Abstract
The role of specific members of the NF-κB family of transcription factors in CD8 T-cell selection and development is largely unknown. Here, we show that mice lacking NF-κB1 develop a unique population of conventional CD8 single-positive (SP) thymocytes with memory T cell-like properties that populate peripheral immune organs. Development of this memory-like population is not due to PLZF+ thymocytes and instead coincides with changes in CD8 T-cell selection. These include a reduction in the efficiency of negative selection and a dependence on MHC class Ia or Ib expressed by haematopoietic cells. These findings indicate that NF-κB1 regulates multiple events in the thymus that collectively inhibit the excess development of CD8+ thymocytes with memory cell characteristics.
Keywords: memory CD8 T cells, NF-κB, NF-κB1, p50, T-cell development
Introduction
The differentiation of thymocytes that express αβ T-cell receptors (TCRs) is dependent on selection steps that are influenced by the strength and duration of TCR signals (Hogquist, 2001). Initially, pre-TCR signals in CD4−CD8− (DN) progenitors initiate TCRα gene rearrangement and the subsequent development of CD4+CD8+ (DP) thymocytes. DP thymocytes are then screened by a process of positive and negative selection involving TCR interactions with self-peptide bound to MHC molecules (Bosselut, 2004) that dictate CD4 and CD8 T-cell differentiation and maturation. Cells bearing TCRs that recognise classical MHC class I (class Ia) become CD8+ T cells, while recognition of MHC class II promotes CD4+ T-cell development (Starr et al, 2003). For CD4+ and CD8+ T cells, DP thymocytes with TCRs that bind peptide–MHC complexes expressed on thymic epithelial cells (TECs) with low-to-moderate affinity, undergo positive selection. At this stage, cells expressing a non-functional TCR fail to receive a differentiation signal and undergo death by neglect. Thymocytes expressing a TCR that binds peptide–MHC complexes with high affinity are potentially auto-reactive and are targeted by negative selection to undergo apoptosis (von Boehmer and Melchers, 2010). Although DP thymocytes are thought to be first subjected to positive selection, and then in an overlapping manner to negative selection as cells move from the cortex to the medulla, the sequence and timing of these thymic selection events appears to be flexible (von Boehmer and Melchers, 2010).
DP thymocytes also serve as precursors for other T-cell lineages, such as CD4 regulatory T cells and innate T lymphocytes, the latter including CD1d restricted natural killer T (NKT) cells and CD8 T cells that express TCRs specific for non-classical MHC class Ib molecules (Berg, 2007). Innate CD8+ thymocytes typically express high levels of the memory T-cell markers CD44 and CD122, require IL-15 for development as well as for survival, and rapidly produce effector cytokines following activation (Dubois et al, 2006; Berg, 2007). Positive selection and differentiation of these cells is dependent on peptides presented by MHC class Ib expressed on haematopoietic cells (Urdahl et al, 2002; Kurepa et al, 2003). Aside from the importance of MHC class Ia- and Ib-dependent selection by thymic epithelial and haematopoietic cells, respectively, little is known about other mechanisms that shape the distinct phenotypes acquired by conventional and innate CD8 T cells. A recent advance in our understanding of innate/memory-like CD8+ T-cell development has emerged from findings that certain thymic T lymphocytes, including NKT and γδ cells that express the transcription factor PLZF, can promote development of CD8 thymocytes with memory characteristics (Weinreich et al, 2010; Lee et al, 2011).
Among the transcription factors activated by TCR engagement during thymocyte development and selection are the NF-κB proteins (Gerondakis and Siebenlist, 2010). These transcription factors comprise dimers of related proteins (c-Rel, RelA, RelB, NF-κB1 and NF-κB2), which in most cells remain in a latent state bound to IκB proteins within the cytoplasm. Signal-dependent activation of an IκB kinase (IKK), leads to IκB phosphorylation and degradation (Ghosh and Karin, 2002), that results in NF-κB proteins entering the nucleus and controlling the transcription of target genes (Ghosh and Karin, 2002). NF-κB proteins are differentially regulated during thymocyte differentiation (Gerondakis and Siebenlist, 2010). Initially, constitutive NF-κB activation during the late stages of DN thymocyte development (Feuillard et al, 2000; Voll et al, 2000) precedes a downregulation of NF-κB in DP thymocytes. Following selection, only mature CD8SP thymocytes have significant levels of NF-κB activity (Hettmann and Leiden, 2000) that comprised RelA/NF-κB1 heterodimers and NF-κB1 homodimers (Moore et al, 1995). While the importance of NF-κB in promoting the survival of DN thymocytes is well recognised (Voll et al, 2000; Mandal et al, 2005), the exact roles of this pathway during and after selection are subject to debate. Although mice expressing T lineage restricted IκB super-repressor transgenes confirm that NF-κB is more important for CD8 than for CD4 T-cell selection and development (Boothby et al, 1997; Mora et al, 1999), the function of NF-κB during positive and negative selection was confused by variable inhibition of NF-κB in different IκB transgenic mouse strains (Gerondakis and Siebenlist, 2010). Recently, these results were accommodated by a study showing that the level of NF-κB activity corresponded to TCR signal strength that sets the threshold for positive and negative selection of CD8+ T cells (Jimi et al, 2008). However, specific roles for the different NF-κB family members in thymocyte differentiation and maturation following TCRαβ repertoire selection remain poorly defined.
Despite functions ascribed to NF-κB for thymocyte survival, selection and differentiation, redundancy limits our understanding of the roles served by each NF-κB transcription factor (Gerondakis et al, 2006). The activation of NF-κB1, RelA and c-Rel in thymocytes by signals that impinge on selection and differentiation (Moore et al, 1995) highlight the likelihood that different NF-κB proteins engaged by the IKKβ-dependent pathway perform distinct functions during and after selection. Notwithstanding limited information about the role of RelA in thymocyte development due to the embryonic lethality of rela−/− mice (Beg et al, 1995), to date roles for c-Rel and NF-κB1 in thymocyte development have been restricted to CD4 regulatory T cells (Zheng et al, 2003; Isomura et al, 2009; Vang et al, 2010) and NKT cells (Sivakumar et al, 2003; Godfrey and Berzins, 2007; Stankovic et al, 2011), respectively.
Here, we report that NF-κB1 regulates the selection and development of conventional TCRαβ CD8SP thymocytes. In naive nfκb1−/− mice, elevated numbers of peripheral CD8 memory-like T cells coincide with the development of a unique population of CD8SP thymocytes that possess the phenotypic and functional characteristics of CD8+ memory T cells. Unlike other mouse strains with similar phenotypes (Lee et al, 2011), the development of nfκb1−/− CD8 memory-like thymocytes is not due to the influence of thymic PLZF+ T cells. Instead, their development coincides with altered patterns of positive and negative selection in NF-κB1-deficient mice. Collectively, our findings indicate that NF-κB1 serves critical functions in the thymus by ensuring CD8SP thymocytes adopt naive characteristics during development and that their selection by MHC class I is restricted to TECs.
Results
CD8 T cells that possess a memory phenotype develop in the thymus of nfκb1−/− mice
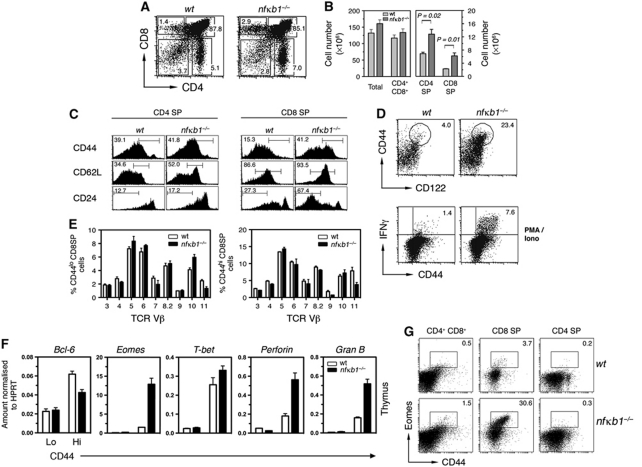
Despite an increasing appreciation of the importance of NF-κB in thymocyte differentiation, an understanding of the roles served by individual members of this family of transcription factors is limited. In particular, little is known about the non-redundant roles of NF-κB1, prompting a detailed analysis of conventional T-cell development in nfκb1−/− mice. Compared with wt mice, the thymocyte cellularity was marginally elevated in 7–10-week-old nfκb1−/− mice, with significant increases in percentages and absolute numbers of CD4 and CD8 single-positive (SP) thymocytes (Figure 1A and B). A significant change in the CD4:CD8 ratio (wt 3.04±0.29 versus nfκb1−/− 2.09±0.19; n=5 per genotype; P=0.05) also suggested that T lineage development was altered. Most nfκb1−/− CD8SP thymocytes displayed low levels of CD24 and high levels of CD62L (Figure 1C), a phenotype associated with increased maturity. The most striking finding was that ∼40% of nfκb1−/− CD8SP thymocytes (compared with only 15% of wt CD8SP cells) expressed high levels of CD44. While CD44hi T cells are typically activated or memory cells, an absence of CD25 and CD69 expression (data not shown) indicated that nfκb1−/− CD44hiCD8SP thymocytes were not activated cells. Coupled with the high expression of CD122 (Figure 1D), these cells instead resembled memory T cells that usually only reside in the periphery. Since a hallmark of memory T cells is an ability to rapidly secrete effector cytokines following activation, we assessed IFN-γ production by nfκb1−/− CD8 thymic T cells. Following PMA plus ionomycin stimulation in vitro, 1% of wt CD44hiCD8SP thymocytes produced IFN-γ, whereas five- to six-fold more nfκb1−/− CD44hiCD8SP cells rapidly secreted this cytokine (Figure 1D). In contrast, nfκb1−/− CD4SP thymocytes exhibited normal CD44 and CD24 expression (Figure 1C), and failed to produce significant levels of IFN-γ when activated (data not shown).
