Abstract
Background:
Immunodeficiency and AIDS-related pulmonary infections have been suggested as independent causes of lung cancer among HIV-infected persons, in addition to smoking.
Methods:
A total of 68 lung cancers were identified in the Swiss HIV Cohort Study (SHCS) or through linkage with Swiss Cancer Registries (1985–2010), and were individually matched to 337 controls by centre, gender, HIV-transmission category, age and calendar period. Odds ratios (ORs) were estimated by conditional logistic regression.
Results:
Overall, 96.2% of lung cancers and 72.9% of controls were ever smokers, confirming the high prevalence of smoking and its strong association with lung cancer (OR for current vs never=14.4, 95% confidence interval (95% CI): 3.36–62.1). No significant associations were observed between CD4+ cell count and lung cancer, neither when measured within 1 year (OR for <200 vs ⩾500=1.21, 95% CI: 0.49–2.96) nor further back in time, before lung cancer diagnosis. Combined antiretroviral therapy was not significantly associated with lung cancer (OR for ever vs never=0.67, 95% CI: 0.29–1.52), and nor was a history of AIDS with (OR=0.49, 95% CI: 0.19–1.28) or without (OR=0.53, 95% CI: 0.24–1.18) pulmonary involvement.
Conclusion:
Lung cancer in the SHCS does not seem to be clearly associated with immunodeficiency or AIDS-related pulmonary disease, but seems to be attributable to heavy smoking.
Keywords: lung cancer, HIV, AIDS, immunodeficiency, smoking, case–control study
Lung cancer is one of the most common non-AIDS-defining cancers to occur among HIV-infected persons (Dal Maso et al, 2009; Franceschi et al, 2010; Shiels et al, 2011), and shows two- to seven-fold excess risks in comparison with the general population (Grulich et al, 2007; Shiels et al, 2009; Franceschi et al, 2010). In the Swiss HIV Cohort Study (SHCS) subjects, lung cancer risk is three times that in the general Swiss population (Franceschi et al, 2010). A large part of this excess can be explained by the high proportion of heavy smokers among HIV-infected persons (Giordano and Kramer, 2005), particularly among intravenous drug users (IDUs) (Clifford et al, 2005; Dal Maso et al, 2009).
However, HIV infection has been suggested to be associated with increased lung cancer incidence even after controlling for individually collected (Shiels et al, 2010) or hypothetically modelled (Engels et al, 2006; Chaturvedi et al, 2007) smoking history data. Further suggesting a link between lung cancer and HIV-related immunodeficiency, large cohorts of HIV-infected persons (Patel et al, 2008; Guiguet et al, 2009; Reekie et al, 2010) have recently reported strong associations between declining CD4+ counts and lung cancer risk. Nevertheless, other record linkage studies of cancer registries with cohorts of HIV-infected persons (Clifford et al, 2005) and/or AIDS registries (Chaturvedi et al, 2007; Polesel et al, 2010) have failed to observe any link between CD4+ cell counts and lung cancer risk, or any change in risk between the pre-combined antiretroviral therapy (cART) and cART era.
Although HIV is not considered to have any direct carcinogenic effects (Bouvard et al, 2009), it has been hypothesised that HIV-associated inflammation in the lungs might predispose to smoking-related lung damage (Engels et al, 2006), and lung cancer in HIV-infected persons has been associated with a history of AIDS-related pulmonary diseases (Kirk et al, 2007; Shebl et al, 2010), which are themselves related to immunodeficiency.
Our aim was to disentangle the independent effects of smoking, HIV-related immunodeficiency and AIDS-related pulmonary diseases on lung cancer among HIV-infected persons in Switzerland.
Materials and methods
The SHCS is an ongoing study that has been enrolling HIV-infected persons since 1984 from seven large hospitals in Switzerland (http://www.shcs.ch) (Swiss HIV Cohort Study et al, 2010), including 103 000 person-years (py) of follow-up until December 2009. Women contribute 31% of py and representation of HIV-transmission categories are balanced between men having sex with men (MSM), IDU and heterosexual/other (35, 29 and 36% of py, respectively). Detailed information on all AIDS-related disease, CD4+ cell count and HIV-related treatments are collected at the time of enrolment, and at each 6-month follow-up visit. Detailed information on smoking history (in number of pack-years) has been routinely collected from all HIV-infected persons under active follow-up in the SHCS since April 2000, for whom current smoking intensity, as well as number of cigarettes smoked per day, is also recorded at each 6-month follow-up visit.
