Abstract
Objective
To characterize the molecular pathways involved in apoptosis following administration of histone deacetylase inhibitors to Type I and II endometrial cancer cells.
Methods
Ark2, Ishikawa, and AN3 cell lines representing both Type I and II endometrial cancers were treated with various concentrations of oxamflatin and HDAC inhibitor-1. Cell apoptosis was determined by flow cytometry, nuclear staining, Western blotting, and mitochondrial membrane potential assays.
Results
Compared to controls, there was a 95% reduction in the growth of Ark2 cells following administration of histone deacetylase inhibitors and this response was dose-dependent. These agents also caused profound morphologic changes and loss of mitochondrial membrane potentials consistent with the induction of apoptosis. Cleavage of PARP, caspase-9, and caspase-8 was detected, confirming the activation of apoptotic cascades in endometrial carcinoma cells. This effect was present in both serous and endometrioid cell types.
Conclusion
Our results suggest that oxamflatin and HDAC inhibitor-1 have potent cytotoxicity in endometrial cancer cells by inducing cell apoptosis. Histone deacetylase inhibitors are promising agents for the treatment of both Type I and II endometrial carcinoma.
Keywords: Histone deacetylase, Endometrial cancer, Apoptosis
Introduction
Endometrial carcinomas are traditionally divided into two types based on their molecular and clinical characteristics [1]. Type I, or endometrioid carcinomas, represents the majority of cases and may be found in premenopausal women exposed to excess levels of estrogen. The most common molecular alterations found in this subtype include PTEN inactivation [2], and mutations of K-ras [3], beta-catenin [4], or hMLH1/MSH2 [5]. These tumors frequently develop in a background of adenomatous hyperplasia. These women are usually diagnosed with early stage disease and have a good prognosis. In contrast, Type II endometrial cancers, the majority of which are classified as serous, arise from atrophic endometrium in older women, are not hormonally dependent, and frequently possess p53 mutations [6], HER2/neu amplification, or display inactivation of p16 and e-cadherin [7]. The clinical course of patients with this histologic subtype is far worse than that observed with Type I cancers, even for the minority who are diagnosed with early stage disease. Chemotherapeutic regimens for patients with Type II cancers or those with advanced Type I endometrial carcinoma include the use of adriamycin and cisplatin [8]. Responses to these toxic regimens are usually partial with a median disease-free survival of less than 12 months for patients with advanced or recurrent disease [9,10].
Epigenetic alterations and the resultant silencing of tumor suppressor and DNA repair genes play an important role in cancer development [11]. In endometrial cancer, DNA hypermethylation and/or histone deacetylation mechanisms are directly involved in the silencing of hMLH1/MSH2, PTEN, and progesterone receptor (PR). hMLH1/MSH2 has been observed in atypical hyperplasia, a finding suggesting that epigenetic alterations may be an early event in carcinogenesis [12,13]. PTEN expression is associated with more aggressive tumors and poor outcomes [14]. The loss of PR expression may also contribute to the development of endometrial cancer as well as resistance to hormonal therapy [15,16]. It has been well recognized that modification of DNA methylation and/or histone modification codes can lead to reactivation of silenced genes. The reversible nature of epigenetic changes in cancer cells by inhibitory agents has been explored as a new avenue for cancer treatment. Histone deacetylase (HDAC) inhibitors were recently found to be well-tolerated in patients with hematologic and solid malignancies [17]. Several classes of HDAC inhibitors exist, and they display diverse effects on cellular functions. These effects include cell cycle arrest, initiation of differentiation, chromatin remodeling, inhibition of angiogenesis, and apoptosis induction [18–21]. Many of these effects were originally thought to be due to hyperacetylation of histones and activation of previously silenced genes. However, it appears that these agents cause hyperacetylation of a variety of proteins, the subject of recent studies [22,23]. It has been suggested that the tumor specificity of these agents is related to their ability to induce apoptosis [24]. Normal cells are sensitive to apoptotic signals such as DNA damage and DNA repair deficiency. Defects in apoptotic pathways are considered contributing factor in tumorigenesis and in the resistance of cancer cells to a variety of therapeutic agents. HDAC inhibitors may cause cells death by restoring the integrity of apoptotic pathways that have been blocked or suppressed in cancers. However, relatively few studies have investigated the apoptotic pathways that are activated by HDAC inhibitors in endometrial cancer, and many aspects of the HDAC effects in endometrial cancer cells remain unknown. Defining these mechanisms is particularly important given that defects in caspase activation and apoptosis have been linked to chemoresistance [25].
