Abstract
Background
The purpose of this research was to compare the effects of niacin extended-release in combination with simvastatin (NER/S) versus atorvastatin monotherapy on high-density lipoprotein (HDL) particle number and size in patients with hyperlipidemia or dyslipidemia from the SUPREME study.
Methods
This was a post hoc analysis of patients (n = 137) who completed the SUPREME study and who had lipid particle number and size measurements at both baseline and at week 12 by nuclear magnetic resonance spectroscopy. Following ≥4 weeks without lipid-modifying therapy (washout period), the patients received NER/S 1000/40 mg/day for 4 weeks followed by NER/S 2000/40 mg/day for 8 weeks, or atorvastatin 40 mg/day for 12 weeks. Median percent changes in HDL particle number and size from baseline to week 12 were compared between the NER/S and atorvastatin treatment groups using the Wilcoxon rank-sum test. Distribution of HDL particle subclasses at week 12 was compared between the treatment groups using the Cochran–Mantel–Haenszel test.
Results
Treatment with NER/S resulted in a significantly greater percent reduction in small HDL particle number at week 12 compared with atorvastatin monotherapy (−1.8% versus 4.2%, P = 0.014), and a numerically greater percent increase in large HDL particle number (102.4% versus 39.2%, P = 0.078) compared with atorvastatin monotherapy. A significantly greater percent increase in HDL particle size from baseline at week 12 was observed with NER/S compared with atorvastatin (6.0% versus 1.3%, P < 0.001). NER/S treatment also resulted in a significant shift in HDL particle size from small and medium at baseline to large at week 12 (P < 0.0001).
Conclusion
Treatment with NER/S resulted in larger favorable changes in number and size of HDL particle subclasses compared with atorvastatin monotherapy, including a numerically greater increase in number of large HDL particles, and a significantly greater decrease in number of small HDL particles compared with atorvastatin monotherapy. In addition, NER/S treatment resulted in a significant change in HDL particle size distribution from small and medium to large.
Keywords: niacin, simvastatin, atorvastatin, lipoprotein particles, dyslipidemia, combination therapy, high-density lipoprotein
Introduction
Atherogenic dyslipidemia is highly prevalent, especially in patients with insulin resistance and diabetes mellitus.1 Atherogenic dyslipidemia increases the risk for coronary heart disease and peripheral vascular disease, and remains a serious public health problem despite efforts to implement lifestyle modification and pharmacologic intervention using lipid-modifying drugs.2 Atherogenic dyslipidemia is comprised of the so-called lipid triad, ie, elevated levels of plasma triglycerides, increased numbers of small, dense low-density lipoprotein (LDL) particles with normal or slightly elevated LDL cholesterol levels, and lower numbers of high-density lipoprotein (HDL) particles, primarily due to fewer large, cholesterol-rich (HDL2) particles with either no change or increased numbers of small, dense (HDL3) particles.3,4
Large, prospective, global, epidemiological studies have observed that high LDL cholesterol and low HDL cholesterol are among the most important predictors of future cardiovascular events, including myocardial infarction, ischemic stroke, and death.5–7 Recent studies employing lipoprotein subclass fractionation and measurement techniques8 have shown that increased numbers of LDL particles are associated with increased risk for coronary heart disease, with small, dense LDL particles usually having greater association with coronary heart disease.9 Furthermore, particle number, rather than LDL cholesterol levels, has been shown to be a better predictor of development of atherosclerotic disease and sustaining acute cardiovascular events.10–12 Reduced numbers of HDL particles independently predict coronary heart disease, and show a strong inverse correlation with risk for developing cardiovascular disease.13,14 Large HDL2 particles appear to have an inverse association, whereas small HDL particle associations are more controversial, with some studies reporting positive associations with coronary heart disease prevalence,15,16 and others suggesting cardioprotective effects of HDL3 particles.4,17
Pharmacological interventions inducing less atherogenic lipid profiles are desirable in dyslipidemic patients who fail to respond to therapeutic lifestyle changes as the initial step in lipid management. Statins are the first-line medication widely prescribed to lower LDL cholesterol levels and lower LDL particle numbers; however, their effect on HDL particles is modest.18 Niacin is the most effective agent currently available for raising HDL cholesterol, and when used in combination with simvastatin, has shown a decrease in atherogenic particle numbers to a greater extent than atorvastatin monotherapy.19 Moreover, niacin monotherapy has been shown to reduce risk of nonfatal myocardial infarction and stroke.20
The objective of this analysis was to compare the effects of niacin extended-release in combination with simvastatin (NER/S) versus atorvastatin monotherapy on HDL particle number and size in a post hoc analysis of patients with hyperlipidemia or dyslipidemia from the SUPREME study (Study to Compare the Lipid Effects of Niacin ER and Simvastatin to Atorvastatin in Subjects with Hyperlipidemia or Mixed Dyslipidemia).