Figure 1.
nfκb1−/− CD8SP thymocytes display memory characteristics. (A) CD4 and CD8 expression by thymocytes from 8-week-old wt and nfκb1−/− mice with numbers showing percentages of cells in each gate. (B) Mean (±s.e.m.) numbers of thymocyte subsets in wt and nfκb1−/− mice. Data consist of one cohort (n=3 mice per genotype). (C) Phenotype of wt and nfκb1−/− CD4 and CD8 SP thymocytes (n=7 mice per genotype). (D) Expression of CD44 and CD122 by wt and nfκb1−/− CD8SP thymocytes. Values indicate percentages of CD44hiCD122hi cells and data represent seven mice per genotype. IFN-γ and CD44 expression gated on wt and nfκb1−/− CD8SP thymocytes after 5 h of stimulation with PMA (10 ng/ml) plus ionomycin (1 μg/ml) (n=6 mice per genotype). (E) Proportion of Vβ TCRs (x axis) gated on CD8SP CD44lo or CD44hi thymocytes for wt and nfκb1−/− mice (mean±s.e.m.; n=3 per genotype). (F) Relative expression of Bcl-6, Eomes, T-bet, Perforin and Granzyme B mRNA in wt and nfκb1−/− CD44lo and CD44hi CD8SP thymocytes (cells were purified from four mice per genotype for each experiment). Graphs show mean±s.d. from triplicate reactions. (G) Expression of Eomes and CD44 by wt and nfκb1−/− thymocyte subsets (n=4 mice per genotype). Values indicate percentages of cells in each gate. Data shown are representative of seven (A), five (B), four (C, G), three (D) and two (E, F) independent experiments. P-values were determined by an unpaired two-tailed Student's t-test.
It remained unclear whether this expanded CD44hiCD8 T-cell population developed in the thymus, or instead represented peripheral CD8 memory T cells that had migrated to the thymus. Although a small proportion of activated or memory T cells normally circulate through the thymus (Michie and Rouse, 1989; Agus et al, 1991), nfκb1−/− CD8+ T cells injected into congenic hosts, while readily detectable in spleen and lymph nodes, were not found in the thymus (Supplementary Figure S1A). This suggested that memory-like CD8SP cells in nfκb1−/− mice were not peripheral cells that had entered the thymus, but instead were of thymic origin. This possibility was assessed using FTOC. While wt and nfκb1−/− thymic precursors readily develop into mature CD4 and CD8 SP thymocytes, a prominent population of CD44hiCD8SP thymocytes was only observed in nfκb1−/− thymi (Supplementary Figure S1B). Collectively, these results reveal that in the absence of NF-κB1, CD8+ T cells acquire memory-like properties during thymic development.
Development of nfκb1−/− CD44hiCD8 thymocytes coincides with increased Eomes expression
The memory-like properties of nfκb1−/− CD8SP thymocytes are a characteristic shared with innate T cells in the CD8 lineage (Berg, 2007; Veillette et al, 2007). These similarities prompted a detailed characterisation of the nfκb1−/− CD44hiCD8SP thymocyte population. Like wt CD44lo and CD44hi CD8SP cells, nfκb1−/− CD44hiCD8SP thymocytes utilise a diverse Vβ TCR repertoire (Figure 1E). In addition to rapidly synthesising IFN-γ, nfκb1−/− CD44hiCD8SP thymocytes express high levels of perforin and granzyme B mRNA (Figure 1F). The T-box transcription factors T-bet and Eomesodermin (Eomes), which contribute to a pattern of gene expression that is characteristic of memory CD8 T cells (Pearce et al, 2003; Intlekofer et al, 2005), were also highly expressed in these cells (Figure 1F). While T-bet levels were high in wt and nfκb1−/− CD44hiCD8SP thymocytes, Eomes was selectively elevated in nfκb1−/− CD44hiCD8SP cells. Further analysis confirmed that Eomes was expressed in nfκb1−/− CD8SP cells, and there was a small but significant increase in the proportion of nfκb1−/− DP thymocytes expressing Eomes (Figure 1G). This indicates that the changes in Eomes expression observed in nfκb1−/− CD44hiCD8SP thymocytes may have been initiated earlier in DP cells.
Development of CD8 memory-like thymocytes depends on the loss of NF-κB1 transcription factor function and not impaired ERK signalling
In addition to being a precursor for the p50NF-κB1 transcription factor, p105NF-κB1 serves as a scaffold for Tpl2, an MAP3K that controls MEK1-dependent ERK activation (Belich et al, 1999). In the absence of p105NF-κB1, Tpl2 is labile (Waterfield et al, 2003), resulting in ERK activation defects in various immune cells (Waterfield et al, 2003; Banerjee et al, 2006). Given that ERK signalling is critical for thymocyte differentiation (Fischer et al, 2005), we examined whether development of nfκb1−/− CD44hiCD8SP thymocytes was due to the absence of p50NF-κB1, or a failure to activate ERK. These two possibilities were distinguished by examining thymocyte development in tpl2−/− mice, which retain normal p50NF-κB1 function (Waterfield et al, 2003). CD8SP thymocyte numbers and CD44 expression were normal in tpl2−/− mice (Supplementary Figure S1C), establishing that impaired NF-κB activity resulting from the loss of p50NF-κB1 must be responsible for generating memory-like CD8SP thymocytes.
The development of nfκb1−/− CD44hiCD8SP thymocytes is independent of IL-4 producing PLZF+ T cells
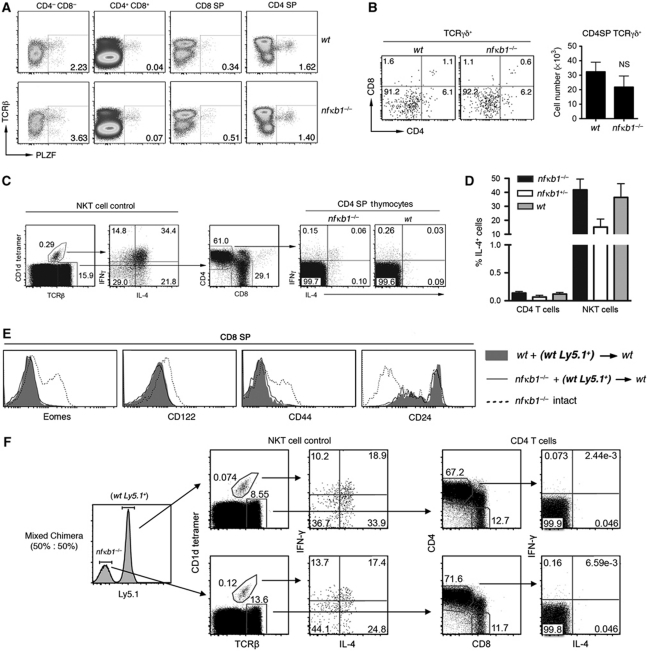
Mouse strains with mutations in T-cell signalling molecules, including ITK, KLF2 and Id3, possess CD8SP thymocytes with memory characteristics (Atherly et al, 2006; Broussard et al, 2006; Verykokakis et al, 2010; Weinreich et al, 2010), which are dependent on the expanded number of IL-4 producing PLZF+ αβ and γδ thymocytes (Verykokakis et al, 2010; Weinreich et al, 2010; Lee et al, 2011). This prompted us to determine if a similar mechanism also accounted for the development of memory-like nfκb1−/− CD8SP thymocytes. The proportion of PLZF+TCRαβ+ thymocytes in wt and nfκb1−/− mice was similar (Supplementary Figure S2A), while the analysis of individual thymocyte subsets revealed a small increase in the proportion of nfκb1−/− PLZF+ DN cells (Figure 2A). While the total number of PLZF+ thymocytes was marginally elevated in the nfκb1−/− thymus, PLZF+CD4SP cell numbers were comparable with wt controls (Supplementary Figure S2B). The proportion of γδ+ thymocytes was also examined in nfκb1−/− mice. Importantly, the number of wt and nfκb1−/− TCRγδ+CD4SP cells was equivalent (Figure 2B).
Figure 2.
nfκb1−/− CD8SP thymocytes acquire memory markers independently of the IL-4 producing PLZF+ population. (A) Expression of PLZF in wt and nfκb1−/− thymocyte subsets. Data represent four mice per genotype. (B) CD4 and CD8 expression by wt and nfκb1−/− TCRγδ+ thymocytes. Mean (±s.e.m.) numbers of wt and nfκb1−/− TCRγδ+CD4SP thymocytes, determined by total numbers of TCRγδ+ thymocytes and proportion of TCRγδ+CD4SP cells (n=3–4 mice per genotype). (C) Expression of IL-4 and IFN-γ in wt, nfκb1+/− and nfκb1−/− thymocytes 2 h after stimulation with PMA plus ionomycin. NKT cells (CD1d tetramer+ TCRβ+) stained with CD1d tetramer loaded with PBS-44 were analysed as an internal positive control for IL-4 and IFN-γ (left panel). CD4SP thymocytes gated on TCRβ+ cells were examined for IL-4 and IFN-γ (right panel). (D) Percentages (mean±s.e.m.) of IL-4-producing CD4 T cells and NKT cells from thymuses of wt (n=4), nfκb1+/− (n=2) and nfκb1−/− (n=7) mice. Results are representative of three (B) and four (A, C, D) experiments. (E) Expression of Eomes, CD122, CD44 and CD24 by wt (Ly5.1+) CD8SP thymocytes (bold) isolated from wt+ (wt Ly5.1+) (shaded histograms) or nfκb1−/− + (wt Ly5.1+) (black lines) chimera mice. Memory phenotype of nfκb1−/− CD8SP cells from intact nfκb1−/− mice (hatched lines) served as concurrent positive control. Data are representative of three different chimera cohorts (n>6 mice per group). BM chimera mice were established by engrafting wt (Ly5.2+) hosts with a mix (50:50) of nfκb1−/− and wt (Ly5.1+) haematopoietic cells. (F) NKT cells (CD1d tetramer+ TCRβ+) and CD4 T cells (TCRβ+CD4+CD8−) were assessed for IL-4 and IFNγ expression. Thymocytes were isolated from chimeras and stimulated in vitro with PMA and ionomycin for 2 h and then analysed for intracellular levels of IL-4 and IFN-γ by flow cytometry. Wt and nfκb1−/− thymocytes were defined as Ly5.1+ and Ly5.1−, respectively (left panel).
