Abstract
Background:
Activating mutations of Fms-like tyrosine kinase 3 (FLT3) constitute a major driver in the pathogenesis of acute myeloid leukaemia (AML). Hence, pharmacological inhibitors of FLT3 are of therapeutic interest for AML.
Methods:
The effects of inhibition of FLT3 activity by a novel potent FLT3 inhibitor, BPR1J-097, were investigated using in vitro and in vivo assays.
Results:
The 50% inhibitory concentration (IC50) of BPR1J-097 required to inhibit FLT3 kinase activity ranged from 1 to 10 nM, and the 50% growth inhibition concentrations (GC50s) were 21±7 and 46±14 nM for MOLM-13 and MV4-11 cells, respectively. BPR1J-097 inhibited FLT3/signal transducer and activator of transcription 5 phosphorylation and triggered apoptosis in FLT3-driven AML cells. BPR1J-097 also showed favourable pharmacokinetic property and pronounced dose-dependent tumour growth inhibition and regression in FLT3-driven AML murine xenograft models.
Conclusion:
These results indicate that BPR1J-097 is a novel small molecule FLT-3 inhibitor with promising in vivo anti-tumour activities and suggest that BPR1J-097 may be further developed in preclinical and clinical studies as therapeutics in AML treatments.
Keywords: acute myeloid leukaemia, FLT3, FLT3-ITD, MOLM-13, MV4-11, kinase inhibitor
Acute myeloid leukaemia (AML) is the most common type of adult leukaemia. It is an aggressive disease that involves rapid growth of abnormal leukaemic cells in the bone marrow, resulting in failure of production of normal blood cells (Dormer et al, 1980). Fms-like tyrosine kinase 3 (FLT3), a cell surface receptor belonging to the class III receptor tyrosine kinase family, has a pivotal role in the differentiation and survival of haematopoietic stem cells in the bone marrow (Scheijen and Griffin, 2002; Markovic et al, 2005). Fms-like tyrosine kinase 3 is frequently overexpressed and mutated in AML patients and is a major driver in the pathogenesis of AML cancer cells (Stirewalt and Radich, 2003; Kiyoi et al, 2005). Activating mutations of FLT3 that result in constitutive FLT3 tyrosine kinase activity lead to activation of downstream signal molecules, including signal transducer and activator of transcription 5 (STAT5), Ras, MAPK, PI3K, and Akt, to subsequently stimulate the survival and proliferation of leukaemic cells (Zhang and Broxmeyer, 1999; Hayakawa et al, 2000; Mizuki et al, 2000; Brandts et al, 2005; Sallmyr et al, 2008). Two types of activating FLT3 mutations, namely FLT3-ITD (an internal tandem duplication mutation in the juxtamembrane domain) and FLT3-KDM (a missense mutation at the Asp835 residue within the kinase domain), account for ∼30% of AML patients (Nakao et al, 1996; Kiyoi et al, 1998; Yamamoto et al, 2001; Gilliland and Griffin, 2002a, 2002b). Clinically, these mutations are associated with poor prognosis for AML patients receiving traditional chemotherapy (Thiede et al, 2002; Yanada et al, 2005). Therefore, activated FLT3 (due to FLT3-ITD and/or FLT3-KDM) is a promising molecular target for AML therapies.
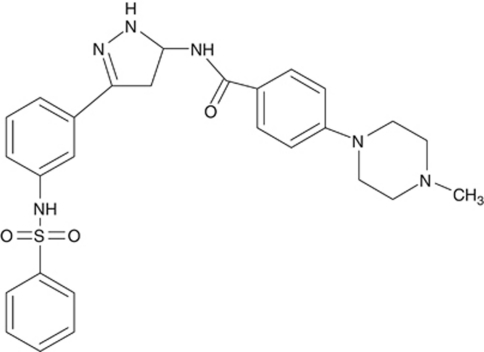
A number of small molecule FLT3 inhibitors have entered clinical trials. These include sorafenib (BAY 43-9006), sunitinib (SU11248), midostaurin (PKC412), lestaurtinib (CEP-701), tandutinib (MLN518), ABT-869, KW-2449, and AC220 (Wong et al, 2009; Kindler et al, 2010; Wiernik, 2010). However, approval of these agents for FLT3-associated diseases is still challenging, which was suspected to be due to the failure to fully inhibit FLT3 in tumours and undesirable drug properties (Ustun et al, 2009). The purpose of this study was to characterise the pharmacological profile of a novel FLT3 kinase inhibitor, BPR1J-097 (N1-(3-3-[(phenylsulphonyl)amino]phenyl-1H-5-pyrazolyl)-4-(4-methylpiperazino) benzamide) (Figure 1), that was discovered by a rational design strategy. BPR1J-097 with a novel sulphonamide pharmacophore exhibits potent FLT3-inhibitory activity and has potent growth-inhibitory effects on FLT3-ITD leukaemic cells. According to our SAR studies, we found that sulphonamide pharmacophore preferred at meta-position of the phenyl ring, and that replacement the sulphonamide group of BPR1J-097 with the urea group resulted in a significant loss in cellular potency (data not shown). Inhibition of FLT3 resulted in blockage of FLT3 and STAT5 phosphorylation and triggered apoptosis in cancer cells relying on FLT3 signalling for survival. In addition, BPR1J-097 showed favourable pharmacokinetic properties and significant dose-dependent tumour reduction in FLT3-ITD murine xenograft models.
Figure 1.
Chemical structure of BPR1J-097 (N1-(3-3-[(phenylsulphonyl)amino]phenyl-1H-5-pyrazolyl)-4-(4-methylpiperazino) benzamide).
These results demonstrate the potential of BPR1J-097 as a therapeutic candidate for treatment of AML patients. Further preclinical and clinical studies in AML patients are warranted.