Materials and methods
Study design
SUPREME was a prospective, randomized, open-label, blinded-endpoint, 12-week, multicenter clinical study in the US comparing the efficacy and safety of NER/S combination therapy with atorvastatin monotherapy. The study was approved by the institutional ethics committee and all patients gave written informed consent.21
The details of the SUPREME study have been described elsewhere.21 In brief, patients eligible for inclusion were men and women ≥21 years of age, with primary type II hyperlipidemia or mixed dyslipidemia (LDL cholesterol ≥130 mg/dL and <250 mg/dL, HDL cholesterol <40/50 mg/dL for men/women, and triglycerides <350 mg/dL) following the National Cholesterol Education Program therapeutic lifestyle changes diet. Patients were required to discontinue lipid medication 4–5 weeks prior to randomization. The major exclusion criteria were creatine phosphokinase ≥3 × the upper limit of normal (ULN), alanine aminotransferase ≥1.3 × ULN, aspartate aminotransferase ≥1.3 × ULN, calculated creatinine clearance <30 mL/minute, glycosylated hemoglobin ≥9%, uric acid levels ≥1.3 × ULN, poorly controlled type 1 or 2 diabetes, persistent, uncontrolled hypertension, and pregnancy. Following 4 weeks of a therapeutic lifestyle changes diet and washout of any pretrial lipid treatment, eligible patients were randomized centrally in a 3:2 ratio to treatment arms (NER/S:atorvastatin). The dosing schedule was NER/S 1000/40 mg/day for 4 weeks, followed by NER/S 2000/40 mg/day for 8 weeks; or atorvastatin 40 mg/day for 12 weeks.
Analysis
Patients who completed the SUPREME study and had lipid particle concentration and size measurements at both baseline and at week 12 were included in this analysis. Fasting plasma samples were analyzed to determine lipoprotein particle size (diameter [nm]) and number (concentration [μmol/L]) by nuclear magnetic resonance spectroscopy (LipoProfile-II Test®, LipoScience Inc, Raleigh, NC).22,23 Differences in distribution of HDL particle subclasses defined as large (>8.8–13 nm), medium (>8.2–8.8 nm), and small (7.3–8.2) at week 12 were compared between treatment groups based on the Cochran–Mantel–Haenszel test. Median percent changes in HDL particle number and size from baseline to week 12 were compared between treatment groups using the Wilcoxon rank-sum test.
