Abstract
Pain modulation is complex, but noradrenergic signalling promotes anti-nociception, with α2-adrenergic agonists used clinically. To better understand the noradrenergic/peptidergic modulation of nociception, we examined the octopaminergic inhibition of aversive behaviour initiated by the Caenorhabditis elegans nociceptive ASH sensory neurons. Octopamine (OA), the invertebrate counterpart of norepinephrine, modulates sensory-mediated reversal through three α-adrenergic-like OA receptors. OCTR-1 and SER-3 antagonistically modulate ASH signalling directly, with OCTR-1 signalling mediated by Gαo. In contrast, SER-6 inhibits aversive responses by stimulating the release of an array of ‘inhibitory’ neuropeptides that activate receptors on sensory neurons mediating attraction or repulsion, suggesting that peptidergic signalling may integrate multiple sensory inputs to modulate locomotory transitions. These studies highlight the complexity of octopaminergic/peptidergic interactions, the role of OA in activating global peptidergic signalling cascades and the similarities of this modulatory network to the noradrenergic inhibition of nociception in mammals, where norepinephrine suppresses chronic pain through inhibitory α2-adrenoreceptors on afferent nociceptors and stimulatory α1-receptors on inhibitory peptidergic interneurons.
Keywords: monoamines, neuropeptides, nociception
Introduction
The modulation of pain is complex and involves multiple signalling pathways, but a clear role for noradrenergic anti-nociception has emerged, with α2-adrenergic agonists clinically used as analgesic agents (Pertovaara, 2006; Giovannoni et al, 2009). To better understand potential modulators of adrenergic signalling in the perception of noxious stimuli, we have examined the modulation of aversive behaviour mediated by the Caenorhabditis elegans nociceptive ASH sensory neurons. C. elegans contains a simple nervous system that can be dissected using well-defined sensory-mediated locomotory assays. In C. elegans, behavioural state or ‘mood’ is largely defined by food availability and is translated by both monoamines and neuropeptides into the modulation of sensory-mediated behaviours. Although C. elegans lacks norepinephrine and epinephrine, it contains two structurally related invertebrate counterparts, octopamine (OA) and tyramine (TA). Importantly, OA signalling is mediated by receptors with homology to mammalian α-adrenergic receptors, suggesting that ASH signalling may be a useful model to characterize interactions between monoaminergic/peptidergic signalling in the modulation of aversive behaviour. For example, serotonin (5-HT) stimulates aversive responses through three distinct 5-HT receptors operating at different levels within the ASH-mediated locomotory circuit; OA inhibits this 5-HT stimulation through an α1-like adrenergic OA receptor, OCTR-1 (Chao et al, 2004; Wragg et al, 2007; Harris et al, 2009, 2010; Ezcurra et al, 2011). OCTR-1 activates Gαo and inhibits the release of both glutamate and stimulatory peptides from the ASHs (Wragg et al, 2007; Harris et al, 2009, 2010). In fact, neuropeptides modulate many of the same behaviours as monoamines, and the C. elegans genome encodes over 100 neuropeptides and 50 peptide receptors, many with clear orthologues in mammals.
In the present study, we have identified complex interactions among three α-adrenergic-like OA receptors that modulate the OA inhibition of ASH-mediated aversive responses. OCTR-1 inhibits signalling from the ASHs directly and at increased OA levels, OCTR-1 signalling is antagonized by SER-3. With increased ASH stimulation, OA/SER-6 stimulates the Gαs-dependent release of multiple peptides encoded from additional sensory neurons that inhibit ASH-mediated aversive responses and perhaps integrate nutritional state and aversive behaviour. These studies suggest that OA inhibits aversive responses by (1) inhibiting the ASH sensory neurons directly and (2) stimulating the release of neuropeptides from additional neurons. Importantly, the peptide receptors mediating this inhibition appear to function on sensory neurons mediating both attraction and repulsion, suggesting that this OA-dependent peptidergic signalling cascade may integrate attractive/repulsive inputs into a subset of interneurons downstream of the sensory neurons to ultimately dictate reversal dynamics. In addition, these studies highlight the similarities of OA modulation to the noradrenergic inhibition of nociception in mammals, where norepinephrine released from descending pathways in the spinal cord suppresses pain through inhibitory α2-adrenoreceptors on primary afferent nociceptors and by the activation of α1-adrenoreceptors on inhibitory interneurons.
Results
OA modulates the food or 5-HT stimulation of ASH-mediated aversive responses to dilute 1-octanol through two OA receptors, OCTR-1 and SER-3
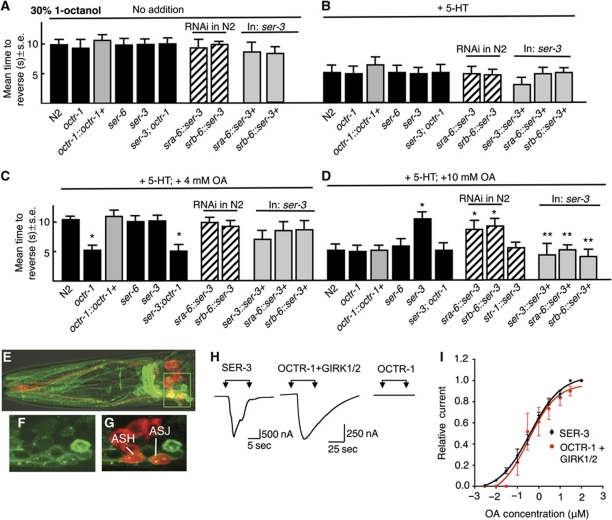
Aversive responses to dilute 1-octanol (30%) mediated by the ASH sensory neurons are more rapid in the presence of food or 5-HT, as measured as the mean time to reverse after exposure to an odorant presented on a hair placed in front of forward moving animals (Figure 1A and B; Wragg et al, 2007; Harris et al, 2009, 2010). In contrast, 4 mM OA prevented this more rapid 5-HT-dependent ‘stimulation’ and this OA-dependent ‘inhibition’ of 5-HT stimulation required the OA receptor, OCTR-1 expressed in the ASHs (Wragg et al, 2007; Harris et al, 2010). Surprisingly, 10 mM OA did not prevent the 5-HT ‘stimulation’ of aversive responses, in contrast to 4 mM OA, suggesting that an additional OA receptor might antagonize the effects of OCTR-1 signalling at higher OA concentrations (Figure 1D). To identify this additional OA receptor, null mutants for putative C. elegans OA receptors were screened for octanol avoidance (Wragg et al, 2007). Indeed, 10 mM OA prevented the 5-HT stimulation of aversive responses in ser-3 null animals to levels observed for 4 mM OA in wild-type animals (Figure 1D). As predicted, OA had no effect on 5-HT stimulation in ser-3;octr-1 animals (Figure 1C and D).
Figure 1.
The OA-dependent delay in the 5-HT stimulation of ASH-mediated aversive responses to dilute 1-octanol is modulated by two OA receptors, OCTR-1 and SER-3 in the ASH sensory neurons. (A) Aversive behaviour to 30% 1-octanol was assayed in wild-type, mutant and transgenic animals after incubation in exogenous 5-HT (4 mM) and/or OA (4 or 10 mM), as described in Materials and methods. Black bars, wild-type or null animals; grey bars, null animals expressing a transgene; hatched bars, RNAi in wild-type animals. Data are presented as a mean±s.e. and analysed by two-tailed Student's t-test. *P<0.001, significantly different from wild-type animals under identical test conditions; **P<0.001, significantly different from null mutant. (A) No addition, (B) +4 mM 5-HT, (C) +4 mM 5-HT and 4 mM OA, (D) +4 mM 5-HT and 10 mM OA. (E–G) Fluorescence from ser-3 null animals expressing a ser-3∷ser-3∷gfp translational fusion using confocal microscopy. (E) Merge of GFP fluorescence, (DiD) staining and DIC, (F, G) Z-section from inset in (E) (F, GFP fluorescence; G, Merge of GFP and DiD staining). (H, I) OCTR-1 and SER-3 exhibit nearly identical affinities for OA when heterologously expressed in Xenopus oocytes. (H) OA activates an inward current in oocytes expressing SER-3 or OCTR-1/GIRK1/2, but not in oocytes expressing OCTR-1 alone. (I) OA dose–response curves for oocytes expressing SER-3 or OCTR-1 and GIRK1/2.