Our findings show that an absence of NF-κB1 does not lead to a pronounced expansion of PLZF+ thymocytes. Nevertheless, a small increase in these cells might produce sufficient IL-4 to promote expression of memory markers on CD8 T cells. To test this possibility, wt and nfκb1−/− thymocytes were stimulated with PMA plus ionomycin and levels of IL-4 plus IFN-γ determined by intracellular FACS staining. Thymic NKT cells (CD1d tetramer+ TCRβ+) were used as a positive control for staining, as this population is a potent producer of IL-4 and IFN-γ after stimulation. Importantly, IL-4 production was comparable between wt and nfκb1−/− thymic NKT cells, while nfκb1−/− and wt TCRαβ+CD4SP cells produced only barely detectable levels of IL-4 (Figure 2C and D). Collectively, these findings demonstrate that the absence of NF-κB1 does not promote a marked expansion of IL-4 producing PLZF+ thymocytes.
Mixed bone marrow (BM) chimeras were generated to determine whether nfκb1−/− PLZF+ thymocytes are able to promote memory characteristics in bystander wt CD8SP cells. Chimeric mice were established by engrafting wt hosts with an equal (50:50) or unequal (85:15) mix of nfκb1−/− (Ly5.2+) and wt (Ly5.1+) BM cells. Analysis of chimeras 5–7 weeks post transplant revealed that wt CD8SP thymocytes had a phenotype resembling naive conventional CD8SP cell rather than memory-like cells (Figure 2E; Supplementary Figure S2C). Consistent with our finding in nfκb1−/− mice, wt and nfκb1−/− NKT cells from chimeras produced equivalent levels of IL-4 when stimulated with PMA plus ionomycin, while CD4SP cells of either genotype produced negligible levels of IL-4 (Figure 2F; Supplementary Figure S2D). Overall, these results demonstrate that unlike some mutant strains, such as the itk−/− mice (Weinreich et al, 2010), the absence of NF-κB1 does not create an expanded population of IL-4 producing PLZF+ thymocytes that can confer memory properties on neighbouring wt CD8SP cells.
The development of nfκb1−/− CD8 memory-like thymocytes requires the loss of NF-κB1 in the haematopoietic compartment and is independent of MHC class I expressed on TECs
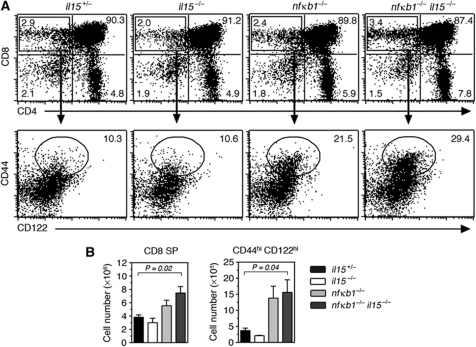
Although nfκb1−/− CD44hiCD8SP thymocytes and various innate CD8 thymic T-cell populations have overlapping phenotypes, certain key features distinguish conventional and innate CD8SP thymocytes (Glimcher et al, 2004; Berg, 2007). One such property is a dependence of innate CD8 thymocytes on IL-15 for development (Dubois et al, 2006; Berg, 2007). The presence of comparable numbers of CD44hiCD122hi CD8SP thymocytes in nfκb1−/− and nfκb1−/−il15−/− mice (Figure 3A and B) established that the development of these cells is IL-15 independent. The memory-like thymocytes in nfκb1−/− mice like conventional CD8 T cells also express CD8αβ dimers (data not shown), but lack NK1.1 expression (unlike CD1d restricted nfκb1−/− NKT cells that are NK1.1+) (Supplementary Figure S3A), a characteristic marker of innate CD8 T cells (Berg, 2007).
Figure 3.
nfκb1−/−CD8SP thymocytes acquire memory markers independently of IL-15. (A) CD4 and CD8 expression by thymocytes from 8-week-old il15+/−, il15−/−, nfκb1−/− and nfkb1−/− il15−/− mice. Values represent percentages of cells in each quadrant. Expression of CD44 and CD122 by CD8SP thymocytes (lower dot plots) with numbers representing the percentages CD44hiCD122hi CD8SP cells. (B) Mean±s.e.m. (n=6 mice per genotype) number of CD8SP and CD44hiCD122hi CD8SP thymocytes. Data are representative of five experiments. P-values were determined by an unpaired two-tailed Student's t-test.
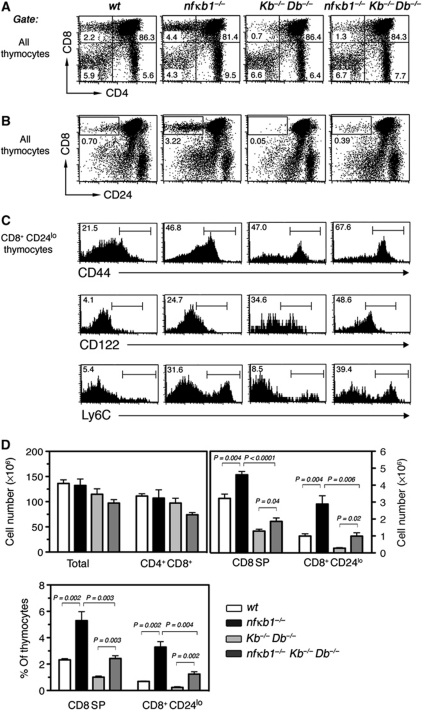
Most importantly, the selection and development of conventional CD8 lineage T cells is dependent on MHC class Ia expressed on TECs, whereas innate CD8 lineage T cells typically require MHC class Ib presented by haematopoietic cells (Urdahl et al, 2002; Kurepa et al, 2003). To determine whether the development of nfκb1−/− CD8SP memory-like thymocytes requires MHC class Ia (KbDb), we generated nfκb1−/−Kb−/−Db−/− mice. A comparison of CD8SP thymocyte populations between wt, nfκb1−/−, Kb−/−Db−/− and nfκb1−/−Kb−/−Db−/− mice is shown in Figure 4. While nfκb1−/− mice had 1.5-fold more CD8SP thymocytes than wt controls, with ∼45% of these cells being CD44hi (Figure 4A and D), the absence of MHC class Ia led to a 60% reduction in the entire nfκb1−/− CD8SP population, including CD44hi cells (Figure 4A and D). CD24loCD8SP cells, which normally represent the most mature CD8SP thymocytes, were also markedly reduced in the nfκb1−/−Kb−/−Db−/− mutants (Figure 4B–D), confirming that the majority of nfκb1−/− CD44hiCD8SP cells was lost in these mice. This establishes that a significant proportion of memory-like CD8SP thymocytes that develop in nfκb1−/− mice depends on MHC class Ia.
Figure 4.
Loss of NF-κB1 promotes memory marker acquisition independently of MHC class Ia. (A) CD4 and CD8 profiles for wt, nfκb1−/−, Kb−/−Db−/− and nfκb1−/−Kb−/−Db−/− thymocytes. Numbers indicate the percent of thymocytes in each quadrant. (B) CD8 and CD24 expression by thymocytes from each genotype. Numbers represent percentages of CD8+CD24lo cells in each gate. (C) CD44, CD122 and Ly6C expression by CD8+CD24lo thymocytes. Percentages are shown for each gate. (D) Absolute numbers (mean±s.e.m.) of total thymocytes, DP, CD8SP and CD8+CD24lo thymocytes for each genotype; Lower graph shows percentages of CD8SP and CD8+CD24lo thymocytes (mean±s.e.m.). Data in (A–D) are representative of four experiments (4–6 mice per genotype). P-values were determined by an unpaired two-tailed Student's t-test.
In Kb−/−Db−/− mice, the remaining CD8SP thymocytes are selected by class Ib expressing haematopoietic cells (Urdahl et al, 2002; Kurepa et al, 2003). Interestingly, the MHC class Ia-independent CD8SP population was 1.5-fold greater in nfκb1−/−Kb−/−Db−/− mice than in Kb−/−Db−/− mutants (Figure 4A and D), with the remaining CD24loCD8SP thymocytes that develop in nfκb1−/−Kb−/−Db−/− mice elevated five-fold (Figure 4B and D). This indicates that the absence of NF-κB1 also increased class Ib-dependent thymocyte differentiation. Further characterisation of these CD24loCD8SP thymocytes revealed that CD44hiCD122hi and Ly6chi cells were enriched in nfκb1−/−Kb−/−Db−/− mice when compared with the equivalent population in Kb−/−Db−/− and nfκb1−/− mutants (Figure 4C). This suggests that the loss of NF-κB1 alters the phenotype of the CD44hiCD8 population irrespective of whether their development is dependent on MHC class Ia or Ib.