Results
A total of 137 patients in the treatment groups of NER/S (n = 74) or atorvastatin (n = 63) were included in the analysis. Baseline demographics and clinical characteristics of the two treatment groups were well matched. The mean age was 53.6 years, 43% of patients were male, and 15% of patients had diabetes mellitus.19 Baseline lipid/lipoprotein values were similar between the NER/S versus atorvastatin treatment groups, with mean LDL cholesterol levels of 162.4 mg/dL versus 168.0 mg/dL, mean HDL cholesterol levels 39.9 mg/dL versus 37.6 mg/dL, and median triglycerides 174.3 mg/dL versus 175.5 mg/dL.19
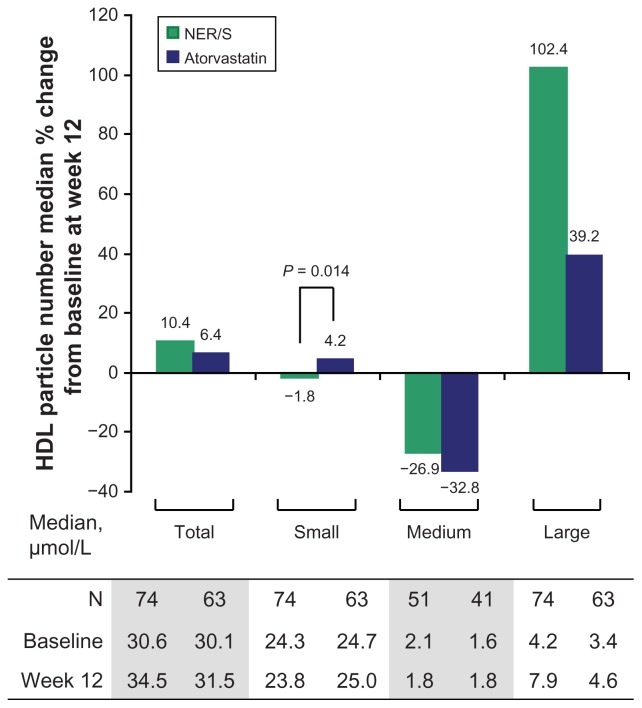
Median percent changes in HDL particle number from baseline following 12 weeks of treatment are shown in Figure 1. NER/S combination therapy resulted in a reduction in small HDL particle number compared with atorvastatin monotherapy, and the difference in median percent change was statistically significant between the two treatment groups (−1.8% versus 4.2%, P = 0.014). The median percent change in the number of large HDL particles was numerically greater for NER/S combination therapy compared with atorvastatin monotherapy, although the difference did not reach statistical significance (102.4% versus 39.2%, P = 0.078). In addition, NER/S treatment resulted in a significantly greater median (interquartile range Q1, Q3) percent increase in HDL particle size from baseline, 6.0% (2.7%, 8.7%) versus 1.3% (−0.5%, 3.0%) compared with atorvastatin (P < 0.001).
Figure 1.
Median percent change in high-density lipoprotein particle number from baseline after 12 weeks of treatment with NER/S combination therapy or atorvastatin monotherapy. P value is from the Wilcoxon rank-sum test and is shown for significant comparison between NER/S combination therapy and atorvastatin monotherapy. The interquartile ranges (Q1, Q3) for the percent changes shown are as follows. Total HDL particles (−1.4%, 24.4%) for NER/S and (−0.6%, 15.9%) for atorvastatin; small HDL particles (−16.8%, 8.8%) for NER/S and (−6.3%, 17.5%) for atorvastatin; medium HDL particles (−74.3%, 194.3%) for NER/S and (−75.7%, 117.8%) for atorvastatin; large HDL particles (32.2%, 176.9%) for NER/S and (4.2%, 167.7%) for atorvastatin.
Notes: For medium HDL particles, patients with a baseline value of 0 were excluded. Reference values for median HDL particle concentrations were total = 30.1 μmol/L, small = 20.8 μmol/L, medium = 1.1 μmol/L, and large = 6.0 μmol/L (Based on inhouse data from a random set of fasting patient plasma samples sent to LipoScience for NMR LipoProfile® analysis in November/December, 2003). The patients are mainly from the southeastern US and are a mix of primary prevention patients without coronary artery disease and secondary prevention patients with coronary artery disease. Age range 10–94 years, median age 58 years.
Abbreviations: HDL, high-density lipoprotein; NMR, nuclear magnetic resonance; NER/S, extended-release niacin-simvastatin; N, number of patients.