Materials and methods
Reagents
The FLT3 inhibitors BPR1J-097 and ABT-869 were synthesised by our laboratory. Sorafenib and PKC412 were obtained from Calbiochem (Darmstadt, Germany). The recombinant human FLT3 ligand (T27-P185) was purchased from R&D Systems (Minneapolis, MN, USA). The anti-phospho and total (FLT3, STAT5, and Erk1/2) (Cell Signaling Technology, Beverly, MA, USA), anti-cleaved poly (ADP-ribose) polymerase (Cell Signaling Technology), and anti-caspase 3 (Imgenex, San Diego, CA, USA), anti-β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) antibodies were purchased for western blotting analysis. The N-terminal GST-tagged human FLT3 (residues Y567-S993), VEGFR1 (residues R781-I1338), and VEGFR2 (residues V789-V1356), expressed in Sf9 insect cells, were purified for kinase assay (Lin et al, 2008). The preparation of recombinant Aurora A (residues R781-I1338) was described in a previous study (Coumar et al, 2008). Aurora B (Upstate, Billerica, MA, USA) was purchased for biochemical kinase assay. Human full-length FLT3 expression clone (pCMV6-Entry-FLT3-WT, RC211459) was purchased from OriGene (Rockville, MD, USA). The pCMV6-Entry-FLT3-ITD plasmid was constructed by introducing the RT–PCR fragment, generated by 5′-TATGCAACAATTGGTGTTTGTCTCC-3′ and 5′-TACGCGTCGAATCTTCGACCTGAGAGCCTGC-3′ primers with MV4-11 cDNA templates, into pCMV6-Entry-FLT3-WT MfeI- and MluI-digested fragments. The pCMV6-Entry-FLT3-D835Y plasmid was constructed by mutagenic primers (5′-TGGATTGGCTCGATATATCATGAGTGA-3′ and 5′-TCACTCATGATATATCGAGCCAATCCA-3′) and pCMV6-Entry-FLT3-WT as template using the QuikChange II Site-Directed Mutagenesis Kit (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer's recommendations.
Cell lines
MOLM-13 cells were purchased from the Deutsche Sammlung von Microorganismen und Zellkulturen GmbH (Braunschweig, Germany). RS4;11, MV4-11, U937, and K562 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). All cell lines were grown in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS) (Fisher Scientific, Pittsburgh, PA, USA). HEK293T- and FLT3-transfected HEK293T cells were cultured in DMEM (Invitrogen) medium with 10% FBS.
Biochemical kinase assays
The FLT3 Kinase-Glo kinase assays were carried out in 96-well plates at 30°C for 4 h in a final volume of 50 μl, including 25 mM Tris pH 7.4, 10 mM MgCl2, 4 mM MnCl2, 1 mM DTT, 0.02% Triton X-100, 0.01% BSA, 1 μM ATP, 20 μM peptide (GGMEDIYFEFMGGKKK), 75 ng recombinant FLT3 proteins, and test compound at the indicated concentration. The VEGFR1 or VEGFR2 kinase assay was carried out in 96-well plates with tested compound in a final volume of 50 μl reaction at 30°C for 2 h with the following components: 25 mM HEPES pH 7.4, 10 mM MgCl2, 4 mM MnCl2, 0.5 mM Na3VO4, 2 mM DTT, 0.02% Triton X-100, 0.01% BSA, 1 μM ATP, 2 μM polyGlu4:Tyr peptide, 100 ng recombinant VEGFR1 or VEGFR2 protein. Aurora kinase A and Aurora kinase B assays were performed as reported by us in an earlier study (Coumar et al, 2008). After incubation, 50 μl Kinase-Glo Plus Reagent (Promega, Madison, WI, USA) was added and incubated at 25°C for 20 min. A 70 μl aliquot of each reaction mixture was transferred to a black microtiter plate and the luminescence was measured on a Wallac Vector 1420 multilabel counter (Perkin-Elmer, Shelton, CT, USA). Each IC50 value was determined by three different experiments. Kinase inhibition profiling and FLT3-D835Y-inhibitory activity were determined by Invitrogen SelectScreen kinase profiling service.
Cellular proliferation assays
Proliferation assays were performed by seeding 10 000 cells per well in a 96-well culture plate. After 16 h, cells were then treated with vehicle or test compounds at various concentrations of the tested compound in medium for 72 h. Cell viability was quantitated using the MTS method (Promega) according to the manufacturer's recommended protocol. The results were determined by measuring absorbance at 490 nm using a plate reader (Victor2; Perkin-Elmer). The GC50 value was defined as the amount of compound that caused 50% reduction in cell viability in comparison with DMSO-treated (vehicle) control and was calculated using Prism version 4 software (GraphPad, San Diego, CA, USA).
Western blotting
Cells were lysed in lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 1 mM sodium orthovanadate, 1 mM PMSF, and 1 mM DTT). Protein lysates were resolved in SDS–PAGE and then transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). Membranes were immunoblotted with appropriate antibodies and reacted with the SuperSignal reagent (Pierce, Rockford, IL, USA), followed by exposure to X-ray film.
Pharmacokinetic studies
Male Sprague–Dawley rats weighing 300–400 g each (8–12 weeks old) were obtained from BioLASCO (Taiwan Co., Ltd, Ilan, Taiwan). Animals were surgically prepared with a jugular-vein cannula one day before dosing and fasted overnight (for approximately 18–20 h) before dosing. Water was available ad libitum throughout the experiment. Food was provided at 4 h after dosing. Single 3.4 mg kg−1 dose of BPR1J-097, as a PEG400/water (80/20, v/v) solution, was separately administered to groups of 3 rats each intravenously (i.v.) by a bolus injection through the jugular-vein cannula. Each animal received 1 ml of the dosing solution per kg of body weight i.v. At 0 (before dosing), 2, 5, 15, and 30 min and at 1, 2, 4, 6, 8, and 24 h after dosing, a blood sample (0.15 ml) was collected from each animal through the jugular-vein cannula and stored in ice (0–4°C). Immediately after collecting the blood sample, 150 μl of physiological saline (containing 30 Units of heparin per ml) was injected into the rat through the jugular-vein cannula. Plasma was separated from the blood by centrifugation (14 000 g for 15 min at 4°C in a Beckman Model AllegraTM 6R centrifuge) and stored in a freezer (−20°C). All samples were analysed for the parent drug by LC-MS/MS. Data were acquired through selected reaction ion monitoring. Plasma concentration data were analysed with non-compartmental method.