In this report we show that the HDAC inhibitors oxamflatin and HDAC inhibitor-1 (HDAC-I1) significantly inhibit the growth of endometrial cancer cells. Furthermore, these agents are found to induce apoptosis in both Type I and Type II endometrial carcinomas. The pathways by which apoptosis is induced is dependent on the particular drug and cell lines used. However, both the mitochondrial and death receptor pathways appear to be activated when oxamflatin is administered to serous endometrial cancer cells. This dual activation may account for the improved efficacy observed with administration of this agent.
Materials and methods
Cell lines and reagents
The human endometrial serous cancer Ark2 cell line was generously provided by Dr. Alessandro Santi (University of Arkansas). These cells were isolated from African American patients harboring advanced stage uterine serous papillary carcinoma (USPC). The well differentiated human endometrioid cancer Ishikawa cell line was generously provided by Dr. Masato Nishida (Kasumigaura National Hospital, Japan). The less well differentiated human endometrioid cancer AN3 was obtained from American Type Culture Collection (Rockville, MD). Ark2, Ishikawa, and AN3 cells were grown in RPMI 1640, MEMα, and F12 media, respectively. All the media were supplemented with 10% fetal calf serum (BioWhittaker, Walkersville, MD), 100 μg/ml streptomycin, 100 units/ml penicillin, and 2 mM glutamine. Cells were maintained at 37 °C in an atmosphere containing 5% CO2 and 100% humidity. Oxamflatin and HDAC inhibitor-1 are products of Calbiochem (San Diego, CA). Antibodies against poly ADP-ribose polymerase (PARP), Caspase-8, and caspase-9 were purchased from Roche (Basel, Switzerland). Rabbit polyclonal antibody for β-actin was purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Western blot analysis
Ark2, Ishikawa, and AN3 cells were treated with oxamflatin or HDAC Inhibitor-1 as indicated in the figure legends. Cellular proteins were isolated and resolved in SDS PAGE (5–10% gradient, Bio-Rad Laboratories, Hercules, CA) and electro-transferred to Immun-Blot™ PVDF membrane (Bio-Rad Laboratories). The membranes were blocked for 2 h in PBS buffer containing 0.1% Tween-20 and 10% nonfat dried milk. Antibodies against PARP, caspase-8, and caspase-9 were diluted following the manufacturer's recommendations. Primary antibody binding was performed at 4 °C overnight with constant shaking. The anti-rabbit or anti-mouse antibodies labeled with horseradish peroxidase (Amersham Corp, Arlington Heights, IL) were used at 1:5000 dilutions. Secondary antibody binding was carried out at room temperature for 1 h. Chemiluminescence detection was carried out with the ECL plus Western Blotting Detection System (Amersham Corp, Arlington Heights, IL). The blots were re-probed with β-actin antibody and the results provided loading controls.
Cell growth assay
Ark2, Ishikawa, and AN3 cells were plated at 20%confluence in 10-cmdishes one day earlier and counted as the base line level. The cells were treated with Oxamflatin (0.25 μM), HDAC-I1 (0.5 μM), or DMSO solvent as control. The cell numbers were counted thereafter once a day for 4 consecutive days. Floating cells were washed away and only the living cells were detached from dishes by trypsin digestion and counted. Growth curves were constructed for individual experimental groups. Average and standard error of each time point was calculated based on three or more parallel experiments.
Apoptosis assays
The Annexin V-FITC kit (BD Biosciences, San Diego, CA) was used to label apoptotic cells. Cells treated with oxamflatin and HDAC-I1 were washed with cold PBS and diluted in 1× Annexin binding buffer at a concentration of 1×106 cells/ml. 1×105 cells were mixed with 5 μl of Annexin V-FITC stock solution and the binding carried out at room temperature for 15 min in the dark. The samples were diluted to 400 μl and immediately analyzed by flow cytometry for apoptotic cells.
For nuclear staining, cells were washed with cold PBS and fixed with 4% paraformaldehyde, and stained for 5 min with Hoechst dye (2 μg/ml in 0.1% triton X-100). The stained cells were washed twice with 0.1% triton X-100, 1×PBS, and observed under a fluorescence microscope. Apoptotic cells with condensed or fragmented nuclei were counted. The results were presented as percentage of apoptotic cells in total population.
