Abstract
The dorsal striatum is critically involved in a variety of motor behaviours, including regulation of motor activity, motor skill learning and motor response to psychostimulant and neuroleptic drugs, but contribution of D2R-striatopallidal and D1R-striatonigral neurons in the dorsomedial (DMS, associative) and dorsolateral (DLS, sensorimotor) striatum to distinct functions remains elusive. To delineate cell type-specific motor functions of the DMS or the DLS, we selectively ablated D2R- and D1R-expressing striatal neurons with spatial resolution. We found that associative striatum exerts a population-selective control over locomotion and reactivity to novelty, striatopallidal and striatonigral neurons inhibiting and stimulating exploration, respectively. Further, DMS-striatopallidal neurons are involved only in early motor learning whereas gradual motor skill acquisition depends on striatonigral neurons in the sensorimotor striatum. Finally, associative striatum D2R neurons are required for the cataleptic effect of the typical neuroleptic drug haloperidol and for amphetamine motor response sensitization. Altogether, these data provide direct experimental evidence for cell-specific topographic functional organization of the dorsal striatum.
Keywords: D1R, D2R, motor control, motor learning, striatum
Introduction
The striatum represents the main input nucleus of the basal ganglia, a system of subcortical nuclei critically involved in motor control and motivational processes and altered in several conditions such as Parkinson's and Huntington's diseases or drug addiction and schizophrenia (Nestler, 2005; Kreitzer and Malenka, 2008). The projection neurons of the striatum are GABAergic medium-sized spiny neurons (MSNs) subdivided into two sub-populations, the striatonigral and striatopallidal neurons (Gerfen et al, 1990; Schiffmann et al, 2007) that form two main efferent pathways. The striatonigral MSNs (direct pathway) co-express dopamine D1 receptor (D1R) and substance P (SP), while striatopallidal MSNs (indirect pathway) co-express dopamine D2 receptor (D2R), adenosine A2A receptor (A2AR) and enkephalin (Enk) (Schiffmann and Vanderhaeghen, 1993). MSNs in the direct and indirect pathways are equal in number and shape, mosaically distributed throughout the striatum (Gerfen, 1992), and are not dissociable with techniques such as surgical or excitotoxic lesions.
The striatum can be functionally divided into dorsal and ventral subregions based on the origin of cortical glutamatergic and midbrain dopaminergic (DA) afferents. The dorsal striatum is thought to be involved mostly in motor behaviours, while ventral striatum is crucial for motivational processes (Robbins and Everitt, 1996; Groenewegen, 2003). The dorsal striatum is often subdivided into an external portion (the dorsolateral striatum (DLS) corresponding to the primate putamen, predominantly innervated by the sensorimotor cortex) and an internal part (the dorsomedial striatum (DMS) homologous to primate caudate nucleus, receiving projections from prefrontal and other association cortices) (Voorn et al, 2004; Graybiel, 2008). Cell-specific functions of the DMS or DLS in motor control and learning or in basal ganglia-related disorders (such as schizophrenia or drug addiction) remain poorly understood.
While the DMS seems more engaged during initial stages of motor skill learning, when the task is more dependent on attention and susceptible to interference (Jueptner and Weiller, 1998; Luft and Buitrago, 2005), the DLS seems required for progressive skill automatization and habit learning (Miyachi et al, 2002; Yin et al, 2004).
Treatment of schizophrenia-positive symptoms with typical antipsychotics, such as haloperidol, often induces a dramatic rigidity and locomotor immobility, called catalepsy (Lieberman et al, 2008). While striatal D2R antagonism is a common characteristic of antipsychotic drugs (Karam et al, 2010), a cell type-specific involvement of dorsal striatum subregions in the haloperidol-induced catalepsy remains elusive.
Behavioural sensitization to psychostimulants provides a model of addictive behaviours such as those associated with craving and relapse (Robinson and Berridge, 1993; Hyman et al, 2006), but involvement of D1R- and D2R-neuron pathways in this process remains controversial (Mattingly et al, 1996; Chen et al, 2003; Karlsson et al, 2008).
To date, to the best of our knowledge, no in-vivo approach has unravelled respective functions of D1R and D2R MSNs in the DMS and DLS. We selectively ablated each class of neurons in adult mice using an inducible diphtheria toxin receptor (DTR)-mediated cell targeting strategy (Durieux et al, 2009) and delineated distinct roles of D1R and D2R MSNs in associative and sensorimotor striatum during novelty- or drug-induced locomotor behaviours and motor skill learning.
Results
We bred lines of Drd1a-EY262-cre+/− (Gong et al, 2007; founder EY262) or Adora2a-cre+/− (Durieux et al, 2009) mice to inducible DTR+/+ (iDTR+/+) (Buch et al, 2005) mice, leading to mice that selectively expressed the DTR in D1R or D2R MSNs (Drd1a-EY262-cre+/− iDTR+/− (D1-DTR+ mice) or Adora2a-cre+/− iDTR+/− (A2A-DTR+ mice), respectively); and control mice (Drd1a-EY262-cre−/− iDTR+/− (D1-DTR− mice) or Adora2a-cre−/− iDTR+/− (A2A-DTR− mice)). This approach allows inducible ablation of D1R or D2R neurons in striatum of adult mice with high spatial resolution, avoiding neuronal ablation in other brain areas or potential developmental adaptations (Drago et al, 1998; Gantois et al, 2007; Durieux et al, 2009). All animal procedures were approved by the Université Libre de Bruxelles School of Medicine Ethical Committee. Diphtheria toxin (DT) was stereotaxically injected into the striatum to ablate the class of neurons throughout the entire striatum (full ablation) or to restrict the ablation to DMS (DMS ablation) or DLS (DLS ablation).
Selective ablation of D1R-striatonigral neurons
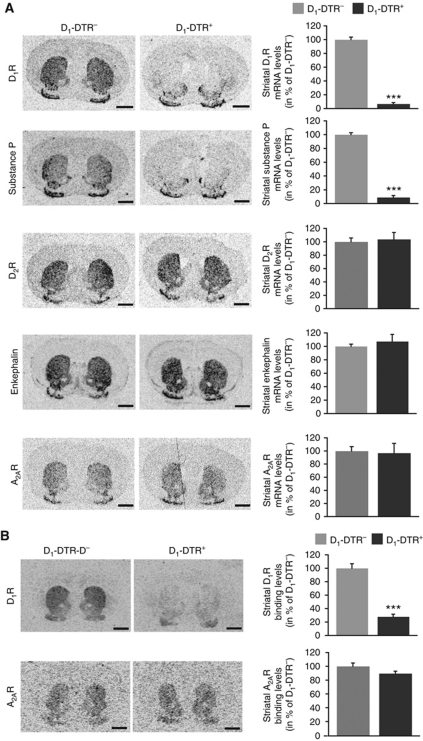
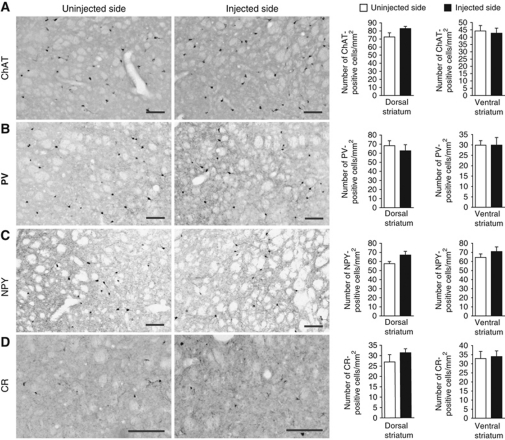
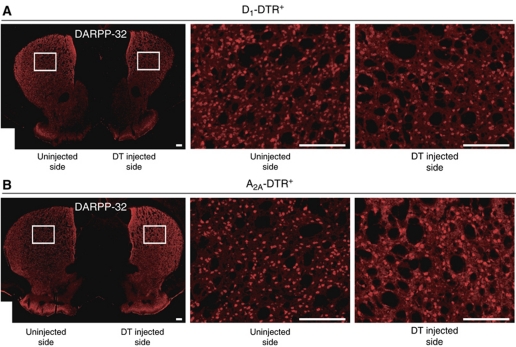
We first characterized full ablation of D1R-striatonigral neurons by DT injections in the entire striatum of D1-DTR mice (Figures 1 and 2). Bilateral full DT-injected D1-DTR+ mice showed specific loss of D1R and SP mRNAs (Figure 1A) as well as D1R binding (Figure 1B) with full preservation of D2R-striatopallidal neurons markers as D2R, A2AR and Enk mRNAs (Figure 1A) and A2AR binding (Figure 1B). The four sub-populations of striatal interneurons were spared in full D1R MSN ablated striatum (Figure 2). We also demonstrated that DT injections in the entire striatum of D1-DTR+ or A2A-DTR+ animals lead to a reduction of nearly 45% of dopamine- and cAMP-regulated phosphoprotein Mr 32 kDa (DARPP-32, a protein expressed in both MSN sub-populations)-positive cells in each model (Figure 3), confirming that striatal D1R and D2R neurons are approximately equal in number (Bertran-Gonzalez et al, 2008). Altogether, these results indicate that striatal DT injections in D1-DTR+ and, as previously described (Durieux et al, 2009), in A2A-DTR+ mice lead to selective elimination of D1R-striatonigral and D2R-striatopallidal neurons, respectively.