Subcutaneously xenograft tumour models
Male nude mice (Nu-Fox1nu) of 8 weeks of age were purchased from BioLASCO (Taipei, Taiwan, R.O.C.). Nude mice (n=5–7 per group) were inoculated subcutaneously with MOLM-13 (1 × 106 per flank) or MV4-11 cells (5 × 106 per flank). All human cancer cells were detected as free of Mycoplasma spp before they were injected into animals. When the tumour size reached 100–200 mm3, animals were grouped and treated with BPR1J-97 at various doses in a 2-week treatment period as indicated. Animals were treated with BPR1J-097 (10 and 25 mg kg−1, i.v.) or vehicle as control at once daily for 5 days per week for 2 weeks. Tumour volumes were measured and calculated with the formula length × width2/2 after initiation of treatments. Tumour size and animal body weight were measured twice a week after tumour cell inoculation. At the end of the study, animals were killed by carbon dioxide inhalation followed by cervical dislocation. The significant difference between drug treatment and vehicle control were analysed using one-way ANOVA and Student–Newman–Keuls test. The level of a statistical significance was set at P<0.05. The uses and experimental procedures in animals were approved by the IACUC (Institutional Animal Care and Use Committee) of the National Health Research Institutes and met the standards required by the UKCCCR (United Kingdom Coordinating Committee on Cancer Research) guidelines (Workman et al, 2010).
Results
BPR1J-097 is a potent inhibitor of FLT3 activity
BPR1J-097 is a novel compound with a novel sulphonamide pharmacophore (Figure 1). BPR1J-097 exhibits potent FLT3-inhibitory activity and has potent growth-inhibitory effects on FLT3-ITD leukaemic cells. As shown in Table 1, BPR1J-097 potently inhibited wild-type FLT3 (FLT3-WT) activity with an IC50 of 11±7 nM. In comparison, the IC50 of ABT-869 was 17±7 nM as measured in this study. The IC50 value of ABT-869 for inhibition of FLT3-WT was 4 nM as previously reported by Shankar et al (2007). BPR1J-097 specifically targets FLT3 kinase with weaker inhibitory activity towards related kinases such as FLT1 (VEGFR1) and KDR (VEGFR2) (Table 2). In a screening assay for kinase inhibition specificity, 59%, and 91% of FLT1 and KDR activities, respectively, were inhibited by BPR1J-097 at 1 μM. Subsequently, the IC50s of BPR1J-097 were determined to be 211 and 129 nM for FLT1 and KDR, respectively. As some FLT3 inhibitors were also found to be potent inhibitors for Aurora kinases (Harrington et al, 2004; McLaughlin et al, 2010), due to the high degree of similarity in their ATP-binding pockets (Pollard and Mortimore, 2009), the IC50s of BPR1J-097 were also determined for Aurora A and B kinases. It is interesting to note that the IC50s of BPR1J-097 were 340 and 876 nM for Aurora A kinase and Aurora B kinase, respectively. Overall, BPR1J-097 is a quite specific FLT3 inhibitor.
Table 1. FLT3 kinase-inhibitory activity of BPR1J-097.
| In vitro kinase inhibition IC50, nM | |
|---|---|
| BPR1J-097 | 11±7 |
| ABT-869 | 17±7 |
| Sorafenib | 44±9 |
| PKC412 | 37±5 |
Abbreviation: FLT3=Fms-like tyrosine kinase 3.
Each IC50 determination was performed with eight concentrations by kinase-Glo assay. Data represent mean±s.d. from three different experiments.
Table 2. Specificity of kinase inhibition of BPR1J-097.
| Kinase | Percentage of inhibition | IC50b, nM |
|---|---|---|
| FLT3 | 96 | 11 |
| FLT3-D835Y | 99 | 3c |
| FLT1 (VEGFR1) | 59 | 211 |
| KDR (VEGFR2) | 91 | 129 |
| PDGFRA (PDGFR-α) | 80 | ND |
| TEK (Tie2) | 61 | ND |
| KIT | 49 | ND |
| AURKA (Aurora A) | 80 | 340 |
| AURKB (Aurora B) | 79 | 876 |
| AURKC (Aurora C) | 26 | ND |
| AMPK A1/B1/G1 | 82 | ND |
| SRC | 81 | ND |
Abbreviations: FLT3=Fms-like tyrosine kinase 3; ND=not determined; PDGFR=platelet-derived growth factor receptor; VEGFR=vascular endothelial growth factor receptor.
Inhibition of kinase at 1 μM, carried out by Invitrogen SelectScreen kinase profiling service.
IC50 determination was performed by kinase-Glo assay.
IC50 determination was performed by Invitrogen SelectScreen kinase assay.