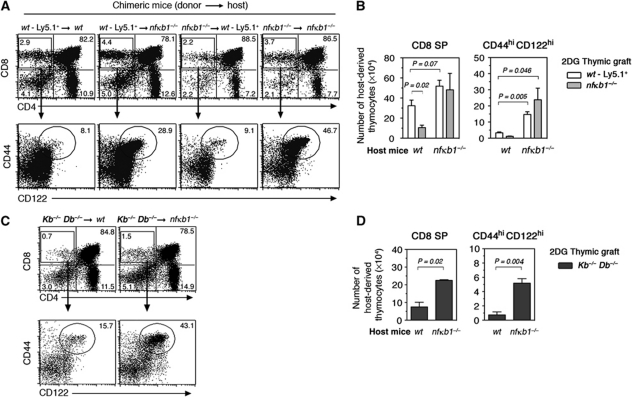
To determine whether a loss of p50NF-κB1 in haematopoietic cells, thymic epithelium or both compartments promote the abnormally elevated development of memory-like CD8SP thymocytes, embryonic thymic lobes (wt or nfκb1−/−) depleted of endogenous thymocytes with 2DG were transplanted under the renal capsule of wt or nfκb1−/− hosts. In this model, host-derived thymocyte progenitors infiltrate the grafted thymus and develop into mature SP thymocytes by interacting with the stroma of the donor thymus (Jenkinson et al, 1992). When wt or nfκb1−/− thymi were engrafted into nfκb1−/− hosts, the proportions and numbers of CD8SP thymocytes were slightly elevated in these thymi compared with equivalent grafts in wt hosts (Figure 5A and B). While CD44hiCD122hi CD8SP thymocyte numbers were comparable in wt and nfκb1−/− thymi grafted into wt hosts, this population was three- to four-fold higher in wt and nfκb1−/− thymi engrafted into nfκb1−/− mice (Figure 5B). Furthermore, the numbers of these cells appeared to be increased when nfκb1−/− thymi were grafted into nfκb1−/− hosts. This indicates that the absence of p50NF-κB1 in haematopoietic cells is a prerequisite for the excessive development of memory-like thymocytes, and that the added loss of NF-κB1 in the stroma enhances this phenotype.
Figure 5.
nfκb1−/− CD8SP thymocytes acquire a memory phenotype independently of MHC class I expressing TECs. Thymic lobes were harvested from wt (Ly5.1+), nfκb1−/− (Ly5.2+) and Kb−/−Db−/− day 15.5 embryos and cultured by FTOC in the presence of 2-DG for depletion of endogenous thymocytes. After 7 days in culture, 2-DG-treated thymi were transplanted under the renal capsule of wt and nfκb1−/− hosts. Grafts were harvested and processed for immunofluorescent staining and cell counting 8 weeks post transplant. (A) CD4 and CD8 expression by host-derived thymocytes isolated from thymic grafts. Values represent percentages of each thymocyte subset. CD44 and CD122 expression by CD8SP thymocytes and numbers indicate the percentage of CD44hiCD122hi CD8SP cells. (B) Absolute numbers (mean±s.e.m.) of host-derived CD8SP and CD144hiCD122hi CD8SP thymocytes from 3 to 5 transplanted thymi per host. (C) CD4 and CD8 profiles of host-derived thymocytes from Kb−/−Db−/− thymi grafted into wt or nfκb1−/− mice. CD44 and CD122 phenotype of host CD8SP thymocytes isolated from Kb−/−Db−/− grafts. (D) Absolute numbers (mean±s.e.m.; n=4 grafts per host) of CD8SP and CD144hiCD122hi CD8SP thymocytes isolated from Kb−/−Db−/− grafts. Data in (A–D) are representative of two independent experiments. P-values were determined by an unpaired two-tailed Student's t-test.
Although the development of conventional TCRαβ+CD8SP thymocytes normally depends on MHC class Ia signals delivered by TECs, emerging evidence indicates that strong or sustained TCR signals from antigen–MHC class I complexes expressed on haematopoietic cells are crucial for acquisition of memory properties by innate thymic T cells (Urdahl et al, 2002). This raised the intriguing possibility that development of nfκb1−/− TCRαβ+CD8 memory-like thymocytes might depend on MHC class I signals presented by haematopoietic cells. This hypothesis was examined by placing 2DG-treated Kb−/−Db−/− thymi under the renal capsule of wt or nfκb1−/− mice. As expected, CD8SP thymocyte numbers that develop in MHC class Ia-deficient thymi grafted in wt mice were markedly reduced (Figure 5C and D), with those remaining CD8SP cells selected by MHC class Ia or Ib expressed on haematopoietic cells. Of these residual cells, ∼15% displayed a CD44hiCD122hi phenotype. The two-fold increase in CD8SP thymocytes seen in KbDb-deficient thymi grafted into nfκb1−/− mice mainly comprising CD44hiCD122hi cells and represented a six-fold increase in this population (Figure 5D). This shows that the enhanced development of nfκb1−/− CD8 memory-like thymocytes can occur in the absence of MHC class Ia expressed on TECs, and regardless of the selecting MHC (Ia or Ib), it is the absence of NF-κB1 in haematopoietic cells that promotes the acquisition of memory characteristics.
Thymocyte negative selection is impaired in nfκb1−/− mice
To further understand how the absence of p50NF-κB1 in the haematopoietic compartment promotes the excessive generation of memory-like CD8 thymocytes, we undertook a detailed study of thymocyte development in nfκb1−/− mice. At embryonic day 18 and neonatal days 3 and 7, CD8SP thymocyte numbers as well as their expression of CD44, CD122 and CD24 were comparable in wt and nfκb1−/− mice (Supplementary Figure S3B and data not shown). However, by 2 weeks of age increased numbers of CD44hiCD122hi CD8SP cells were present in nfκb1−/− thymi, coinciding with an increased proportion of IFN-γ producing CD8SP thymocytes (Supplementary Figure S3C). In 4–7-week-old nfκb1−/− mice, the proportions and absolute numbers of DN and DP thymocytes were normal (data not shown). With the expression of Eomes elevated in nfκb1−/− DP cells (Figure 1G), this suggested that CD8 thymocyte development was altered during transition from a DP to CD8SP cell, a phase that coincides with T-cell selection.
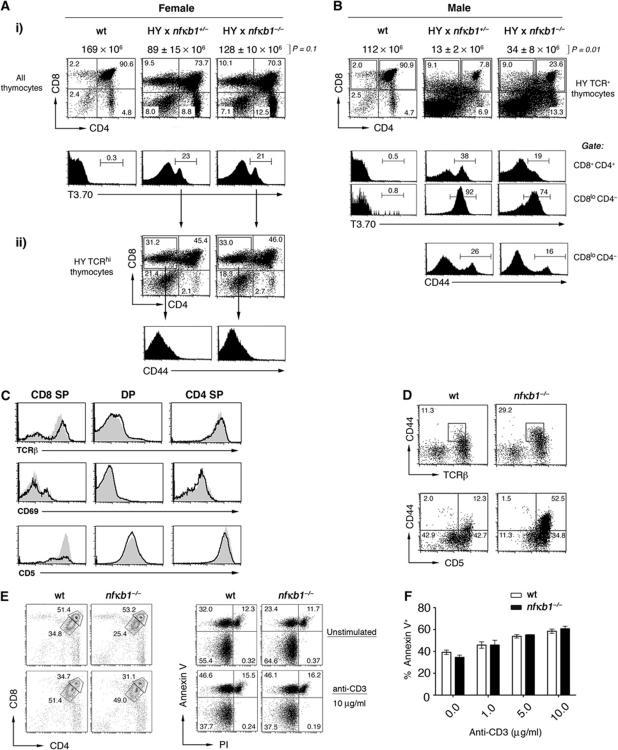
To assess whether the loss of NF-κB1 alters positive or negative CD8 T-cell selection, we utilised transgenic mice (HYTg) expressing a TCR specific for the male HY antigen presented by MHC class I H-2Db (Teh et al, 1988). In female mice (on a C57BL/6 background), thymocytes expressing the HY TCR (T3.70) undergo MHC class I restricted positive selection and develop into mature CD8 T cells. While total thymocyte cellularity was slightly elevated (Figure 6A), the loss of NF-κB1 had minimal effects on positive selection. Examination of thymic subsets revealed equivalent profiles in female wt and nfκb1−/− HYTg mice, with comparable numbers of HYTg TCRhi cells (Figure 6A), indicating that NF-κB1 was dispensable for positive selection. In male H-2Db restricted HYTg mice, thymocytes expressing the HY TCR recognise a self-antigen, resulting in their elimination by negative selection (Teh et al, 1988; von Boehmer, 1990). Negative selection was less efficient in the absence of NF-κB1, with male nfκb1−/− HYTg mice displaying an increase in overall thymocyte cellularity and ∼3-fold more DP thymocytes than wt male HYTg controls (Figure 6B). A greater proportion of the remaining male nfκb1−/− HYTg DP and CD8SP thymocytes expressed low levels of the HY TCR (Figure 6B), a finding consistent with reduced TCR expression being one mechanism by which auto-reactive DP thymocytes can escape negative selection (von Boehmer and Melchers, 2010). Interestingly, nfκb1−/− CD8SP HYTg thymocytes in female and male mice developed without acquiring memory markers (Figure 6A and B).
Figure 6.
