Abstract
Background
Chronic obstructive pulmonary disease (COPD) is frequently associated with comorbid depression and anxiety. Managing COPD symptoms and exacerbations through use of appropriate and adequate pharmacotherapy in this population may result in better COPD-related outcomes.
Methods
This retrospective, observational study used administrative claims of patients aged 40 years and older with COPD and comorbid depression/anxiety identified from January 1, 2004 through June 30, 2008. Patients were assigned to fluticasone propionate/salmeterol 250/50 mcg combination (FSC) or anticholinergics (AC) based on their first (index) prescription. The risks of COPD exacerbations and healthcare utilization and costs were compared between cohorts during 1 year of follow-up.
Results
The adjusted risk of a COPD-related exacerbation during the 1-year follow-up period was 30% higher in the AC cohort (n = 2923) relative to the FSC cohort (n = 1078) (odds ratio [OR]: 1.30, 95% confidence interval [CI]: 1.08–1.56) after controlling for baseline differences in covariates. The risks of COPD-related hospitalizations and emergency department visits were 56% and 65% higher, respectively, in the AC cohort compared with the FSC cohort. The average number of COPD-related hospitalizations during the follow-up period was 46% higher for the AC cohort compared with the FSC cohort (incidence rate ratio [IRR]: 1.46, 95% CI: 1.01–2.09, P = 0.041). The savings from lower COPD-related medical costs ($692 vs $1042, P < 0.050) kept the COPD-related total costs during the follow-up period comparable to those in the AC cohort ($1659 vs $1677, P > 0.050) although the pharmacy costs were higher in the FSC cohort.
Conclusions
FSC compared with AC was associated with more favorable COPD-related outcomes and lower COPD-related utilization and medical costs among patients with COPD and comorbid anxiety/depression.
Keywords: COPD, fluticasone propionate/salmeterol, anticholinergics, depression, comorbidity
Introduction
Chronic obstructive pulmonary disease (COPD) affects an estimated 24 million individuals and incurred an estimated annual $49.9 billion cost in the US in 2010. Addressing this issue is important because of the increasing number of diagnosed cases.1–4 Neuropsychiatric symptoms and disorders including major depression, depressive symptoms, and anxiety are commonly comorbid with COPD.5,6 Estimates of prevalence of depression in COPD vary but are generally higher than those reported in some other advanced chronic diseases.5 A cross-sectional study of 18,588 individuals in the 2004 US-based Health and Retirement Survey found that depressive symptoms were more common in COPD than in coronary heart disease, stroke, diabetes, arthritis, hypertension, and cancer.7 Of the 1736 individuals with self-reported COPD, 40% had at least three depressive symptoms. In another general practice study, the risk of depression was 2.5 times greater for patients with severe COPD than for controls without COPD or asthma.8 The risk of comorbid depression was not increased in mild-to-moderate COPD. The authors concluded that patients with severe COPD are at heightened risk of depression and that the results highlight the importance of reducing symptoms and improving physical functioning in patients with COPD.
Depression and anxiety in COPD may additionally adversely increase the burden of COPD. Depression independently predicts poor quality of life in patients with COPD.9–11 Depression was among several predictors of lack of adherence to the recommended criteria for use of inhaled corticosteroids in a sample of 10,711 patients with COPD in the primary care setting.12 Furthermore, the presence of depression predicted noncompletion of pulmonary rehabilitation in COPD.13
The impact of COPD therapies on outcomes and costs in patients with COPD who have comorbid anxiety and/or depression is not fully understood. Bronchodilators (including beta-agonists and anticholinergics) are central to COPD management as they can reduce the frequency and severity of acute exacerbations; however, their impact in a cohort of COPD patients with comorbid depression has not been studied.14 Evidence suggests that combination long-acting beta agonist/inhaled corticosteroid therapy may be more beneficial than long-acting anticholinergic therapy with respect to clinical and economic outcomes.15,16 This study was conducted to compare the risk of COPD exacerbations and COPD-related health care utilization and costs between patients initiating inhaled corticosteroid/long-acting beta agonist combination therapy (fluticasone propionate/salmeterol 250/50 mcg combination [FSC]) and those initiating anticholinergics (ACs) in patients with COPD and comorbid anxiety/depression.