BPR1J-097 inhibits the phosphorylation of FLT3 and STAT5 in cells
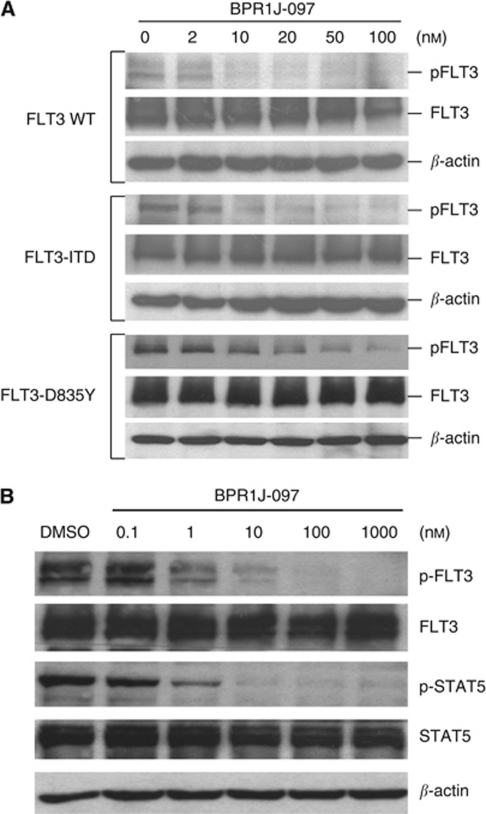
To examine whether BPR1J-097 was able to inhibit the phosphorylation of FLT3, HEK293T cells were transfected with plasmids encoding the FLT3-WT or mutants FLT3 (FLT3-ITD, FLT3-D835Y). 293T cells were treated with BPR1J-097 at various concentrations for 2 h and then the FLT3 ligand (50 ng ml−1) was added for 5 min to prepare cell lysates for western analysis. As shown in Figure 2A, phosphorylation of all FLT3-WT, FLT3-IDT, and FLT3-D835Y were inhibited by BPR1J-097 at a concentration as low as 10 nM. The constitutive activation of STAT5 has a pivotal role in FLT3-ITD leukaemia cell expansion and survival (Hayakawa et al, 2000). To characterise the effects of BPR1J-097 on the FLT3 signalling pathway in FLT3-driven cells, MOLM-13 and MV4-11 cells were cultured in the presence of different concentrations of BPR1J-097 for 2 h, and inhibition of the phosphorylation of FLT3 and STAT5 was examined by western blot analysis. As shown in Figure 2B, BPR1J-097 suppressed the phosphorylation of FLT3 and STAT5 in a dose-dependent manner. The observed IC50 was ∼10 nM for FLT3-ITD activity and 1 nM for STAT5 phosphorylation.
Figure 2.
BPR1J-097 inhibits FLT3-dependent signalling. (A) HEK 293T cells were transfected with FLT-WT-, FLT3-ITD-, or FLT3-D835Y-expressing plasmids for 24 h and then incubated with various concentrations of BPR1J-097 for 2 h. (B) MV4-11 cells, which are homozygous for FLT-ITD, were treated with BPR1J-097 at the indicated concentrations for 2 h. The phosphorylation states of FLT3 and STAT5 were evaluated by western blot.
BPR1J-097 inhibits proliferation of FLT3-dependent cells
Potent anti-proliferative activity of BPR1J-097 was observed in FLT3-driven MOLM-13 and MV4-11 AML cell lines containing the FLT-ITD-activating mutation. The IC50 values of BPR1J-097 on MOLM-13 and MV4-11 cells were 21±7 and 46±14, respectively. In contrast, RSV4;11, U937, and K562 cells the growth of which is independent of FLT3 signalling (Yee et al, 2002; Pallis et al, 2003; Odgerel et al, 2008), were weakly inhibited by BPR1J-097 (Table 3). Thus, BPR1J-097 is a potent inhibitor for the proliferation for FLT3-driven cells and BPR1J-097 is not generally cytotoxic.
Table 3. Growth-inhibitory activities of BPR1J-097 on various leukaemia cell lines.
|
Proliferation GI50, nM
|
|||||
|---|---|---|---|---|---|
| Cell lines | Characterisation | BPR1J-097 | ABT-869 | Sorafenib | PKC412 |
| MOLM-13 | AML-FLT3-ITD (heterozygous) | 21±7 | 38±14 | 82±37 | 55±18 |
| MV4-11 | AML-FLT3-ITD (homozygous) | 46±14 | 82±17 | 43±10 | 38±12 |
| U937 | AML-FLT3-negative | >20 000 | >18 000 | 3350±1200 | 1400±900 |
| RSV;11 | ALL-WT-FLT3 (homozygous) | 9400±700 | 9200±2700 | 9300±1200 | 400±100 |
| K562 | CML-Bcr-Abl FLT3 –negative | >20 000 | >20 000 | 7300±2700 | >20 000 |
Abbreviations: ALL=acute lymphoblastic leukaemia; AML=acute myelocytic leukaemia; CML=chronic myelogenous leukaemia; WT=wild-type.
Data represent means±s.d. from three different experiments.
BPR1J-097 induces apoptosis in FLT3-ITD leukaemic cells
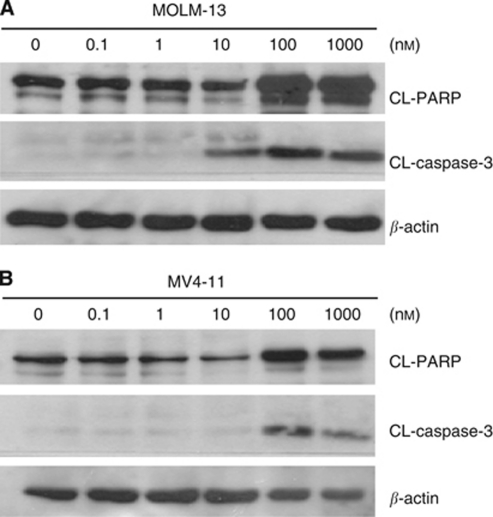
Fms-like tyrosine kinase 3 inhibition would trigger apoptosis in cells dependent on FLT3 for cell growth and survival. We then evaluated whether BPR1J-097 induced cell death in relevant cells. Both MOLM-13 and MV4-11 cells were treated with BPR1J-097 at different concentrations for 48 h before western blot analysis. The emergence of active caspase-3 (CL-caspase-3) was observed in MOLM-13 cells treated with BPR1J-097 at 10 nM (Figure 3A). However, the effect of BPR1J-097 seems to be weaker in MV4-11 cells as CL-caspase-3 was not evident until 100 nM of BPR1J-097 was applied to treat cells (Figure 3B). It seemed that the anti-proliferation activity of BPR1J-097 is more pronounced in MOLM-13 than in MV4-11 cells.
Figure 3.
BPR1J-097 induces apoptosis in MOLM-13 and MV4-11 cells. Western blotting showed that BPR1J-097 was able to induce apoptosis in FLT3-driven AML cells. MOLM-13 (A) and MV4-11 (B) cells were treated with BPR1J-097 at the indicated concentrations for 48 h, and then cell lysates were subjected to western blot analysis with cleavage of poly(ADP-ribose) polymerase (PARP) and caspase 3.