To determine where ser-3 was functioning to modulate aversive responses, the expression of a ser-3∷ser-3∷gfp translational fluorescent reporter was analysed (Figure 1E–G). ser-3∷ser-3∷gfp was expressed in multiple neurons, including the ASHs. Importantly, 10 mM OA prevented the 5-HT stimulation of aversive responses in wild-type animals with ser-3 knocked down in the ASHs by RNAi, and the ASH expression of ser-3 rescued ser-3 null animals, suggesting that SER-3 functioned directly in the ASHs to antagonize OCTR-1 signalling (Figure 1C and D). Presumably, at higher exogenous OA concentrations, ASH SER-3 was activated and acted antagonistically to OCTR-1.
The observation that SER-3 signalling only antagonizes OCTR-1 at higher exogenous OA concentrations might be explained if OCTR-1 had a higher affinity for OA than SER-3. To test this hypothesis, both receptors were expressed in Xenopus oocytes and OA-dependent currents were examined electrophysiologically. In oocytes expressing SER-3, OA elicited an inward current, presumably mediated by endogenous Ca2+-gated Cl− channels, with an EC50 of 0.33 μM (Figure 1H and I). In contrast, in oocytes expressing OCTR-1, OA had no effect, but did elicit an inward current in oocytes co-expressing OCTR-1 and the mammalian G-protein inwardly rectifying K+ channel subunits (GIRK) 1 and 2, with an EC50 of 0.39 μM (Figure 1H and I). GIRK channels are activated by G-protein βγ subunits and a variety of mammalian Gαo-coupled receptors have been assayed in Xenopus oocytes using this approach (Saugstad et al, 1996; Zhang et al, 2002; Ohana et al, 2006). These observations suggest that OCTR-1 and SER-3 are Gαo and Gαq coupled, respectively, and have similar affinities for OA when expressed in Xenopus oocytes. These data suggest that the differential effects of exogenous OA on OCTR-1 and SER-3 signalling in vivo may be dependent on other factors, such as local differences in OA pools. However, ligand affinities measured after heterologous expression may not effectively describe the in-vivo situation, as ligand affinity in heterologous systems may be dependent on additional factors, including expression levels, G-protein coupling, etc. For example, both receptors appeared to exhibit higher affinities for OA when expressed in mammalian cells, with OA inhibiting LSD binding to membranes from COS-7 cells expressing OCTR-1 with an IC50 of 46 nM and as little as 10 nM activating Ca2+ signalling in HEK cells expressing SER-3 (Petrascheck et al, 2007; Wragg et al, 2007).
The OA inhibition of aversive responses to 100% 1-octanol requires a third OA receptor, SER-6
In contrast to aversive responses to 30% 1-octanol, responses to 100% 1-octanol are more rapid and are not stimulated by food or 5-HT (Chao et al, 2004; Wragg et al, 2007). OA dramatically delays the initiation of reversal in response to 100% 1-octanol and in future discussion this OA-dependent delay in aversive responses to 100% 1-octanol will be referred to as ‘OA inhibition’. This OA inhibition appears to mimic, at least in part the starvation response, as the incubation of animals off food for 50 min inhibits aversive responses to 100% 1-octanol and this starvation-dependent inhibition is reduced in tdc-1 or tbh-1 animals that have reduced TA/OA and OA levels, respectively (Supplementary Figure S1; Alkema et al, 2005). Conversely, as predicted, the overexpression of tdc-1 dramatically inhibits aversive behaviour (Supplementary Figure S1). Interestingly, the overexpression of tbh-1 that catalyses the conversion of TA to OA has no effect on aversive behaviour, presumably because TA formation is a rate-limiting step in OA biosynthesis.
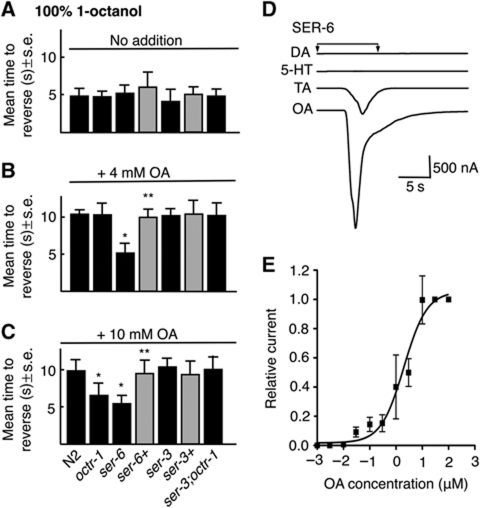
Surprisingly, OCTR-1 does not appear to be involved in the OA inhibition of aversive responses to 100% 1-octanol, that is, 4 mM OA still inhibited aversive responses to 100% 1-octanol in octr-1 null animals (Figure 2A and B; Wragg et al, 2007). In fact, none of the previously identified OA/TA receptors appear to modulate the OA-mediated inhibition of aversive responses to 100% 1-octanol (Wragg et al, 2007). Indeed, in the present study, we have identified an additional OA receptor, SER-6, that was required for the OA inhibition of aversive responses to 100% 1-octanol, that is, OA did not inhibit aversive responses to 100% 1-octanol in ser-6 null animals, and OA inhibition was rescued by the expression of a full-length ser-6∷ser-6∷gfp transgene in ser-6 null animals (Figure 2A and B). These results suggest that the OA inhibition of aversive responses to 100% 1-octanol is mediated by SER-6, not by OCTR-1.
Figure 2.
The OA receptor, SER-6, is required for the OA inhibition of aversive responses to 100% 1-octanol. (A–C) Aversive behaviour to 100% 1-octanol was assayed off food in wild-type, mutant and transgenic animals after incubation in exogenous OA (4 or 10 mM). Black bars, wild-type or null animals; grey bars, null animals expressing a transgene. Data are presented as a mean±s.e. and analysed by two-tailed Student's t-test. *P<0.001, significantly different from wild-type animals under identical test conditions; **P<0.001, significantly different from null mutant. (D) OA is the preferred ligand for SER-6. SER-6 was heterologously expressed in Xenopus oocytes and oocytes were exposed to DA, TA, OA and 5-HT (1 μM) for up to 30 s. (E) OA dose–response curve for oocytes expressing SER-6.
As noted above, SER-3 appears to antagonize the effects of OCTR-1 inhibition in the ASHs. As predicted, although OCTR-1 was not required for the inhibition of aversive responses to 100% 1-octanol at 4 mM OA, both OCTR-1 and SER-6 were involved in OA inhibition at 10 mM OA (Figure 2C). As predicted based on the results described above, 10 mM OA did inhibit aversive responses to 100% 1-octanol in ser-3;octr-1 animals, emphasizing the balance of OCTR-1/SER-3 signalling on the modulation ASH-mediated behaviours at higher OA concentrations (Figure 2).
These results suggest that SER-6 functions as an OA receptor. Indeed, OA robustly activated an inward current in Xenopus oocytes expressing SER-6, presumably mediated by endogenous Ca2+-gated chloride channels, with an EC50 of about 2 μM (Figure 2D and E). In contrast, TA initiated a much smaller current at 2 μM (never >20% of the OA current for a given oocyte), and dopamine and 5-HT were inactive (Figure 2D). In contrast, mock-injected oocytes showed no response (unpublished observations). These results support the identification of SER-6 as an OA receptor.
SER-6 functions in the ADL, AWB and ASI sensory neurons to inhibit ASH-mediated aversive responses
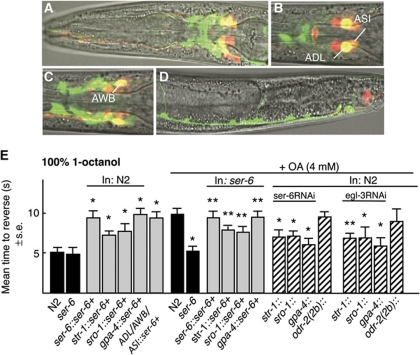
A ser-6∷ser-6∷gfp transgene is expressed in head neurons, including the AWB, ADL and ASI sensory neurons, posterior ventral cord motor neurons and the intestine (Figure 3A–D). This ser-6 transgene rescued OA inhibition in ser-6 null animals and its overexpression in wild-type animals inhibited basal aversive responses off food (Figure 3E). To identify the specific neurons involved in OA inhibition, ser-6 was selectively expressed in the ADLs, AWBs or ASIs of ser-6 null or wild-type animals (Figure 3E). The expression of ser-6 in either the ADLs or AWBs of ser-6 null animals partially rescued OA inhibition, with full rescue observed after expression in the ASIs. Conversely, the expression of ser-6 in the ADLs, AWBs or ASIs of wild-type animals inhibited basal aversive responses off food, with expression in the ASIs alone decreasing basal responses to those observed in the presence of OA (Figure 3E). Interestingly, expressing ser-6 in the AWBs, ADLs and ASIs of the same animal did not inhibit aversive responses to levels greater than those observed by expression in the ASIs alone (Figure 3E). To confirm these observations, ser-6 expression was also selectively knocked down, using neuron-selective RNAi. As predicted, the RNAi knockdown of ser-6 in the ADLs, AWBs or ASIs significantly reduced OA inhibition, but had no effect on basal responses off food (Figure 3E, unpublished observations).