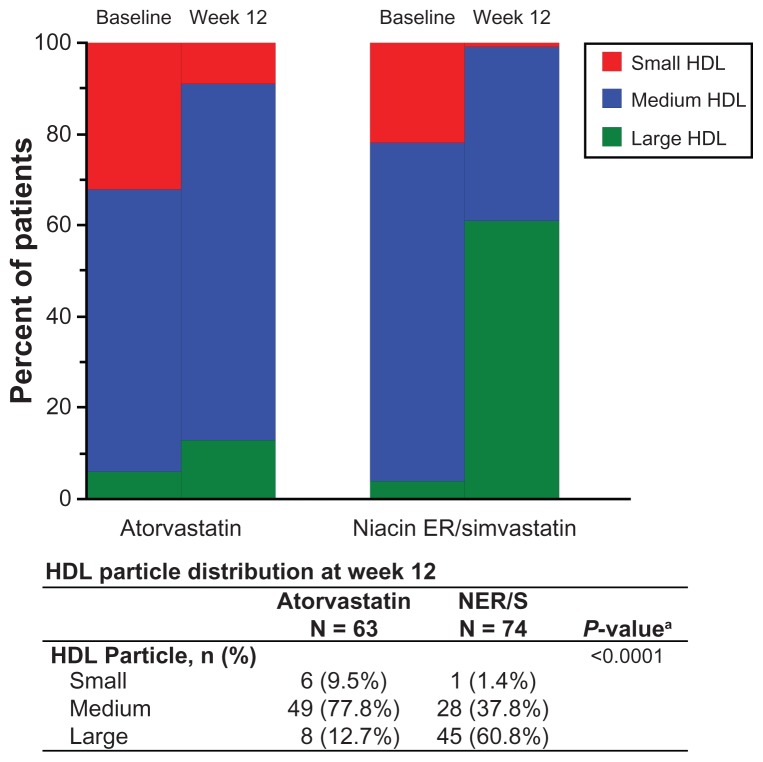
NER/S treatment resulted in a significant shift in HDL particle size from small and medium at baseline to large at week 12 (P < 0.0001, Figure 2). A higher proportion of patients with large HDL particles was observed at week 12 after combination NER/S treatment (60.8%) compared with atorvastatin monotherapy (12.7%). Similarly, a lower proportion of patients with small HDL particles after NER/S treatment (1.4%) was observed compared with atorvastatin monotherapy (9.5%) at week 12.
Figure 2.
Distribution of HDL particles in groups treated with NER/S (2000/40 mg/day) combination therapy and atorvastatin monotherapy (40 mg/day) at baseline and week 12. The difference in HDL particle size distribution at week 12 between NER/S and atorvastatin after adjusting for HDL particle size at baseline is highly significant (P < 0.0001). Size ranges of HDL particle subclasses were large (8.8–13 nm), medium (8.2–8.8 nm), and small (7.3–8.2). The P valuea is from the Cochran–Mantel–Haenszel test and is shown for significant comparison in HDL particle size distribution between NER/S combination therapy and atorvastatin monotherapy.
Abbreviations: HDL, high-density lipoprotein; NER/S, extended-release niacinsimvastatin.
Safety
The safety profiles of NER/S combination therapy and atorvastatin monotherapy were consistent with the established safety profiles of these medications as reported earlier for the overall population from the SUPREME study.21 In the NER/S group, 82% of patients experienced treatment-emergent adverse events versus 41% of patients in the atorvastatin monotherapy group (P < 0.001). The adverse event of flushing primarily accounted for the higher percentage of patients who experienced treatment-emergent adverse events in the NER/S group compared with the atorvastatin group (66.2% versus 11.1%, P < 0.001).19
Discussion
In this post hoc analysis of patients with dyslipidemia, NER/S treatment resulted in larger favorable changes in antiatherogenic HDL particles than atorvastatin monotherapy, as evidenced by a significantly greater decrease in small HDL particle number, a numerically greater increase in large HDL particle number, and a significantly greater increase in HDL particle size. NER/S treatment also resulted in a favorable shift in the distribution of HDL particles from small and medium to large. Along with previous results from the same study population, which showed that NER/S produced greater percent reductions in number of atherogenic LDL particles, very low-density lipoprotein (VLDL) and chylomicron particles, greater increases in particle size for LDL and VLDL, and a significant increase of apoprotein A-I levels by 7.2- fold compared with atorvastatin,19 these effects of NER/S demonstrated favorable overall changes in both atherogenic and antiatherogenic lipoprotein particles and apolipoproteins. Further insights into the effects of niacin extended-release on HDL particles should come with the results from an ongoing nuclear magnetic resonance substudy of AIM-HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL Cholesterol/High Triglyceride and Impact on Global Health Outcomes) patients.24
Lipoprotein heterogeneity in structure and particle subclass distribution profiles largely reflect changes in lipid metabolism and lipoprotein particle maturation reactions. In patients with atherogenic dyslipidemia and insulin resistance, there is excessive production and secretion of large triglyceride-rich VLDL1 particles by the liver.25 If this is accompanied by a lipoprotein lipase deficiency state (as occurs, for example, in patients with insulin resistance or loss of function mutations in the gene for lipoprotein lipase), cholesteryl ester transfer protein catalyzes the transfer of triglycerides out of VLDL in exchange for cholesteryl ester from LDL and HDL particles. As these latter particles become progressively more enriched with triglycerides, they become better substrates for lipolysis by hepatic lipase. Hepatic lipase activity produces increased numbers of small, dense LDL particles and promotes the catabolism and elimination of HDL particles. HDL catabolism results in lower serum levels of HDL cholesterol and HDL2 and a larger number of HDL3.26 In the setting of excess triglyceride loading, HDL3 particles do not mature and are highly prone to catabolism and elimination by the kidney.