Figure 1.
Characterization of D1R-striatonigral neuron ablation after full striatum DT injections in D1-DTR+ mice. (A) In-situ hybridization autoradiograms (coronal sections, level +1.2 mm relative to bregma) of D1R-striatonigral (D1R, SP) and D2R-striatopallidal (D2R, Enk and A2AR) neuron mRNAs and respective levels in the striatum of D1-DTR− and D1-DTR+ mice 2 weeks after bilateral full DT injections (n=6 per group). (B) Autoradiograms (coronal sections, level +1.2 mm relative to bregma) of D1 and A2A receptors and respective striatal binding levels of D1-DTR− and D1-DTR+ mice 2 weeks after bilateral full striatum DT injections (n=5–6 per group). Scale bars=1 mm. Data are reported as mean±s.e.m. ***P<0.001.
Figure 2.
Preservation of striatal interneurons after ablation of striatonigral neurons in D1-DTR+ mice. (A–D) Immunostaining and quantitative analysis of (A) choline acetyltransferase (ChAT), (B) parvalbumin (PV), (C) neuropeptide Y (NPY) and (D) calretinin (CR)-positive cells in full DT-injected striatum as compared with uninjected striatum of D1-DTR+ mice (day 22 after unilateral full DT injections). Scale bars=100 μm. Columns represent the mean±s.e.m. (n=7).
Figure 3.
Reduction of striatal DARPP-32-positive neurons in DT-injected D1-DTR+ or A2A-DTR+ mice. (A, B) DARPP-32 immunostaining 1 month after unilateral DT injection in the entire striatum showing a 44.5±2.5 and 42.9±2.62% reduction of DARPP-32-positive cells in the injected striatum as compared with the uninjected striatum of D1-DTR+ (A) (6870±244 cells versus 3807±40 cells, n=2) and A2A-DTR+ (B) (6046±1094 cells versus 3481.5±783.5 cells, n=2) mice, respectively. Scale bars=200 μm.
Motor activity and rotarod learning after D1R- or D2R-neuron removal in the entire striatum
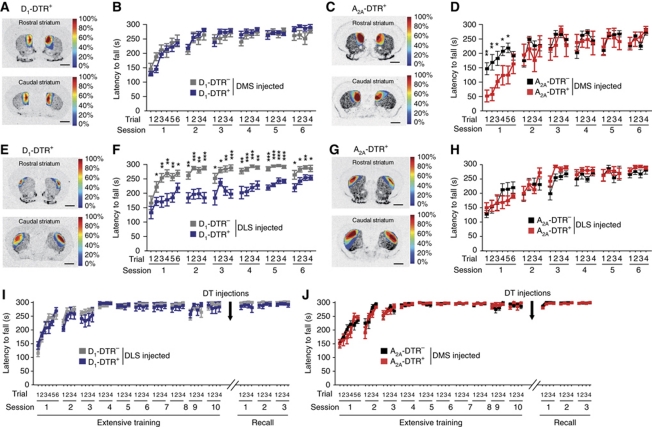
We next compared the respective functions of D1R or D2R MSNs during locomotion and motor skill learning following full ablation of D1-positive or D2-positive MSNs (Figure 4) that was verified by quantitative in-situ hybridization after completion of behavioural observations (Figure 4A and B). As shown in A2A-DTR+ mice (Durieux et al, 2009), the reduction of striatopallidal A2AR binding and behavioural abnormalities appear 1 week after DT injections. For that reason, all the behavioural experiments were conducted after a minimum delay of 1 week post DT injections. Spontaneous locomotion was recorded in a videotracked open field and mice were trained in a motor skill learning task (accelerating rotarod). In this task, mice have to learn a novel sequence of movements to maintain balance on a rotating rod in constant acceleration and receive several trials per day for consecutive days (Buitrago et al, 2004). While A2A-DTR+ mice exhibited persistent hyperactivity as previously described (Durieux et al, 2009) (Figure 4E, genotype: F(1,12)=208.55, P<0.001; time × genotype: F(11,12)=2.48, P=0.007), D1-DTR+ mice showed reduced locomotion (Figure 4C, genotype: F(1,14)=12.88, P=0.003; time × genotype: F(11,14)=2.60, P=0.005) that was still present 2 weeks after DT injections (39±8.9% of D1-DTR− distance moved in 60 min, P=0.03, data not shown). Full ablation of D2-positive MSN resulted in early impairments in the rotarod task, but progressive improvement of performance and finally performance equivalent to control level (Figure 4F, genotype: F(1,12)=16.45, P=0.002; trials × genotype: F(33,12)=3.13, P<0.001). In contrast, full DT-injected D1-DTR+ mice were unable to learn the task and displayed a permanent deficit (Figure 4D, genotype: F(1,14)=206.446, P<0.001; trials × genotype: F(33,14)=4.30, P<0.001).
Figure 4.
Motor activity and rotarod learning in D1R and D2R MSN full ablation mice. (A, B) In-situ hybridization for substance P (SP) (A) and enkephalin (Enk) (B) mRNA and respective quantitation, in rostral (bregma +1.2 mm) and caudal (bregma −0.1 mm) coronal brain sections of full striatum DT-injected D1-DTR−/D1-DTR+ (A) and A2A-DTR−/A2A-DTR+ (B) mice. Data are expressed as optical density values of the injected striatum in DTR+ as a percentage of the respective DTR− mice. Scale bars represent 1 mm. (C, E) Locomotor activity over 60 min in an open field of D1-DTR+ (C) and A2A-DTR+ (E) mice, and respective controls, 1 week after full DT injections. (D, F) Rotarod performance in D1-DTR−/D1-DTR+ (D) and A2A-DTR−/A2A-DTR+ (F) mice 1 week after full DT injections (n=6–10 per group). (G, H) Ten days of rotarod training and three days of performance recall one week after full DT injections in D1-DTR−/D1-DTR+ (G) and A2A-DTR−/A2A-DTR+ (H) mice (n=6–8 per group). Data are reported as mean±s.e.m. Statistical comparisons were made between DTR+ and respective DTR− control mice. *P<0.05, **P<0.01, ***P<0.001.
In view of these cell type-specific deficits during the rotarod acquisition, we investigated impact of D1R- or D2R-MSN removal after extensive rotarod training. D1-DTR and A2A-DTR mice were overtrained on the rotarod for 10 days before receiving full DT injections (Figure 4G and H). One week after DT injections, these mice were retested on the rotarod. While D1R neuron-ablated mice displayed profound rotarod impairments (Figure 4G, genotype: F(1,12)=12.57, P=0.004; time × genotype: F(53,12)=18.82, P<0.001), mice lacking D2R neurons showed similar performances as compared with controls (Figure 4H, genotype: F(1,12)=0.55, P=0.472; time × genotype: F(53,12)=1.65, P=0.003). Thus, after extensive training, D2R MSNs from the entire striatum are not required for rotarod task execution while D1R neurons are still necessary for performance.