Methods
Study design
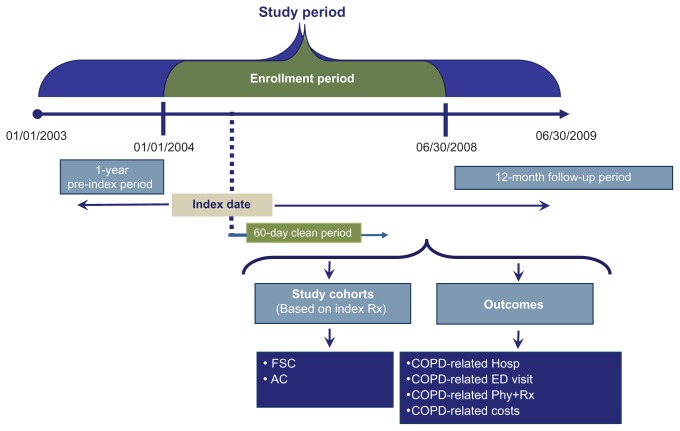
Figure 1 shows the design of this observational, retrospective cohort study. The study period for this analysis ranged from January 1st, 2003 through June 30th, 2009. The population was patients aged 40 years and older with COPD and comorbid depression/anxiety enrolled in health plans. The index date was defined as the date of the first prescription for maintenance COPD treatment (FSC; ACs including tiotropium, ipratropium, or combination ipratropium-albuterol; inhaled corticosteroid; long-acting beta-agonist) during the period from January 1st, 2004 to June 30th, 2008, termed as the enrollment period. Of patients with at least one pharmacy claim for a maintenance medication used to treat COPD, patients were considered to have comorbid depression or anxiety if they had at least one prescription claim for a medication used to treat depression or anxiety along with a diagnosis code for depression during or anxiety, respectively, 1 year pre-index or within 60 days after the index date. The 12-month period before the index date (pre-index period) was used to characterize the study population at baseline, and the 12-month period after the index date (follow-up period) was used to assess all study outcomes. The first 60 days of the follow-up period after the index date (the clean period) was used to ensure receipt of monotherapy by requiring no use of other COPD maintenance medications. This study was exempt from an institutional review board approval as it was retrospective in nature, did not involve an intervention, and utilized anonymized data.
Figure 1.
Study design.
Abbreviations: FSC, fluticasone propionate/salmeterol 250/50 mcg combination; AC, anticholinergics; COPD, chronic obstructive pulmonary disease; Hosp, hospitalization; ED, emergency department; Phy+Rx, physician visit followed within 5 days by a prescription for an antibiotic or oral corticosteroid.
Data source
Data were obtained from the Ingenix Impact National Benchmark database (formerly, IHCIS), a comprehensive US medical claims database generally representative of the insured US population aged <65 years. The data are collected from more than 46 health care plans serving members across nine census regions. It does not include Medicaid or Medicare information. The database contains inpatient/outpatient and pharmacy claims, lab results, and enrollment information on more than 98 million lives from 1997 to 2010. Roughly 30.8 million patients in the IHCIS database have at least 2 years of both medical and pharmacy benefits; roughly 18.3 million patients have at least 3 years of both medical and pharmacy benefits. The data are fully de-identified and compliant with the Health Insurance Portability and Accountability Act.
Sample
The target population had a diagnosis of COPD (ICD-9-CM codes 491.xx, 492.xx, 496.xx) in any field in the pre-index period or 60 days after the index date; absence of exclusionary comorbid conditions (respiratory cancer, cystic fibrosis, fibrosis due to tuberculosis, and bronchiectasis, pneumoconiosis, pulmonary fibrosis, pulmonary tuberculosis, sarcoidosis) during the 1 year pre-index or post-index (follow-up) periods; and an index date occurring during the enrollment period. In addition, a diagnosis of depression or anxiety in any field, along with a medication for treating depression or anxiety was also required. Finally, patients could not have received maintenance medications for COPD other than the index medication on or 60 days after the index date and had to be continuously eligible to receive health care services during the 1-year pre-index and post-index (follow-up) periods.
Patients were categorized on the index date into the FSC cohort or the AC cohort depending on their medication use. Patients who initiated therapy with an inhaled corticosteroid or a long-acting beta-agonist were excluded from the study as only 8% of the sample received these drug classes. It was thought that statistical and scientific conclusions based on the small number of patients who initiated therapy with an inhaled corticosteroid or a long-acting beta-agonist would not be justified.
Outcomes and costs
Clinical outcomes were risk of and number of COPD exacerbations during the 1-year follow-up period. A COPD exacerbation was defined as an emergency department visit with a primary diagnosis code for COPD, a hospitalization with a primary discharge diagnosis for COPD, or a physician visit with a primary diagnosis code for COPD plus a prescription for an oral corticosteroid or antibiotic within 5 days of the visit. When computing the number of exacerbations, an exacerbation within 45 days of a previous exacerbation was not counted as a separate exacerbation.17 COPD-related total (medical plus pharmacy), medical, and pharmacy costs were also determined during the 1-year follow-up period and summed to yield annual costs, which were standardized to 2009 $US using the Consumer Price Index for US medical care. COPD-related medical costs were computed from the paid amounts of medical claims with a primary diagnosis code for COPD. COPD-related pharmacy costs were computed from paid amounts of COPD-related prescription medications (including ACs, short-acting beta agonists, long-acting beta-agonists, inhaled corticosteroids, combination inhaled corticosteroids/long-acting beta-agonists, methylxanthines, oral corticosteroids, and antibiotics for respiratory infections) identified using national drug codes, health care common procedure coding system codes beginning with the letter J, or current procedural terminology codes as appropriate.