A widely held view is that large HDL2 particles are atheroprotective.27 The role of HDL3 particles in preventing atherogenesis on the other hand is less clear.27,28 Some studies have reported that HDL3 is significantly associated with coronary heart disease, particularly in patients with metabolic syndrome because small HDL particles coincide with low HDL cholesterol levels and can be functionally compromised in patients with metabolic syndrome.4,16 Other studies associate the small HDL3 particle with atheroprotective functions, including an ability to mature into larger HDL2 and promote reverse cholesterol transport, and to antagonize and inhibit inflammation and oxidation within blood vessel walls.29–31 Discordance in these data may reflect the complex relationships between HDL subclasses, methods of fractionation, physiological versus pathological conditions (such as metabolic diseases or presence of coronary artery disease), or treatment effects with different lipid-modifying drugs. Nevertheless, niacin therapy results in a shift from small to large HDL particles, suggesting that niacin promotes more lipidation and maturation of HDL particles.32
Several cardiovascular outcomes and imaging studies with extended-release niacin in combination with a statin, such as HATS (HDL-Atherosclerosis Treatment Study) and ARBITER-6-HALTS (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis), have shown favorable effects on hard clinical outcomes or on surrogate endpoints (such as carotid intima media thickness) in patients at high risk and on statin therapy.33,34 However, the recent premature termination of the AIM-HIGH study due to futility raised the question about whether adding extended-release niacin is an effective treatment strategy for reducing the risk of cardiovascular events in patients with established cardiovascular disease and already very low LDL cholesterol (mean 71 mg/dL) and non-HDL cholesterol (mean 106 mg/dL) levels on statin or statin/ezetimibe therapy. Despite such a debate, use of NER/S in certain patient populations affords an opportunity for comprehensive management of all modifiable lipid risk factors in the prevention/treatment of cardiovascular disease. Advantages of such combination therapy may include its use in patients needing treatment for the atherogenic lipid triad in insulin resistance, type 2 diabetes, and in cardiovascular disease, in patients who need additional LDL cholesterol and/or non-HDL cholesterol reduction following implementation of the maximum dose of statins, and the use of lower doses of statins in patients who cannot tolerate higher doses because of adverse events (eg, myopathy).
In conclusion, treatment with NER/S resulted in a numerically greater increase in numbers of large HDL particles, and a significantly greater decrease in small HDL particles compared with atorvastatin monotherapy. In addition, NER/S treatment resulted in a significant change in HDL particle size distribution from small and medium to large. In patients with hyperlipidemia or dyslipidemia, NER/S was associated with significant improvements in multiple components of the lipid profile, including HDL cholesterol.
Footnotes
Disclosure
Rhea Parreno of Abbott provided assistance with the statistical analysis and Amy Y Xia provided medical writing assistance. These services were funded by Abbott. PPT is a consultant and member of the speaker’s bureau for Abbott. KMT, PJ, and RJP are employees and stockholders of Abbott. Abbott is the financial sponsor of the SUPREME clinical trial.