Figure 3.
OA acts on SER-6 in the AWB, ADL and ASI sensory neurons. (A–D) Fluorescence from ser-6 null animals expressing a ser-6∷gfp transcriptional fusion using confocal microscopy. Merge of GFP fluorescence, DiD staining of a subset of sensory neurons and DIC. (E) Aversive responses to 100% 1-octanol were assayed off food in the presence or absence of exogenous OA (4 mM) in transgenic animals expressing either ser-6 or ser-6RNAi under the AWB (str-1); ADL (sro-1) or ASI (gpa-4) neuron-selective promoters. Black bars, wild-type or null animals; grey bars, wild-type or null animals expressing a transgene; hatched bars, RNAi in wild-type animals. Data are presented as a mean±s.e. and analysed by two-tailed Student's t-test. *P<0.001, significantly different from wild-type animals under identical test conditions; **P<0.01, significantly different from null animals under identical test conditions.
These results have each been confirmed in multiple independent lines and suggest that at least three additional sensory neurons, the ADLs, AWBs and ASIs are involved in the OA/SER-6-mediated inhibition of nociception. Interestingly, the expression of ser-6 in the ASIs is sufficient to rescue OA inhibition in ser-6 animals, but the RNAi knockdown of ser-6 in the AWBs or ADLs significantly inhibit OA inhibition. This apparent contradiction is discussed more fully below, but is not an artifact of ADL or AWB ser-6 RNAi knockdown interfering with ser-6 expression in the ASIs, as these same promoters were insufficient for full rescue and AWB or ADL∷gfp RNAi has no effect on ASI GFP expression (unpublished observations). As predicted, off target ser-6RNAi knockdown, using the odr-2(2b) promoter had no effect on OA inhibition (Figure 3E).
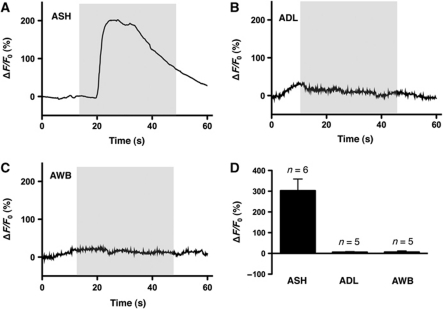
Interestingly, the role for the ADLs and AWBs in aversive responses to 100% octanol has been identified previously (Chao et al, 2004). For example, ablation of the ASHs reduces, but does not abolish, responses to 100% octanol off food. In contrast, responses to 100% octanol are absent after co-ablation of the ASHs, ADLs and AWBs, suggesting that all three pairs of sensory neurons may sense octanol directly. To assess the direct octanol responsiveness of these neurons, we expressed the calcium sensor GCaMP3 in the ASHs, ADLs and AWBs, as has been described previously in other C. elegans sensory-mediated assays (Ha et al, 2010; McGrath et al, 2011). As predicted, the ASHs exhibited a robust ‘on’ response to octanol, as has been observed previously for other ASH ligands (Figure 4A and D; Hilliard et al, 2005; Ezak and Ferkey, 2010; Ezcurra et al, 2011). In contrast, neither the ADLs nor the AWBs responded to octanol (Figure 4B–D). These data suggest that the ADLs and AWBs do not directly respond to octanol, at least with changes in calcium dynamics, and instead may function downstream of as yet unidentified sensory neurons, or, alternatively that ablation of the ASHs causes compensatory changes in the octanol responsiveness of the ADLs and AWBs.
Figure 4.
Octanol responses of ASH, ADL and AWB sensory neurons (A–C) Acute octanol exposure triggers a rise in intracellular Ca2+ level in ASH neurons, but not in ADL or AWB neurons. Representative traces of GCaMP3 fluorescence intensity from single neurons are shown, relative to baseline. Grey boxes indicate application of stimulus. (D) Quantitative comparison of octanol responses from ASH, ADL and AWB neurons. Bars represent mean±s.e.m.
SER-6-mediated OA inhibition of aversive responses requires a diverse group of peptides
Many putative peptide-encoding genes are expressed in the AWBs, ADLs and ASIs. Therefore, neuron-selective RNAi was used to knockdown the expression of egl-3 that encodes a proprotein convertase that is essential for neuropeptide processing (Lee et al, 1999; Kass et al, 2001). OA inhibited aversive responses much less robustly in egl-3 null animals or animals with egl-3 selectively knocked down in the AWBs, ADLs or ASIs, suggesting that signalling from these neurons was primarily peptidergic, at least in the inhibition of ASH-mediated aversive responses (Figure 3E).
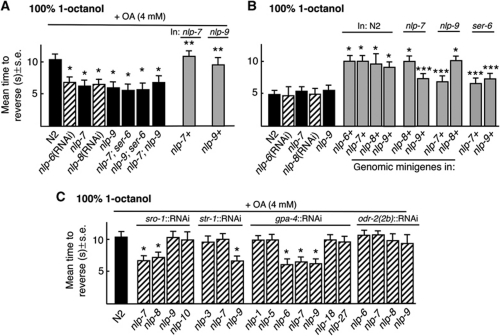
The AWBs (nlp-3,-9), ADLs (nlp-7, -8, -10, flp-21) and ASIs (nlp-1, -5, -6, -7, -9, -14, -18, -24, -27) express a number of genes predicted to encode neuropeptides (Nathoo et al., 2001; Li and Kim, 2008). Therefore, putative null animals for these peptide-encoding genes were screened for OA inhibition. Alternatively, feeding or neuron-selective RNAi was used to knockdown expression. Aversive responses to 100% 1-octanol were not inhibited by OA in nlp-7, nlp-9, ser-6; nlp-7, ser-6; nlp-9 and nlp-9;nlp-7 null animals or in animals with nlp-6 or 8 knocked down by RNAi feeding (Figure 5A). Importantly, these animals exhibited wild-type basal responses to 100% 1-octanol, suggesting that their octanol-sensing machinery was intact (Figure 5B). OA inhibition also was significantly reduced by the neuron-selective RNAi knockdown of nlp-7 or 8 in the ADLs, nlp-9 in the AWBs or nlp-6, 7 or 9 in the ASIs of wild-type animals (Figure 5C). OA inhibition could be rescued by the expression of full-length nlp-7∷nlp-7 or nlp-9∷nlp-9 transgenes in nlp-7 or 9 null animals, respectively, and the overexpression of full-length nlp-6, 7, 8 or 9 transgenes in wild-type animals significantly inhibited basal responses to 100% 1-octanol (Figure 5A and B). The overexpression of nlp-7 in nlp-9 null animals or nlp-9 in nlp-7 null animals inhibited basal responses to a lesser extent than their overexpression in wild-type animals, suggesting that the inhibition associated with the nlp-7 or nlp-9 peptides required basal levels of signalling from peptides encoded by the other gene (Figure 5B). Finally, as predicted, peptides encoded by nlp-7 or nlp-9 did not appear to have any effect on aversive responses to 30% 1-octanol (unpublished observations).
Figure 5.
OA inhibition requires neuropeptides encoded by nlp-6, 7, 8 and 9 released from multiple sensory neurons. (A–C) Wild-type, mutant and transgenic animals were examined for aversive responses to 100% 1-octanol off food in the presence and absence of OA (4 mM). Black bars, wild-type or null animals; grey bars, wild-type or null animals expressing a transgene; hatched bars, RNAi in wild-type animals. Data are presented as a mean±s.e. and analysed by two-tailed Student's t-test. *P<0.001, significantly different from wild-type animals under identical test conditions; **P<0.001, significantly different from null animals under identical test conditions; ***P<0.01, significantly different from wild-type animals under identical test conditions.