Pharmacokinetic parameters of BPR1J-097
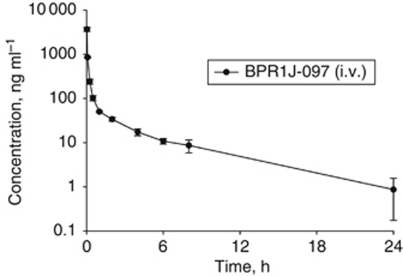
To assess the pharmacokinetic properties of BPR1J-097, the plasma concentration of BPR1J-097 over a 24-h period was measured after a single i.v. administration (Figure 4 and Table 4). BPR1J-097, after a 3.4 mg kg−1 i.v. dose administered to rats, achieved a maximum plasma level maximum concentration of 7.1 μM (3670 ng ml−1) at 2 min of dosing and, at 24 h after dosing, the estimated BPR1J-097 plasma concentration remained at a concentration of 1 ng ml−1 (1.9 nM). The total body clearance was 102.4±9.8 ml min−1 per kg and the volume of distribution at the steady state (Vss) was 15.5±4.8 l kg−1 for BPR1J-097 in rats. The apparent plasma half-life was ∼4.5 h.
Figure 4.
Pharmacokinetic profile of BPR1J-097 in rats. A single intravenous bolus dose (3.4 mg kg−1) of BPR1J-097 was administered to the adult male Sprague–Dawley rats (n=3). Data illustrate mean values (n=3)±s.d. for each time of plasma concentrations of BPR1J-097.
Table 4. Pharmacokinetic parameters of BPR1J-097 in rats.
|
Rat IV (dose: 3.4 mg kg−1)
|
||||
|---|---|---|---|---|
| Compound | T1/2 (h) | CL (ml min−1 per kg) | Vss (l/kg) | AUC(0–24) (ng ml−1 × h) |
| BRP1J-097 | 4.5±1.5 | 102.4±9.8 | 15.5±4.8 | 551±51 |
Abbreviations: AUC=area under the curve; CL=Clearance; VSS=volume of distribution at the steady state.
In vivo tumour growth-suppressing activities of BPR1J-097
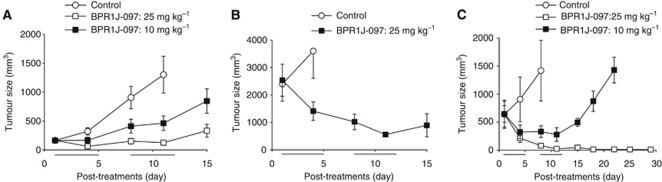
To examine whether BPR1J-097 exhibited anti-tumour activity in vivo, MOLM-13 or MV4-11 cells were subcutaneously implanted into nude mice. In the MOLM-13 model, tumours were allowed to grow to a size of approximately 100–200 mm3. After i.v. administration of mice with BPR1J-097 at two cycles of 10 or 25 mg kg−1, a clear dose-dependent anti-tumour effect was observed (Figure 5A). Tumours in mice treated with 25 mg kg−1 per day stopped growing. After the treatment was terminated, tumours continued to grow. ABT-869 was shown to be unable to shrink the MOLM-13 xenograft tumours (Shankar et al, 2007). To examine whether BPR1J-097 can shrink established tumour mass, the MOLM-13 xenograft was allowed to grow to a size over 2000 mm3. In contrast to ABT-869, BPR1J-097 (25 mg kg−1) showed a significant tumour shrinkage effect on the subcutaneously growing MOLM-13 tumours in a size of >2000 mm3 (Figure 5B). Tumours started to grow after the termination of BPR1J-097 treatment. Compared with ABT-869, BPR1J-097 seemed to be more efficacious in the MOLM-13 xenograft model. Furthermore, BPR1J-097 (10 and 25 mg kg−1) also produced a dose-dependent growth reduction and shrinkage of another model using MV4-11 cells. It is noted that a prolonged disappearance of MV4-11 tumours was observed in mice treated with BPR1J-097 at 25 mg kg−1 (Figure 5C). There was little (3%) or no body weight loss of BPR1J-097-treated nude mice during the observation periods in these in vivo studies. It is interesting to note that although BPR1J-097 was able to trigger more apoptosis in MOLM-13 cells than in MV4-11 cells (Figure 3A), BPR1J-097 seemed more effective for MV4-11 than for MOLM-13 xenograft tumours (Figure 5C). Further studies are required to elucidate the underlying mechanism.
Figure 5.
Anti-tumour activity of BPR1J-097 against FLT3-driven leukaemia tumour growth in nude mice. (A) In vivo anti-tumour effect of BPR1J-097 in the MOLM-13 xenograft nude mice model. Growth of the tumour xenograft was inhibited by BPR1J-097 (10 or 25 mg kg−1, i.v.); P<0.05 compared with vehicle treatment. (B) The subcutaneously growing MOLM-13 tumour of large size (>2000 mm3) in nude mice could be significantly shrunk by BPR1J-097 (25 mg kg−1, i.v.). (C) BPR1J-097 at 10 and 25 mg kg−1 (i.v.) showed significant suppression of MV4-11 tumour growth; P<0.05, n=5–6 per group. Mean tumour volumes±s.e.m. are shown. Vehicle (○), 10 mg kg−1 (▪), 25 mg kg−1 (□).
Discussion
Aberrant regulation of tyrosine kinase has been implicated as a causal factor in tumourigenesis, cancer progression, and drug resistance in many tumours. Pharmacological inhibition of dysregulated kinases is an attractive therapeutic approach for treatment of cancers. Imatinib, a Bcr-Abl kinase inhibitor, and gefitinib, an EGFR tyrosine kinase inhibitor, showed remarkable efficacy in chronic myelogenous leukaemia and in a subset of non-small cell lung cancer, respectively (Cohen et al, 2003; Schiffer, 2007). Fms-like tyrosine kinase 3 is the tyrosine kinase that is commonly found in an aberrant form, including overexpression and activating mutant forms, to contribute to AML oncogenesis. Two principal types of aberrant FLT3-activating mutations, namely FLT3-ITD and FLT3-KDM, were shown to account for almost 30% of AML patients, and these mutations are associated with poor prognosis in patients receiving chemotherapy. Therefore, FLT3 is an attractive therapeutic target for discovery of novel therapeutics for AML treatment.