Reduced negative selection in HY TCR transgenic nfκb1−/− mice. (A) Expression of CD4 and CD8 by thymocytes from female (gate: all thymocytes) and (B) male (gate: HY+ TCR) nfκb1+/− and nfκb1−/− HY TCR transgenic mice. Values indicate percentages of cells in each quadrant. Expression of HY TCR (T3.70) is shown for all thymocytes (Ai) or individual thymocyte subsets (B). Wt thymocytes were stained concurrently with T3.70 as a staining control. Values in histograms represent percentages of HY TCRhi thymocytes in gated areas. (Aii) CD4 and CD8 profiles by HY TCRhi thymocytes in female transgenic mice. Total thymocyte cellularity is shown for each genotype (above dot plots; mean±s.e.m.). P-values were determined by an unpaired two-tailed Student's t-test. (Aii, B) CD44 expression by HY TCRhiCD8SP thymocytes. Data in (A) and (B) are representative four experiments (n>4 mice per genotype). (C) Phenotype of wt (black lines) and nfκb1−/− (grey shaded) thymocyte subsets for TCRβ, CD69 and CD5 expression. (D) Analysis of CD44 in combination with TCRβ or CD5 gated on wt and nfκb1−/− CD8SP thymocytes. Data in (C) and (D) are representative of three experiments (n=5 mice per genotype). (E) CD4 and CD8 expression of wt and nfκb1−/− thymocytes 24 h after in-vitro stimulation with anti-CD3 Ab (μg/ml). Values indicate percentages of cells expressing high or low levels of CD4 and CD8. Annexin V and PI staining of wt and nfκb1−/− thymocytes at the corresponding time point. (F) Percentages of Annexin V+ cells (mean±s.e.m.) for wt and nfκb1−/− thymocytes cultured for 24 h at the indicated concentrations of anti-CD3 (μg/ml) Ab or media alone (>95% of wt and nfκb1−/− thymocytes were viable at T0). Data in (E) and (F) are from three experiments.
Importantly, features of the nfκb1−/− HYTg model indicative of a defect in negative, but not positive selection were also observed in nfκb1−/− mice with a polyclonal TCR repertoire. First, the pattern of CD69 expression on nfκb1−/− DP and SP thymocytes was normal (Figure 6C). Second, in nfκb1−/− mice reduced TCRβ levels were detected on CD8SP but not on CD4SP thymocytes (Figure 6C). Third, CD5, a cell surface marker whose expression levels on thymocytes reflect the strength of TCR signals received during development and selection (Azzam et al, 1998, 2001; Stojakovic et al, 2008), differs in NF-κB1-deficient mice. A prominent population of nfκb1−/− CD8SP cells expressed higher levels of CD5 (Figure 6C), whereas CD5 expression on wt and nfκb1−/− DP and CD4SP thymocytes was similar. While high CD5 expression on nfκb1−/− CD44hiCD8SP thymocytes supports the notion that this memory-like population had received a stronger TCR signal, elevated CD5 levels were also observed on a prominent population of nfκb1−/− CD44loCD8SP cells (Figure 6D). This suggests that despite nfκb1−/− CD8SP thymocytes receiving a stronger TCR signal, this event alone is insufficient to promote the development of a memory phenotype.
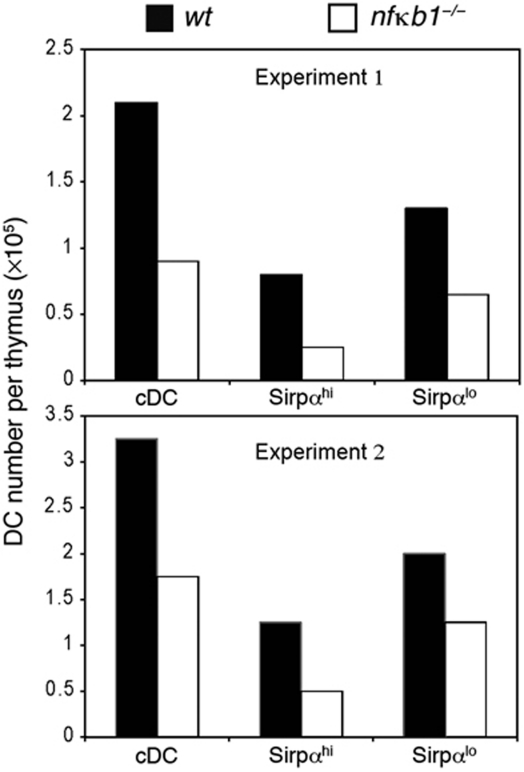
To determine whether there is a thymocyte-intrinsic defect in negative selection, the levels of apoptosis were examined in cultures of wt and nfκb1−/− thymocytes stimulated with plate-bound anti-CD3 antibodies (Figure 6E and F). The absence of NF-κB1 had no impact on the death of DP thymocytes, suggesting that any role of NF-κB1 in this process is not intrinsic to thymocytes undergoing negative selection. This finding prompted an examination of CD11c+ CD8lo Sirpα+ thymic DCs, a population known to be important in negative selection (Li et al, 2009; Proietto et al, 2009). We had previously shown that the splenic CD11c population is reduced two- to three-fold in nfκb1−/− mice (O’Keeffe et al, 2005) and consistent with this result, the nfκb1−/− Sirpα+ subset of thymic conventional (c) DC was also reduced three-fold (Figure 7). This indicates that the reduced efficiency of negative selection in nfκb1−/− mice is most likely due to a reduction in a specific subset of DCs.
Figure 7.
Decreased numbers of Sirpαhi DCs in the thymus of nfκb1−/− mice. Comparison of DC numbers (conventional, Sirpαhi and Sirpαlo) from the thymus of wt and nfκb1−/− mice. DCs were analysed on four pooled thymi per genotype, staining for CD11c, Sirpα and CD45R: cDCs were gated as CD11chiCD45− cells and analysed for Sirpα expression. Data show two independent experiments using a total of eight mice per genotype.
The production of CD8+ memory-like T cells in the thymus contributes to the elevated levels of peripheral CD44hi CD8+ T cells in nfκb1−/− mice
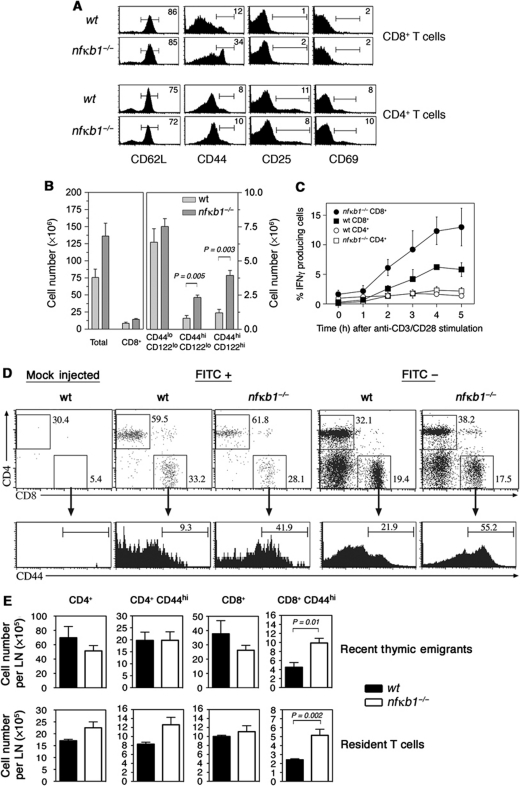
Although abnormally elevated numbers of CD44hi CD8SP thymocytes were generated in nfκb1−/− mice, it was unclear whether these cells could be exported from the thymus and contribute to the peripheral CD8 T-cell pool. An examination of the splenic T-cell population in young nfκb1−/− mice revealed that CD44hiCD25loCD69lo CD8 T-cell numbers were abnormally elevated (Figure 8A and B) and that these cells were capable of rapidly synthesising IFN-γ when activated in culture (Figure 8C). Direct evidence that CD44hiCD8SP thymocytes contribute to the peripheral CD44hi CD8 T-cell population was obtained by showing that FITC-labelled CD44hi CD8 T cells were present in the spleen and lymph nodes of nfκb1−/− mice that had been given an intrathymic FITC injection (Figure 8D and E).
Figure 8.
Thymic memory-like CD8+ T cells populate the periphery of nfκb1−/− mice. (A) Phenotype of wt and nfκb1−/− CD4+ and CD8+ splenic T cells. Values indicate percentages of cells in gated areas. Data represent at least nine mice per genotype. (B) Mean (±s.e.m.) numbers of total splenocytes, CD8+ T cells, naive (CD44loCD122lo) and memory-like (CD44hiCD122lo and CD44hiCD122hi) CD8+ T cells. Expression of CD44 and CD122 by wt and nfκb1−/− splenic CD8+ T cells is shown in Supplementary Figure S3E. Data in (A) and (B) are representative of three experiments (n>9 mice per genotype). (C) Percentages of IFN-γ producing T cells from wt and nfκb1−/− mice. Splenocytes were stimulated with plate-bound anti-CD3/anti-CD28 Abs (both 10 μg/ml) and stained for IFN-γ, CD4 and CD8 expression. Stains were performed on duplicate samples every hour and sample flow profiles shown in Supplementary Figure S3E. Data are representative of two independent experiments. (D) Memory-like CD8+ T cells home to the periphery of nfκb1−/− mice. Wt and nfκb1−/− mice were injected intrathymically with FITC or PBS (mock injected). After 20 h, mice were euthanised and mesenteric lymph nodes harvested and processed for immunofluorescent staining. FITC+ (Recent Thymic Emigrants) and FITC− (Resident) T cells were examined for CD4 and CD8 expression. Values indicate percentages of cells in each gated region. CD44 expression gated on Recent Thymic Emigrants or Resident CD8+ T cells from wt and nfκb1−/− mice. (E) Absolute numbers of RTE and resident T cells (mean±s.e.m.; n=5 per genotype) calculated from T-cell percentages and total lymph node cell numbers. P-values were determined by an unpaired two-tailed Student's t-test.