As noted above, the overexpression of ser-6 in the AWBs, ADLs or ASIs of wild-type animals inhibits basal aversive responses to 100% 1-octanol off food. Interestingly, ser-6 expression in the ASIs alone was completely sufficient to rescue OA inhibition in ser-6 null animals and ser-6 overexpression in the ASIs is sufficient to inhibit basal aversive responses to levels observed in the presence of OA (Figure 3E). Given that the ASIs express at least three of the four peptides involved in SER-6-dependent OA inhibition, this result is probably not surprising. Similarly, the overexpression of nlp-6, 7, 8 or 9 also inhibits basal aversive responses, as long as basal signalling from the other peptides is maintained, that is, inhibition from nlp-7 or nlp-9 overexpression is significantly reduced in nlp-9 or nlp-7 null animals, respectively. This result implies that many of these peptides may also be tonically released and, as predicted, the inhibition of basal responses off food associated with nlp-7 and nlp-9 overexpression are substantially reduced in ser-6 null animals (Figure 5B). Indeed, since these peptides most likely act at extrasynaptic receptors, these results also imply that physiological peptide levels and functional rescue may be achieved by expression in multiple neurons or overexpression in individual neurons, as long as basal signalling of other peptides is maintained. These data also raise another question that may have broad application for the interpretation of neuron-specific RNAi and rescue, that is, how can one get an RNAi knockdown phenotype in one pair of neurons in wild-type animals and yet get rescue in a different pair of neurons in null animals, assuming that the appropriate controls for potential RNAi spreading are in place. This observation was at first perplexing, but we have also made similar observations in other signalling pathways. Our current thinking is that, since much of the monoaminergic and peptidergic signalling appears to be humoral, that is, the receptors are not expressed on cells directly innervated by the ligand-releasing neurons, the ‘pool’ of ligand released from multiple neurons may dictate the overall modulatory effect, so that RNAi knockdown in one neuron pair in wild-type animals is sufficient to diminish the pool and alter behaviour, but, conversely, the overexpression of the same ligand from a different neuron pair is sufficient to restore the ligand pool and rescue null animals. Interestingly, two-fold changes in tyra-3 expression (tyra-3 encodes a G-protein coupled TA receptor) are sufficient to significantly modify foraging behaviour, suggesting that subtle changes in the levels of individual signalling molecules and/or receptors are sufficient to initiate significant changes in behaviour (Bendesky et al, 2011).
Gαs signalling in the ADLs is essential for the OA/SER-6 inhibition of aversive responses
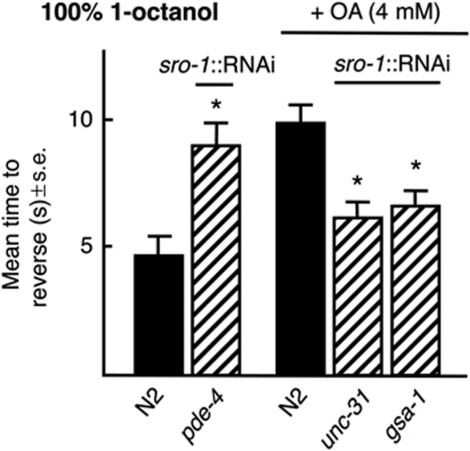
Since signalling from ADLs, AWBs and ASIs in the modulation of ASH-mediated aversive responses appears to be primarily peptidergic, the pathways modulating peptide release from the ADLs were examined by neuron-selective RNAi, as we have described previously for the ASHs (Figure 6; Harris et al, 2010). The ADL RNAi knockdown of unc-31, that encodes a DAG binding protein that plays a key role in dense core vesicle (DCV), but not synaptic vesicle release dramatically reduced OA inhibition, supporting the hypothesis that signalling from the ADLs is primarily peptidergic, at least with respect to OA inhibition (Figure 6; Speese et al, 2007; Zhou et al, 2007).
Figure 6.
Gαs signalling is essential for the release of inhibitory peptides from the ADLs. Wild-type animals expressing RNAi under ADL (sro-1) cell-selective promoter were assayed for aversive responses to 100% 1-octanol off food in the presence and absence of exogenous OA (4 mM). Black bars, wild type; hatched bars, RNAi in wild-type animals. Data are presented as a mean±s.e. and analysed by two-tailed Student's t-test. *P<0.001, significantly different from wild-type animals under identical test conditions.
Gαs (and PKA activation) enhances exocytosis from DCVs (Charlie et al, 2006; Zhou et al, 2007) and ADL∷gsa-1(Gαs) RNAi significantly reduced the OA inhibition of aversive responses to 100% 1-octanol (Figure 6). As predicted, ADL∷RNAi knockdown of pde-4, a 5′ phosphodiesterase, predicted to increase cAMP levels, decreased basal aversive responses in the absence of OA (Figure 6). These results suggest that ADL Gαs signaling stimulates peptide release.
Multiple peptide receptors appear to modulate the SER-6-dependent OA inhibition of aversive responses
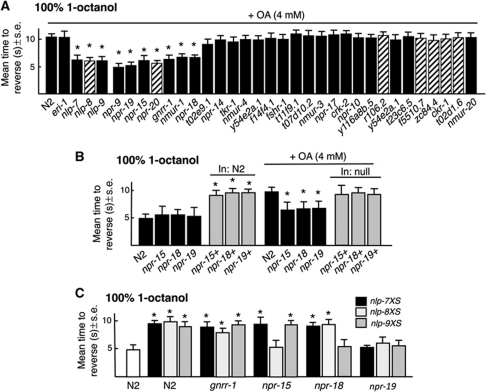
To identify GPCRs activated by peptides encoded by nlp-6, 7, 8 or 9 aversive responses to 100% 1-octanol were examined in peptide receptor null animals, on the assumption that peptide and cognate receptor null animals would exhibit similar phenotypes. Putative null alleles are available for most of the over 50 predicted peptide receptors; others were examined using feeding RNAi (Harris et al, 2010). OA inhibition was reduced in putative t27d1.3 (npr-15), c43c3.2 (npr-18), c02h7.2 (npr-19), gnrr-1, nmur-1 and npr-9 null animals and after RNAi knockdown of t07d4.1 (npr-20) in wild-type animals (Figure 7A). However, npr-9 and nmur-1 animals also exhibited faster basal responses to 30% 1-octanol off food, a phenotype not shared by nlp-6-9 animals; therefore, npr-9 and nmur-1 animals were not examined further (unpublished observations). As predicted, OA inhibition in npr-15, npr-18 and npr-19 animals could be rescued by the expression of the appropriate receptors (no npr-20 null was available) and receptor overexpression in wild-type animals significantly inhibited basal aversive responses, as noted above for peptide overexpression, suggesting either that these peptides are tonically released or that the receptors exhibit constitutive activity in the absence of ligand (Figure 7B). To identify the individual receptors activated by these peptides, each peptide-encoding gene was expressed in receptor null animals, on the assumption that the slower basal responses associated with peptide overexpression would be absent or significantly reduced in animals lacking the cognate receptors (Figure 7C). This approach has been used previously to tentatively identify ligands for NPR-17 (Harris et al, 2010). The expression of nlp-7 or 9 in npr-15 animals inhibited basal aversive responses, but the expression of nlp-8 did not, suggesting that nlp-8 might encode ligands for NPR-15 (Figure 7C). Similarly, the expression of nlp-7 or 8 in npr-18 animals still inhibited basal responses, but the expression of nlp-9 did not, suggesting that nlp-9 might encode peptides for NPR-18 (Figure 7C). In contrast, the expression of nlp-7, nlp-8 or nlp-9 still inhibited basal responses in gnrr-1 animals, suggesting that GNRR-1 was not activated by peptides encoded by these genes (Figure 7C). Conversely, the expression of nlp-7, nlp-8 or nlp-9 in npr-19 null animals failed to increase basal aversive responses (Figure 7C). These results have tentatively identified seven peptide receptors potentially involved in the OA inhibition of aversive responses; two of which, NPR-15 and NPR-18 appear to be activated by peptides encoded by nlp-8 and nlp-9, respectively.
Figure 7.
Identification of potential ligands for the neuropeptide receptors involved in the OA inhibition of ASH-mediated aversive responses. (A) Neuropeptide receptor null mutants and eri-1 animals with feeding RNAi were examined for aversive responses to 100% 1-octanol off food in the presence 4 mM OA. Black bars, wild-type or null animals; hatched bars, RNAi in eri-1 animals. (B, C) Animals overexpressing individual neuropeptide-encoding genes in wild-type and mutant backgrounds were examined for the OA inhibition of aversive responses to 4 mM OA. (B) Black bars, wild-type or null animals; grey bars, wild-type or null animals expressing a transgene. (C) Overexpression of different neuropeptide transgenes in wild-type and null animals as noted in figure. Data are presented as a mean±s.e. and analysed by two-tailed Student's t-test. *P<0.001, significantly different from wild-type animals under identical conditions.