A total of 107 lung cancer cases were identified in SHCS participants, of which 84 were identified from the SHCS database, and 23 additional cases were identified through record linkage with 8 Swiss Cantonal Cancer Registries (Franceschi et al, 2010). In all, 10 patients with Kaposi sarcoma (KS) and 13 with lymphoma localised in the lung were excluded. In addition, 6 prevalent cases occurring before, or within 1 month of, SHCS enrolment and 10 diagnosed >6 months after the last SHCS follow-up date were excluded, leaving 68 eligible incident cases occurring during active SHCS follow-up (median follow-up from SHCS enrolment to lung cancer diagnosis=7 years; interquartile range, 3–12 years). Confirmation of histological subtype was available for 65 (94%) of the 68 cases, including 21 adenocarcinoma (International Classification of Disease in Oncology morphology codes (ICD-O) codes 81403; 82523; 82533; 84813), 15 large cell carcinoma (80103; 80123; 80203; 80313; 80823), 14 squamous cell carcinoma (80703; 80713; 80723), 8 small cell carcinoma (80413) and 7 other specified carcinoma (80463; 84303).
For each lung cancer case, five control subjects were matched at random from eligible SHCS participants without lung cancer. Eligible controls had at least the same length of follow-up as did matched cases. Matching criteria were: (1) SHCS centre; (2) gender; (3) HIV-transmission category (IDU, MSM, heterosexual/other); (4) age at enrolment (as close as possible, up to a maximum of 9 years difference); (5) year at reference date (as close as possible, but within the following calendar periods: 1985–1991; 1992–1996; 1997–April 2000; May 2000–2010). April 2000 was included as a key date to match lung cancer cases and controls with respect to the beginning of availability of smoking information. For 2 cases, only 3 and 4 controls, respectively, could be matched, leaving 337 control subjects for this study (Table 1).
Table 1. Distribution of 68 lung cancer cases and 337 controls, according to matching variables.
|
Lung cancer
|
Controls
|
|||
|---|---|---|---|---|
| N | (%) | N | (%) | |
| Centre | ||||
| Basel | 11 | 16.2 | 55 | 16.3 |
| Bern | 9 | 13.2 | 42 | 12.5 |
| Geneva | 15 | 22.1 | 75 | 22.3 |
| St Gallen | 7 | 10.3 | 35 | 10.4 |
| Vaud | 13 | 19.1 | 65 | 19.3 |
| Zurich | 13 | 19.1 | 65 | 19.3 |
| Gender | ||||
| Male | 54 | 79.4 | 270 | 80.1 |
| Female | 14 | 20.6 | 67 | 19.9 |
| HIV-transmission category | ||||
| MSM | 19 | 27.9 | 95 | 28.2 |
| IDU | 25 | 36.8 | 125 | 37.1 |
| Het/other | 24 | 35.3 | 117 | 34.7 |
| Age at lung cancera (years) | ||||
| 25–44 | 24 | 35.3 | 132 | 39.2 |
| 45–54 | 26 | 38.2 | 122 | 36.2 |
| 55–64 | 13 | 19.1 | 61 | 18.1 |
| ⩾65 | 5 | 7.4 | 22 | 6.5 |
| Duration of follow-up before lung cancera (months) | ||||
| <24 | 11 | 16.2 | 55 | 16.3 |
| 24–59 | 13 | 19.1 | 65 | 19.3 |
| ⩾60 | 44 | 64.7 | 217 | 64.4 |
| Calendar period at lung cancer a | ||||
| 1985–1991 | 3 | 4.4 | 15 | 4.5 |
| 1992–1996 | 6 | 8.8 | 30 | 8.9 |
| 1997–April 2000 | 6 | 8.8 | 31 | 9.2 |
| May 2000b–2005 | 28 | 41.2 | 146 | 43.3 |
| 2006–2010 | 25 | 36.8 | 115 | 34.1 |
Abbreviations: Het=heterosexual; IDU=intravenous drug user; MSM=men having sex with men.
Or reference date for controls (date after a similar length of follow-up in the SHCS as matched cases).
Truncation in mid-2000 to match for availability of smoking information.
Markers of immunodeficiency (CD4+ cell count; CD8+ cell count; CD4+/CD8+ ratio; HIV viral load) were extracted from the SHCS database at two time periods (1–2 years and <1 year) before the reference date, defined for cases as the date of lung cancer diagnosis, and for controls as that occurring after a similar length of SHCS follow-up (to the exact day) as matched cases before lung cancer. We additionally extracted CD4+ cell counts at 2–3, 3–4, 4–5, 5–6, 6–7, 7–8, 8–9 and 9–10 years before the reference date and calculated mean CD4+ cell counts restricted to cases and controls who (1) were under active follow-up and (2) had a valid CD4+ cell count, in each time period. If more than one measurement for any marker of immunodeficiency was available during any one time period, that closest to the reference date was used. Matching was not retained in the long-term comparison and numbers of cases and controls obviously decreased substantially as follow-up went back in time. The nadir CD4+ cell count, defined as the lowest ever reported CD4+ cell count while under active SHCS follow-up, was also extracted for each subject.
Here, cART use was defined as the prescription of at least three antiretroviral drugs, including a protease inhibitor or a non-nucleoside reverse transcriptase inhibitor or three nucleosides, including abacavir. Only persons who had used cART for >1 month before the reference date were classified as users.