D1R and D2R MSNs in the DMS or DLS oppositely regulate locomotor activity and novelty exploration
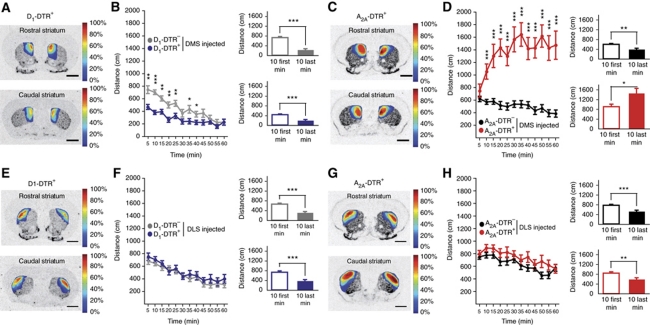
To further investigate the roles of D2R and D1R MSNs in functionally distinct portions of the dorsal striatum, A2A-DTR+ and D1-DTR+ mice were bilaterally injected with DT in either the DMS or the DLS (Figures 5, 6, 7, 8, 9 and 10). The cell-type ablation was characterized (Figure 5) and its regional location measured and topographically represented after behavioural observations were completed (see respective figures). Elimination of D1R-striatonigral neurons in the DMS (Figure 6A and B) induced a reduction in ambulation (genotype: F(1,38)=14.096, P=0.001) that was not observed following the DLS lesion (Figure 6E and F, genotype: F(1,49)=1.38, P=0.245). In contrast, DMS D2R-striatopallidal neuron-ablated mice displayed hyperlocomotion over the trial (Figure 6D, genotype: F(1,40)=48.78, P<0.001; time: F(11,40)=3.01, P=0.001) while D2R-striatopallidal neuron loss in the DLS did not produce any locomotor activity increase (Figure 6H, genotype: F(1,41)=3.18, P=0.082). It is worth to note that the increased locomotor activity observed in DMS D2R-striatopallidal neuron ablated mice exhibited an incremental kinetics in contrast to the decremental locomotor activity observed in all other groups (Figure 6B, time: F(11,38)=24.1, P<0.001; Figure 6F, time: F(11,49)=16.85, P<0.001; Figure 6H, time: F(11,41)=9.11, P<0.001, see also histograms on each figure).
Figure 5.
Ablation of D1R and D2R MSNs in the dorsomedial (DMS) or dorsolateral (DLS) striatum. (A–D) In-situ hybridization and quantitation of substance P (SP) (A, C) and enkephalin (Enk) (B, D) mRNA in rostral (bregma +1.2 mm) and caudal (bregma −0.1 mm) coronal brain sections of DMS (A, B) or DLS (C, D) DT-injected D1-DTR−/D1-DTR+ (A, C) and A2A-DTR−/A2A-DTR+ (B, D) mice (topographical representation of lesions can be found on Figure 6A, C, E and G). Data are expressed as optical density values of the injected striatum in DTR+ as a percentage of respective DTR− mice. Scale bars represent 1 mm. Data are reported as mean±s.e.m. (n=7–9 per group). Statistical comparisons were made between DTR+ and respective DTR− control mice. *P<0.05, ***P<0.001.
Figure 6.
Locomotor behaviour after ablation of D1R or D2R MSNs in the dorsomedial (DMS) or dorsolateral (DLS) striatum. (A, C, E, G) Topographic representation of the lesioned areas in D1-DTR+ (A, E) and A2A-DTR+ (C, G) DT-injected mice into the DMS (A, C) or the DLS (E, G). Colours represent percent of superimposed lesioned areas. (B, D, F, H) Locomotion of DMS DT-injected D1-DTR+ (B) and A2A-DTR+ (D) or DLS DT-injected D1-DTR+ (F) and A2A-DTR+ (H) mice, and respective controls over 60 min in an open field; histograms represent mean ambulation during the first 10 min and the 10 last minutes of the open field. Data are reported as mean±s.e.m. (n=19–28 per group). Statistical comparisons were made between DTR+ and respective DTR− control mice (dot chart) or between first and last open field 10 min for the same genotype (histograms). *P<0.05, **P<0.01, ***P<0.001.
Figure 7.
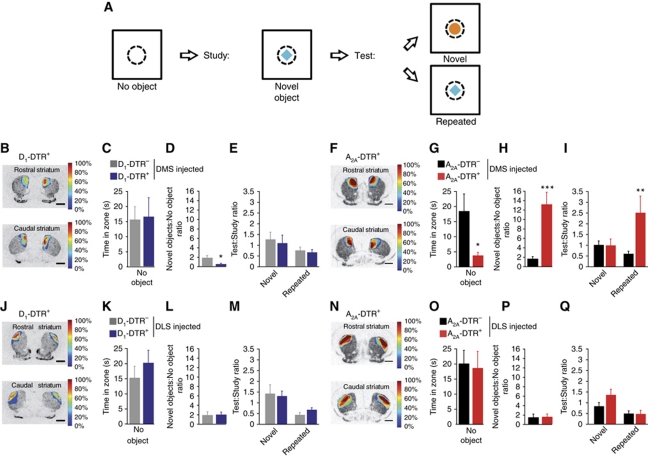
Object recognition task of DMS or DLS D1R- and D2R-MSN ablated mice. (A) Decoupled delayed spontaneous object recognition task, in which time spent in an open field core zone (dot line) is recorded without object, with novel object or repeated object. (B, F, J, N) Topographic representation of the lesioned areas in D1-DTR+ (B, J) and A2A-DTR+ (F, N) DT-injected mice into the DMS (B, F) or the DLS (J, N). Colours represent percent of superimposed lesioned areas. (C–E, G–I, K–M, O–Q) Time spent in the open field core in the absence of object (C, G, K, O), time spent in open field core in the presence of novel objects divided by the time spent in the open field core without object (D, H, L, P) and time spent in the open field core during the test phase divided by time spent in the open field core during the study phase in novel or repeated conditions (F, I, M, Q) of DMS D1-DTR (C–E) and A2A-DTR (G–I) or DLS D1-DTR (K–M) and A2A-DTR (O–Q) DT-injected mice. Data are reported as mean±s.e.m. (n=6–10 per group). Statistical comparisons were made as described in Materials and methods. *P<0.05, **P<0.01, ***P<0.001.
Figure 8.
Rotarod performance after ablation of D1R and D2R MSNs in the DMS or DLS. (A, C, E, G) Topographic representation of the lesioned areas in D1-DTR+ (A, E) and A2A-DTR+ (C, G) DT-injected mice into the DMS (A, D) or the DLS (E, G). Colours represent percent of superimposed lesioned areas. (B, D, F, H) Rotarod performance of DMS D1-DTR+ (B) and A2A-DTR+ (D) and DLS D1-DTR+ (F) and A2A-DTR+ (H) DT-injected mice and respective controls. (I, J) Ten days of rotarod training and three days of performance recall one week after DT injections in DLS of D1-DTR (I) and DMS of A2A-DTR (J) mice; respective topographic representation of the lesioned areas can be found on Figure 5F and J. Data are reported as mean±s.e.m. (n=7–9 per group). Statistical comparisons were made between DTR+ and respective DTR− control mice. *P<0.05, **P<0.01, ***P<0.001.
Figure 9.
Haloperidol-induced immobility and catalepsy in mice lacking D1R or D2R MSNs in the DMS or DLS. (A, B, D, E, G, H, J, K) Locomotor activity in a 60-min open field after saline or haloperidol (1.5 mg/kg) administration in DMS D1-DTR (A, B) and A2A-DTR (D, E) or DLS D1-DTR (G, H) and A2A-DTR (J, K) DT-injected mice. (C, F, I, L) Catalepsy score (latency to move) 30 min after haloperidol (1.5 mg/kg) administration in D1-DTR+ (C, I) and A2A-DTR+ (F, L) DT-injected mice into the DMS (C, F) or the DLS (I, L). Respective topographic representation of the lesioned areas can be found on Figure 5B, F, J and N. Data are reported as mean±s.e.m. (n=6–11 per group). Statistical comparisons were made between haloperidol and saline treatment (dot chart) or DTR+ and respective DTR− control mice (histograms). *P<0.05, **P<0.01, ***P<0.001.