Several existing drugs or experimental compounds with inhibitory activity against FLT3 kinase are being evaluated for treatment of AML patients (Weisberg et al, 2002; O’Farrell et al, 2003; Griswold et al, 2004; Smith et al, 2004; Stone et al, 2005; Auclair et al, 2007; Shankar et al, 2007; Shiotsu et al, 2009; Zarrinkar et al, 2009). Clinical studies have shown that treatment with a single FLT3 inhibitor does not yield therapeutic benefits in AML patients, suggesting that they may not be optimal to fully inhibit FLT3 in tumours. Alternatively, current FLT3 inhibitors may not be active enough to inhibit pre-existing drug-resistant FLT3 or new drug-resistant FLT3 mutants, which can be readily acquired after initiation of treatment (Breitenbuecher et al, 2009a, 2009b). Thus, continuous efforts are warranted to design and discover novel and more effective inhibitors of FLT3.
Through rational design, we discovered a potent FLT3 inhibitor, BPR1J-097, which effectively inhibits FLT3 activity in vitro and in vivo. The inhibitory activity of BPR1J-097 was characterised using various assays including in vitro kinase activity, cell-based phosphorylation of FLT3 and a major downstream signalling modulator, STAT5, and proliferation of FLT3-driven leukaemic cells under in vitro and in vivo conditions. We found that BPR1J-097 potently inhibits FLT3 activity in the in vitro kinase assay compared with other FLT3 inhibitors such as ABT-869, sorafenib, and PKC412 (Table 1). In addition, BPR1J-097 inhibited proliferation of FLT3-driven cells (MOLM-13 and MV4-11), but not FLT3-independent cells (U937, RSV;11, and K562), with equal or better potency and selectivity than other FLT3 inhibitors (Table 3). To assess the FLT3-ITD-inhibitory activity of BPR1J-097, we measured FLT3 phosphorylation in transfected 293T-FLT3-ITD and FLT3-ITD-homozygous MV4-11 cells. Results showed that BPR1J-097 decreased FLT3-ITD phosphorylation levels with an observed IC50 of approximately 1–10 nM. Transfected cells with the FLT3-D835Y mutant was also inhibited by BPR1J-097 with similar IC50 values in 293T-FLT3-ITD cells. Proliferation and aberrant FLT3 signalling were both inhibited by BPR1J-097, with an IC50 of 10 nM. Treatment of FLT3-driven cell lines with BPR1J-097 led to induction of apoptosis.
The maximum achievable plasma concentration of BPR1J-097 after a single dose of 3.4 mg kg−1 administration to rats is >645-fold above the IC50 for FLT3-ITD inhibition in the biochemical and cellular assays. Even at 24 h after single dosing, plasma levels of BPR1J-097 were high enough for complete inhibition of FLT3-ITD. In addition, the high Vss indicated that the distribution of BPR1J-097 into a deep, including tumour, tissue compartment is expected. These good pharmacokinetic properties indicated that BPR1J-097 dosing once a day is sufficient for continuous inhibition of FLT3 activity in rats or mice.
To evaluate the anti-tumour efficacy of BPR1J-097 in vivo, we used MOLM-13 and MV4-11 murine xenograft models. Results showed that BPR1J-097 administration resulted in significant tumour regression in those two models. In a previous report, ABT-869 was not able to produce regressions of tumours in the MOLM-13 model (Shankar et al, 2007). In contrast, in our study, BPR1J-097 reduced the size of tumours even when they were allowed to grow to a size of >2000 mm3 in the MOLM-13 xenograft tumour model (Figure 5B). In the MV4-11 model, BPR1J-097 completely eliminated tumours after two cycles of treatment (Figure 5C). The precise mechanism of its strong anti-tumour efficacy in animals will require further investigation.
In conclusion, these data demonstrate that BPR1J-097 exhibits potent FLT3-inhibitory activity in both in vitro and in vivo assays, excellent selectivity among the kinases examined, and favourable pharmacokinetic properties. Further studies of the clinical features of BPR1J-097 will be required to evaluate whether BPR1J-097 may have therapeutic benefit for AML patients.
Acknowledgments
This study was supported by grants from the National Science Council (NSC 99-2323-B-400-013- to Weir-Torn Jiaang and Chiung-Tong Chen) and the National Health Research Institutes (to John T-A Hsu).