NPR-15 and NPR-18 function in the AWC and ASER sensory neurons to inhibit aversive responses
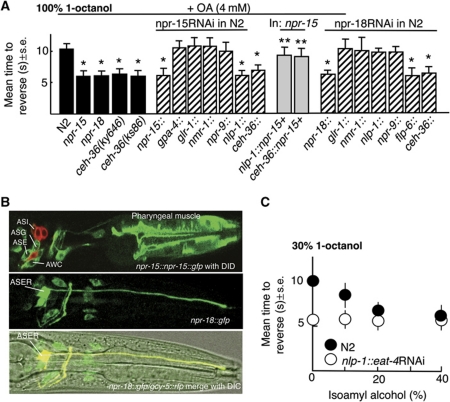
An npr-15∷npr-15∷gfp full-length transgene that rescued the OA inhibition of aversive responses in npr-15 null animals was expressed in pharyngeal muscle, and the AWC, ASG, ASE sensory neurons, based on GFP fluorescence (Figure 8B). Weak fluorescence was also observed in the ASI and ASJ sensory neurons and four other unidentified head neurons (Figure 8B). The OA inhibition of aversive responses was dramatically reduced by npr-15∷npr-15RNAi, suggesting that expression from the minimal npr-15 promoter was sufficient for knockdown (Figure 8A). In addition, AWC∷ npr-15 RNAi knockdown using either the nlp-1 or ceh-36 promoters significantly reduced OA inhibition. nlp-1 is expressed in the AWCs, ASIs, PHBs, BDUs and four other sensory neurons; ceh-36 in the AWCs and ASER. Although npr-15 was expressed in both the AWCs and ASIs, ASI∷npr-15RNAi knockdown had no effect on OA inhibition, (Figure 8A). This ASI∷npr-15 RNAi also serves as an effective control for RNAi spreading. Finally, as predicted, the expression of npr-15 in the AWCs partially rescued OA inhibition in npr-15 null animals (Figure 8A). Together, these data strongly suggest that NPR-15 functions in the AWCs.
Figure 8.
npr-15 and npr-18 function in the AWC and ASER sensory neurons mediating attraction to inhibit aversive responses to 100% 1-octanol. (A) Aversive behaviour to 100% 1-octanol was assayed off food in wild-type, mutant and transgenic animals after incubation in 4 mM OA, as described in Materials and methods. Black bars, wild-type or null animals; grey bars, animals expressing a transgene; hatched bars, RNAi in wild-type animals. Data are presented as a mean±s.e. and analysed by two-tailed Student's t-test. *P<0.001, significantly different from wild-type animals under identical test conditions. **P<0.001, significantly different from null animals under identical test conditions. (B) GFP fluorescence from an npr-15∷npr-15∷gfp and npr-18∷gfp transgenes, Top: Anterior portion of animal expressing a pnpr-15∷npr-15∷gfp translational fusion stained with DiD. Middle and bottom: Colocalization (yellow) of anterior portion of animal expressing a npr-18∷gfp transcriptional fusion and gcy-5∷rfp. (C) The AWC-dependent attractant, isoamyl alcohol stimulates basal aversive responses to 30% 1-octanol. Aversive responses were assayed at 30% 1-octanol mixed with various concentrations of isoamyl alcohol, as indicated.
An npr-18∷gfp transgene was robustly expressed in ASER and more faintly in a small number of additional neurons (Figure 8B). The OA inhibition of aversive responses was significantly reduced by npr-18∷npr-18RNAi (Figure 8A), suggesting that expression from the minimal npr-18 promoter was sufficient for knockdown. Indeed, RNAi knockdown of npr-18 in the ASEs also significantly reduced OA inhibition (Figure 8A). The results suggest that NPR-15 and NPR-18 function in the AWCs and ASER, respectively, to inhibit aversive responses.
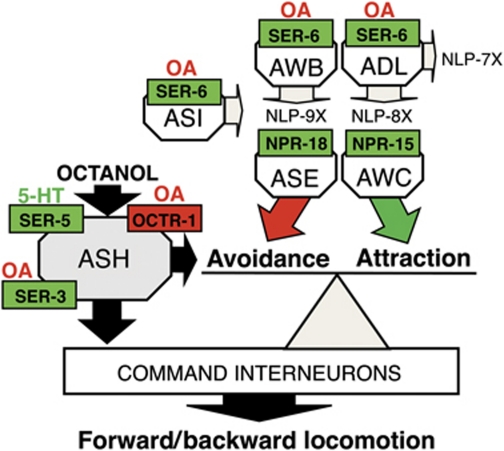
No role for the ASEs or AWCs in octanol avoidance had been observed previously. Interestingly, ceh-36 mutant animals that are defective for AWC and ASE-mediated chemotaxis exhibited more rapid basal aversive responses to 30% 1-octanol (5.2±0.4 s, n=3) and no OA inhibition of responses to 100% 1-octanol, supporting a role for these sensory neurons in the modulation ASH-mediated reversal (Figure 8A; Koga and Ohshima, 2004). In addition, AWC RNAi knockdown of eat-4 that abolishes AWC signalling (Chalasani et al, 2007) also stimulated aversive responses to 30% 1-octanol (Figure 8C). The AWCs are ‘odor off’ neurons and the attractive odorant, isoamyl alcohol (IAA), decreases signalling in both AWCs, based on the decreased fluorescence from GCaMP, a genetically encoded calcium sensor (Chalasani et al, 2007, 2010). Therefore, we reasoned that if inhibiting AWC signalling enhanced aversive responses, then the simultaneous addition of both 30% 1-octanol and IAA to the assay should increase aversive responses relative to 30% 1-octanol alone. As predicted, IAA increased basal aversive responses to 30% 1-octanol in a dose-dependent manner (Figure 8C). Importantly, animals did not reverse in response to 100% IAA in these assays (unpublished observations). In contrast, activation of ASER appears to initiate avoidance behaviour, suggesting that NPR-15 may inhibit tonic ASER signalling (Suzuki et al, 2008; Bretscher et al, 2011). These results suggest that OA not only inhibits ASH signalling directly, but also stimulates the release of an array of neuropeptides that potentially modulate tonic signalling from other sensory neurons mediating both attraction and repulsion to modulate ASH-mediated aversive inputs (Figure 9).
Figure 9.
The OA inhibition of ASH-mediated aversive responses involves the OCTR-1-dependent inhibition of the ASHs and the SER-6-dependent stimulation of peptide release from the AWB, ADL and ASI sensory neurons. The ASHs mediate aversive responses and extensively innervate the command interneurons that control forward/backward locomotion, stimulating reversal. OA inhibits the ASHs directly through OCTR-1. In addition, OA through SER-6 stimulates the release of an array of neuropeptides from the ASIs, AWBs and ADLs that activate receptors on the AWCs and ASER. The AWCs and ASER mediate chemoattraction, not repulsion, and do not make extensive synaptic contact with the command interneurons, but instead along with the ASHs synapse extensively on a layer of interneurons that integrate multiple sensory inputs to modulate turning and reversal. The results of the present study suggest that these OA- and SER-6-dependent neuropeptides act on receptors in sensory neurons mediating chemoattraction to inhibit aversive responses and integrate chemoattractive and chemorepulsive signalling.
Discussion
The present study demonstrates that the OA modulation of nociception in C. elegans is complex and involves multiple OA receptors and neuropeptidergic signalling. This complexity was unanticipated and highlights the sensitivity of this simple aversive assay. OA is only released from two RIC interneurons and two non-neuronal gonadal sheath cells, based on the expression of tbh-1, the rate-limiting enzyme in OA biosynthesis, but OA still modulates many key aspects of C. elegans behaviour (Alkema et al, 2005). In spite of its limited release, OA appears to activate a global signalling cascade that involves not only multiple OA receptors, but also the activation of a wide range of neuropeptide receptors that translate the OA signal throughout the nervous system. This OA initiated cascade has the potential not only to amplify the OA signal, but also to provide multiple levels for modulation and interaction with other sensory-mediated circuits. It will now be important to determine whether these monoamine/peptide receptors play a developmental role and how they potentially modulate the octanol-sensing circuit directly and tonic release from other sensory neurons involved in locomotory decision making. However, with most of these key receptors now localized to individual neurons, their complex interactions in the modulation of neuronal excitability, neurotransmitter release and/or post-synaptic sensitivity can be characterized in cell-based assays. Interestingly, in this and previous studies on the modulation of ASH-mediated aversive responses to octanol, animals appear to reverse in either about 5 or 10 s, with intermediate responses (7–8 s) only more rarely observed (Wragg et al, 2007; Harris et al, 2009, 2010). This observation suggests that at least some components of this modulatory network may exist in two activity states with modulatory input potentially integrating the switch from one state to the other, as has been suggested for the ‘bistable switch’ predicted to operate in the command interneurons to modulate the decision to move forward or backward (Chalfie et al, 1985; Zheng et al, 1999; Brockie et al, 2001).