Cohorts were also compared with respect to pretreatment characteristics including demographics (age, sex, US census region), comorbidities during the pre-index period (Charlson comorbidity index score,18 asthma), and proxies for COPD severity during the pre-index period (number of canisters of inhaled short-acting beta-agonists, number of prescriptions for oral corticosteroids, use of home oxygen therapy, number of hospitalizations/emergency department visits for COPD, number of physician visits with a prescription for COPD). Age, sex, and US geographic region at the index date were obtained from enrollment files. The Dartmouth–Manitoba adaptation of the Charlson comorbidity index score18 – a weighted index of 19 chronic medical conditions that predict mortality, postoperative complications, and length of hospital stay – was calculated for each patient based on diagnoses reported during the pre-index period (excluding COPD codes). The number of canisters of short-acting beta-agonists was computed by dividing the quantity dispensed in mg by mg per canister. The use of home oxygen therapy was categorized as a binary variable (use, no use) based on current procedural terminology codes for home oxygen therapy on medical claims.
Statistical analysis
Pretreatment characteristics were summarized with descriptive statistics. Inferential statistics (chi-square test for categorical variables, t-test or Mann–Whitney test for continuous variables) were used to quantify differences between cohorts.
Multivariate regression models were used to examine the association between the initiation of index drugs and outcomes while controlling for potential confounders. Covariates were chosen a priori for all models on the basis of clinical relevance and significant differences at baseline. The covariates were age, male gender, Charlson comorbidity index, upper respiratory tract infection, cardiovascular disease, asthma, presence of short-acting beta-agonist, presence of oral corticosteroid, presence of home oxygen therapy, and presence of COPD-related hospitalizations. A logistic regression model was used to analyze differences between cohorts in the risk of COPD exacerbations. A zero-inflated negative binomial regression model was used to analyze differences between cohorts in the number of COPD-related exacerbations.
Differences between cohorts in COPD-related costs were evaluated with a generalized linear model using a gamma distribution with a log link function while controlling for covariates. This method has the advantage of estimating the adjusted costs directly without the need for retransformation while simultaneously using log-transformed costs in its estimation. The method of recycled predictions was used to obtain predicted costs for each cohort.
Results
Sample
Among patients with a diagnosis of COPD and a prescription filled for COPD maintenance medications during the study period, 34,189 (11.5%) patients had a diagnosis of depression or anxiety and a prescription filled for depression or anxiety medications between 1 year pre-index and 60 days post-index date. Of these 34,189 patients, 30,188 (88.3%) were excluded from the study mainly because of noncontinuous eligibility (44.4%) and no COPD diagnosis (25.5%) during the pre-index period through 60 days after the index date. After applying exclusion criteria, the final sample comprised 4001 patients (n = 1078 FSC; n = 2923 AC).
Table 1 shows demographics and pre-index clinical characteristics, health care use, and costs. In the FSC cohort compared with the AC cohort, patients were younger, more likely to be female, had lower Charlson comorbidity index at baseline, and were more likely to have asthma. Pre-index COPD was more severe in the FSC cohort than the AC cohort reflected by the higher use of short-acting beta-agonist canisters and oral corticosteroid prescriptions. During the 1-year pre-index period, the proportion of patients having a COPD-related hospitalization and COPD-related medical costs was significantly lower in the FSC cohort than the AC cohort. Pre-index COPD-related pharmacy costs were significantly higher for the FSC cohort than the AC cohort.
Table 1.