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
References
- Auclair D, Miller D, Yatsula V, Pickett W, Carter C, Chang Y, Zhang X, Wilkie D, Burd A, Shi H, Rocks S, Gedrich R, Abriola L, Vasavada H, Lynch M, Dumas J, Trail PA, Wilhelm SM (2007) Antitumor activity of sorafenib in FLT3-driven leukemic cells. Leukemia 21: 439–445 [DOI] [PubMed] [Google Scholar]
- Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J, Buerger H, Muller-Tidow C, Choudhary C, McMahon M, Berdel WE, Serve H (2005) Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res 65: 9643–9650 [DOI] [PubMed] [Google Scholar]
- Breitenbuecher F, Markova B, Kasper S, Carius B, Stauder T, Bohmer FD, Masson K, Ronnstrand L, Huber C, Kindler T, Fischer T (2009a) A novel molecular mechanism of primary resistance to FLT3-kinase inhibitors in AML. Blood 113: 4063–4073 [DOI] [PubMed] [Google Scholar]
- Breitenbuecher F, Schnittger S, Grundler R, Markova B, Carius B, Brecht A, Duyster J, Haferlach T, Huber C, Fischer T (2009b) Identification of a novel type of ITD mutations located in nonjuxtamembrane domains of the FLT3 tyrosine kinase receptor. Blood 113: 4074–4077 [DOI] [PubMed] [Google Scholar]
- Cohen MH, Williams GA, Sridhara R, Chen G, Pazdur R (2003) FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist 8: 303–306 [DOI] [PubMed] [Google Scholar]
- Coumar MS, Wu JS, Leou JS, Tan UK, Chang CY, Chang TY, Lin WH, Hsu JT, Chao YS, Wu SY, Hsieh HP (2008) Aurora kinase A inhibitors: identification, SAR exploration and molecular modeling of 6,7-dihydro-4H-pyrazolo-[1,5-a]pyrrolo[3,4-d]pyrimidine-5,8-dione scaffold. Bioorg Med Chem Lett 18: 1623–1627 [DOI] [PubMed] [Google Scholar]
- Dormer P, Lau B, Wilmanns W (1980) Kinetics of bone marrow cell production in human acute and chronic myeloid leukemias. Leuk Res 4: 231–237 [DOI] [PubMed] [Google Scholar]
- Gilliland DG, Griffin JD (2002a) Role of FLT3 in leukemia. Curr Opin Hematol 9: 274–281 [DOI] [PubMed] [Google Scholar]
- Gilliland DG, Griffin JD (2002b) The roles of FLT3 in hematopoiesis and leukemia. Blood 100: 1532–1542 [DOI] [PubMed] [Google Scholar]
- Griswold IJ, Shen LJ, La Rosee P, Demehri S, Heinrich MC, Braziel RM, McGreevey L, Haley AD, Giese N, Druker BJ, Deininger MW (2004) Effects of MLN518, a dual FLT3 and KIT inhibitor, on normal and malignant hematopoiesis. Blood 104: 2912–2918 [DOI] [PubMed] [Google Scholar]
- Harrington EA, Bebbington D, Moore J, Rasmussen RK, Ajose-Adeogun AO, Nakayama T, Graham JA, Demur C, Hercend T, Diu-Hercend A, Su M, Golec JM, Miller KM (2004) VX-680, a potent and selective small-molecule inhibitor of the Aurora kinases, suppresses tumor growth in vivo. Nat Med 10: 262–267 [DOI] [PubMed] [Google Scholar]
- Hayakawa F, Towatari M, Kiyoi H, Tanimoto M, Kitamura T, Saito H, Naoe T (2000) Tandem-duplicated Flt3 constitutively activates STAT5 and MAP kinase and introduces autonomous cell growth in IL-3-dependent cell lines. Oncogene 19: 624–631 [DOI] [PubMed] [Google Scholar]
- Kindler T, Lipka DB, Fischer T (2010) FLT3 as a therapeutic target in AML: still challenging after all these years. Blood 116: 5089–5102 [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, Naoe T (1998) Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia 12: 1333–1337 [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Yanada M, Ozekia K (2005) Clinical significance of FLT3 in leukemia. Int J Hematol 82: 85–92 [DOI] [PubMed] [Google Scholar]
- Lin WH, Song JS, Chang TY, Chang CY, Fu YN, Yeh CL, Wu SH, Huang YW, Fang MY, Lien TW, Hsieh HP, Chao YS, Huang SF, Tsai SF, Wang LM, Hsu JT, Chen YR (2008) A cell-based high-throughput screen for epidermal growth factor receptor pathway inhibitors. Anal Biochem 377: 89–94 [DOI] [PubMed] [Google Scholar]
- Markovic A, MacKenzie KL, Lock RB (2005) FLT-3: a new focus in the understanding of acute leukemia. Int J Biochem Cell Biol 37: 1168–1172 [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Markovtsov V, Li H, Wong S, Gelman M, Zhu Y, Franci C, Lang DW, Pali E, Lasaga J, Low C, Zhao F, Chang B, Gururaja TL, Xu W, Baluom M, Sweeny D, Carroll D, Sran A, Thota S, Parmer M, Romane A, Clemens G, Grossbard E, Qu K, Jenkins Y, Kinoshita T, Taylor V, Holland SJ, Argade A, Singh R, Pine P, Payan DG, Hitoshi Y (2010) Preclinical characterization of Aurora kinase inhibitor R763/AS703569 identified through an image-based phenotypic screen. J Cancer Res Clin Oncol 136: 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Muller C, Gruning W, Kratz-Albers K, Serve S, Steur C, Buchner T, Kienast J, Kanakura Y, Berdel WE, Serve H (2000) Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 96: 3907–3914 [PubMed] [Google Scholar]
- Nakao M, Yokota S, Iwai T, Kaneko H, Horiike S, Kashima K, Sonoda Y, Fujimoto T, Misawa S (1996) Internal tandem duplication of the flt3 gene found in acute myeloid leukemia. Leukemia 10: 1911–1918 [PubMed] [Google Scholar]
- O’Farrell AM, Abrams TJ, Yuen HA, Ngai TJ, Louie SG, Yee KW, Wong LM, Hong W, Lee LB, Town A, Smolich BD, Manning WC, Murray LJ, Heinrich MC, Cherrington JM (2003) SU11248 is a novel FLT3 tyrosine kinase inhibitor with potent activity in vitro and in vivo. Blood 101: 3597–3605 [DOI] [PubMed] [Google Scholar]
- Odgerel T, Kikuchi J, Wada T, Shimizu R, Futaki K, Kano Y, Furukawa Y (2008) The FLT3 inhibitor PKC412 exerts differential cell cycle effects on leukemic cells depending on the presence of FLT3 mutations. Oncogene 27: 3102–3110 [DOI] [PubMed] [Google Scholar]