Mitochondrial membrane potential (MMP) assay
The changes in mitochondrial membrane potential were measured by flow cytometry using cell-permeable mitochondrial-sensitive dye MitoTracker red CMX (CMXRos, Molecular Probes, Eugene OR). 2×106 cells were washed twice with cold PBS, and stained in 1 ml of 25 nM CMXRos diluted in serum free medium. The staining was performed at 37 °C for 30 min. The cells were collected by centrifugation and washed three times, each with 2 ml cold PBS. The cells were resuspended in PBS and subject to flow cytometry (Becton Dickinson, San Jose, CA) measurement on FL3 (EM=599 nm). The data were analyzed by FACScan program and the results were presented as the percentage of cells with mitochondrial membrane permeability transition.
Data analysis
All data groups are analyzed by analysis of variance (ANOVA) to determine if there is significance (p<0.05) among the groups. For experimental groups that satisfied the initial ANOVA criterion, individual comparisons between each experimental group and control group are performed with the use of post hoc Bonferroni t tests, based on the assumption of two-tail distribution and two samples with equal variance. Statistical significance is indicated by asterisks in the figures.
Results
Oxamflatin and HDAC-I1 inhibit endometrial cancer cell growth
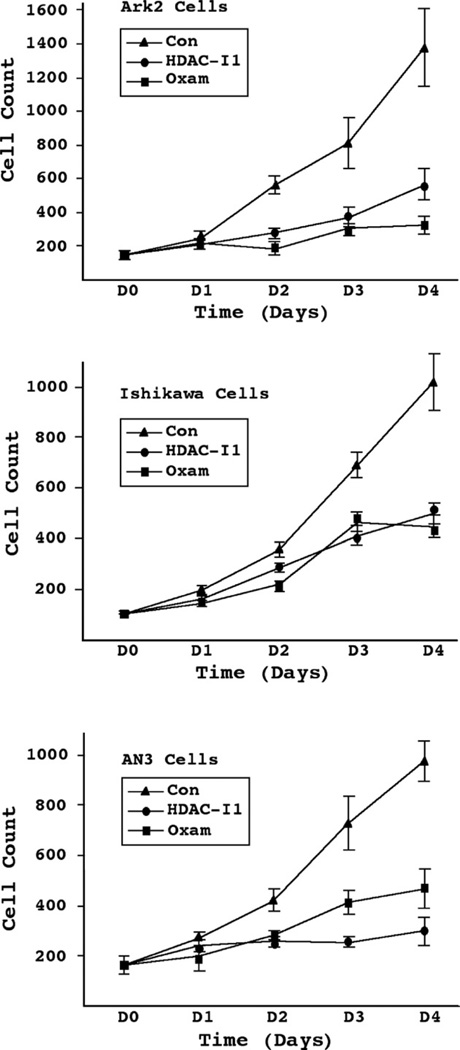
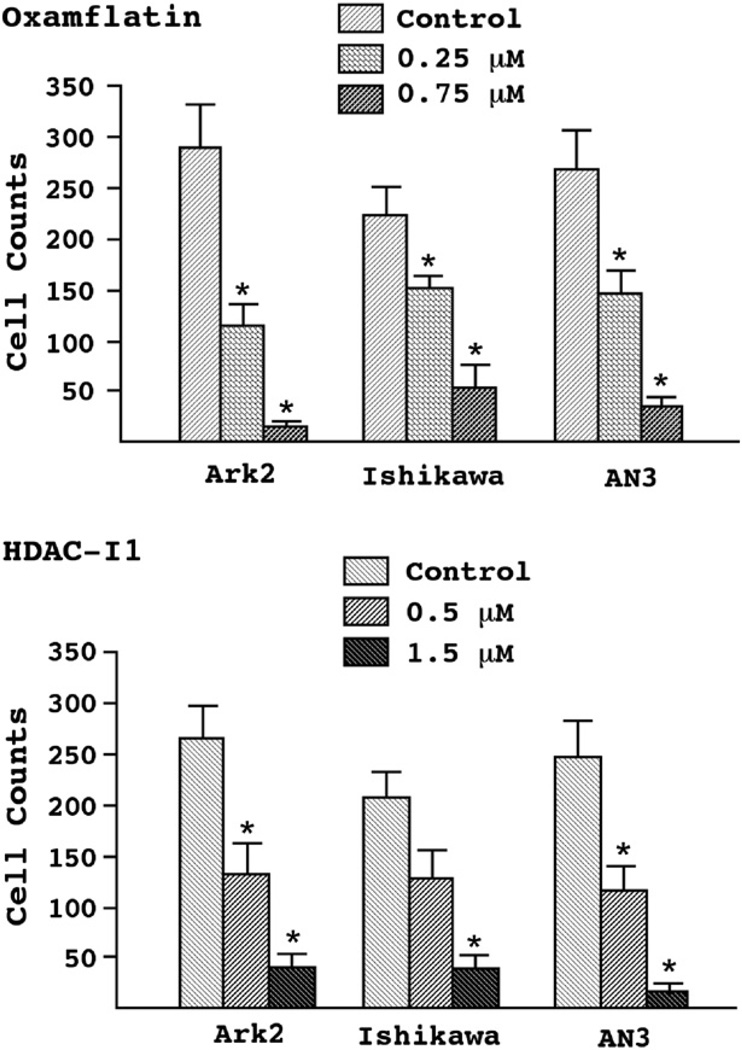
We began by examining the effects of HDAC inhibitors on the growth of both Type I and II endometrial cancer cells in vitro. Sub-micromolar concentrations of oxamflatin and HDAC-I1 exerted strong growth inhibition on the endometrioid carcinoma cell lines Ishikawa and AN3 (Fig. 1). This effect was particularly evident in the serous endometrial cancer cell line Ark2. Over the course of 4 days, there was a 78% and 60% reduction in Ark2 cell counts by oxamflatin and HDAC-I1 