Baseline characteristics
| Characteristics | Total (n = 4001) | FSC (n = 1078) | AC (n = 2923) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Age in years (mean, SD) | 59.6 (10.2) | 57.8 (9.8) | 60.3 (10.3) | <0.001 |
| Female (n, %) | 2652 (66.3%) | 742 (68.8%) | 1910 (65.3%) | 0.038 |
| Region (n, %) | 0.539 | |||
| Northeast | 1580 (39.5%) | 437 (40.5%) | 1143 (39.1%) | |
| Midwest | 701 (17.5%) | 174 (16.1%) | 527 (18.0%) | |
| South | 1279 (32.0%) | 350 (32.5%) | 929 (31.8%) | |
| West | 441 (11.0%) | 117 (10.9%) | 324 (11.1%) | |
| Overall disease burden (1-year pre-index) | ||||
| Charlson index (mean, SD) | 2.2 (2.5) | 2.0 (2.2) | 2.2 (2.6) | 0.006 |
| Upper respiratory tract infection (n, %) | 1287 (32.2%) | 400 (37.1%) | 887 (30.4%) | <0.001 |
| Lower respiratory tract infection (n, %) | 1707 (42.7%) | 448 (41.6%) | 1,259 (43.1%) | 0.390 |
| Cardiovascular disease (n, %) | 2,743 (68.6%) | 694 (64.4%) | 2,049 (70.1%) | 0.001 |
| Asthma (n, %) | 904 (22.6%) | 367 (34.0%) | 537 (18.4%) | <0.001 |
| COPD severity (1-year pre-index) | ||||
| Number of short-acting beta-agonist canisters (mean, SD) | 0.8 (2.4) | 1.0 (2.8) | 0.7 (2.2) | 0.001 |
| Use of short-acting beta-agonist (n, %) | 1111 (27.8%) | 384 (35.6%) | 727 (24.9%) | <0.001 |
| Number of oral corticosteroid prescriptions (mean, SD) | 0.6 (1.4) | 0.7 (1.4) | 0.5 (1.4) | |
| Use of oral corticosteroid (n, %) | 1129 (28.2%) | 366 (34.0%) | 763 (26.1%) | <0.001 |
| Use of home oxygen therapy (n, %) | 343 (8.6%) | 77 (7.1%) | 266 (9.1%) | 0.050 |
| Hospitalization/emergency visit for COPD (n, %) | 382 (9.6%) | 89 (8.3%) | 293 (10.0%) | 0.091 |
| Hospitalization | 231 (5.8%) | 49 (4.6%) | 182 (6.2%) | 0.043 |
| Emergency department visit | 177 (4.4%) | 49 (4.6%) | 128 (4.4%) | 0.820 |
| Number of office visits with a prescription for COPD (mean, SD) | 0.1 (0.4) | 0.1 (0.4) | 0.1 (0.4) | 0.977 |
| COPD-related costs (1-year pre-index) | ||||
| Total (mean, SD) | $1150 ($4720) | $977 ($3774) | $1213 ($5023) | 0.559 |
| Pharmacy (mean, SD) | $139 ($219) | $161 ($233) | $131 ($214) | <0.001 |
| Medical (mean, SD) | $1010 ($4701) | $817 ($3740) | $1082 ($5007) | 0.021 |
| Hospitalization | $640 ($3,943) | $543 ($3534) | $676 ($4083) | 0.058 |
| Emergency department visit | $51 ($437) | $39 ($289) | $55 ($480) | 0.843 |
| Physician visit | $72 ($175) | $73 ($174) | $72 ($176) | 0.908 |
| Outpatient visit | $81 ($401) | $65 ($307) | $87 ($431) | 0.093 |
| Other | $166 ($2302) | $97 ($866) | $191 ($2640) | 0.005 |
Note: Bold values indicate statistically significant difference between cohorts (P < 0.050); all costs in USD.
Abbreviations: FSC, fluticasone propionate/salmeterol 250/50 mcg combination; AC, anticholinergics; SD, standard deviation; COPD, chronic obstructive pulmonary disease.
Clinical and economic outcomes
Unadjusted outcomes
Table 2 shows unadjusted data on COPD-related outcomes and costs. In the unadjusted dataset, the proportion of patients with any COPD-related exacerbation in the follow-up period was lower in the FSC cohort compared with the AC cohort (18.6% vs 23.1%, P = 0.002). The annual number of COPD-related hospitalizations was significantly lower for the FSC cohort compared with the AC cohort (0.04 vs 0.07, P < 0.001). Despite higher annual COPD-related pharmacy costs in the FSC cohort compared with the AC cohort (US$934 vs US$684, P < 0.001), COPD-related annual total costs (US$1604 vs US$1687, P < 0.001) were significantly lower for the FSC cohort driven because of lower medical costs (US$670 vs US$1003, P = 0.001).
Table 2.