The definition of AIDS-related pulmonary disease includes recurrent bacterial pneumonia, pulmonary tuberculosis (TB), or Pneumocystis jiroveci pneumonia, recorded at any time before the reference date.
This study was approved by the local ethical committees of the seven SHCS sites and of the International Agency for Research on Cancer. Written informed consent was obtained from all SHCS participants.
Statistical analysis
Logistic regression, conditioned on matching variables, was used to calculate odds ratios (ORs) and corresponding 95% confidence intervals (95% CIs). Models were also adjusted for smoking status (never/former, current with <30 pack-years, current with ⩾30 pack-years, unknown).
Results
Table 1 shows the distribution of the 68 lung cancer cases and controls by matching variables. A majority of lung cancer cases were male (79.4%), had been followed up in the SHCS for >5 years before lung cancer diagnosis (64.7%) and were diagnosed after 1996 (86.8%). Intravenous drug users accounted for 36.8% of cases. As a result of matching on these criteria, these proportions were similar among controls. Lung cancer occurred at a mean age of 50 years. Of the 64 lung cancer cases with follow-up post-cancer, only 9 (14%) were still alive at 2 years after lung cancer diagnosis.
The associations of smoking, cART use, AIDS and nadir CD4+ cell count with lung cancer risk are shown in Table 2. Smoking status was known for 52 lung cancer cases (76.5%) and 262 controls (77.7%) (i.e., those followed up in the SHCS after April 2000), of whom 96.2% (11.5% former, 84.6% current) of lung cancer cases and 72.9% (24.0% former, 48.9% current) of controls were smokers. Among controls, the prevalence of smoking was 96.0, 60.0 and 66.4% among IDU, MSM and heterosexual/others, respectively. Lung cancer risk was very strongly associated with current smoking (OR vs never=14.4, 95% CI: 3.36–62.1), and was also elevated, although not significantly so, among the few former smokers (OR vs never=3.22, 95% CI: 0.63–16.6). Former smokers were at significantly lower risk than current smokers (OR=0.22, 95% CI: 0.08–0.59). Odds ratios were slightly higher among current smokers who had smoked ⩾30 (OR vs never=15.9, 95% CI: 3.67–69.1) than <30 (OR vs never=11.5, 95% CI: 2.42–54.6) pack-years. History of cART use was not significantly associated with lung cancer risk (OR for ever vs never=0.67, 95% CI: 0.29–1.52). History of AIDS-related diseases, whether with pulmonary disease (OR=0.49, 95% CI: 0.19–1.28) or without pulmonary disease (OR=0.53, 95% CI: 0.24–1.18), was not more frequent among cases than controls. Three, three and zero cases of recurrent bacterial pneumonia, P. jiroveci pneumonia and TB, respectively, were previously diagnosed among lung cancer cases. No significant associations or trends with lung cancer were observed for nadir CD4+ cell count (OR for <50 vs ⩾200=0.73, 95% CI: 0.34–1.55).
Table 2. Relative risk for lung cancer by selected characteristics at reference datea.
|
Lung cancer
|
Controls
|
|||||
|---|---|---|---|---|---|---|
| N | % | N | % | ORb (95% CI) | Smoking-adjusted ORc (95% CI) | |
| Overall | 68 | 337 | ||||
| Smoking status | ||||||
| Never | 2 | 3.8 | 71 | 27.1 | 1 | |
| Former | 6 | 11.5 | 63 | 24.0 | 3.22 (0.63–16.6) | |
| Current | 44 | 84.6 | 128 | 48.9 | 14.4 (3.36–62.1) | |
| Unknown | 16 | 75 | ||||
| Pack-yearsd | ||||||
| <30 | 16 | 36.4 | 62 | 50.8 | 11.5 (2.42–54.6) | |
| ⩾30 | 28 | 63.6 | 60 | 49.2 | 15.9 (3.67–69.1) | |
| Unknown | 0 | 6 | ||||
| History of cART use | ||||||
| Never | 18 | 26.5 | 77 | 22.8 | 1 | 1 |
| Ever | 50 | 73.5 | 260 | 77.2 | 0.67 (0.29–1.52) | 0.73 (0.31–1.70) |
| History of AIDS-defining disease | ||||||
| No | 54 | 79.4 | 229 | 68.0 | 1 | 1 |
| Yes, without pulmonary diseasee | 8 | 11.8 | 62 | 18.4 | 0.53 (0.24–1.18) | 0.60 (0.27–1.36) |
| Yes, with pulmonary diseasee | 6 | 8.8 | 46 | 13.6 | 0.49 (0.19–1.28) | 0.62 (0.22–1.72) |
| Nadir CD4+ cell count, cells per μl | ||||||
| ⩾200 | 31 | 45.6 | 142 | 42.4 | 1 | 1 |
| 50–199 | 26 | 38.2 | 124 | 37.0 | 0.96 (0.54–1.71) | 1.07 (0.57–2.02) |
| <50 | 11 | 16.2 | 69 | 20.6 | 0.73 (0.34–1.55) | 0.87 (0.39–1.90) |
| Unknown | 0 | 2 | ||||
Abbreviations: cART=combined antiretroviral therapy; CI=confidence interval; OR=odds ratio.