- Pallis M, Seedhouse C, Grundy M, Russell N (2003) Flow cytometric measurement of phosphorylated STAT5 in AML: lack of specific association with FLT3 internal tandem duplications. Leuk Res 27: 803–805 [DOI] [PubMed] [Google Scholar]
- Pollard JR, Mortimore M (2009) Discovery and development of aurora kinase inhibitors as anticancer agents. J Med Chem 52: 2629–2651 [DOI] [PubMed] [Google Scholar]
- Sallmyr A, Fan J, Datta K, Kim KT, Grosu D, Shapiro P, Small D, Rassool F (2008) Internal tandem duplication of FLT3 (FLT3/ITD) induces increased ROS production, DNA damage, and misrepair: implications for poor prognosis in AML. Blood 111: 3173–3182 [DOI] [PubMed] [Google Scholar]
- Scheijen B, Griffin JD (2002) Tyrosine kinase oncogenes in normal hematopoiesis and hematological disease. Oncogene 21: 3314–3333 [DOI] [PubMed] [Google Scholar]
- Schiffer CA (2007) BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia. N Engl J Med 357: 258–265 [DOI] [PubMed] [Google Scholar]
- Shankar DB, Li J, Tapang P, Owen McCall J, Pease LJ, Dai Y, Wei RQ, Albert DH, Bouska JJ, Osterling DJ, Guo J, Marcotte PA, Johnson EF, Soni N, Hartandi K, Michaelides MR, Davidsen SK, Priceman SJ, Chang JC, Rhodes K, Shah N, Moore TB, Sakamoto KM, Glaser KB (2007) ABT-869, a multitargeted receptor tyrosine kinase inhibitor: inhibition of FLT3 phosphorylation and signaling in acute myeloid leukemia. Blood 109: 3400–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiotsu Y, Kiyoi H, Ishikawa Y, Tanizaki R, Shimizu M, Umehara H, Ishii K, Mori Y, Ozeki K, Minami Y, Abe A, Maeda H, Akiyama T, Kanda Y, Sato Y, Akinaga S, Naoe T (2009) KW-2449, a novel multikinase inhibitor, suppresses the growth of leukemia cells with FLT3 mutations or T315I-mutated BCR/ABL translocation. Blood 114: 1607–1617 [DOI] [PubMed] [Google Scholar]
- Smith BD, Levis M, Beran M, Giles F, Kantarjian H, Berg K, Murphy KM, Dauses T, Allebach J, Small D (2004) Single-agent CEP-701, a novel FLT3 inhibitor, shows biologic and clinical activity in patients with relapsed or refractory acute myeloid leukemia. Blood 103: 3669–3676 [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Radich JP (2003) The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 3: 650–665 [DOI] [PubMed] [Google Scholar]
- Stone RM, DeAngelo DJ, Klimek V, Galinsky I, Estey E, Nimer SD, Grandin W, Lebwohl D, Wang Y, Cohen P, Fox EA, Neuberg D, Clark J, Gilliland DG, Griffin JD (2005) Patients with acute myeloid leukemia and an activating mutation in FLT3 respond to a small-molecule FLT3 tyrosine kinase inhibitor, PKC412. Blood 105: 54–60 [DOI] [PubMed] [Google Scholar]
- Thiede C, Steudel C, Mohr B, Schaich M, Schakel U, Platzbecker U, Wermke M, Bornhauser M, Ritter M, Neubauer A, Ehninger G, Illmer T (2002) Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: association with FAB subtypes and identification of subgroups with poor prognosis. Blood 99: 4326–4335 [DOI] [PubMed] [Google Scholar]
- Ustun C, DeRemer DL, Jillella AP, Bhalla KN (2009) Investigational drugs targeting FLT3 for leukemia. Expert Opin Investig Drugs 18: 1445–1456 [DOI] [PubMed] [Google Scholar]
- Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, Gilliland DG, Griffin JD (2002) Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 1: 433–443 [DOI] [PubMed] [Google Scholar]
- Wiernik PH (2010) FLT3 inhibitors for the treatment of acute myeloid leukemia. Clin Adv Hematol Oncol 8: 429–436, 444 [PubMed] [Google Scholar]
- Wong CI, Koh TS, Soo R, Hartono S, Thng CH, McKeegan E, Yong WP, Chen CS, Lee SC, Wong J, Lim R, Sukri N, Lim SE, Ong AB, Steinberg J, Gupta N, Pradhan R, Humerickhouse R, Goh BC (2009) Phase I and biomarker study of ABT-869, a multiple receptor tyrosine kinase inhibitor, in patients with refractory solid malignancies. J Clin Oncol 27: 4718–4726 [DOI] [PubMed] [Google Scholar]
- Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, Double JA, Everitt J, Farningham DA, Glennie MJ, Kelland LR, Robinson V, Stratford IJ, Tozer GM, Watson S, Wedge SR, Eccles SA (2010) Guidelines for the welfare and use of animals in cancer research. Br J Cancer 102: 1555–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Kiyoi H, Nakano Y, Suzuki R, Kodera Y, Miyawaki S, Asou N, Kuriyama K, Yagasaki F, Shimazaki C, Akiyama H, Saito K, Nishimura M, Motoji T, Shinagawa K, Takeshita A, Saito H, Ueda R, Ohno R, Naoe T (2001) Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 97: 2434–2439 [DOI] [PubMed] [Google Scholar]
- Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T (2005) Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia 19: 1345–1349 [DOI] [PubMed] [Google Scholar]
- Yee KW, O’Farrell AM, Smolich BD, Cherrington JM, McMahon G, Wait CL, McGreevey LS, Griffith DJ, Heinrich MC (2002) SU5416 and SU5614 inhibit kinase activity of wild-type and mutant FLT3 receptor tyrosine kinase. Blood 100: 2941–2949 [DOI] [PubMed] [Google Scholar]
- Zarrinkar PP, Gunawardane RN, Cramer MD, Gardner MF, Brigham D, Belli B, Karaman MW, Pratz KW, Pallares G, Chao Q, Sprankle KG, Patel HK, Levis M, Armstrong RC, James J, Bhagwat SS (2009) AC220 is a uniquely potent and selective inhibitor of FLT3 for the treatment of acute myeloid leukemia (AML). Blood 114: 2984–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Broxmeyer HE (1999) p85 subunit of PI3 kinase does not bind to human Flt3 receptor, but associates with SHP2, SHIP, and a tyrosine-phosphorylated 100-kDa protein in Flt3 ligand-stimulated hematopoietic cells. Biochem Biophys Res Commun 254: 440–445 [DOI] [PubMed] [Google Scholar]