The OA inhibition of ASH-mediated aversive responses involves at least three distinct OA receptors
The OA modulation of aversive responses to volatile repellents involves three different α-adrenergic-like OA receptors (Figure 9). At submaximal stimulation, the ASH sensory neurons are primarily responsible for octanol avoidance and although specific octanol receptors have not yet been identified, ablation of the ASHs abolishes responses to 30% 1-octanol (Chao et al, 2004). OA prevents the 5-HT sensitization of aversive responses through ASH OCTR-1 and Gαo-mediated signalling pathways, with ASH SER-3 directly antagonizing OCTR-1 signalling (Wragg et al, 2007; Harris et al, 2010). As noxious stimulation increases, the AWBs and ADLs also appear to be involved in octanol avoidance. For example, responses to 100% octanol off food are reduced, but not abolished, by ablation of the ASHs (Chao et al, 2004). In contrast, responses to 100% octanol are absent after co-ablation of the ASHs, AWBs and ADLs, but since the ADLs and AWBs do not appear to respond directly to octanol, at least by changes in calcium dynamics, their role in octanol sensing is unclear. Whether the ADLs, AWBs and/or ASIs function primarily to mediate the nutritional modulation of aversive behaviours, as increasing OA levels are associated with starvation, and/or to prevent overstimulation of the ASH-mediated circuit during elevated/chronic stimulation is unclear. Clearly, endogenous OA inhibits aversive responses, tdc-1 and tbh-1 null animals that are incapable of OA synthesis have increased basal aversive responses to dilute 1-octanol and starvation and tdc-1 overexpression both inhibit aversive responses to 100% 1-octanol (Wragg et al, 2007). The ASHs form multiple gap junctions with the octopaminergic RIC interneurons, so that increases in ASH signalling would be predicted to increase OA release (Alkema et al, 2005). However, tbh-1 null and wild-type animals respond identically to 100% 1-octanol, suggesting that ASH-stimulated OA release is not essential to maintain rapid aversive responses. Whether ASH-stimulated OA release modulates ASH signalling during chronic stimulation remains to be determined.
The SER-6-dependent OA inhibition of aversive responses requires an array of neuropeptides from multiple sensory neurons
As noted above, the activation of SER-6 in the AWB, ADL and ASI sensory neurons stimulates the release of a large and diverse group of peptides that ultimately inhibit ASH-mediated aversive responses to 100% 1-octanol. At least some of the peptides released by the AWBs and ADLs act at sites outside the circuit innervated directly by the ASHs. Certainly, the gustatory ASIs only weakly innervate the ASH-mediated circuit, so that ASI-derived peptides most probably act humorally. In fact, most monoamine receptors are expressed on neurons that are not directly innervated by monoaminergic neurons, suggesting that a significant portion of monoaminergic signalling may be humoral and extrasynaptic. This also appears to be true for peptidergic signalling, suggesting that both monoaminergic and peptidergic signalling have a tonic component, with neuronal ‘activity states’ maintained by a complex mixture of humoral ligands, and an acute component with monoamine and peptide release changing rapidly in response to environmental stimuli. The peptide receptors mediating the inhibition of ASH-mediated responses are also diverse and little is known about their modulatory roles. Importantly, these receptors are similar to vertebrate somatostatin receptors (NPR-18), cannabinoid receptors (NPR-19), galanin/cholecystokinin receptors (NPR-20) that are involved in pain modulation and arthropod allatostatin receptors (NPR-15) that have been targeted for insecticide development (Walker et al, 1999; Cazzamali and Grimmelikhuijzen, 2002; Blakeman et al, 2003; Bar et al, 2004).
Neuropeptides appear to integrate sensory signalling to modulate locomotory behaviour
NPR-15 and NPR-18 appear to activate receptors on the AWC and ASER sensory neurons, but not on neurons innervated directly by the ASHs. The roles of the AWCs and ASER in the modulation of locomotory behaviour are complex. For example, the AWCs mediate attraction and ASER has been implicated in the avoidance of salt and CO2 (Bargmann and Horvitz, 1991; Bargmann et al, 1993; Suzuki et al, 2008; Bretscher et al, 2011). Although the ASHs extensively innervate the command interneurons that control forward/backward locomotion, the AWCs and ASER do not, and instead, along with the ASHs, synapse extensively on a layer of interneurons that integrate multiple sensory inputs to modulate turning and reversal (White et al, 1986; Gray et al, 2005). For example, the AIB interneurons receive sensory input from the ASHs, AWCs and ASEs and modulate locomotory transitions associated with nutritional state (Chalasani et al, 2007, 2010). Presumably, each of these sensory neurons contributes individually to AIB signalling and theoretically input from any of these neurons could differentially modulate locomotory behaviour. For example, the AWCs exhibit basal activity at rest and appear to tonically modulate AIA/AIB signalling (Chalasani et al, 2010). In the present study, basal aversive responses off food were dramatically increased in ceh-36 animals that lack AWCs or by addition of an AWC-specific ligand, IAA, predicted to decrease AWC signalling, supporting a role for the AWCs in the modulation of ASH-mediated aversive responses. Together, our results suggest that the OA-dependent release of neuropeptides may inhibit ASH-mediated aversive responses by interfering with tonic signalling from other sensory neurons both favouring and opposing reversal. Recently, additional neuropeptides have been implicated in the modulation of both ASH and AWC-mediated behaviours, suggesting that the peptidergic modulation of sensory output observed in the present study may be a general mechanism for integrating attractive/repulsive sensory stimuli in the modulation of locomotory behaviours (Chalasani et al, 2010; Harris et al, 2010).
The OA inhibition of aversive responses in C. elegans is similar to the noradrenergic inhibition of nociception in mammals
The network of OA receptors modulating aversive responses in C. elegans mimics the noradrenergic inhibition of nociception in mammals, where norepinephrine released from descending pathways suppresses pain through inhibitory α2-adrenoreceptors (Gαi coupled) on afferent nociceptors and by the activation of α1-receptors on inhibitory peptidergic interneurons. Under basal conditions, the noradrenergic system has little effect on nociception, but sustained pain induces noradrenergic feedback inhibition (Obata et al, 2005). Similarly, in C. elegans, OA has no effect on basal aversive responses to submaximal ASH stimulation, but as the intensity of ASH stimulation increases, OA inhibition becomes more robust, with the OA inhibition associated with ‘inhibitory’ peptides only apparent at increased ASH stimulation. Pro-nociceptive adrenergic pathways have also been identified, with norepinephrine apparently increasing excitability of dorsal root ganglion neurons in nerve injured animals through the α2-adrenergic receptor-mediated blockade of N-type Ca2+ channels and subsequent inhibition of Ca2+-activated K+ channels (Abdulla and Smith, 1997; Honma et al, 1999). Whether the antagonistic effects of SER-3 on OCTR-1-mediated OA inhibition in the ASHs mimics the pro-nociceptive action of α2-adrenoreceptors on Ca2+ dynamics remains to be determined.
These studies highlight the complexity of octopaminergic modulation, the importance of ‘inhibitory’ peptidergic neurons in octopaminergic signalling, and the utility of C. elegans as a model to study the role of individual neurons in the monoaminergic/peptidergic modulation of complex behaviours, such as nociception.
Materials and methods
Nematode growth and strains
Strains were maintained as described (Brenner, 1974). The following strains were used: N2, ser-3(ad1774),ser-6 (tm2146) octr-1(ok371), tdc-1(n3419), tbh-1(n3247), npr-18(ok1387), npr-19(ok2068), nmur-3(ok2295), npr-15(ok1626), nmur-1(ok1387), fshr-1(ok778), nmur-4(ok1381), to2e9.1(ok2008), t11f9.1(ok2284), npr-10(ok1541), c49a9.7(ok1620), t11f9.1(ok2284), eri-1(kp3948), ckr-1(ok2502), nmur-2(ok3502) and f14f4.1(tm2243), nlp-7(tm2184), nlp-9(tm3572), npr-9(tm1652), tkr-1(tm1765), t07d10.2(tm2765), npr-14(tm2974), ckr-2(tm3082), npr-17(tm3210), gnnr-1(tm238), ser-6(tm2104) and to7d12.2(tm2765).