Unadjusted COPD-related outcomes and costs for patients receiving FSC versus AC
| Outcome | FSC (n = 1078) | AC (n = 2923) | P value |
|---|---|---|---|
| Risk of COPD exacerbation (n, %) | |||
| Hospitalization | 35 (3.3%) | 168 (5.8%) | 0.002 |
| Emergency department visits | 37 (3.4%) | 171 (5.9%) | 0.002 |
| Phy+Rx | 156 (14.5%) | 448 (15.3%) | 0.503 |
| Hospitalization/emergency department visit | 63 (5.8%) | 307 (10.5%) | <0.001 |
| Hospitalization/emergency department visit/Phy+Rx | 200 (18.6%) | 674 (23.1%) | 0.002 |
| Number of COPD exacerbations (mean, SD) | |||
| Hospitalization | 0.04 (0.2) | 0.07 (0.3) | <0.001 |
| Emergency department visits | 0.06 (0.4) | 0.07 (0.4) | 0.208 |
| Phy+Rx | 0.19 (0.6) | 0.20 (0.6) | 0.671 |
| Hospitalization/emergency department visit | 0.09 (0.5) | 0.14 (0.5) | 0.009 |
| Hospitalization/emergency department visit/Phy+Rx | 0.29 (0.8) | 0.34 (0.8) | 0.052 |
| COPD-related annual costs (mean, SD) | |||
| Total costs | $1604 ($3187) | $1687 ($4299) | <0.001 |
| Pharmacy costs | $934 ($814) | $684 ($724) | <0.001 |
| Index drug | $636 ($612) | $397 ($471) | <0.001 |
| Other study drug | $298 ($475) | $287 ($478) | 0.529 |
| Medical costs | $670 ($2923) | $1003 ($4095) | 0.001 |
| Hospitalization | $229 ($1983) | $453 ($3543) | 0.001 |
| Emergency department visit | $44 ($438) | $51 ($356) | 0.003 |
| Physician visit | $127 ($361) | $133 ($257) | 0.335 |
| Outpatient visit | $113 ($562) | $162 ($680) | 0.014 |
| Other | $158 ($1203) | $206 ($1126) | <0.001 |
Note: Bold values indicate statistically significant difference between cohorts (P < 0.050); all costs in USD.
Abbreviations: COPD, chronic obstructive pulmonary disease; FSC, fluticasone propionate/salmeterol 250/50 mcg combination; AC, anticholinergics; Phy+Rx, physician visit followed within 5 days by a prescription for an antibiotic or oral corticosteroid; SD, standard deviation.
Adjusted outcomes
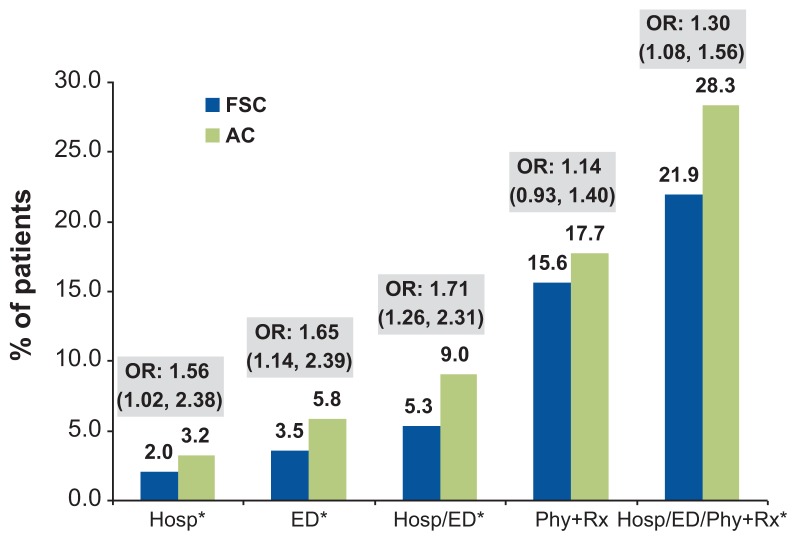
After controlling for differences in baseline covariates between the cohorts, the adjusted overall risk of having any COPD-related exacerbation during the 1-year follow-up period was 30% higher in the AC cohort relative to the FSC cohort (odds ratio [OR]: 1.30, 95% confidence interval [CI]: 1.08–1.56) (Figure 2). The risks of COPD-related hospitalizations and emergency department visits were 56% and 65% higher, respectively, in the AC cohort compared with the FSC cohort (Figure 2).
Figure 2.
Risk of COPD exacerbations in the AC cohort relative to the FSC cohort (reference group).
Note: *P<0.050; ORs were derived from logistic regression models.
Abbreviations: COPD, chronic obstructive pulmonary disease; AC, anticholinergics; FSC, fluticasone propionate/salmeterol 250/50 mcg combination; OR, odds ratio; Hosp, hospitalization; ED, emergency department visit; Phy+Rx, physician visit followed within 5 days by a prescription for an antibiotic or oral corticosteroid.
The average number of COPD-related hospitalizations during the 1-year follow-up period was 46% higher for the AC cohort compared with the FSC cohort (incidence rate ratio [IRR]: 1.46, 95% CI: 1.01–2.09, P = 0.041). The average number of combined COPD-related hospitalizations and emergency department visits was 31% higher in the AC cohort compared with the FSC cohort (IRR: 1.31, 95% CI: 1.05–1.72, P = 0.045). The cohorts did not differ significantly with respect to the numbers of COPD-related emergency department visits or COPD-related physician office visits with an oral corticosteroid/ antibiotic dispensed within 5 days of the visit.