Discussion
The strength and duration of TCR signals delivered to developing thymocytes by antigen/MHC class I complexes expressed on thymic epithelial or haematopoietic cells is thought to be important in determining whether thymocytes acquire naive or memory cell characteristics (Hogquist, 2001; Berg, 2007). Little is known about the signalling pathways that influence CD8 thymocyte acquisition of these distinct properties (Carpenter and Bosselut, 2010). Here, we show that a loss of NF-κB1 function promotes the development of TCRαβ+CD8SP thymocytes with memory properties. Unlike some other mouse strains (itk−/− and klf2−/−) with expanded CD8 memory-like thymocyte populations, the generation of these cells in nfκb1−/− mice is not the result of an expanded population of PLZF+ thymic T cells. Instead this phenotype in nfκb1−/− mice coincided with defects in both positive and negative selection. Importantly, our findings identify a novel NF-κB1 regulated pathway that influences TCRαβCD8 thymocyte differentiation by preventing the acquisition of memory-like characteristics.
To date, non-redundant roles for NF-κB1 in conventional T cells have been confined to mature lymphocyte function (Gerondakis and Siebenlist, 2010). Our finding that nfκb1−/− mice had increased numbers of CD4 and CD8 SP thymocytes indicates that NF-κB1 also regulates T-cell development. Although NF-κB activity had previously been linked to thymocyte survival (Voll et al, 2000; Mandal et al, 2005), the normal size and viability of nfκb1−/− DN and DP thymocyte populations suggested that early T-cell differentiation was not perturbed. Instead, NF-κB1 appears to be important in regulating later stages of thymocyte development. While both nfκb1−/− CD4 and CD8 SP thymocytes displayed phenotypic changes, the presence of a CD44hiCD24lo subset of TCRαβ+CD8SP thymocytes comprising ∼40% of CD8SP cells was the most striking feature of nfκb1−/− mice. These CD44hiCD8SP cells, which develop in the thymus, resembled innate and conventional CD8 memory T cells, possessing the ability to rapidly produce IFN-γ when activated and expressing increased levels of IL-15Rβ, granzyme B, perforin and Eomes. Elevated Eomes expression in these cells is consistent with its role in controlling the transcription of genes expressed in memory T cells (Glimcher et al, 2004; Intlekofer et al, 2005). Although nfκb1−/− CD44hiTCRαβ+ CD8SP thymocytes share characteristics with innate CD8 thymic T cells, differences in cell surface marker expression, including NK1.1 and a lack of dependence on IL-15 for their development, instead indicate that nfκb1−/− CD44hiCD8SP thymocytes are conventional CD8 T cells that had acquired a memory-like phenotype during differentiation.
Recent studies have shown that the enhanced development of innate/memory CD8SP thymocytes in many mouse mutants is due to increased levels of IL-4 produced by elevated populations of CD4 thymic T cells, NKT and γδ T cells that express PLZF (Verykokakis et al, 2010; Weinreich et al, 2010). Unlike itk−/− and id3−/− mice, strains with aberrant PLZF+ thymic T cells that can induce a memory phenotype in wt CD8 thymocytes, nfκb1−/− haematopoietic cells failed to provide such a by-stander effect in mixed BM chimeras. Consistent with this finding PLZF+ cells were not markedly elevated in the pool of CD4SP thymocytes that include NKT and γδ T cells. Combined with the observation that the proportion of IL-4 producing CD4 thymic T cells in nfκb1−/− mice and mixed BM chimeras was normal, our data indicate that the development of memory-like CD44hiCD8SP thymocytes in NF-κB1-deficient mice was not due to the influence of IL-4 producing PLZF+ T cells. However, a potential role for IL-4 cannot be unequivocally ruled out since IL-4 could not be entirely eliminated in these studies.
Our subsequent analysis established that an absence of NF-κB1 in haematopoietic cells was sufficient to generate CD44hiCD8SP thymocytes, although the added loss of NF-κB1 in the thymic stroma augmented their development. One key change in the nfκb1−/− haematopoietic compartment that is essential for the development of CD44hiTCRαβ+CD8SP thymocytes is the role of MHC class I-dependent positive selection by haematopoietic cells. Contrary to reports indicating that MHC class Ib selectively promotes the development of innate/memory-like cells, our data indicate that there is nothing unique about MHC class Ib as opposed to class Ia in directing the development of memory properties in conventional TCRαβ+CD8 thymic T cells. While the requirements for MHC class I will need to be assessed further by examining the development of nfκb1−/− CD8SP cells on a B2m-deficient background. Our findings and those of others (Urdahl et al, 2002; Broussard et al, 2006) emphasise the importance of haematopoietic cells. Despite this altered mode of CD8 T-cell selection in nfκb1−/− mice, nfκb1−/− TECs still retain the capacity to promote the MHC class Ia-dependent selection and development of naive CD44loCD8 thymocytes. This suggests that in the absence of NF-κB1, functional changes in existing haematopoietic APC or the generation of a new haematopoietic APC promotes the development of memory-like CD8SP thymocytes. This interpretation is consistent with the inability of normal thymic haematopoietic APC expressing MHC class Ia to promote the positive selection of conventional T cells (Bix and Raulet, 1992). This differs from NKT and innate CD8 T cells that undergo CD1d and MHC class Ib-dependent selection by haematopoietic cells (Godfrey and Berzins, 2007). In the case of NKT cells, DP thymocytes serve as the APC responsible for positive selection. To overcome any inherent reduced capacity that haematopoietic cells may have in promoting positive selection, the higher affinity Type 1 NKT cells and presumably innate CD8 T cells have for self-ligands are believed to be a prerequisite for their positive selection (Godfrey and Berzins, 2007).
Examination of conventional CD8 T-cell selection in nfκb1−/− mice using a transgenic TCR specific for an HY peptide presented by MHC class I revealed that while NF-κB1 is dispensable for positive selection it is required for efficient negative selection. Although the levels of NF-κB activity in DP cells set a threshold for determining whether conventional CD8 T cells undergo positive or negative selection (Jimi et al, 2008), a specific contribution by NF-κB1 was unknown. A requirement for NF-κB1 is supported by data showing that nfκb1 mRNA expression is induced during negative selection (Schmitz et al, 2003; Baldwin and Hogquist, 2007) and contrasts with that of c-Rel, which is dispensable for both CD8 positive and negative selection (Strasser et al, 1999). While the mechanism(s) by which NF-κB1 promotes negative selection of CD8 T cells remains to be determined, the failure to detect enhanced cell death in cultures of anti-CD3 antibody activated nfκb1−/− DP thymocytes indicates that this defect is not intrinsic to thymocytes undergoing selection. Instead, it points to NF-κB1 regulating other aspects of thymic function that influence negative selection. This notion is consistent with a reduction in thymic CD8 Sirpα+ DCs in nfκb1−/− mice, an APC population that is important in promoting negative selection (Li et al, 2009; Proietto et al, 2009). Despite impaired CD8 T-cell selection, nfκb1−/− mice have not been reported to be prone to autoimmune disease. This anomaly may reflect a defect in the effector function of nfκb1−/− CD8 T cells or that other mechanisms act as a safeguard against autoimmunity.
Our finding that the CD44loCD8SP thymocyte population in nfκb1−/− mice remained intact indicates that additional memory-like CD8 thymic T cells were not generated from the pool of precursors destined to become naive CD8SP thymocytes. Instead, this indicates that these cells emerge from distinct precursors. A clue as to the origin of these cells is that the majority of nfκb1−/− CD44hiCD8SP thymocytes have a CD5hi phenotype, indicating that during selection these cells have received a strong TCR signal. Given that the majority of immature thymocytes with high-affinity TCRs are normally deleted during negative selection, our findings raise the intriguing possibility that impaired negative selection in nfκb1−/− mice creates an additional pool of thymocytes with higher affinity TCRs that are targeted by haematopoietic APC to differentiate into memory-like CD8SP thymocytes. However, generating CD8 thymic precursors possessing higher affinity TCRs for self-antigens, along with the ability of nfκb1−/− haematopoietic cells to promote MHC class Ia-dependent selection, does not guarantee development of memory-like CD8 T cells. Although OT-I TCR transgenic mice represent a model for MHC class I-dependent selection of CD8 T cells with high-affinity TCRs, CD44hiCD8SP thymocytes were absent in nfκb1−/− OT-I and HY TCR transgenic mice (Supplementary Figure S3D). Instead, selection of CD8SP T cells expressing these TCRs is thought to require TECs rather than haematopoietic cells. Together, these findings support the notion that certain TCRs are better suited to class I selection by either TECs or haematopoietic cells.
What emerges from our study is that the NF-κB1 transcription factor serves multiple functions in the thymus that include ensuring efficient negative selection and determining which thymic APC can promote MHC Ia-dependent positive selection, events that determine the outcome of conventional CD8 thymocyte development. While altered positive selection due to the loss of NF-κB1 function plays an essential part in the altered development of CD8 T cells, we are currently unable to discern what impact the absence of NF-κB1 might have on the post-selection differentiation of CD8SP thymocytes. Nevertheless, our findings establish that a key role of NF-κB1 in the thymus is to prevent conventional CD8 T cells developing memory-like properties. With our study showing that this phenotype in nfκb1−/− mice is independent of the role played by IL-4 producing PLZF+ CD4 T cells, this report highlights the existence of a distinct NF-κB1 regulated pathway controlling CD8 thymic T-cell differentiation. Future studies will focus on identifying other components in this NF-κB1 regulated pathway and the specific target genes controlled by NF-κB1 in thymic haematopoietic populations that influence the development of naive CD8 T cells.