See the ‘Materials and Methods’ section for definition of reference date.
Conditioned upon matching variables.
Conditioned upon matching variables and adjusted for smoking status (never/former, current with <30 pack-years, current with ⩾30 pack-years, unknown).
Current smokers only.
Includes recurrent bacterial pneumonia, pulmonary tuberculosis, or Pneumocystis carinii pneumonia.
The associations of various measures of immunodeficiency with lung cancer risk are shown in Table 3, measured at two different time periods with respect to lung cancer diagnosis (within 1 year and 1–2 years before), and according to two statistical models (unadjusted and adjusted for smoking). No significant associations were observed between lung cancer and CD4+ cell counts, neither when measured within 1 year (OR for <200 vs ⩾500=1.21, 95% CI: 0.49–2.96) or 1–2 years (OR=0.96, 95% CI: 0.41–2.24) before lung cancer diagnosis. Similarly, no significant associations or trends with lung cancer were observed for CD8+ cell counts (Table 3). A CD4+/CD8+ ratio lower than 25 within 1 year of lung cancer diagnosis showed an association with lung cancer risk of borderline statistical significance (OR=2.15, 95% CI: 1.00–4.59), but this relationship was not seen at 1–2 years before lung cancer diagnosis (OR=1.07, 95% CI: 0.49–2.36). Although data on HIV viral load were available for a smaller number of cases (n=54, 79%) and controls (n=269, 80%), no evidence of an association of lung cancer with higher viral load was observed within 1 year of lung cancer diagnosis (OR for ⩾10,000 vs <500=1.10, 95% CI: 0.44–2.75).
Table 3. Relative risk of lung cancer, by markers of immunodeficiency at two different time periods before cancer diagnosis.
|
One to two years before lung cancera
|
Within one year before lung cancera
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Lung cancer
|
Controls
|
Lung cancer
|
Controls
|
|||||||||
| N | % | N | % | ORb (95% CI) | Smoking-adjusted ORc (95% CI) | N | % | N | % | ORb (95% CI) | Smoking-adjusted ORc (95% CI) | |
| Overall | 68 | 337 | 68 | 337 | ||||||||
| CD4+ cell count, cells per μl | ||||||||||||
| ⩾500 | 23 | 39.0 | 100 | 34.2 | 1 | 1 | 20 | 29.4 | 119 | 36.4 | 1 | 1 |
| 200–499 | 26 | 44.1 | 146 | 50.0 | 0.76 (0.41–1.43) | 0.59 (0.30–1.16) | 38 | 55.9 | 159 | 48.6 | 1.41 (0.78–2.54) | 1.11 (0.59–2.10) |
| <200 | 10 | 16.9 | 46 | 15.8 | 0.96 (0.41–2.24) | 0.97 (0.40–2.34) | 10 | 14.7 | 49 | 15.0 | 1.21 (0.49–2.96) | 1.19 (0.47–3.04) |
| Unknown | 9 | 45 | 0 | 10 | ||||||||
| CD8+ cell count, cells per μl | ||||||||||||
| ⩾1000 | 28 | 47.5 | 122 | 41.9 | 1 | 1 | 26 | 38.2 | 132 | 40.4 | 1 | 1 |
| 500–999 | 25 | 42.4 | 128 | 44.0 | 0.84 (0.46–1.53) | 1.05 (0.55–1.98) | 35 | 51.5 | 156 | 47.7 | 1.15 (0.65–2.05) | 1.11 (0.60–2.04) |
| <500 | 6 | 10.2 | 41 | 14.1 | 0.64 (0.25–1.67) | 0.57 (0.20–1.58) | 7 | 10.3 | 39 | 11.9 | 0.92 (0.37–2.29) | 0.77 (0.30–1.97) |
| Unknown | 9 | 46 | 0 | 10 | ||||||||
| CD4+/CD8+ ratio, % | ||||||||||||
| ⩾0.50 | 25 | 42.4 | 125 | 43.0 | 1 | 1 | 22 | 32.4 | 140 | 42.8 | 1 | 1 |
| 0.25–0.49 | 22 | 37.3 | 109 | 37.5 | 1.01 (0.53–1.90) | 0.78 (0.39–1.55) | 26 | 38.2 | 120 | 36.7 | 1.38 (0.74–2.57) | 1.14 (0.58–2.23) |
| <0.25 | 12 | 20.3 | 57 | 19.6 | 1.07 (0.49–2.36) | 0.92 (0.40–2.09) | 20 | 29.4 | 67 | 20.5 | 2.15 (1.00–4.59) | 2.12 (0.94–4.77) |
| Unknown | 9 | 46 | 0 | 10 | ||||||||
| HIV viral load, copies per ml | ||||||||||||
| <500 | 37 | 68.5 | 192 | 71.4 | 1 | 1 | 40 | 72.7 | 207 | 74.5 | 1 | 1 |
| 500–9999 | 12 | 22.2 | 36 | 13.4 | 1.79 (0.83–3.86) | 2.05 (0.90–4.67) | 8 | 14.5 | 37 | 13.3 | 1.15 (0.49–2.70) | 1.27 (0.50–3.21) |
| ⩾10 000 | 5 | 9.3 | 41 | 15.2 | 0.66 (0.24–1.79) | 0.44 (0.15–1.29) | 7 | 12.7 | 34 | 12.2 | 1.10 (0.44–2.75) | 0.81 (0.32–2.07) |
| Unknown | 14 | 68 | 13 | 59 | ||||||||
Abbreviations: CI=confidence interval; OR=odds ratio.
