecarboxylic acid desensitizes behavioral responses to compounds acting at the N-methyl-d-aspartate receptor complex. Psychopharmacology. 1992;107:489–496. doi: 10.1007/BF02245261. [DOI] [PubMed] [Google Scholar]
- 136.Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur. J. Pharmacol. 1990;185:1–10. doi: 10.1016/0014-2999(90)90204-j. [DOI] [PubMed] [Google Scholar]
- 137.Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 138.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-d-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 139.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M. STAR*D Study Team, Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am. J. Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 140.Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-d-aspartate antagonist in treatment-resistant bipolar depression. Arch. Gen. Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Matthew SJ. Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol. Psychiatry. 2010;67:139–145. doi: 10.1016/j.biopsych.2009.08.038. [DOI] [PubMed] [Google Scholar]
- 142.Phelps LE, Brutsche N, Moral JR, Luckenbaugh DA, Manji HK, Zarate CA., Jr Family history of alcohol dependence and initial antidepressant response to an N-methyl-d-aspartate antagonist. Biol. Psychiatry. 2009;65:181–184. doi: 10.1016/j.biopsych.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Price RB, Nock MK, Charney D, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol. Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J. Clin. Psychopharmacol. 2008;28:631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 145.Salvadore G, Cornwell BR, Colon-Rosario V, Coppola R, Grillon C, Zarate CA, Jr, Manji HK. Increased anterior cingulate cortical activity in response to fearful faces: A neurophysiological biomarker that predicts rapid antidepressant response to ketamine. Biol. Psychiatry. 2009;65:289–295. doi: 10.1016/j.biopsych.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Salvadore G, Cornwell BR, Sambataro F, Latov D, Colon-Rosario V, Carver F, Holroyd T, DiazGranados N, Machado-Vieira R, Grillon C, Drevets WC, Zarate CA., Jr Anterior cingulate desynchronization and functional connectivity with the amygdala during a working memory task predict rapid antidepressant response to ketamine. Neuropsychopharmacology. 2010;35:1415–1422. doi: 10.1038/npp.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang LW, Berry-Kravis E, Hagerman RJ. Fragile X: Leading the way for targeted treatments in autism. Neurotherapeutics. 2010;7:264–274. doi: 10.1016/j.nurt.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Erickson CA, Mullett JE, McDougle CJ. Brief report: Acamprosate in fragile X syndrome. J. Autism Dev. Disord. 2010;40:1412–1416. doi: 10.1007/s10803-010-0988-9. [DOI] [PubMed] [Google Scholar]
- 150.Jacquemont S, Curie A, des Portes V, Torrioli MG, Berry-Kravis E, Hagerman RJ, Ramos FJ, Cornish K, He Y, Paulding C, Neri G, Chen F, Hadjikhani N, Martinet D, Meyer J, Beckmann JS, Delange K, Brun A, Bussy G, Gasparini F, Hilse T, Floesser A, Branson J, Bilbe G, Johns D, Gomez-Mancilla B. Epigenetic modification of the FMR1 gene in fragile X syndrome is associated with differential response to themGluR5 antagonist AFQ056. Sci. Transl. Med. 2011;3:64ra1. doi: 10.1126/scitranslmed.3001708. [DOI] [PubMed] [Google Scholar]
- 151.Gazerani P, Dong X, Wang M, Kumar U, Cairns BE. Sensitization of rat facial cutaneous mechanoreceptors by activation of peripheral N-methyl-d-aspartate receptors. Brain Res. 2010;1319:70–82. doi: 10.1016/j.brainres.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 152.Castrillon EE, Cairns BE, Ernberg M, Wang K, Sessle BJ, Arendt-Nielsen L, Svensson P. Effect of peripheral NMDA receptor blockade with ketamine on chronic myofascial pain in temporomandibular disorder patients: A randomized, double-blinded, placebo-controlled trial. J. Orofac. Pain. 2008;22:122–130. [PubMed] [Google Scholar]
- 153.Walsh T, McClellan JM, McCarthy SE, Addington AM, Pierce SB, Cooper GM, Nord AS, Kusenda M, Malhotra D, Bhandari A, Stray SM, Rippey CF, Roccanova P, Makarov V, Lakshmi B, Findling RL, Sikich L, Stromberg T, Merriman B, Gogtay N, Butler P, Eckstrand K, Noory L, Gochman P, Long R, Chen Z, Davis S, Baker C, Eichler EE, Meltzer PS, Nelson SF, Singleton AB, Lee MK, Rapoport JL, King MC, Sebat J. Rare structural variants disrupt multiple genes in neurodevelopmental pathways in schizophrenia. Science. 2008;320:539–543. doi: 10.1126/science.1155174. [DOI] [PubMed] [Google Scholar]
- 154.Allen NC, Bagade S, McQueen MB, Ioannidis JP, Kavvoura FK, Khoury MJ, Tanzi RE, Bertram L. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: The SzGene database. Nat. Genet. 2008;40:827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 155.Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]