treatments, respectively, as compared to controls treated with DMSO solvent. Although oxamflatin was applied at half the concentration of HDAC-I1, this drug induced a significantly greater reduction in Ark2 cells proliferation than did HDAC-I1. This relationship was opposite to that seen in AN3 cells, while Ishikawa cells appeared to be equally sensitive to both reagents. Similar response patterns were observed in the dose–response studies (Fig. 2). Most striking observation is the 95% reduction in cell count following administration of 0.75 μM oxamflatin to Ark2 cells.
Figure 1.
Effects of HDAC inhibitors on endometrial cancer cell growth. Ark2, Ishikawa, and AN3 cells were treated with oxamflatin (Oxam, 0.25 μM), HDAC inhibitor-1 (HDAC-I1, 0.5 μM), or DMSO solvent as control (Con). Cell number was counted once a day following treatment. While both drugs significantly inhibited cell proliferation, the three cell lines exhibited different sensitivity profiles. Oxamflatin appears to be especially effective in Ark2 cells; Ishikawa cells are equally sensitivity to the two regents; AN3 cells showed stronger response to HDAC-I1 than to oxamflatin.
Figure 2.
Dose–response of endometrial cancer cells to HDAC inhibitors. Ark2, Ishikawa, and AN3 cells were treated with increasing concentrations of oxamflatin (upper panel, 0, 0.25, and 0.75 μM) and HDAC-I1 (lower panel, 0, 0.5, and 1.5 μM) for 3 days and survival cells were harvested and counted. The cell number was compared between experiment and control groups and statistical significance (p<0.05) was marked by asterisks on the top of standard error bars.
HDAC inhibitors induce apoptosis
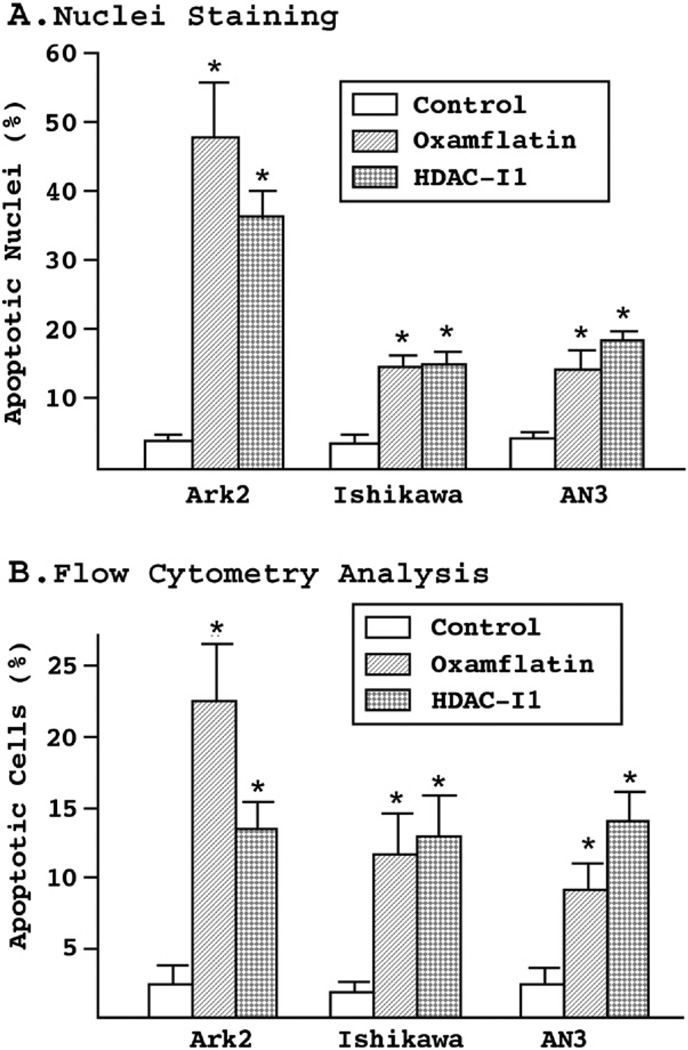