Figure 10.
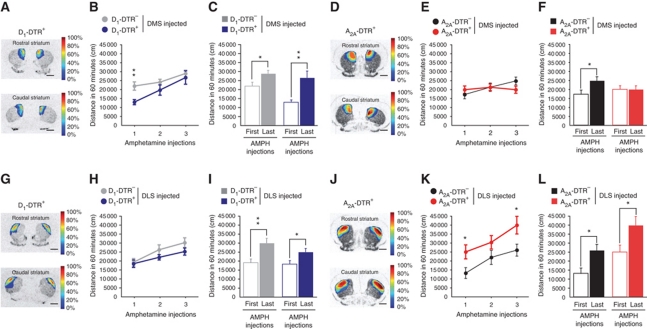
Amphetamine locomotor sensitization after D1R or D2R MSN ablation in the DMS or DLS. (A, D, G, J) Topographic representation of the lesioned areas in D1-DTR+ (A, G) and A2A-DTR+ (D, J) DT-injected mice into the DMS (A, D) or the DLS (G, J). Colours represent percent of superimposed lesioned areas. (B, C, E, F, H, I, K, L) Locomotor activity in a 60-min open field after repeated d-amphetamine (3 mg/kg) administration in DMS D1-DTR (B, C) and A2A-DTR (E, F) or DLS D1-DTR (H, I) and A2A-DTR (K, L) DT-injected mice. Histograms represent ambulation following first and last d-amphetamine administration. Data are reported as mean±s.e.m. (n=10–17 per group). Statistical comparisons were made between DTR+ and respective DTR− control mice (dot chart) or between first and last amphetamine injection (histograms). *P<0.05, **P<0.01.
In view of this impairment in novel environment habituation, we tested whether or not novel object exploration and recognition (Hughes, 2007) were also altered (Figure 7). Each ablated group was tested in a decoupled delayed spontaneous object recognition task (McTighe et al, 2010; see Materials and methods) in which mice have to explore an object placed in the middle of an open field. DMS D2R neuron-ablated mice spent less time in the open field core in the absence of object (Figure 7G, P=0.0497), but exhibited a dramatic increase in novel object exploration (Figure 7H, P<0.001) and were unable to reduce their exploratory behaviour when an object was repeatedly presented (Figure 7E, I, M and Q, condition: F(1,57)=5.211, P=0.026; condition × group: F(7,57)=3.284, P=0.005; Figure 7I, group: P=0.027). On the other hand, DMS D1R neuron-ablated mice showed no difference in exploring the open field core without object (Figure 7C, P=0.899), but displayed a reduced novel object exploration (Figure 7D, P=0.033). These effects were specific of the associative striatum, since neither DLS D2R neuron (Figure 7O, P=0.833; Figure 7P, P=0.90) nor DLS D1R neuron (Figure 7K, P=0.386; Figure 7L, P=0.912) ablations altered the task.
Thus, DMS-specific ablations, but not the DLS lesions, demonstrate a cell type-specific modulation of locomotor activity and novel object exploration in which D2R MSNs and D1R MSNs inhibit and stimulate locomotion and novelty exploration, respectively.
DMS D2R neurons and DLS D1R neurons are required for early motor learning and progressive skill acquisition, respectively
Evaluation of involvement of DLS and DMS D1R and D2R neurons in motor skill learning by the accelerating rotarod (Figure 8) showed that DMS D2R neuron-ablated mice (Figure 8D, genotype: F(1,14)=1.17, P=0.297; trials × genotype: F(25,14)=2.87, P<0.001) were impaired during initial trials but gradually improved their performances to reach control levels from the second learning day, while D2R-neuron elimination in the DLS did not affect the task (Figure 8H, genotype: F(1,14)=0.32, P=0.58; trials × genotype: F(25,14)=1.51, P=0.06). On the other hand, mice lacking D1R-expressing neurons in the DLS (Figure 8F) showed rotarod impairments until 6 days of learning (genotype: F(1,16)=39.96, P<0.001; trials × genotype: F(33,16)=2.50, P<0.001), an effect totally absent in DMS D1R neuron-ablated mice (Figure 8B, genotype: F(1,14)=0.16, P=0.69; trials × genotype: F(25,14)=1.19, P=0.244). Importantly, after extensive rotarod training, ablation of DMS D2R neurons (Figure 8J, genotype: F(1,14)=0.285, P=0.602) or DLS D1R neurons (Figure 8I, genotype: F(1,18)=0.308, P=0.586) did not induce performance deficit, indicating that rotarod learning impairments (see Figure 8D and F) are not due to task execution disruption.
Distinct contribution of DMS and DLS direct or indirect pathways in haloperidol-induced catalepsy and amphetamine sensitization
Treatment of schizophrenia-positive symptoms with typical neuroleptic drugs is often associated with motor side effects such as catalepsy (Lieberman et al, 2008). We then evaluated involvement of each neuronal population in motor responses to neuroleptic drugs (Figure 9). Investigation of these locomotor responses revealed that D2R-striatopallidal neuron removal selectively in the associative striatum completely abolished haloperidol-induced immobility (Figure 9E, treatment: F(1,12)=0.127, P=0.728) and catalepsy (Figure 9F, P<0.001). Neither ablation of D2R neurons in the DLS (Figure 9K, treatment: F(1,10)=11.81, P=0.006; Figure 9L, P=0.17) nor D1R-neuron ablations in both dorsal striatum subregions (Figure 9B, treatment: F(1,10)=99.26, P<0.001; Figure 9C, P=0.32; Figure 9H, treatment: F(1,16)=56.15, P<0.001; Figure 9I, P=0.59) altered haloperidol responses, indicating that D2R antagonism in striatopallidal neurons of the DMS is crucial for the motor effects of haloperidol.
Locomotor sensitization to psychostimulants is produced by repeated drug administration and is defined as an increase in the locomotor effect of the drug upon readministration. We evaluated involvement of each neuronal population in these motor responses to psychostimulant (Figure 10). Mice lacking D1R neurons in the DMS (Figure 10B, genotype: F(2,24)=4.56, P=0.043), but not in the DLS (Figure 10H, genotype: F(1,29)=1.3, P=0.26) showed a reduced acute amphetamine-induced hyperlocomotion as compared with controls; but repeated amphetamine injections sensitized this locomotor response (Figure 10B, repeated injections: F(2,24)=13.47, P<0.001; repeated injections × genotype: F(2,24)=1.45, P=0.246; Figure 10C, P=0.003). In contrast, ablation of DMS D2R neurons totally disrupted amphetamine locomotor sensitization (Figure 10E, repeated injections × genotype: F(2,24)=4.345, P=0.018; Figure 10F, P=0.995). On the other hand, DLS lesions of D2R neurons induced an increase in amphetamine acute locomotor activation (Figure 10K, genotype: F(1,21)=5.926, P=0.024) but sensitization was preserved in these mice (Figure 10K, repeated injections: F(2,21)=12.085, P<0.001; repeated injections × genotype: F(2,21)=0.482, P=0.621; Figure 10L, P=0.026).
Discussion
While it is currently accepted that dorsal striatum plays an important role in locomotor behaviours and motor learning (Graybiel, 1991; Mink and Thach, 1993; Groenewegen, 2003), MSN-type and dorsal striatum subregion involvement in these behaviours remains difficult to decipher without cell-type targeting tools. In the present work, we produced selective and inducible D2R and D1R MSN ablation in Drd1a-cre+/− iDTR+/− and Adora2a-Cre+/− iDTR+/− mice with a spatial resolution allowing their functional dissection selectively in the DMS and DLS (see Table I for a synthetic view of the observed behaviours in the different ablation subtypes).