References
- 1.Vinik AI. The metabolic basis of atherogenic dyslipidemia. Clin Cornerstone. 2005;7(2–3):27–35. doi: 10.1016/s1098-3597(05)80065-1. [DOI] [PubMed] [Google Scholar]
- 2.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 3.Adiels M, Olofsson SO, Taskinen MR, Boren J. Overproduction of very low-density lipoproteins is the hallmark of the dyslipidemia in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2008;28(7):1225–1236. doi: 10.1161/ATVBAHA.107.160192. [DOI] [PubMed] [Google Scholar]
- 4.Kontush A, Chapman MJ. Antiatherogenic small, dense HDL – guardian angel of the arterial wall? Nat Clin Pract Cardiovasc Med. 2006;3(3):144–153. doi: 10.1038/ncpcardio0500. [DOI] [PubMed] [Google Scholar]
- 5.Brown SA, Hutchinson R, Morrisett J, et al. Plasma lipid, lipoprotein cholesterol, and apoprotein distributions in selected US communities. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb. 1993;13(8):1139–1158. doi: 10.1161/01.atv.13.8.1139. [DOI] [PubMed] [Google Scholar]
- 6.Castelli WP, Garrison RJ, Wilson PW, Abbott RD, Kalousdian S, Kannel WB. Incidence of coronary heart disease and lipoprotein cholesterol levels. The Framingham Study. JAMA. 1986;256(20):2835–2838. [PubMed] [Google Scholar]
- 7.Gotto AM, Jr, Brinton EA. Assessing low levels of high-density lipoprotein cholesterol as a risk factor in coronary heart disease: a working group report and update. J Am Coll Cardiol. 2004;43(5):717–724. doi: 10.1016/j.jacc.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 8.Rosenson RS, Brewer HB, Jr, Chapman MJ, et al. HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem. 2011;57(3):392–410. doi: 10.1373/clinchem.2010.155333. [DOI] [PubMed] [Google Scholar]
- 9.El Harchaoui K, van der Steeg WA, Stroes ES, et al. Value of low-density lipoprotein particle number and size as predictors of coronary artery disease in apparently healthy men and women: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007;49(5):547–553. doi: 10.1016/j.jacc.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 10.Toft-Petersen AP, Tilsted HH, Aaroe J, et al. Small dense LDL particles – a predictor of coronary artery disease evaluated by invasive and CT-based techniques: a case-control study. Lipids Health Dis. 2011;10:21. doi: 10.1186/1476-511X-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krauss RM. Is the size of low-density lipoprotein particles related to the risk of coronary heart disease? JAMA. 2002;287(6):712–713. doi: 10.1001/jama.287.6.712. [DOI] [PubMed] [Google Scholar]
- 12.Cromwell WC, Otvos JD, Keyes MJ, et al. LDL particle number and risk of future cardiovascular disease in the Framingham Offspring Study – implications for LDL management. J Clin Lipidol. 2007;1(6):583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otvos JD, Collins D, Freedman DS, et al. Low-density lipoprotein and high-density lipoprotein particle subclasses predict coronary events and are favorably changed by gemfibrozil therapy in the Veterans Affairs High-Density Lipoprotein Intervention Trial. Circulation. 2006;113(12):1556–1563. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 14.Arsenault BJ, Lemieux I, Despres JP, et al. HDL particle size and the risk of coronary heart disease in apparently healthy men and women: the EPIC-Norfolk prospective population study. Atherosclerosis. 2009;206(1):276–281. doi: 10.1016/j.atherosclerosis.2009.01.044. [DOI] [PubMed] [Google Scholar]
- 15.Freedman DS, Otvos JD, Jeyarajah EJ, Barboriak JJ, Anderson AJ, Walker JA. Relation of lipoprotein subclasses as measured by proton nuclear magnetic resonance spectroscopy to coronary artery disease. Arterioscler Thromb Vasc Biol. 1998;18(7):1046–1053. doi: 10.1161/01.atv.18.7.1046. [DOI] [PubMed] [Google Scholar]
- 16.Asztalos BF, Cupples LA, Demissie S, et al. High-density lipoprotein subpopulation profile and coronary heart disease prevalence in male participants of the Framingham Offspring Study. Arterioscler Thromb Vasc Biol. 2004;24(11):2181–2187. doi: 10.1161/01.ATV.0000146325.93749.a8. [DOI] [PubMed] [Google Scholar]
- 17.Lamarche B, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Despres JP. Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 1997;17(6):1098–1105. doi: 10.1161/01.atv.17.6.1098. [DOI] [PubMed] [Google Scholar]