Behavioural assays
Responses to 1-octanol were assayed as previously described (Chao et al, 2004). For all behavioural assays, L4 stage animals were picked 24 h before testing. NGM plates were prepared the morning of the experiment by adding either 5-HT and/or OA (final concentration as noted) to liquid NGM before pouring. Dilute (30%) 1-octanol was prepared daily using 100% ethanol (vol/vol). Briefly, the blunt end of a hair was dipped in 1-octanol and the hair placed in front of an animal exhibiting forward sinusoidal locomotion (Zhao et al, 2003; Chao et al, 2005). For assays in the absence of food or exogenous 5-HT, animals were first transferred to intermediate (non-seeded) plates and left for 1 min, then transferred to assay plates and tested after 10 min. For assays in the presence of 5-HT/OA, animals were transferred to plates and assayed after 30 min. In all, 20–25 animals were examined for each strain and condition. Statistical analysis was performed using mean±s.e. and Student's t-test.
Expression and electrophysiology in Xenopus laevis oocytes
The ser-6, octr-1 or ser-3 cDNAs were cloned into a Xenopus expression vector containing a T7 promoter and the Xenopus 5′ and 3′ β-globin untranslated regions. Linearized plasmids were transcribed using an Ambion mMessage mMachine T7 kit (Applied Biosystems, Carlsbad, CA). Xenopus laevis ovaries were obtained from Nasco Co (Fort Atkinson, WI, USA). Oocytes were mechanically separated before incubation in ND-96 (Ca2+ free) medium (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM HEPES, pH 7.6) containing 1 mg/ml of collagenase for 45 min to defolliculate. Defolliculated oocytes were incubated in modified Barth′s medium (Barth′s medium with 1 mM Na Pyruvate, 0.01 mg/ml Gentamicin, and 1 × Antibiotic-antimycotic; Gibco, Invitrogen, Carlsbad, CA, USA) at 18°C overnight. Receptor cRNAs were injected at 5 ng per 50 nl injection volume (1 ng for each of the GIRK subunits). Oocytes were incubated at 18°C for 24–72 h post injection and then transferred to 4°C. Two-Electrode Voltage-Clamp (TEVC) recordings were performed 1–4 days post injection using an Axon Gene Clamp 500 Amplifier (Molecular Devices, Sunnyvale, CA) as previously described (Stuhmer, 1998; Bamber et al, 2003). Cells were clamped at −60 mV and all recordings were performed at RT using standard Ringers solution (115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES, pH 7.2). To examine OCTR-1 signalling, ooctyes were co-injected with GIRK1/2 subunits, clamped at −80 mV, and recorded using a High K+ Ringers solution (96 mM KCl, 2 mM NaCl, 1.8 mM CaCl2, 10 mM HEPES, pH 7.2). Ligands were applied by gravity perfusion, initially at 1 μM. Oocytes expressing SER-6 were tested with OA, TA, DA and 5-HT at 5 min intervals to determine ligand specificity. OA dose–response curves were fitted with the equation: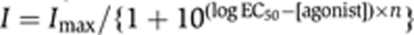 where I is current at a given OA concentration, Imax is current at saturation, EC50 is the OA concentration required to produce half-maximal current and n is the slope coefficient. Curve fitting was performed using GraphPad Prism software (San Diego).
where I is current at a given OA concentration, Imax is current at saturation, EC50 is the OA concentration required to produce half-maximal current and n is the slope coefficient. Curve fitting was performed using GraphPad Prism software (San Diego).
Molecular biology and transgenesis
cDNA or genomic regions corresponding to the entire coding sequences of octr-1, ser-3, ser-6, nlp-6, nlp-7, nlp-8, nlp-9 were amplified by PCR and expressed under cell-selective promoters. The following promoters were used: ceh-36 (AWC, AWC), flp-6 (ASE, AFD, ASG, PVT), gcy-5 (ASER), gpa-4 (ASI), nlp-1 (ASI, AWC, PHB, BDU, four head neurons), nmr-1 (AVA, AVD, AVE, RIM, AVG, PVG), npr-9 (AIB), odr-2(2b) (AIB, AIZ, ASG, AVG, RIF, RIV, PVP, SIAV, IL2), sra-6 (ASH, ASI, PVQ), srb-6 (ASH, ADL), sro-1 (ADL, SIA), and str-1 (AWB). Neuron-selective rescue constructs were created by overlap fusion PCR (Hobert, 2002). Neuron-selective RNAi transgenes were created as previously described (Esposito et al, 2007). PCR products were pooled from at least three separate PCRs and co-injected with a selectable marker (myo-3∷gfp or rol-6) and carrier DNA into gonads of C. elegans wild-type or null mutant animals by standard techniques (Kramer et al, 1990; Esposito et al, 2007).
Calcium imaging
Worms were glued to cover slips coated with Sylgard and immersed in NaCl 150 mM, KCl 5 mM, CaCl2 5 mM, MgCl2 1 mM, glucose 10 mM and HEPES 15 mM, pH 7.30, <330 mOsm, using WormGlu cyanoacrylate glue (GluStitch, Delta, Canada). After gluing, the cover slip was transferred to a laminar flow chamber (RC26G, Warner Instruments, Hamden, CT), positioned under the microscope with saline perfusion. Saline containing 2.3 mM 1-octanol (a saturating concentration) was applied using a multi-barrel fast perfusion system (Warner SF77B, barrel width 300 μm) controlled by pCLAMP10 acquisition software (Molecular Devices). Adjacent barrels were loaded with control saline, and saline plus octanol, such that octanol could be rapidly applied and washed out by lateral motions of the tubing (300 μm step size). GCaMP3 recordings were performed on an Axioskop 2 FS Plus upright compound microscope ( × 40 Achroplan water immersion objective, GFP filter set #38), fitted with an Orca ER CCD camera and programmable shutter (Uniblitz, Vincent Associates, Rochester, NY). Fluorescence images were acquired using MetaVue 7.6.5 (MDS Analytical Technologies, Sunnyvale, CA), and analysed using Jmalyze software (R Kerr). Samples were obtained at ∼10 Hz (100 ms exposure time) with 4 × binning.
Supplementary Material
Acknowledgments
We thank the C. elegans Genetics Center and the National Bioresources Center for strains; Yifan Xu for the ASH∷GCaMP3 strain; Dr Y Zhang for the AWB∷GCaMP strain and Drs W Schafer and J Cregg for assistance with the video microscopy setup. This work was supported by NIH Grant AI-145147 awarded to RK and funds from the Joan L and Julius H Jacobson Biomedical Professorship.