Table 1. Summary of the observed behaviours in each ablation model.
| DMS ablation |
DLS ablation |
Entire striatum ablation |
||||
|---|---|---|---|---|---|---|
| D1 neurons | D2 neurons | D1 neurons | D2 neurons | D1 neurons | D2 neurons | |
| Open field | ||||||
| Total locomotion | ↓ | ↑ | ↔ | ↔ | ↓ | ↑ |
| Habituation | ↔ | Inverted | ↔ | ↔ | ↔ | Absent |
| Rotarod | ||||||
| Learning curve | ↔ | Initial ↓ | ↓ | ↔ | ↓ | Initial ↓ |
| Performance after learning | ND | ↔ | ↔ | ND | ↓ | ↔ |
| Novel object | ||||||
| Exploration of novel object | ↓ | ↑ | ↔ | ↔ | ND | ND |
| Exploration of old object | ↔ | ↑ | ↔ | ↔ | ND | ND |
| Object memory | ↔ | ↔ | ↔ | ↔ | ND | ND |
| Haloperidol-induced catalepsy | ↔ | ↓ | ↔ | ↔ | ND | ND |
| Amphetamine-induced locomotion | ||||||
| Acute effect | ↓ | ↔ | ↔ | ↑ | ND | ND |
| Sensitization | ↔ | ↓ | ↔ | ↔ | ND | ND |
| ND, not determined. | ||||||
First, the selective loss of D1R and SP in D1-DTR+, taken together with the previously described loss of D2R, A2AR and Enk in A2A-DTR+ DT-injected mice (Durieux et al, 2009), confirms that expression of these receptors and neuropeptides is largely segregated in the two MSN populations (Gerfen and Young, 1988; Gerfen et al, 1990; Lobo et al, 2006; Bateup et al, 2008; Bertran-Gonzalez et al, 2008; Heiman et al, 2008).
Full D2R-MSN ablation produced hyperlocomotion in contrast to the reduction of ambulation observed in D1R neuron full ablated mice. These data provide direct experimental evidence for an opposite control of the two populations over motor activity in freely moving animals, showing that D2R and D1R MSNs inhibit and stimulate motor activity respectively. Moreover, this modulatory influence on locomotion was partially recapitulated in DMS, but not in DLS, restricted ablations, indicating that associative striatum area exerts an MSN population-dependent control over spontaneous locomotion. Consistently, recent works using optogenetics showed that stimulation of D1R or D2R neurons selectively in the DMS increases and reduces spontaneous ambulation, respectively (Kravitz et al, 2010), while cell-type stimulation in the ventral striatum had no effect on spontaneous locomotion (Lobo et al, 2010).
Mice with selective DMS neuron ablation show alterations in several behaviours related to novelty. In D1R neuron-ablated mice, a decrease in novelty-induced exploration of objects (novel object test) and environment (open field) was observed, indicating that the direct pathway in the associative cortico-striatal loop is necessary for novelty-induced exploration. In D2R neuron-ablated mice, the hyperlocomotion in the open field and increased exploration of a novel object when it is repeatedly presented, can be, at least in part, interpreted as a form of hyperexploration, that is, the reciprocal of the behaviours of DMS D1R neuron-ablated mice. In mice lacking DMS D2R neurons, hyperlocomotion/hyperexploration becomes evident after an initial inhibition (see progressive increase in locomotion in the open field test and increased exploration from novel to repeated object in the novel object test), suggesting the existence in these mice of a higher sensitivity to the aversive effects of novelty. These results point to DMS as a crucial component of the circuits mediating novelty reactivity. Interestingly, it has been shown that novelty exposure preferentially induces immediate early gene expression in DMS and other brain regions of the executive prefrontocortico-striatal loop, in particular when novel objects are involved (Rinaldi et al, 2010). DMS neurons would select the access to the limited motor and cognitive resources of the conflicting sensory stimuli associated with the increased number of objects or environmental cues that become salient by virtue of their novelty (Redgrave and Gurney, 2006). Within the DMS, Go units and No-Go units mediated by D1 and D2 MSNs, respectively (Maia and Frank, 2011), are physiologically balanced. After selective cell ablations, the prevalence of Go or No-Go units would cause hypoexploration/hypolocomotion or hyperexploration/hyperlocomotion. In particular, in D2R neuron-ablated mice, a continuous translation of sensory stimuli to locomotion would lead to a state of continuous exploration/locomotion.
Rotarod motor skill learning experiments demonstrated that D1R MSNs are crucially required to acquire a motor task as their entire striatum removal disrupts permanently skill performance in naive animals. In contrast, mice lacking the D2R MSNs showed task impairments only during the initial trials and displayed late performances similar to controls. After extensive rotarod training, D1R neurons in the entire striatum are still required for task performance while D2R MSNs can be ablated. These findings demonstrate that execution of a previously learned motor sequence is not dependent on the D2R MSN pathway (Ashby et al, 2010). In a previous work (Gantois et al, 2007), ablation of D1R neurons led to locomotor hyperactivity and normal rotarod performances. It is worth to note that ablation in Gantois et al model was achieved by DT expression at 1–2 weeks after birth in forebrain D1R neurons, including the striatum, the cortex and the hippocampus. These differences in the neuronal population targeted and the age of ablation onset could explain the behavioural discordances between Gantois et al model and the present work.
DMS D2R neuron-ablated mice showed early rotarod deficits, while a permanent rotarod performance deficit was found in DLS D1R neuron-ablated mice. After extensive rotarod training, DMS D2R neurons or DLS D1R neuron can be ablated without affecting task performances, indicating that rotarod defects observed in naive animals cannot be attributed to task execution disruption. These results suggest that in naive animals, when task performance is more susceptible to interference and more dependent on attention (Luft and Buitrago, 2005), D1R and D2R MSNs work in concert to promote acquisition of a new motor skill: activation of D1R MSNs in the sensorimotor striatum is required for progressive automaticity of task performance, by development of correct motor strategies (Graybiel, 2008), while activation of D2R MSNs in associative striatum inhibits competing exploratory activity. During later skill learning stage, attention to action is less required (Jueptner and Weiller, 1998) and DMS D2R MSNs progressively disengage the process while DLS D1R MSN pathway is still required for skill automatization. Recent work (Yin et al, 2009) showed a potentiation of synaptic strength in the DMS after early rotarod learning that returns back to naive animal level after extensive training. Our results are in accord with this dynamic recruitment of DMS during motor skill learning. The authors further found a DLS increase in long-lasting potentiation only after rotarod overtraining that was more important in D2R than in D1R MSNs (Yin et al, 2009), a result which is in contrast to the present results, as D2R MSN elimination in the DLS did not disrupt rotarod learning, and full ablation of D2R MSNs interfered neither with late rotarod trials nor with the recall of a rotarod task previously learned through extensive training. On the other hand, DLS D1R MSN ablation disrupts rotarod performances at both initial and late motor skill learning stages. This view is supported by a recent in-vivo electrophysiological study showing that task-related ensemble activity in the DLS can emerge early during procedural learning (Thorn et al, 2010).
Neuroleptic-induced catalepsy represents a dramatic side effect of schizophrenia-positive symptom treatment (Lieberman et al, 2008). While most of neuroleptic drugs are D2R antagonists (Karam et al, 2010), identification of a restricted neuronal population involved in their motor effects remained elusive because of the wide D2R distribution (Beaulieu and Gainetdinov, 2011). Using genetic models, previous works have suggested that striatopallidal neurons mediate the cataleptic effect of haloperidol (Bertran-Gonzalez et al, 2008; Bateup et al, 2010). The complete insensitivity of DMS D2R neuron-ablated mice to haloperidol, strongly indicates that, in normal subject, a selective activation of striatopallidal neurons in the associative striatum is critical for the haloperidol hypolocomotor effect.