Or before the reference date in controls (see the ‘Materials and Methods’ section for definition).
Conditioned upon matching variables.
Conditioned upon matching variables and adjusted for smoking (never/former, current with <30 pack-years, current with ⩾30 pack-years, unknown).
Adjustment for smoking had no material effect on any of the above findings (Table 3), nor did a sensitivity analysis excluding subjects of unknown smoking status (e.g., smoking adjusted OR for <200 vs ⩾500 CD4+ cell counts at 1–2 years before lung cancer=0.93, 95% CI: 0.31–2.76). Among controls, there were no significant correlations between any of the markers of immunodeficiency with smoking status (data not shown).
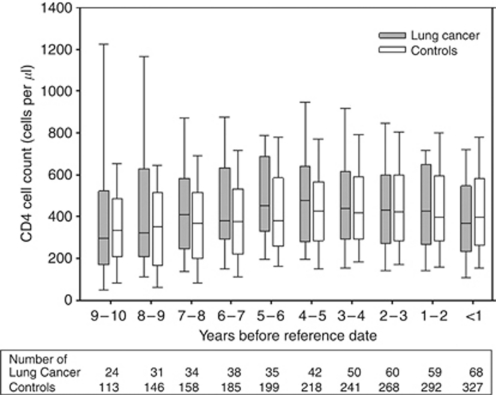
Figure 1 shows mean CD4+ cell counts from 10 years to <1 year before the reference date in lung cancer cases and controls. There was no evidence of any difference in CD4+ cell counts between cases and controls in any time period before the reference date.
Figure 1.
Box plotsa of CD4+ cell counts at yearly intervals prior to reference dateb, among lung cancer cases and controls. aHorizontal lines in box plots represent 10th, 25th, 50th (median), 75th and 90th (percentiles). bSee Materials and Methods section for definition of reference date.
Discussion
Our carefully matched case–control study within the SHCS suggests no evidence for a significant effect of HIV-related immunodeficiency on lung cancer risk in this high-risk population (Franceschi et al, 2010). None of the classic markers of HIV-related immunodeficiency, including low CD4+ cell counts, high HIV viral load nor history of AIDS or AIDS-related pulmonary disease, showed any clear association with lung cancer in the SHCS.
A strong relationship between declining CD4+ cell counts and lung cancer risk was recently reported by the French Hospital HIV Database (FHVD) (Guiguet et al, 2009), with a relative risk of 4.8 (95% CI: 2.8–8.0) for 100–199 vs >500 latest CD4+. Similarly strong relationships with CD4+ cell counts have been reported in two additional studies from the United States and Europe (Patel et al, 2008; Reekie et al, 2010). Although the CIs around our risk estimates are not entirely incompatible with those of previous studies, the findings from the SHCS show no or little association. The reasons for these inconsistencies are unclear, but not all previous studies were supplemented by data linkage with cancer registries to the same extent of the SHCS. Indeed, the FHVD has since been estimated to be only 67% complete with respect to lung cancer diagnosis (Lanoy et al, 2011). Thus, in HIV cohorts that do not obtain comprehensive cancer ascertainment, the more immunosuppressed patients may be overrepresented among lung cancer cases as a consequence of investigations of AIDS-related pulmonary diseases. Alternatively, the inability to completely rule out KS and lymphoma localised in the lung, which contributed up to 50% of lung neoplasms in the pre-cART era (Parker et al, 1998), could lead to erroneous interpretations, given the strong associations of these two AIDS-defining cancers with declining CD4+ cell counts. In this study, histological verification allowed the exclusion of 10 lung KS and 13 lung lymphomas. Otherwise, the distribution of histological types were similar to those reported in other series of HIV-positive lung cancer, as well as in age-matched series of HIV-negative cases (Lavole et al, 2006).