- 18.Asztalos BF, Le Maulf F, Dallal GE, et al. Comparison of the effects of high doses of rosuvastatin versus atorvastatin on the subpopulations of high-density lipoproteins. Am J Cardiol. 2007;99(5):681–685. doi: 10.1016/j.amjcard.2006.09.117. [DOI] [PubMed] [Google Scholar]
- 19.Insull W, Jr, Toth PP, Superko HR, et al. Combination of niacin extended-release and simvastatin results in a less atherogenic lipid profile than atorvastatin monotherapy. Vasc Health Risk Manag. 2010;6:1065–1075. doi: 10.2147/VHRM.S14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berge KG, Canner PL. Coronary drug project: experience with niacin. Coronary Drug Project Research Group. Eur J Clin Pharmacol. 1991;(40 Suppl 1):S49–51. [PubMed] [Google Scholar]
- 21.Insull W, Jr, Basile JN, Vo AN, Jiang P, Thakkar R, Padley RJ. Efficacy and safety of combination therapy with niacin extended-release and simvastatin versus atorvastatin in patients with dyslipidemia: The SUPREME Study. J Clin Lipidol. 2009;3(2):109–118. doi: 10.1016/j.jacl.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Freedman DS, Otvos JD, Jeyarajah EJ, et al. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: the Framingham Study. Clin Chem. 2004;50(7):1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 23.Otvos JD. Measurement of lipoprotein subclass profiles by nuclear magnetic resonance spectroscopy. Clin Lab. 2002;48(3–4):171–180. [PubMed] [Google Scholar]
- 24.AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol: Rationale and study design. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: Impact on Global Health outcomes (AIM-HIGH) Am Heart J. 2011;161(3):471–477. e472. doi: 10.1016/j.ahj.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan CE, Foster L, Caslake MJ, et al. Relations between plasma lipids and postheparin plasma lipases and VLDL and LDL subfraction patterns in normolipemic men and women. Arterioscler Thromb Vasc Biol. 1995;15(11):1839–1848. doi: 10.1161/01.atv.15.11.1839. [DOI] [PubMed] [Google Scholar]
- 26.Kontush A, Chapman MJ. Functionally defective high-density lipoprotein: a new therapeutic target at the crossroads of dyslipidemia, inflammation, and atherosclerosis. Pharmacol Rev. 2006;58(3):342–374. doi: 10.1124/pr.58.3.1. [DOI] [PubMed] [Google Scholar]
- 27.Morgan J, Carey C, Lincoff A, Capuzzi D. High-density lipoprotein subfractions and risk of coronary artery disease. Curr Atheroscler Rep. 2004;6(5):359–365. doi: 10.1007/s11883-004-0047-0. [DOI] [PubMed] [Google Scholar]
- 28.Barter P, Kastelein J, Nunn A, Hobbs R. High density lipoproteins (HDLs) and atherosclerosis; the unanswered questions. Atherosclerosis. 2003;168(2):195–211. doi: 10.1016/s0021-9150(03)00006-6. [DOI] [PubMed] [Google Scholar]
- 29.Asztalos B, Zhang W, Roheim PS, Wong L. Role of free apolipoprotein A-I in cholesterol efflux. Formation of pre-alpha-migrating high-density lipoprotein particles. Arterioscler Thromb Vasc Biol. 1997;17(9):1630–1636. doi: 10.1161/01.atv.17.9.1630. [DOI] [PubMed] [Google Scholar]
- 30.Kontush A, Chantepie S, Chapman MJ. Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol. 2003;23(10):1881–1888. doi: 10.1161/01.ATV.0000091338.93223.E8. [DOI] [PubMed] [Google Scholar]
- 31.Ashby DT, Rye KA, Clay MA, Vadas MA, Gamble JR, Barter PJ. Factors influencing the ability of HDL to inhibit expression of vascular cell adhesion molecule-1 in endothelial cells. Arterioscler Thromb Vasc Biol. 1998;18(9):1450–1455. doi: 10.1161/01.atv.18.9.1450. [DOI] [PubMed] [Google Scholar]
- 32.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) trial. Am J Cardiol. 2002;90(2):89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 33.Villines TC, Stanek EJ, Devine PJ, et al. The ARBITER 6-HALTS Trial (Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol 6-HDL and LDL Treatment Strategies in Atherosclerosis): final results and the impact of medication adherence, dose, and treatment duration. J Am Coll Cardiol. 2010;55(24):2721–2726. doi: 10.1016/j.jacc.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583–1592. doi: 10.1056/NEJMoa011090. [DOI] [PubMed] [Google Scholar]