Repeated drug of abuse administration can lead to a gradual increase in behavioural responsiveness, such as locomotor activation in rodents, called behavioural sensitization (Robinson and Berridge, 1993). While striatum DA transmission is crucial for psychostimulant locomotor effects (Swerdlow et al, 1986; Nestler, 2005), a selective involvement of distinct dorsal striatum area neuronal subtypes in these processes remained elusive. Acute amphetamine locomotor response was increased in mice lacking DLS striatopallidal neurons while DMS D2R neuron-ablated mice displayed a deficit in amphetamine response sensitization, suggesting that sensorimotor and associative striatum indirect pathways are normally involved in regulation of acute amphetamine locomotor response and its sensitization respectively. In contrast, dorsal striatum D1R-neuron ablations did not affect amphetamine sensitization, but DMS direct pathway neuron removal reduces acute locomotor response to amphetamine. The interpretation of amphetamine acute effects is not straightforward due to the effects of region-selective neuron ablations on spontaneous locomotion. On the other hand, the lack of amphetamine sensitization in DMS D2R neuron-ablated mice but its maintenance in DLS D2R neuron-ablated mice as well as DMS or DLS D1R neuron-ablated mice highlights the relevance of the former neuronal population for amphetamine sensitization. Experiments with receptor antagonists and knockout mice indicate an involvement of both D1R and D2R in addictive drug sensitization (Vanderschuren and Kalivas 2000; Karlsson et al, 2008; Harrison and Nobrega, 2009). These approaches do not allow a precise identification of striatal target cell populations that may include multiple dorsal as well as ventral D1R- or D2R-expressing striatal sub-populations. Much evidence points to the ventral tegmental area and nucleus accumbens as main striatal regions contributing to the development of psychostimulant sensitization (Steketee and Kalivas 2011), although an involvement of caudate-putamen, especially its medial portion, has been proposed (see, e.g, Conversi et al, 2008). While present results are compatible with a main role of ventral striatal circuits, they strongly point to a necessary contribution of DMS D2R neurons in amphetamine sensitization.
Recently, other groups have targeted striatonigral and striatopallidal neurons in mice (Hikida et al, 2010) or rats (Ferguson et al, 2011). These authors developed inducible approaches with viral transgenesis to inhibit neurotransmission by cell-type expression of a tetanus toxin (Hikida et al, 2010) or to silence neuronal activity by selective expression of a Gi/o-coupled receptor in Enk- or SP-expressing neurons and pharmacological agent injections (Ferguson et al, 2011). Surprisingly, the resulting animals did not show a decrease or increase of spontaneous locomotor activity (Hikida et al, 2010; Ferguson et al, 2011), in contrast to the results obtained in other models (Durieux et al, 2009; Bateup et al, 2010; Kravitz et al, 2010), while acute locomotor response (Hikida et al, 2010) or sensitization (Ferguson et al, 2011) to amphetamine was altered. These differences could be explained by the different proportion of neurons targeted in the different models (see Durieux et al, 2011 for an extended discussion) and/or by the ability of each approach to effectively strongly and durably inhibit or stimulate neuronal activity.
Taken together, these results provide direct in-vivo experimental evidence for dissociation between neuronal subtypes and striatal subregions in the regulation of novelty- or drug-induced motor responses and motor learning.
Materials and methods
Animal care
All procedures were performed according to the Institutional Animal Care Committee guidelines and were approved by the Local Ethical Committee.
Animal breeding
C57BL/6 Adora2a-Cre+/− 2M strain mice were crossed with C57BL/6 iDTR+/+ mice (Durieux et al, 2009) resulting in 50% double heterozygous Adora2a-Cre+/− iDTR+/− (A2A-DTR+ mice) and 50% Adora2a-Cre−/− iDTR+/− (A2A-DTR− mice), which were used as controls. Outbred Drd1a-cre+/− EY262 strain (http://www.gensat.org; Gong et al, 2007) mice were mated with C57BL/6 iDTR+/+ mice resulting in 50% of Drd1a-cre+/− iDTR+/− (D1-DTR+ mice) and 50% of Drd1a-cre−/− iDTR+/− (D1-DTR−) control mice. For the two models, all experiments were then performed on animals with the same strain background. Experiments were conducted on male mice, but to increase the group size in some experiments (Figure 6), females were added in a counterbalanced way between double transgenic and control groups without gender effect (Figure 6B, F(1,12)=0.364, P=0.557; Figure 6F, F(1,14)=1.128, P=0.306).
Stereotaxic injections
Adora2a-Cre+/− iDTR+/−, Drd1a-cre+/− iDTR+/− and respective control mice were deeply anaesthetized at the age of 16–20 weeks, and placed in a stereotaxic apparatus. DT (Sigma-Aldrich) was diluted in 0.01 M PBS (pH 7.4) to a concentration of 100 pg/μl and was stereotaxically injected with a blunt needle in both sides of the striatum over 10 min. For the full striatum ablation, 1 μl of DT was injected at two sites with the coordinates (adapted from atlas of Paxinos and Franklin, 2001, with bregma and dura as references): anterior +1.2 mm, lateral ±1.5 mm, ventral +3 mm, and anterior +0.5 mm, lateral ±1.8 mm, ventral +3 mm.
For the DLS and DMS ablation, 0.2 μl of DT was injected at two sites with the coordinates:
DLS: anterior +1.2 mm, lateral ±2.6 mm, ventral +1.6 mm; anterior +0.1 mm, lateral ±3.2 mm, ventral +1.6 mm;
DMS: anterior +1.2 mm, lateral ±1.1 mm, ventral +1.9 mm; anterior +0.1 mm, lateral ±1.3 mm, ventral +1.9 mm.
Locomotor activity and object recognition tasks
Locomotor activity was assessed by videotracking (Ethovision, Noldus). Mice were placed for 60 min in open field locomotor activity boxes (20 cm × 40 cm) under non-stressful conditions (15 lux illumination) and horizontal ambulation was recorded.
Object recognition task was adapted from McTighe et al (2010). Decoupled delayed spontaneous object recognition was conducted in a 40 × 40 cm open field coupled to a videotracking system (Ethovision, Noldus). The objects used in the experiment differed in shape and colour, had a 5–6 cm diameter and were placed in the centre of the open field. During each trial, the mouse explored the open field for 20 min and the time spent in a central zone (10 cm in diameter) was recorded. At the end of the trial, the mouse was removed from the open field and placed in its home cage. The experiment started with a trial without object in the open field, followed by sessions consisting of two phases: a study and a test trial. During the study trial, a novel object was placed in the centre of the open field. One hour after the study, the test phase could take one of two forms: the novel condition or the repeated condition. In the novel condition, a novel object is placed in the apparatus and in the repeated condition, the mouse explores a new identical copy of the object used in the study trial. Mice were tested in novel and repeated conditions in a counterbalanced order. Which object of the pair was viewed in the study trial was also counterbalanced and both objects were viewed an equal number of times in the study phase in novel and repeated trials.
Haloperidol and amphetamine challenges
Mice were placed in open field locomotor activity boxes (20 cm × 40 cm) under non-stressful conditions (15 lux illumination) and horizontal ambulation was recorded.
For haloperidol-induced hypolocomotion, locomotor activity was first assessed for 60 min following saline (NaCl 0.9%) intraperitoneal (i.p.) injection and mice were placed in their home cage at the end of the challenge. Twenty-four hours later, locomotor activity was recorded during 60 min after haloperidol (JANSSEN-CILAG, 1.5 mg/kg) i.p. administration.
For haloperidol-induced catalepsy, mice received an injection of haloperidol (1.5 mg/kg, i.p.) in their home cage. After a delay of 30 min, mice forepaws were placed on a bar (0.2 cm in diameter) 4 cm above the tabletop. The latency to remove both forepaws from the bar was recorded with a maximum cutoff time of 180 s.
For amphetamine locomotor response trials, mice were first placed in the open field for 1 h (habituation) and then received an i.p. d-amphetamine injection (Certa, Waregem, Belgium; 3 mg/kg). Horizontal distance was recorded for 60 min. Mice received three trials with a 48-h interval between each trial.