Although confirmed as a very strong risk factor for lung cancer (16-fold increase in risk for ⩾30 pack-years), smoking behaviour was not statistically related to markers of immunodeficiency in the SHCS and hence would appear not to be a strong confounder of the association between these markers and lung cancer. However, confounding by smoking behaviour is more problematic when comparing lung cancer between HIV-positive and HIV-negative subjects (Engels et al, 2006; Chaturvedi et al, 2007; Shiels et al, 2010; Engsig et al, 2011). In the face of such a strong relationship, even with adjustment for smoking measures at an individual level (Levine et al, 2010; Shiels et al, 2010), there remains potential for residual confounding through unmeasured differences in the patterns (e.g., duration of the habit and time since quitting among former smokers) of tobacco use between HIV-positive and HIV-negative ever smokers. A two-fold excess risk of lung cancer is also consistently seen among immunosuppressed kidney transplant recipients (Grulich et al, 2007; van Leeuwen et al, 2010), which might suggest a role of immunodeficiency. However, smoking is also associated with indications for kidney transplant, so that confounding by smoking history is also difficult to rule out in this scenario (Wen et al, 2008).
History of AIDS-related pulmonary disease, and in particular recurrent pneumonia, was recently linked to an increase in lung cancer risk in the large HIV/AIDS Cancer Match study (Shebl et al, 2010), suggesting that HIV-related chronic inflammation might potentiate the carcinogenic effects of smoking in the lung (Engels et al, 2006). However, this study had to use hypothetical scenarios to address the problem of confounding by smoking behaviour (Shebl et al, 2010), which is a risk factor for both pulmonary infections (notably TB (Lin et al, 2007; Gajalakshmi and Peto, 2009)) and lung cancer. Although our sample size was much smaller, we were unable to reproduce evidence of such an effect in the SHCS, where only 8.8% of lung cancer cases had a history of AIDS-related pulmonary disease (and none with TB). This proportion was actually slightly lower than among matched controls, as was the proportion of patients with a history of AIDS.
In agreement with the lack of association with CD4+ cell counts and history of AIDS, there was no evidence for an effect of cART use on lung cancer in the SHCS. Although other studies have suggested that lung cancer incidence is increasing in the era of cART (Bower et al, 2003), this phenomenon may be largely an artefact of the increased survival of HIV-infected persons and the inability to fully adjust for ageing and corresponding exponential increase in lung cancer by age (Franceschi et al, 2010). Indeed, other studies have suggested that the age-standardised incidence of lung cancer is decreasing over time in persons infected with HIV (Silverberg et al, 2009; Shiels et al, 2011).
If confirmed, the lack of an effect of immunodeficiency on lung cancer risk would not lend support to a role of infection in lung cancer aetiology. Although infection with human papillomavirus has been suggested to have a role in lung cancer, recent large studies in non-HIV infected persons have provided evidence against this hypothesis, particularly among smokers (Simen-Kapeu et al, 2010; Koshiol et al, 2011).
The SHCS has many strengths, including the duration and regularity of follow-up and comprehensiveness of clinical and laboratory information. Approximately half of HIV-infected persons in Switzerland have been enrolled in the SHCS, and both genders and different risk categories are well represented. The supplementation of cancer diagnoses through linkage with cancer registries (Clifford et al, 2005) meant a more comprehensive registration of lung cancer, and the availability of histological and/or cytological confirmation for a majority of cases. The use of a nested case–control approach allowed careful matching for many important correlates of lung cancer risk, smoking and immune status. The principal weakness of the study is the relative small number of lung cancer cases that have accrued in the SHCS, which limits the extent to which small effects of HIV-related immunodeficiency can be ruled out.
As repeatedly noted in HIV-infected cohorts, we observed a high prevalence of smoking in the SHCS (73% among matched controls), and the expected large increased risks for lung cancer among smokers. However, an important finding of this study was the confirmation that, although the lung cancer risk for former smokers did not disappear, it was considerably less than that among current smokers, as seen previously in a cohort of HIV-infected females (Levine et al, 2010) and a number of large studies in the general population (IARC, 2007). Thus, the beneficial effects of quitting smoking appear, in relative terms, as important in HIV-infected persons as in the general population (Peto et al, 2000), although more important in absolute terms on account of their heavy burden of lung cancer.
As HIV-infected persons live longer in the era of cART, it can be expected that smoking will increasingly manifest its long-term oncogenic potential and that this lethal cancer becomes an increasingly important cause of death. Focusing on ways to help to quit smoking (Elzi et al, 2006) would be effective in reducing lung cancer in this high-risk population.
Acknowledgments
This study was performed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (Grant 3347-069366), assisted by grants from OncoSuisse (ICP OCS 01355-03-2003, KFS-02478-08-2009). Mauro Lise received a fellowship from the Associazione Italiana per la Ricerca sul Cancro carried out at the International Agency for Research on Cancer.