Materials and methods
Mouse strains
nfκb1−/− mice (Sha et al, 1995) were backcrossed for 10 generations onto a C57BL/6 background. nfκb1−/−il15−/− and nfκb1−/−Kb−/−Db−/− mice were generated by intercrossing the nfκb1−/− mice with il15−/− and Kb−/−Db−/− mice (B6 background; Taconic), respectively. The tpl-2−/− mice (Dumitru et al, 2000) were obtained from Thomas Jefferson University (Philadelphia, PA). The OT-I and HY TCR transgenic mouse lines were crossed to nfκb1−/− mice, with transgene-negative littermates used as experimental controls. C57BL/6 (Ly5.1+) congenic mice were obtained from The Walter and Eliza Hall Institute animal facility (Kew, Victoria). All animals were used at 7–10 weeks of age (unless stated otherwise), were bred and housed under SPF conditions at The Walter and Eliza Hall Institute or the Alfred Medical Research and Education Precinct, with all experiments approved by the respective Animal Ethics Committee.
Mixed BM chimeras
BM was flushed from the femur and tibia. BM cells from nfκb1−/− (Ly5.2+) and wt (Ly5.1+) mice were mixed at an equal (50:50) or unequal (85:15) ratio and injected intravenously into lethally irradiated (2 × 550 rads) wt (Ly5.2+) hosts. Mice were killed and analysed 5–7 weeks after transplant.
Antibodies and flow cytometry
Single cell suspensions were prepared from thymus, lymph node and spleen using standard protocols. Red blood cells in spleen suspensions were lysed by performing a brief incubation with an ammonium chloride-based solution. Immunofluorescent staining was performed as previously described (Pohl et al, 2002) with a list of antibodies provided in Supplementary Materials and Methods. For intracellular staining, cells were fixed and permeabilised using the Foxp3 Staining Buffer Set (eBioscience) and stained for 1 h with Alexa647-anti-Eomes (Dan11mag; eBioscience) or Alexa647-anti-PLZF (clone D-9; a gift from Taras Kreslavsky, Daner Faber Cancer Institute, USA) antibodies. Data were acquired on a FACSCalibur, LSR-II or FACS Canto II (BD Biosciences) and analysed with Cell Quest Software (BD Biosciences), Weasel (WEHI) or FlowJo software (Tree Star Inc.).
Stimulation and intracellular cytokine staining
Thymocytes and splenocytes were stimulated in vitro for 2–5 h with PMA (10 ng/ml) plus ionomycin (1 μg/ml) or with plate-bound anti-CD3/anti-CD28 antibodies (both 10 μg/ml) respectively. The cells were cultured at 37oC in the presence of Golgi Stop™ (BD Biosciences). Cells were surface stained, fixed and permeabilised using the BD Biosciences Cytofix/Cytoperm Plus Fixation/Permeabilisation Kit, and then stained with PECy7-anti-IFNγ (XMG 1.2) and/or APC-anti-IL-4 (11B11) antibodies.
Thymic transplantation under the renal capsule
wt, nfκb1−/− and Kb−/−Db−/− fetal thymi were harvested on day 15 of gestation, cultured for 7 days by FTOC in the presence of 2-Deoxyguanosine (2-DG) (1.3 mg/ml) to deplete endogenous thymocytes and then grafted under the renal capsule of wt or nfκb1−/− hosts. Grafts were removed and processed for flow cytometric analysis 8 weeks after transplantation.
Real-time quantitative PCR
Total RNA was extracted from 1 to 2 × 106 sorted thymocytes (Mo-Flo; Cytomation) using the RNeasy Mini-kit (Qiagen) with an on-column DNAse digest was reverse transcribed using SuperScript III RNaseH Reverse Transcriptase (Invitrogen) with random hexamers (Promega). Quantitative RT–PCR was performed using the Roche LightCycler 480 System (primers, Supplementary Table 1). For each sample, the starting quantity of each target gene was normalised to the housekeeping gene Hprt.
Dendritic cell isolation
Thymic dendritic cells were isolated using previously established methods (Dakic et al, 2004). Purified cell populations were stained for CD11c, CD45R and CD172a (Sirpα). cDC was gated as CD11chi CD45R cells, and then gated as Sirpαlo or Sirpαhi populations and enumerated.
Examination of thymic emigrants
Wt and nfκb1−/− mice were injected intrathymically with FITC as described (Uldrich et al, 2006). The animals were anaesthetised by i.p. injection of ∼0.3 mg of xylazine hydrochloride (Ilium xylazil; Troy Laboratories) and 1.5 mg of ketamine hydrochloride (Ketalar; Parke-Davis) in 300 μl of PBS, and subsequently administered s.c. with 50 μg/10 g (0.5 μg/kg) body weight of the analgesic carprofen (Rimadyl; Pfizer). The thoracic cavity was opened, each thymic lobe was injected with ∼10 μl of an FITC solution (1 mg/ml in sterile PBS) and then the wound was closed with surgical staples. Mice were euthanised 20 h post injection, thymuses, spleens and mesenteric lymph nodes were removed and processed for flow cytometry. Only mice with >70% of FITC-labelled thymocytes were assessed. Thymic emigrants were identified as live-gated FITC+ cells expressing either CD4 or CD8 and quantified by analysing the percentage of FITC+ cells, total cell counts of immune organs, and the percentage of CD4+ and CD8+ T cells that were FITC+.
Statistical analysis
All statistical analysis was performed with Prism software (Graphpad) using the unpaired, two-tailed t-test to analyse the data and generate P-values.
Supplementary Material
Acknowledgments
We thank Lorraine O’Reilly, Gayle Davey and Jonathan Coquet for technical advice and assistance. This work was supported by grants from the National Health and Medical Research Council (NHMRC) Australia (R Gugasyan, project grants 356219 and 603713; DG and SPB, Career Development Awards; and GB, DIG, AS and SG NHMRC Fellowships). PNT is supported by NIH grant R01 CA124835. We gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program.
Author contributions: RG designed and performed the experiments, analysed the data and wrote the paper; EH, SAK, FR, DG, MO, GTB and RJG performed the experiments; GG, AB, AS and DG analysed the data and reviewed the paper; AS, DG and PNT provided reagents; SPB designed the experiments, analysed the data and reviewed the paper; SG designed the experiments, analysed the data and wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Agus DB, Surh CD, Sprent J (1991) Reentry of T cells to the adult thymus is restricted to activated T cells. J Exp Med 173: 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherly LO, Lucas JA, Felices M, Yin CC, Reiner SL, Berg LJ (2006) The Tec family tyrosine kinases Itk and Rlk regulate the development of conventional CD8+ T cells. Immunity 25: 79–91 [DOI] [PubMed] [Google Scholar]
- Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE (2001) Fine tuning of TCR signaling by CD5. J Immunol 166: 5464–5472 [DOI] [PubMed] [Google Scholar]
- Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE (1998) CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med 188: 2301–2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin TA, Hogquist KA (2007) Transcriptional analysis of clonal deletion in vivo. J Immunol 179: 837–844 [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gugasyan R, McMahon M, Gerondakis S (2006) Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci USA 103: 3274–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D (1995) Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature 376: 167–170 [DOI] [PubMed] [Google Scholar]
- Belich MP, Salmeron A, Johnston LH, Ley SC (1999) TPL-2 kinase regulates the proteolysis of the NF-κB-inhibitory protein NF-κB1 p105. Nature 397: 363–368 [DOI] [PubMed] [Google Scholar]
- Berg LJ (2007) Signalling through TEC kinases regulates conventional versus innate CD8(+) T-cell development. Nat Rev Immunol 7: 479–485 [DOI] [PubMed] [Google Scholar]
- Bix M, Raulet D (1992) Inefficient positive selection of T cells directed by hematopoietic cells. Nature 359: 330–333 [DOI] [PubMed] [Google Scholar]
- Boothby MR, Mora AL, Scherer DC, Brockman JA, Ballard DW (1997) Perturbation of the T lymphocyte lineage in transgenic mice expressing a constitutive repressor of nuclear factor (NF)-κB. J Exp Med 185: 1897–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosselut R (2004) CD4/CD8-lineage differentiation in the thymus: from nuclear effectors to membrane signals. Nat Rev Immunol 4: 529–540 [DOI] [PubMed] [Google Scholar]
- Broussard C, Fleischacker C, Horai R, Chetana M, Venegas AM, Sharp LL, Hedrick SM, Fowlkes BJ, Schwartzberg PL (2006) Altered development of CD8+ T cell lineages in mice deficient for the Tec kinases Itk and Rlk. Immunity 25: 93–104 [DOI] [PubMed] [Google Scholar]
- Carpenter AC, Bosselut R (2010) Decision checkpoints in the thymus. Nat Immunol 11: 666–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakic A, Shao QX, D’Amico A, O’Keeffe M, Chen WF, Shortman K, Wu L (2004) Development of the dendritic cell system during mouse ontogeny. J Immunol 172: 1018–1027 [DOI] [PubMed] [Google Scholar]
- Dubois S, Waldmann TA, Muller JR (2006) ITK and IL-15 support two distinct subsets of CD8+ T cells. Proc Natl Acad Sci USA 103: 12075–12080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN (2000) TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell 103: 1071–1083 [DOI] [PubMed] [Google Scholar]