Accelerated rotarod task
The rotarod apparatus (mouse rotarod, Ugo Basile) consisted of a plastic roller with small grooves running along its turning axis (Bearzatto et al, 2005). One week after injections, mice were tested for six to eight consecutive daily sessions (Figures 4D, F, 8B, D, F and H). To increase the number of early learning trials, mice received six trials on the first session and then four trials per day for 5–7 days. On the first day, mice were habituated by placement on the rotarod at a constant speed (4 r.p.m.) for 60 s. During each trial, animals were placed on the rod rotating at a constant speed (4 r.p.m.), then the rod started to accelerate continuously from 4 to 40 r.p.m. over 300 s. The latency to fall off the rotarod was recorded. Animals that stayed on the rod for 300 s were removed from the rotarod and recorded as 300 s. Mice that clung to the rod for two complete revolutions were removed from the rod and time was recorded. Between each trial, mice were placed in their home cage for a 15–20-min interval. For the recall paradigm (Figures 4G, H, 8I and J), mice received 10 rotarod sessions before full DT injections. One week after DT injections, mice were retested on the rotarod for three consecutive daily sessions.
Immunohistochemistry
Interneuron immunostainings (Figure 2) were performed as previously described (Durieux et al, 2009). Striatal coronal 30 μm free-floating sections were incubated overnight at 4°C with a rabbit primary antibody: anti-NPY (1/6000) (a gift of Pr Pelletier), anti-calretinin (1/3000) (Chemicon), anti-ChAT (1/1000) (Chemicon) or anti-parvalbumin (1/1000) (Chemicon). The sections were then incubated with donkey anti-rabbit biotinylated antibody (1/500, Jackson ImmunoResearch) and visualized with the ABC-diaminobenzidine reaction.
DARPP-32 immunohistochemistry (Figure 3) was performed as previously described (Bertran-Gonzalez et al, 2008). Striatal coronal 30 μm free-floating sections were incubated overnight at 4°C with a mouse primary antibody anti-DARPP-32 (1/2000) (a gift of Dr Jean-Antoine Girault) and were then incubated with donkey anti-mouse IgG Dylight 549 conjugated (1/200) (Jackson ImmunoResearch). Slides were examined under an AxioImager Z1 (Zeiss, Oberkochen, Germany) equipped with a × 5 objective and band-pass filter sets nr 15 for red fluorochromes, using AxioVision software (Zeiss). Entire striatum images were obtained by juxtaposition of successive acquired images (1388 × 1040 pixels; 1.29 μm/pixel; fixed exposure) and DARPP-32-positive cells were counted with cell counter software (ImageJ).
In-situ hybridization histochemistry and in-vitro receptor autoradiography
Striatal 18 μm coronal sections were cut from fresh-frozen brain and mounted onto glass slides. In-situ hybridization was performed as described previously (Schiffmann and Vanderhaeghen, 1993; Dassesse et al, 2001; Blum et al, 2004). After the hybridization procedure, sections were exposed to Kodak Biomax MR film for 1–2 week(s) depending on the marker studied and digitalized images were generated from the autoradiograms. For D1R and A2AR binding, [3H]SCH23390 and [3H]CGS21680 (Perkin-Elmer), respectively, were used as previously described (Blum et al, 2002). After the binding procedure, slides were exposed to a 3H storage phosphor screen (Amersham) for 2–3 days and autoradiographic signals were detected using a Cyclone storage phosphor scanner (Packard Instruments, Meridian, CT). For quantitation of in-situ hybridization and binding (ImageJ software), averaged optical density (OD) was measured in different areas of interest and background level was subtracted to obtain corrected values. In the bilateral full ablation models, a small reduction of the entire striatum area was found in A2A-DTR+ and D1-DTR+ mice as compared with respective controls (about 10% of the DTR area) and average OD measured in the striatum of these animals was corrected to account for this slight shrinkage. Two to three sections were used for each animal to calculate the mean OD. As Drd1a-cre EY262 mice show some level of cre-recombinase expression in cerebral cortical regions surrounding the striatum (http://www.gensat.org), we controlled for cortical integrity in DT-injected D1-DTR mice by performing in-situ hybridization for the cortical marker cholecystokinin (Schiffmann and Vanderhaeghen, 1991) at the level of the two injection sites. Animals demonstrating reductions in cortical cholecystokinin mRNA levels (superior to two times the standard deviation of the control mean) were excluded from further analysis.
Statistical analysis
Rotarod experiments were analysed by a two-way ANOVA with genotype as between factor and trials as within factor. Open field locomotor behaviours (Figures 4C, E, 6B, D, F, H, 9A, B, D, E, F, G, H, J and K) were analysed by a two-way ANOVA with genotype as between factor and 5 min time bins as within factor. Amphetamine sensitization tests were analysed by a two-way ANOVA with genotype as between factor and amphetamine injection as within factor. Depending on the two-way ANOVA results, trial-by-trial, bin-by-bin or injection-by-injection one-way ANOVA was performed (see P-values on respective figures). Object recognition test in novel or repeated condition was analysed by a two-way ANOVA with groups as between factor and condition as within factor, followed by Bonferroni post hoc comparisons.
All other comparisons were subjected to one-way ANOVA analysis.
Supplementary Material
Acknowledgments
We thank Dr Ari Waisman for providing iDTR mice, Dr Jean-Antoine Girault and Denis Hervé for providing DARPP-32 antibody, Dr Marina Picciotto and Dr Michele Zoli for the helpful and critical comments on the manuscript; Patrick Bishop for informatics support; and Souad Laghmiri, Laetitia Cuvelier and Delphine Houtteman for expert technical assistance. PFD is Research Fellow of the FRS–FNRS (chargé de recherche FNRS) and AdKdE Research Associate of the FRS–FNRS (Belgium). This study was supported by FMRE (Belgium), FRS–FNRS (Belgium), FER from ULB, Action de Recherche Concertée from the CFWB. Author contributions: PFD, SNS and AdKdE conceived and designed the experiments; PFD performed the experiments; PFD, SNS and AdKdE analysed the data and wrote the paper.
Footnotes
The authors declare that, except for income received from our primary employer, no financial support or compensation has been received from any individual or corporate entity over the past 3 years for research or professional service and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Ashby FG, Turner BO, Horvitz JC (2010) Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn Sci 14: 208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Santini E, Shen W, Birnbaum S, Valjent E, Surmeier DJ, Fisone G, Nestler EJ, Greengard P (2010) Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc Natl Acad Sci USA 107: 14845–14850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup HS, Svenningsson P, Kuroiwa M, Gong S, Nishi A, Heintz N, Greengard P (2008) Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat Neurosci 11: 932–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearzatto B, Servais L, Cheron G, Schiffmann SN (2005) Age dependence of strain determinant on mice motor coordination. Brain Res 1039: 37–42 [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR (2011) The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev 63: 182–217 [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J, Bosch C, Maroteaux M, Matamales M, Hervé D, Valjent E, Girault JA (2008) Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J Neurosci 28: 5671–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum D, Galas MC, Cuvelier L, Schiffmann SN (2004) Chronic intoxication with 3-nitropropionic acid in rats induces the loss of striatal dopamine terminals without affecting nigral cell viability. Neurosci Lett 354: 234–238 [DOI] [PubMed] [Google Scholar]
- Blum D, Galas MC, Gall D, Cuvelier L, Schiffmann SN (2002) Striatal and cortical neurochemical changes induced by chronic metabolic compromise in the 3-nitropropionic model of Huntington's disease. Neurobiol Dis 10: 410–426 [DOI] [PubMed] [Google Scholar]
- Buch T, Heppner FL, Tertilt C, Heinen TJ, Kremer M, Wunderlich FT, Jung S, Waisman A (2005) A Cre-inducible diphtheria toxin receptor mediates cell lineage ablation after toxin administration. Nat Methods 2: 419–426 [DOI] [PubMed] [Google Scholar]
- Buitrago MM, Schulz JB, Dichgans J, Luft AR (2004) Short and long-term motor skill learning in an accelerated rotarod training paradigm. Neurobiol Learn Mem 81: 211–216 [DOI] [PubMed] [Google Scholar]
- Chen JF, Moratalla R, Yu L, Martín AB, Xu K, Bastia E, Hackett E, Alberti I, Schwarzschild MA (2003) Inactivation of adenosine A2A receptors selectively attenuates amphetamine-induced behavioral sensitization. Neuropsychopharmacology 28: 1086–1095 [DOI] [PubMed] [Google Scholar]