APPENDIX
The members of the Swiss HIV Cohort Study are J Barth, M Battegay, E Bernasconi, J Böni, HC Bucher, P Bürgisser, C Burton-Jeangros, A Calmy, M Cavassini, M Egger, L Elzi, J Fehr, M Flepp, P Francioli (President of the SHCS), H Furrer (Chairman of the Clinical and Laboratory Committee), CA Fux, M Gorgievski, H Günthard (Chairman of the Scientific Board), B Hasse, HH Hirsch, B Hirschel, I Hösli, C Kahlert, L Kaiser, O Keiser, C Kind, T Klimkait, H Kovari, B Ledergerber, G Martinetti, B Martinez de Tejada, N Müller, D Nadal, G Pantaleo, A Rauch, S Regenass, M Rickenbach (Head of Data Center), C Rudin (Chairman of the Mother and Child Substudy), P Schmid, D Schultze, F Schöni-Affolter, J Schüpbach, R Speck, P Taffé, A Telenti, A Trkola, P Vernazza, V von Wyl, R Weber and S Yerly.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
The authors declare no conflict of interest.
References
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V (2009) A review of human carcinogens – Part B: biological agents. Lancet Oncol 10: 321–322 [DOI] [PubMed] [Google Scholar]
- Bower M, Powles T, Nelson M, Shah P, Cox S, Mandelia S, Gazzard B (2003) HIV-related lung cancer in the era of highly active antiretroviral therapy. AIDS 17: 371–375 [DOI] [PubMed] [Google Scholar]
- Chaturvedi AK, Pfeiffer RM, Chang L, Goedert JJ, Biggar RJ, Engels EA (2007) Elevated risk of lung cancer among people with AIDS. AIDS 21: 207–213 [DOI] [PubMed] [Google Scholar]
- Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, Rapiti E, Levi F, Jundt G, Fisch T, Bordoni A, De Weck D, Franceschi S, Swiss HIV Cohort Study (2005) Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst 97: 425–432 [DOI] [PubMed] [Google Scholar]
- Dal Maso L, Polesel J, Serraino D, Lise M, Piselli P, Falcini F, Russo A, Intrieri T, Vercelli M, Zambon P, Tagliabue G, Zanetti R, Federico M, Limina RM, Mangone L, De Lisi V, Stracci F, Ferretti S, Piffer S, Budroni M, Donato A, Giacomin A, Bellu F, Fusco M, Madeddu A, Vitarelli S, Tessandori R, Tumino R, Suligoi B, Franceschi S, for the Cancer and AIDS Registry Linkage (CARL) Study (2009) Pattern of cancer risk in persons with AIDS in Italy in the HAART era. Br J Cancer 100: 840–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzi L, Spoerl D, Voggensperger J, Nicca D, Simcock M, Bucher HC, Spirig R, Battegay M (2006) A smoking cessation programme in HIV-infected individuals: a pilot study. Antivir Ther 11: 787–795 [PubMed] [Google Scholar]
- Engels EA, Brock MV, Chen J, Hooker CM, Gillison M, Moore RD (2006) Elevated incidence of lung cancer among HIV-infected individuals. J Clin Oncol 24: 1383–1388 [DOI] [PubMed] [Google Scholar]
- Engsig FN, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Obel N (2011) Lung cancer in HIV patients and their parents: a Danish cohort study. BMC Cancer 11: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi S, Lise M, Clifford GM, Rickenbach M, Levi F, Maspoli M, Bouchardy C, Dehler S, Jundt G, Ess S, Bordoni A, Konzelmann I, Frick H, Dal Maso L, Elzi L, Furrer H, Calmy A, Cavassini M, Ledergerber B, Keiser O (2010) Changing patterns of cancer incidence in the early- and late-HAART periods: the Swiss HIV Cohort Study. Br J Cancer 103: 416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajalakshmi V, Peto R (2009) Smoking, drinking and incident tuberculosis in rural India: population-based case-control study. Int J Epidemiol 38: 1018–1025 [DOI] [PubMed] [Google Scholar]
- Giordano TP, Kramer JR (2005) Does HIV infection independently increase the incidence of lung cancer? Clin Infect Dis 40: 490–491 [DOI] [PubMed] [Google Scholar]
- Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM (2007) Incidence of cancers in people with HIV/AIDS compared with immunosupressed transplant recipients: a meta-analysis. Lancet 370: 59–67 [DOI] [PubMed] [Google Scholar]
- Guiguet M, Boue F, Cadranel J, Lang JM, Rosenthal E, Costagliola D (2009) Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study. Lancet Oncol 10: 1152–1159 [DOI] [PubMed] [Google Scholar]
- IARC (2007) IARC Handbooks of Cancer Prevention, Volume 11: Tobacco Control: Reversal of Risk after Quitting Smoking. International Agency for Research on Cancer: Lyon [Google Scholar]