- Feuillard J, Memet S, Goudeau B, Lilienbaum A, Schmidt-Ullrich R, Raphael M, Israel A (2000) In vivo identification of lymphocyte subsets exhibiting transcriptionally active NF-κB/Rel complexes. Int Immunol 12: 613–621 [DOI] [PubMed] [Google Scholar]
- Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM (2005) The role of erk1 and erk2 in multiple stages of T cell development. Immunity 23: 431–443 [DOI] [PubMed] [Google Scholar]
- Gerondakis S, Grumont R, Gugasyan R, Wong L, Isomura I, Ho W, Banerjee A (2006) Unravelling the complexities of the NF-κB signalling pathway using mouse knockout and transgenic models. Oncogene 25: 6781–6799 [DOI] [PubMed] [Google Scholar]
- Gerondakis S, Siebenlist U (2010) Roles of the NF-κB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol 2: a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Karin M (2002) Missing pieces in the NF-κB puzzle. Cell 109(Suppl): S81–S96 [DOI] [PubMed] [Google Scholar]
- Glimcher LH, Townsend MJ, Sullivan BM, Lord GM (2004) Recent developments in the transcriptional regulation of cytolytic effector cells. Nat Rev Immunol 4: 900–911 [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Berzins SP (2007) Control points in NKT-cell development. Nat Rev Immunol 7: 505–518 [DOI] [PubMed] [Google Scholar]
- Hettmann T, Leiden JM (2000) NF-kappa B is required for the positive selection of CD8+ thymocytes. J Immunol 165: 5004–5010 [DOI] [PubMed] [Google Scholar]
- Hogquist KA (2001) Signal strength in thymic selection and lineage commitment. Curr Opin Immunol 13: 225–231 [DOI] [PubMed] [Google Scholar]
- Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL (2005) Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol 6: 1236–1244 [DOI] [PubMed] [Google Scholar]
- Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, McNally A, Steptoe RJ, Thomas R, Shannon MF, Gerondakis S (2009) c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med 206: 3001–3014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson EJ, Anderson G, Owen JJ (1992) Studies on T cell maturation on defined thymic stromal cell populations in vitro. J Exp Med 176: 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E, Strickland I, Voll RE, Long M, Ghosh S (2008) Differential role of the transcription factor NF-κB in selection and survival of CD4+ and CD8+ thymocytes. Immunity 29: 523–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa Z, Su J, Forman J (2003) Memory phenotype of CD8+ T cells in MHC class Ia-deficient mice. J Immunol 170: 5414–5420 [DOI] [PubMed] [Google Scholar]
- Lee YJ, Jameson SC, Hogquist KA (2011) Alternative memory in the CD8 T cell lineage. Trends Immunol 32: 50–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Park J, Foss D, Goldschneider I (2009) Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med 206: 607–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal M, Borowski C, Palomero T, Ferrando AA, Oberdoerffer P, Meng F, Ruiz-Vela A, Ciofani M, Zuniga-Pflucker JC, Screpanti I, Look AT, Korsmeyer SJ, Rajewsky K, von Boehmer H, Aifantis I (2005) The BCL2A1 gene as a pre-T cell receptor-induced regulator of thymocyte survival. J Exp Med 201: 603–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie SA, Rouse RV (1989) Traffic of mature lymphocytes into the mouse thymus. Thymus 13: 141–148 [PubMed] [Google Scholar]
- Moore NC, Girdlestone J, Anderson G, Owen JJ, Jenkinson EJ (1995) Stimulation of thymocytes before and after positive selection results in the induction of different NF-κB/Rel protein complexes. J Immunol 155: 4653–4660 [PubMed] [Google Scholar]
- Mora AL, Chen D, Boothby M, Rubin DH (1999) Lineage-specific differences among CD8+ T cells in their dependence of NF-κB/Rel signaling. Eur J Immunol 29: 2968–2980 [DOI] [PubMed] [Google Scholar]
- O’Keeffe M, Grumont RJ, Hochrein H, Fuchsberger M, Gugasyan R, Vremec D, Shortman K, Gerondakis S (2005) Distinct roles for the NF-κB1 and c-Rel transcription factors in the differentiation and survival of plasmacytoid and conventional dendritic cells activated by TLR-9 signals. Blood 106: 3457–3464 [DOI] [PubMed] [Google Scholar]
- Pearce EL, Mullen AC, Martins GA, Krawczyk CM, Hutchins AS, Zediak VP, Banica M, DiCioccio CB, Gross DA, Mao CA, Shen H, Cereb N, Yang SY, Lindsten T, Rossant J, Hunter CA, Reiner SL (2003) Control of effector CD8+ T cell function by the transcription factor Eomesodermin. Science 302: 1041–1043 [DOI] [PubMed] [Google Scholar]
- Pohl T, Gugasyan R, Grumont RJ, Strasser A, Metcalf D, Tarlinton D, Sha W, Baltimore D, Gerondakis S (2002) The combined absence of NF-kappa B1 and c-Rel reveals that overlapping roles for these transcription factors in the B cell lineage are restricted to the activation and function of mature cells. Proc Natl Acad Sci USA 99: 4514–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proietto AI, van Dommelen S, Wu L (2009) The impact of circulating dendritic cells on the development and differentiation of thymocytes. Immunol Cell Biol 87: 39–45 [DOI] [PubMed] [Google Scholar]
- Schmitz I, Clayton LK, Reinherz EL (2003) Gene expression analysis of thymocyte selection in vivo. Int Immunol 15: 1237–1248 [DOI] [PubMed] [Google Scholar]
- Sha WC, Liou HC, Tuomanen EI, Baltimore D (1995) Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80: 321–330 [DOI] [PubMed] [Google Scholar]
- Sivakumar V, Hammond KJ, Howells N, Pfeffer K, Weih F (2003) Differential requirement for Rel/nuclear factor-κB family members in natural killer T cell development. J Exp Med 197: 1613–1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic S, Gugasyan R, Kyparissoudis K, Grumont R, Banerjee A, Tsichlis P, Gerondakis S, Godfrey DI (2011) Distinct roles in NKT cell maturation and function for the different transcription factors in the classical NF-κB pathway. Immunol Cell Biol 89: 294–303 [DOI] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA (2003) Positive and negative selection of T cells. Annu Rev Immunol 21: 139–176 [DOI] [PubMed] [Google Scholar]
- Stojakovic M, Salazar-Fontana LI, Tatari-Calderone Z, Badovinac VP, Santori FR, Kovalovsky D, Sant’Angelo D, Harty JT, Vukmanovic S (2008) Adaptable TCR avidity thresholds for negative selection. J Immunol 181: 6770–6778 [DOI] [PubMed] [Google Scholar]
- Strasser A, Grumont RJ, Stanley ML, Gerondakis S (1999) The transcriptional regulator Rel is essential for antigen receptor-mediated stimulation of mature T cells but dispensable for positive and negative selection of thymocytes and T cell apoptosis. Eur J Immunol 29: 928–935 [DOI] [PubMed] [Google Scholar]
- Teh HS, Kisielow P, Scott B, Kishi H, Uematsu Y, Bluthmann H, von Boehmer H (1988) Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature 335: 229–233 [DOI] [PubMed] [Google Scholar]
- Uldrich AP, Berzins SP, Malin MA, Bouillet P, Strasser A, Smyth MJ, Boyd RL, Godfrey DI (2006) Antigen challenge inhibits thymic emigration. J Immunol 176: 4553–4561 [DOI] [PubMed] [Google Scholar]
- Urdahl KB, Sun JC, Bevan MJ (2002) Positive selection of MHC class Ib-restricted CD8(+) T cells on hematopoietic cells. Nat Immunol 3: 772–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vang KB, Yang J, Pagan AJ, Li LX, Wang J, Green JM, Beg AA, Farrar MA (2010) Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. J Immunol 184: 4074–4077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veillette A, Dong Z, Latour S (2007) Consequence of the SLAM-SAP signaling pathway in innate-like and conventional lymphocytes. Immunity 27: 698–710 [DOI] [PubMed] [Google Scholar]
- Verykokakis M, Boos MD, Bendelac A, Kee BL (2010) SAP protein-dependent natural killer T-like cells regulate the development of CD8(+) T cells with innate lymphocyte characteristics. Immunity 33: 203–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll RE, Jimi E, Phillips RJ, Barber DF, Rincon M, Hayday AC, Flavell RA, Ghosh S (2000) NF-κB activation by the pre-T cell receptor serves as a selective survival signal in T lymphocyte development. Immunity 13: 677–689 [DOI] [PubMed] [Google Scholar]
- von Boehmer H (1990) Developmental biology of T cells in T cell-receptor transgenic mice. Annu Rev Immunol 8: 531–556 [DOI] [PubMed] [Google Scholar]
- von Boehmer H, Melchers F (2010) Checkpoints in lymphocyte development and autoimmune disease. Nat Immunol 11: 14–20 [DOI] [PubMed] [Google Scholar]
- Waterfield MR, Zhang M, Norman LP, Sun SC (2003) NF-κB1/p105 regulates lipopolysaccharide-stimulated MAP kinase signaling by governing the stability and function of the Tpl2 kinase. Mol Cell 11: 685–694 [DOI] [PubMed] [Google Scholar]
- Weinreich MA, Odumade OA, Jameson SC, Hogquist KA (2010) T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol 11: 709–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Vig M, Lyons J, Van Parijs L, Beg AA (2003) Combined deficiency of p50 and cRel in CD4+ T cells reveals an essential requirement for nuclear factor kappaB in regulating mature T cell survival and in vivo function. J Exp Med 197: 861–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.