To determine if the cell death observed following administration of these inhibitors was due to apoptosis induction, Hoechst dye was used to detect nuclei condensation and fragmentation. As shown in Fig. 3A, the proportion of apoptotic nuclei increased up to 8-fold in Ark2 cells after treatment with oxamflatin. Smaller, but statistically significant increases on the order of three-to four-fold were observed in the endometrioid Ishikawa and AN3 cell lines. To confirm these effects, cells were analyzed using flow cytometry. Following treatment with either of the two reagents for 3 days, the cells were stained with biotin-labeled Annexin V, a phospholipid binding protein that specifically recognizes phosphatidylserine exposed on the cell surface, an early event in apoptosis (Fig. 3B). The results indicated that a significantly increased number of cells died following oxamflatin or HDAC-I1 treatment, confirming the potency of these reagents in activating cell death pathways. The relative proportions of cells undergoing apoptosis following oxamflatin and HDAC-I1 are consistent with the sensitivity profiles established by cell growth curves (Fig. 1).
Figure 3.
(A) Nuclei staining assay. The three endometrial cancer cell lines were treated with oxamflatin (0.5 μM) and HDAC-I1 (1.0 μM) for 3 days. Nuclei condensation and fragmentation were detected by Hoechst staining of genomic DNA. Depending on cell type and drug used, a three- to nine-fold increase in apoptotic nuclei was observed. (B) Flow cytometry analysis of cell apoptosis. Following three days of treatment with oxamflatin (0.25 μM) and HDAC-I1 (0.5 μM), the cells were stained with biotin-labeled Annexin V and apoptotic cells counted by flow cytometry. The results confirm significant cell death induced by oxamflatin and HDAC-I1 treatment. Similar drug sensitivity profiles to those observed in cell growth experiments (Fig. 1) were found in the nuclei staining and flow cytometry experiments. Statistical significance between experimental and control groups (p<0.05) is marked by asterisks on the top of standard error bars.