- Conversi D, Bonito-Oliva A, Orsini C, Colelli V, Cabib S (2008) DeltaFosB accumulation in ventro-medial caudate underlies the induction but not the expression of behavioral sensitization by both repeated amphetamine and stress. Eur J Neurosci 27: 191–201 [DOI] [PubMed] [Google Scholar]
- Dassesse D, Ledent C, Parmentier M, Schiffmann SN (2001) Acute and chronic caffeine administration differentially alters striatal gene expression in wild-type and adenosine A(2A) receptor-deficient mice. Synapse 42: 63–76 [DOI] [PubMed] [Google Scholar]
- Drago J, Padungchaichot P, Wong JY, Lawrence AJ, McManus JF, Sumarsono SH, Natoli AL, Lakso M, Wreford N, Westphal H, Kola I, Finkelstein DI (1998) Targeted expression of a toxin gene to D1 dopamine receptor neurons by cre-mediated site-specific recombination. J Neurosci 18: 9845–9857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux PF, Bearzatto B, Guiducci S, Buch T, Waisman A, Zoli M, Schiffmann SN, de Kerchove d’Exaerde A (2009) D2R striatopallidal neurons inhibit both locomotor and drug reward processes. Nat Neurosci 12: 393–395 [DOI] [PubMed] [Google Scholar]
- Durieux PF, Schiffmann SN, de Kerchove d’Exaerde A (2011) Targeting neuronal populations of the striatum. Front Neuroanat 5: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PEM, Dong Y, Roth BL, Neumaier JF (2011) Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14: 22–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantois I, Fang K, Jiang L, Babovic D, Lawrence AJ, Ferreri V, Teper Y, Jupp B, Ziebell J, Morganti-Kossmann CM, O’Brien TJ, Nally R, Schütz G, Waddington J, Egan GF, Drago J (2007) Ablation of D1 dopamine receptor-expressing cells generates mice with seizures, dystonia, hyperactivity, and impaired oral behavior. Proc Natl Acad Sci USA 104: 4182–4187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR (1992) The neostriatal mosaic: multiple levels of compartmental organization. Trends Neurosci 15: 133–139 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ Jr, Sibley DR (1990) D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250: 1429–1432 [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Young WS (1988) Distribution of striatonigral and striatopallidal peptidergic neurons in both patch and matrix compartments: an in situ hybridization histochemistry and fluorescent retrograde tracing study. Brain Res 460: 161–167 [DOI] [PubMed] [Google Scholar]
- Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR (2007) Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci 27: 9817–9823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM (1991) Basal ganglia-input, neural activity, and relation to the cortex. Curr Opin Neurobiol 1: 644–651 [DOI] [PubMed] [Google Scholar]
- Graybiel AM (2008) Habits, rituals, and the evaluative brain. Annu Rev Neurosci 31: 359–387 [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ (2003) The basal ganglia and motor control. Neural Plast 10: 107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison SJ, Nobrega JN (2009) Differential susceptibility to ethanol and amphetamine sensitization in dopamine D3 receptor-deficient mice. Psychopharmacology (Berl) 204: 49–59 [DOI] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suárez-Fariñas M, Schwarz C, Stephan DA, Surmeier DJ, Greengard P, Heintz N (2008) A translational profiling approach for the molecular characterization of CNS cell types. Cell 135: 738–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S (2010) Distinct roles of synaptic transmission in direct and indirect striatal pathway to reward and aversive behavior. Neuron 66: 896–907 [DOI] [PubMed] [Google Scholar]
- Hughes RN (2007) Neotic preferences in laboratory rodents: issues, assessment and substrates. Neurosci Biobehav Rev 31: 441–464 [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29: 565–598 [DOI] [PubMed] [Google Scholar]
- Jueptner M, Weiller C (1998) A review of differences between basal ganglia and cerebellar control of movements as revealed by functional imaging studies. Brain 121: 1437–1449 [DOI] [PubMed] [Google Scholar]
- Karam CS, Ballon JS, Bivens NM, Freyberg Z, Girgis RR, Lizardi-Ortiz JE, Markx S, Lieberman JA, Javitch JA (2010) Signaling pathways in schizophrenia: emerging targets and therapeutic strategies. Trends Pharmacol Sci 31: 381–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Hefner KR, Sibley DR, Holmes A (2008) Comparison of dopamine D1 and D5 receptor knockout mice for cocaine locomotor sensitization. Psychopharmacology (Berl) 200: 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC (2010) Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature 466: 622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC (2008) Striatal plasticity and basal ganglia circuit function. Neuron 60: 543–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman JA, Bymaster FP, Meltzer HY, Deutch AY, Duncan GE, Marx CE, Aprille JR, Dwyer DS, Li XM, Mahadik SP, Duman RS, Porter JH, Modica-Napolitano JS, Newton SS, Csernansky JG (2008) Antipsychotic drugs: comparison in animal models of efficacy, neurotransmitter regulation, and neuroprotection. Pharmacol Rev 60: 358–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ (2010) Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330: 385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW (2006) FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci 9: 443–452 [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM (2005) Stages of motor skill learning. Mol Neurobiol 32: 205–216 [DOI] [PubMed] [Google Scholar]
- Maia TV, Frank MJ (2011) From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci 14: 154–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingly BA, Rowlett JK, Ellison T, Rase K (1996) Cocaine-induced behavioral sensitization: effects of haloperidol and SCH 23390 treatments. Pharmacol Biochem Behav 53: 481–486 [DOI] [PubMed] [Google Scholar]
- McTighe SM, Cowell RA, Winters BD, Bussey TJ, Saksida LM (2010) Paradoxical false memory for objects after brain damage. Science 330: 1408–1410 [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT (1993) Basal ganglia intrinsic circuits and their role in behavior. Curr Opin Neurobiol 3: 950–957 [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, Lu X (2002) Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res 146: 122–126 [DOI] [PubMed] [Google Scholar]
- Nestler E (2005) Is there a common molecular pathway for addiction? Nat Neurosci 11: 1445–1449 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ (2001) The Mouse Brain in Stereotaxic Coordinates, 2nd edn. San Diego: Academic [Google Scholar]
- Redgrave P, Gurney K (2006) The short-latency dopamine signal: a role in discovering novel actions? Nat Rev Neurosci 7: 967–975 [DOI] [PubMed] [Google Scholar]
- Rinaldi A, Romeo S, Agustín-Pavón C, Oliverio A, Mele A (2010) Distinct patterns of Fos immunoreactivity in striatum and hippocampus induced by different kinds of novelty in mice. Neurobiol Learn Mem 94: 373–381 [DOI] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ (1996) Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol 6: 228–236 [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 18: 247–291 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Fisone G, Moresco R, Cunha RA, Ferré S (2007) Adenosine A2A receptors and basal ganglia physiology. Prog Neurobiol 83: 277–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ (1991) Cholecystokinin mRNA detection in rat spinal cord motoneurons but not in dorsal root ganglia neurons. J Comp Neurol 304: 219–233 [DOI] [PubMed] [Google Scholar]
- Schiffmann SN, Vanderhaeghen JJ (1993) Adenosine A2 receptors regulate the gene expression of striatopallidal and striatonigral neurons. J Neurosci 13: 1080–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW (2011) Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev 63: 348–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Vaccarino FJ, Amalric M, Koob GF (1986) The neural substrates for the motor-activating properties of psychostimulants: a review of recent findings. Pharmacol Biochem Behav 25: 233–248 [DOI] [PubMed] [Google Scholar]
- Thorn CA, Atallah H, Howe M, Graybiel AM (2010) Differential dynamics of activity changes in dorsolateral and dorsomedial striatal loops during learning. Neuron 66: 781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW (2000) Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berl) 151: 99–120 [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM (2004) Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci 27: 468–474 [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ, Balleine BW (2004) Lesions of dorsolateral striatum preserve outcome expectancy, but disrupt habit formation in instrumental learning. Eur J Neurosci 19: 181–189 [DOI] [PubMed] [Google Scholar]
- Yin HH, Mulcare SP, Hilário MR, Clouse E, Holloway T, Davis MI, Hansson AC, Lovinger DM, Costa RM (2009) Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci 12: 333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.