Table 3 shows adjusted COPD-related costs by cohort. The savings from lower COPD-related medical cost (US$692 vs US$1042, P < 0.050) kept the COPD-related total costs during the 1-year follow-up period comparable to those in the AC cohort (US$1659 vs US$1677, P > 0.050) although the FSC group had higher COPD-related pharmacy costs (US$941 vs US$683, P < 0.050).
Table 3.
Adjusted COPD-related costs by cohort
| Cost outcome | FSC | AC | Difference (95% CI) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Mean | 95% CI | Mean | 95% CI | ||
| COPD-related costs | |||||
| Total costs | $1659 | ($757, $3754) | $1677 | ($710, $4087) | −$18 ($47, −$333) |
| Pharmacy costs | $941 | ($526, $1708) | $683 | ($360, $1315) | $258 ($167, $392) |
| Medical costs | $692 | ($84, $4377) | $1042 | ($103, $7511) | −$350 (−$19, −$3134) |
| Hospitalization | $231 | ($5, $2118) | $446 | ($7, $4538) | −$215 (−$2, −$2420) |
| Emergency department visit | $46 | ($3, $502) | $50 | ($2, $525) | −$4 ($1, −$23) |
| Physician visit | $128 | ($25, $477) | $132 | ($21, $554) | −$5 ($4, −$77) |
| Outpatient visit | $118 | ($4, $2651) | $156 | ($4, $4503) | −$38 ($0, −$1852) |
| Other | $176 | ($21, $790) | $199 | ($20, $908) | −$23 ($1, −$118) |
Notes: Bold values indicate statistically significant difference between cohorts (P < 0.05); all costs in USD.
Abbreviations: COPD, chronic obstructive pulmonary disease; FSC, fluticasone propionate/salmeterol 250/50 mcg combination; AC, anticholinergics; CI, confidence interval.
Discussion
This study is, to the authors’ knowledge, the first to assess a cohort of COPD patients with comorbid anxiety/depression initiating maintenance therapy with FSC or AC. The use of FSC was associated with better clinical and economic COPD-related outcomes. The AC cohort compared with the FSC cohort had a significantly higher risk of experiencing any COPD-related exacerbation, exacerbations requiring hospitalization, and exacerbations requiring an emergency department visit during the 1-year study period. The number of COPD-related hospitalizations was lower for patients who initiated COPD maintenance therapy with FSC compared with AC. The lower risks of COPD-related hospitalizations and emergency department visits and the lower number of hospitalizations in the FSC cohort were associated with a reduction in COPD-related medical costs for the FSC cohort compared with the AC cohort.
In clinical studies comparing FSC and tiotropium, lung function improvements and exacerbation rates were similar whereas quality of life was better in the FSC groups.19 In addition, patients taking inhaled corticosteroids tended to have fewer exacerbations that required treatment with oral corticosteroids compared with subjects taking tiotropium. These differences (ie, an improved quality of life and a reduction in dyspnea during exacerbations) may reflect the effects of the inhaled corticosteroid-associated improvement in health in these patients and may help to explain why this therapy might improve outcomes in a depressed population.
The present study adds to a body of literature, from studies of patients with COPD selected without regard to the presence of comorbid depression, that supports the benefits of FSC versus both short- and long-acting anticholinergic bronchodilators in reducing the risk of COPD-related events and/or reducing medical and total costs.16,20–22 In an observational study of 14,689 patients aged ≥65 years with COPD in a commercial Medicare health maintenance organization plan, initial maintenance treatment with FSC was associated with total COPD-related cost savings (medical plus pharmacy) of US$110 versus tiotropium, US$295 versus ipratropium/albuterol, and US$1235 versus ipratropium (all of which are ACs) over a 1-year follow-up period.16 This reduction in total costs with FSC versus tiotropium more than offsets the higher pharmacy costs of FSC. In a retrospective, observational study involving Texas Medicaid beneficiaries aged 40 to 64 years (n = 6793), FSC compared with ipratropium was associated with a 27% lower risk of COPD-related hospitalization or emergency department visit and with similar COPD-related total costs (medical plus pharmacy costs) over 12 months.20 In another study, initial maintenance therapy with FSC compared with ipratropium was associated with a 56% lower risk of COPD-related hospitalization or emergency department visit and similar COPD-related total costs (with lower all-cause total costs) in patients ≥40 years with COPD.21 Finally, in a retrospective claims analysis involving 1051 adults ≥65 years with COPD, FSC compared with ipratropium was associated with a 45% reduction in the risk of COPD-related hospitalization or emergency department visit, and lower COPD-related medical costs.22 In several of these studies,16,21,22 FSC-associated reductions in medical costs appeared to more than offset the increase in pharmacy costs such that total costs (medical plus pharmacy) were lower with FSC (although total costs were not reported in one study);21 however, pharmacy costs were higher with FSC than with the short-acting anticholinergic bronchodilator. Across studies comparing FSC with short- and long-acting anticholinergic bronchodilators in patients selected without regard to presence of comorbid depression, FSC was associated with significantly lower risk of COPD-related events and, generally, lower total medical costs.