- Kirk GD, Merlo C, O’Driscoll P, Mehta SH, Galai N, Vlahov D, Samet J, Engels EA (2007) HIV infection is associated with an increased risk for lung cancer, independent of smoking. Clin Infect Dis 45: 103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiol J, Rotunno M, Gillison ML, van Doorn LJ, Chaturvedi AK, Tarantini L, Song H, Quint WG, Struijk L, Goldstein AM, Hildesheim A, Taylor PR, Wacholder S, Bertazzi PA, Landi MT, Caporaso NE (2011) Assessment of human papillomavirus in lung tumor tissue. J Natl Cancer Inst 103: 501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanoy E, Spano JP, Bonnet F, Guiguet M, Boue F, Cadranel J, Carcelain G, Couderc LJ, Frange P, Girard PM, Oksenhendler E, Poizot-Martin I, Semaille C, Agut H, Katlama C, Costagliola D (2011) The spectrum of malignancies in HIV-infected patients in 2006 in France: the ONCOVIH study. Int J Cancer 129: 467–475 [DOI] [PubMed] [Google Scholar]
- Lavole A, Wislez M, Antoine M, Mayaud C, Milleron B, Cadranel J (2006) Lung cancer, a new challenge in the HIV-infected population. Lung Cancer 51: 1–11 [DOI] [PubMed] [Google Scholar]
- Levine AM, Seaberg EC, Hessol NA, Preston-Martin S, Silver S, Cohen MH, Anastos K, Minkoff H, Orenstein J, Dominguez G, Watts DH (2010) HIV as a risk factor for lung cancer in women: data from the Women's Interagency HIV Study. J Clin Oncol 28: 1514–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Ezzati M, Murray M (2007) Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med 4: e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker MS, Leveno DM, Campbell TJ, Worrell JA, Carozza SE (1998) AIDS-related bronchogenic carcinoma: fact or fiction? Chest 113: 154–161 [DOI] [PubMed] [Google Scholar]
- Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, Holmberg SD, Brooks JT (2008) Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med 148: 728–736 [DOI] [PubMed] [Google Scholar]
- Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R (2000) Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 321: 323–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polesel J, Franceschi S, Suligoi B, Crocetti E, Falcini F, Guzzinati S, Vercelli M, Zanetti R, Tagliabue G, Russo A, Luminari S, Stracci F, De Lisi V, Ferretti S, Mangone L, Budroni M, Limina RM, Piffer S, Serraino D, Bellu F, Giacomin A, Donato A, Madeddu A, Vitarelli S, Fusco M, Tessandori R, Tumino R, Piselli P, Dal Maso L, for the Cancer and AIDS Registries Linkage (CARL) Study (2010) Cancer incidence in people with AIDS in Italy. Int J Cancer 127: 1437–144520049835 [Google Scholar]
- Reekie J, Kosa C, Engsig F, Monforte AD, Wiercinska-Drapalo A, Domingo P, Antunes F, Clumeck N, Kirk O, Lundgren JD, Mocroft A (2010) Relationship between current level of immunodeficiency and non-acquired immunodeficiency syndrome-defining malignancies. Cancer 116: 5306–5315 [DOI] [PubMed] [Google Scholar]
- Shebl FM, Engels EA, Goedert JJ, Chaturvedi AK (2010) Pulmonary infections and risk of lung cancer among persons with AIDS. J Acquir Immune Defic Syndr 55: 375–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Cole SR, Kirk GD, Poole C (2009) A meta-analysis of the incidence of non-AIDS cancers in HIV-infected individuals. J Acquir Immune Defic Syndr 52: 611–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Cole SR, Mehta SH, Kirk GD (2010) Lung cancer incidence and mortality among HIV-infected and HIV-uninfected injection drug users. J Acquir Immune Defic Syndr 55: 510–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, Bhatia K, Uldrick TS, Yarchoan R, Goedert JJ, Engels EA (2011) Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst 103: 753–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverberg MJ, Chao C, Leyden WA, Xu L, Tang B, Horberg MA, Klein D, Quesenberry Jr CP, Towner WJ, Abrams DI (2009) HIV infection and the risk of cancers with and without a known infectious cause. AIDS 23: 2337–2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen-Kapeu A, Surcel HM, Koskela P, Pukkala E, Lehtinen M (2010) Lack of association between human papillomavirus type 16 and 18 infections and female lung cancer. Cancer Epidemiol Biomarkers Prev 19: 1879–1881 [DOI] [PubMed] [Google Scholar]
- Swiss HIV Cohort Study, Schoeni-Affolter F, Ledergerber B, Rickenbach M, Rudin C, Gunthard HF, Telenti A, Furrer H, Yerly S, Francioli P (2010) Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 39: 1179–1189 [DOI] [PubMed] [Google Scholar]
- van Leeuwen MT, Webster AC, McCredie MR, Stewart JH, McDonald SP, Amin J, Kaldor JM, Chapman JR, Vajdic CM, Grulich AE (2010) Effect of reduced immunosuppression after kidney transplant failure on risk of cancer: population based retrospective cohort study. BMJ 340: c570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen CP, Cheng TY, Tsai MK, Chang YC, Chan HT, Tsai SP, Chiang PH, Hsu CC, Sung PK, Hsu YH, Wen SF (2008) All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 371: 2173–2182 [DOI] [PubMed] [Google Scholar]