Author contributions: RW, GH and RK designed research; RW, HM, MC, VH, PS, JZ, WL and AK performed research; BB contributed reagents/analytic tools; RW, HM, BB and RK analysed data; RW, HM and RK wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abdulla FA, Smith PA (1997) Ectopic alpha2-adrenoceptors couple to N-type Ca2+ channels in axotomized rat sensory neurons. J Neurosci 17: 1633–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkema MJ, Hunter-Ensor M, Ringstad N, Horvitz HR (2005) Tyramine functions independently of octopamine in the Caenorhabditis elegans nervous system. Neuron 46: 247–260 [DOI] [PubMed] [Google Scholar]
- Bamber BA, Twyman RE, Jorgensen EM (2003) Pharmacological characterization of the homomeric and heteromeric UNC-49 GABA receptors in C. elegans. Br J Pharmacol 138: 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar KJ, Schurigt U, Scholze A, Segond Von Banchet G, Stopfel N, Brauer R, Halbhuber KJ, Schaible HG (2004) The expression and localization of somatostatin receptors in dorsal root ganglion neurons of normal and monoarthritic rats. Neuroscience 127: 197–206 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Horvitz HR (1991) Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 7: 729–742 [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Hartwieg E, Horvitz HR (1993) Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 74: 515–527 [DOI] [PubMed] [Google Scholar]
- Bendesky A, Tsunozaki M, Rockman MV, Kruglyak L, Bargmann CI (2011) Catecholamine receptor polymorphisms affect decision-making in C. elegans. Nature 472: 313–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeman KH, Hao JX, Xu XJ, Jacoby AS, Shine J, Crawley JN, Iismaa T, Wiesenfeld-Hallin Z (2003) Hyperalgesia and increased neuropathic pain-like response in mice lacking galanin receptor 1 receptors. Neuroscience 117: 221–227 [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77: 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher AJ, Kodama-Namba E, Busch KE, Murphy RJ, Soltesz Z, Laurent P, de Bono M (2011) Temperature, oxygen, and salt-sensing neurons in C. elegans are carbon dioxide sensors that control avoidance behavior. Neuron 69: 1099–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockie PJ, Mellem JE, Hills T, Madsen DM, Maricq AV (2001) The C. elegans glutamate receptor subunit NMR-1 is required for slow NMDA-activated currents that regulate reversal frequency during locomotion. Neuron 31: 617–630 [DOI] [PubMed] [Google Scholar]
- Cazzamali G, Grimmelikhuijzen CJ (2002) Molecular cloning and functional expression of the first insect FMRFamide receptor. Proc Natl Acad Sci USA 99: 12073–12078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI (2010) Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci 13: 615–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalasani SH, Chronis N, Tsunozaki M, Gray JM, Ramot D, Goodman MB, Bargmann CI (2007) Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature 450: 63–70 [DOI] [PubMed] [Google Scholar]
- Chalfie M, Sulston JE, White JG, Southgate E, Thomson JN, Brenner S (1985) The neural circuit for touch sensitivity in Caenorhabditis elegans. J Neurosci 5: 956–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MY, Larkins-Ford J, Tucey TM, Hart AC (2005) lin-12 Notch functions in the adult nervous system of C. elegans. BMC Neurosci 6: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC (2004) Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci USA 101: 15512–15517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlie NK, Schade MA, Thomure AM, Miller KG (2006) Presynaptic UNC-31 (CAPS) is required to activate the G alpha(s) pathway of the Caenorhabditis elegans synaptic signalling network. Genetics 172: 943–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Di Schiavi E, Bergamasco C, Bazzicalupo P (2007) Efficient and cell specific knock-down of gene function in targeted C. elegans neurons. Gene 395: 170–176 [DOI] [PubMed] [Google Scholar]
- Ezak MJ, Ferkey DM (2010) The C. elegans D2-like dopamine receptor DOP-3 decreases behavioral sensitivity to the olfactory stimulus 1-octanol. PLoS One 5: e9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezcurra M, Tanizawa Y, Swoboda P, Schafer WR (2011) Food sensitizes C. elegans avoidance behaviours through acute dopamine signalling. EMBO J 30: 1110–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni MP, Ghelardini C, Vergelli C, Dal Piaz V (2009) Alpha2-agonists as analgesic agents. Med Res Rev 29: 339–368 [DOI] [PubMed] [Google Scholar]
- Gray JM, Hill JJ, Bargmann CI (2005) A circuit for navigation in Caenorhabditis elegans. Proc Natl Acad Sci USA 102: 3184–3191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HI, Hendricks M, Shen Y, Gabel CV, Fang-Yen C, Qin Y, Colon-Ramos D, Shen K, Samuel AD, Zhang Y (2010) Functional organization of a neural network for aversive olfactory learning in Caenorhabditis elegans. Neuron 68: 1173–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris G, Mills H, Wragg R, Hapiak V, Castelletto M, Korchnak A, Komuniecki RW (2010) The monoaminergic modulation of sensory-mediated aversive responses in Caenorhabditis elegans requires glutamatergic/peptidergic cotransmission. J Neurosci 30: 7889–7899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GP, Hapiak VM, Wragg RT, Miller SB, Hughes LJ, Hobson RJ, Steven R, Bamber B, Komuniecki RW (2009) Three distinct amine receptors operating at different levels within the locomotory circuit are each essential for the serotonergic modulation of chemosensation in Caenorhabditis elegans. J Neurosci 29: 1446–1456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard MA, Apicella AJ, Kerr R, Suzuki H, Bazzicalupo P, Schafer WR (2005) In vivo imaging of C. elegans ASH neurons: cellular response and adaptation to chemical repellents. EMBO J 24: 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobert O (2002) PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32: 728–730 [DOI] [PubMed] [Google Scholar]
- Honma Y, Yamakage M, Ninomiya T (1999) Effects of adrenergic stimulus on the activities of Ca2+ and K+ channels of dorsal root ganglion neurons in a neuropathic pain model. Brain Res 832: 195–206 [DOI] [PubMed] [Google Scholar]
- Kass J, Jacob TC, Kim P, Kaplan JM (2001) The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J Neurosci 21: 9265–9272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga M, Ohshima Y (2004) The C. elegans ceh-36 gene encodes a putative homemodomain transcription factor involved in chemosensory functions of ASE and AWC neurons. J Mol Biol 336: 579–587 [DOI] [PubMed] [Google Scholar]
- Kramer JM, French RP, Park EC, Johnson JJ (1990) The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol 10: 2081–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RY, Sawin ER, Chalfie M, Horvitz HR, Avery L (1999) EAT-4, a homolog of a mammalian sodium-dependent inorganic phosphate cotransporter, is necessary for glutamatergic neurotransmission in Caenorhabditis elegans. J Neurosci 19: 159–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Kim K (2008) Neuropeptides, Worm Book, pp 1–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath PT, Xu Y, Ailion M, Garrison JL, Butcher RA, Bargmann CI (2011) Parallel evolution of domesticated Caenorhabditis species targets pheromone receptor genes. Nature 477: 321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathoo AN, Moeller RA, Westlund BA, Hart AC (2001) Identification of neuropeptide-like protein gene families in Caenorhabditis elegans and other species. Proc Natl Acad Sci USA 98: 14000–14005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata H, Conklin D, Eisenach JC (2005) Spinal noradrenaline transporter inhibition by reboxetine and Xen2174 reduces tactile hypersensitivity after surgery in rats. Pain 113: 271–276 [DOI] [PubMed] [Google Scholar]
- Ohana L, Barchad O, Parnas I, Parnas H (2006) The metabotropic glutamate G-protein-coupled receptors mGluR3 and mGluR1a are voltage-sensitive. J Biol Chem 281: 24204–24215 [DOI] [PubMed] [Google Scholar]
- Pertovaara A (2006) Noradrenergic pain modulation. Prog Neurobiol 80: 53–83 [DOI] [PubMed] [Google Scholar]
- Petrascheck M, Ye X, Buck LB (2007) An antidepressant that extends lifespan in adult Caenorhabditis elegans. Nature 450: 553–556 [DOI] [PubMed] [Google Scholar]
- Saugstad JA, Segerson TP, Westbrook GL (1996) Metabotropic glutamate receptors activate G-protein-coupled inwardly rectifying potassium channels in Xenopus oocytes. J Neurosci 16: 5979–5985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese S, Petrie M, Schuske K, Ailion M, Ann K, Iwasaki K, Jorgensen EM, Martin TF (2007) UNC-31 (CAPS) is required for dense-core vesicle but not synaptic vesicle exocytosis in Caenorhabditis elegans. J Neurosci 27: 6150–6162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhmer W (1998) Electrophysiologic recordings from Xenopus oocytes. Methods Enzymol 293: 280–300 [DOI] [PubMed] [Google Scholar]
- Suzuki H, Thiele TR, Faumont S, Ezcurra M, Lockery SR, Schafer WR (2008) Functional asymmetry in Caenorhabditis elegans taste neurons and its computational role in chemotaxis. Nature 454: 114–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JM, Huang SM, Strangman NM, Tsou K, Sanudo-Pena MC (1999) Pain modulation by release of the endogenous cannabinoid anandamide. Proc Natl Acad Sci USA 96: 12198–12203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode Caenorhabditis Elegans. Philos T Roy Soc B 314: 1–340 [DOI] [PubMed] [Google Scholar]
- Wragg RT, Hapiak V, Miller SB, Harris GP, Gray J, Komuniecki PR, Komuniecki RW (2007) Tyramine and octopamine independently inhibit serotonin-stimulated aversive behaviors in Caenorhabditis elegans through two novel amine receptors. J Neurosci 27: 13402–13412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pacheco MA, Doupnik CA (2002) Gating properties of GIRK channels activated by Galpha(o)- and Galpha(i)-coupled muscarinic m2 receptors in Xenopus oocytes: the role of receptor precoupling in RGS modulation. J Physiol 545: 355–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Khare P, Feldman L, Dent JA (2003) Reversal frequency in Caenorhabditis elegans represents an integrated response to the state of the animal and its environment. J Neurosci 23: 5319–5328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Brockie PJ, Mellem JE, Madsen DM, Maricq AV (1999) Neuronal control of locomotion in C. elegans is modified by a dominant mutation in the GLR-1 ionotropic glutamate receptor. Neuron 24: 347–361 [DOI] [PubMed] [Google Scholar]
- Zhou KM, Dong YM, Ge Q, Zhu D, Zhou W, Lin XG, Liang T, Wu ZX, Xu T (2007) PKA activation bypasses the requirement for UNC-31 in the docking of dense core vesicles from C. elegans neurons. Neuron 56: 657–669 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.