In the current study, definitions for COPD exacerbations included emergency department visits, hospitalizations, and physician visits with a prescription for an oral corticosteroid or antibiotic for respiratory infections. A similarly inclusive definition was used in recent studies of COPD exacerbations.23–26 Emergency department visits and hospitalizations reflect severe exacerbations, and physician visits with a prescription for an oral corticosteroid or antibiotic may reflect moderate exacerbations. The use of these definitions for COPD exacerbations provides a sensitive measure of treatment outcomes and reflects the spectrum of manifestations of COPD exacerbations in clinical practice.
The results of this study should be interpreted in the context of its limitations. Although analyses controlled for differences in baseline disease severity and other baseline characteristics in both adjusted cost models and risk models in the current study, such adjustment is imperfect and does not obviate the need for further assessment in cohorts balanced with respect to baseline characteristics. The possibility remains of residual confounding due to between-cohort differences in patient characteristics that were not controlled for in multivariate analysis. Other limitations of this study include potential errors in the coding of claims, the inability to verify the accuracy of diagnosis codes, and the absence of a means of assessing patient compliance. The potential for these biases is inherent in observational studies. If these biases were operating, they are likely to have been operating similarly between cohorts.
Patients with COPD are at increased risk of depression and anxiety relative to those with other chronic disorders.5–7 This observational study is believed to be the first to examine the impact of pharmacotherapy on outcomes and costs in patients with COPD and depression or anxiety. The results show that FSC compared with AC was associated with more favorable COPD-related outcomes and lower COPD-related utilization and medical costs among patients with COPD and comorbid depression or anxiety. Further study of the influence of therapeutic intervention on outcomes, utilization, and costs in COPD and comorbid depression or anxiety is warranted.
Acknowledgments
The authors acknowledge Jane Saiers, PhD (The Write-Medicine, Inc) for assistance with writing the manuscript. Dr Saiers’ work was funded by GlaxoSmithKline. This study was sponsored by GlaxoSmithKline (study number ADC113902).
Footnotes
Author contributions
All authors contributed to the design of the study, the interpretation of the data, the writing and review of the manuscript, and approval of the manuscript for submission.
Disclosures
Authors Dalal and Crater are employed by GlaxoSmithKline and own stock in GlaxoSmithKline. Authors Shah, D’Souza, and Chaudhari are employed by Xcenda, which received funds from GlaxoSmithKline for work on the study described in this manuscript.
References
- 1.Mannino DM. Chronic obstructive pulmonary disease: definition and epidemiology. Respir Care. 2003;48:1185–1191. [PubMed] [Google Scholar]
- 2.Barnes PJ. Chronic obstructive pulmonary disease. New Engl J Med. 2006;343:269–280. doi: 10.1056/NEJM200007273430407. [DOI] [PubMed] [Google Scholar]
- 3.US Department of Health and Human Services. Morbidity and Mortality: 2007 Chartbook on Cardiovascular, Lung and Blood Diseases. National Institutes of Health [Bethesda, MD]. National Heart Lung and Blood Institute. [Google Scholar]
- 4.Valente S, Pasciuto G, Bernabei R, Corbo G. Do we need different treatments for very elderly COPD patients. Respiration. 2010;80:357–368. doi: 10.1159/000320221. [DOI] [PubMed] [Google Scholar]
- 5.Maurer J, Rebbapragada V, Borson S, et al. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(Suppl 4):43S–56S. doi: 10.1378/chest.08-0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Miguel Díez J, Hernández Barrera V, Puente Maestu L, Carrasco Garrido P, Gómez García T, Jiménez García R. Prevalence of anxiety and depression among chronic bronchitis patients and the associated factors. Respirology. 2011;16(7):1103–1110. doi: 10.1111/j.1440-1843.2011.02015.x. [DOI] [PubMed] [Google Scholar]
- 7.Schane RE, Walter LC, Dinno A, Covinsky KE, Woodruff PG. Prevalence and risk factors for depressive symptoms in persons with chronic obstructive pulmonary disease. J Gen Intern Med. 2008;23:1757–1762. doi: 10.1007/s11606-008-0749-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Manen JG, Bindels PJ, Dekker FW, Ijzermans CJ, van der Zee JS, Schadé E. Risk of depression in patients with chronic obstructive pulmonary disease and its determinants. Thorax. 2002;57:412–416. doi: 10.1136/thorax.57.5.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sundh J, Stallberg B, Lisspers K, Montgomery SM, Janson C. Comorbidity, body mass index and quality of life in COPD using the Clinical COPD Questionnaire. COPD. 2011;8:173–181. doi: 10.3109/15412555.2011.560130. [DOI] [PubMed] [Google Scholar]
- 10.Tsiligianni I, Kocks J, Tzanakis N, Siafakas N, van der Molen T. Factors that influence disease-specific quality of life or health status in patients with COPD: a review and meta-analysis of Pearson correlations. Prim Care Respir J. 2011;20:257–268. doi: 10.4104/pcrj.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Leupoldt A, Taube K, Lehmann K, Fritzsche A, Magnussen H. The impact of anxiety and depression on outcomes of pulmonary rehabilitation in patients with COPD. Chest. 2011;140:730–736. doi: 10.1378/chest.10-2917. [DOI] [PubMed] [Google Scholar]
- 12.de Miguel-Diez J, Carrasco-Garrido P, Rejas-Gutierrez J, et al. Inappropriate overuse of inhaled corticosteroids for COPD patients: impact on health costs and health status. Lung. 2011;189:199–206. doi: 10.1007/s00408-011-9289-0. [DOI] [PubMed] [Google Scholar]
- 13.Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis. 2011;8:89–99. doi: 10.1177/1479972310393756. [DOI] [PubMed] [Google Scholar]
- 14.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Updated. 2009 Medical Communications Resources, Inc; 2009. [Accessed February 15, 2010]. Available from: http://www.GOLDCOPD.com. [Google Scholar]
- 15.Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA for the INSPIRE Investigators. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/ fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 16.Dalal AA, Petersen H, Simoni-Wastila L, Blanchette CM. Healthcare costs associated with initial maintenance therapy with fluticasone propionate 250 mcg/salmeterol 50 mcg combination versus anticholinergic bronchodilators in elderly US Medicare-eligible beneficiaries with COPD. J Med Econ. 2009;12:339–347. doi: 10.3111/13696990903369135. [DOI] [PubMed] [Google Scholar]
- 17.Seemungal TA, Donaldson GC, Bhowmik A, Jeffries DJ, Wedzicha JA. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1608–1613. doi: 10.1164/ajrccm.161.5.9908022. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Bateman ED, van Dyk M, Sagriotis A. Comparable spirometric efficacy of tiotropium compared with salmeterol plus fluticasone in patients with COPD: a pilot study. Pulm Pharmacol Ther. 2008;21:20–25. doi: 10.1016/j.pupt.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Rascati KL, Akazawa M, Johnsrud M, Stanford RH, Blanchette CM. Comparison of hospitalizations, emergency department visits, and costs in a historical cohort of Texas Medicaid patients with chronic obstructive pulmonary disease, by initial medication regimen. Clin Ther. 2007;29:1203–1213. doi: 10.1016/j.clinthera.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Akazawa M, Hayflinger DC, Stanford RH, Blanchette CM. Economic assessment of initial maintenance therapy for chronic obstructive pulmonary disease. Am J Manag Care. 2008;14:438–448. [PubMed] [Google Scholar]
- 22.Blanchette CM, Akazawa M, Dalal A, Simoni-Wastila L. Risk of hospitalizations/emergency department visits and treatment costs associated with initial maintenance therapy using fluticasone propionate 500 μg/salmeterol 50 μg compared with ipratropium for chronic obstructive pulmonary disease in older adults. Am J Geriatr Pharmacother. 2008;6:138–146. doi: 10.1016/j.amjopharm.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Anzueto A, Ferguson GT, Feldman G, et al. Effect of fluticasone propionate/salmeterol (250/50) on COPD exacerbations and impact on patient outcomes. COPD. 2009;6:320–329. doi: 10.1080/15412550903140881. [DOI] [PubMed] [Google Scholar]
- 24.Ferguson GT, Anzueto A, Fei R, Emmett A, Knobi lK, Kalberg C. Effect of fluticasone propionate/salmeterol (250/50 microg) or salmeterol (50 microg) on COPD exacerbations. Respir Med. 2008;102:1099–1108. doi: 10.1016/j.rmed.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Lee TA, Wilke C, Joo M, et al. Outcomes associated with tiotropium use in patients with chronic obstructive pulmonary disease. Arch Intern Med. 2009;169:1403–1410. doi: 10.1001/archinternmed.2009.233. [DOI] [PubMed] [Google Scholar]
- 26.Akazawa M, Biddle AK, Stearns SC. Economic assessment of early initiation of inhaled corticosteroids in chronic obstructive pulmonary disease using propensity score matching. Clin Ther. 2008;30(Spec No:):1003–1016. doi: 10.1016/j.clinthera.2008.05.020. [DOI] [PubMed] [Google Scholar]