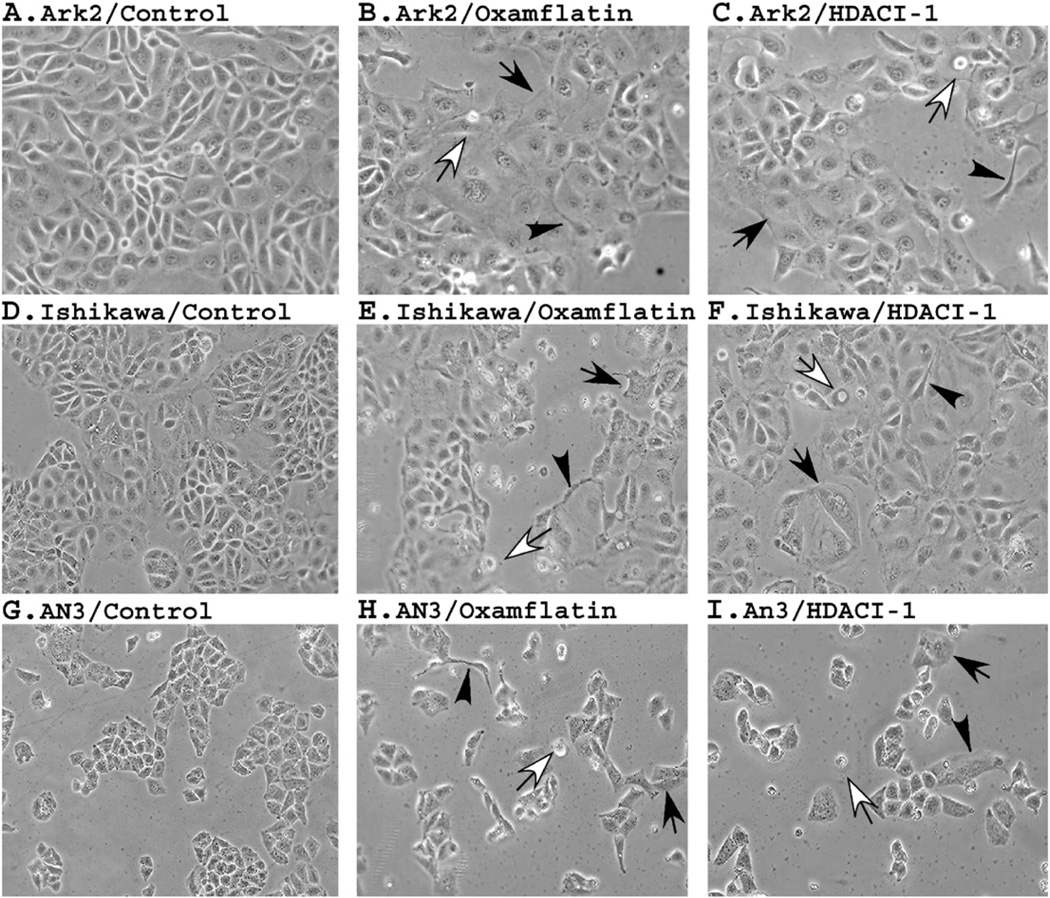
Morphologic changes associated with HDAC inhibitors
Profound morphologic changes are observed in cells treated by oxamflatin and HDAC-I1. As shown in Fig. 4, after 3 days of treatment many floating dead cells are seen in cultures treated with oxamflatin and HDAC-I1. Remaining viable cells became round and enlarged, while others formed digitiform processes. Visible vacuoles are found in an increased density in oxamflatin- or HDAC-I1-treated cells. Both reagents appear to induce similar changes in all three cell lines, suggesting similar mechanisms of action.
Figure 4.
Morphological changes induced by HDAC inhibitors. The three cell cultures were treated with oxamflatin (0.25 μM) and HDAC-I1 (0.5 μM) and pictures were taken after 3 days' treatment. Generally, upon treatment cells tend to become round and enlarged (indicated by solid arrows). Many dead cells can be seen floating in the medium (open arrows). Some treated cells form digitiform processes (indicated by arrowheads). The two reagents appear to induce similar morphological changes in given cell lines.
HDAC inhibitors activate the apoptotic cascade in endometrial cancer cells
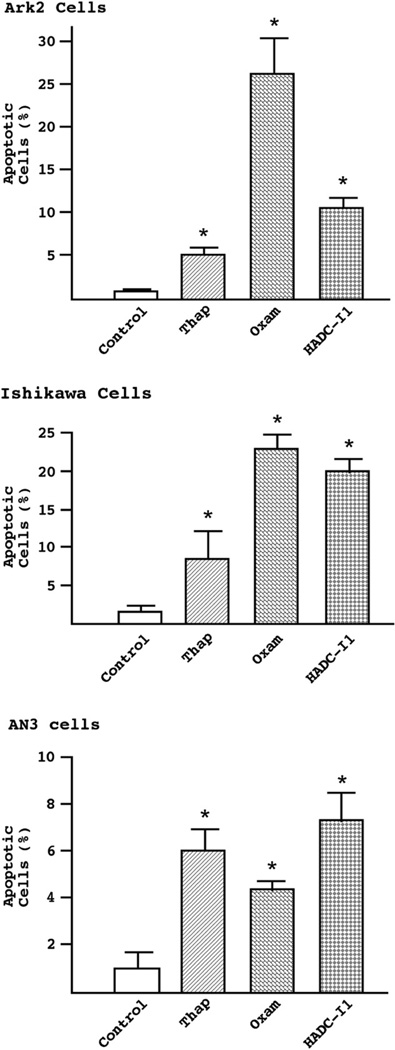
The mitochondrial respiratory chain produces energy which is stored as a transmembrane electrochemical gradient. This source of electrical energy is used to drive the biosynthesis of ATP, a crucial molecule for a variety of intracellular processes. Dissipation of the mitochondrial membrane potential is believed to be a key upstream event during apoptosis. We examined the effects of HDAC inhibitors on mitochondrial function by applying a membrane permeable lipophilic cationic dye that is retained by living cells (Fig. 5). Thapsigargin, an endoplasmid reticulum Ca2+-ATPase inhibitor known to cause mitochondria-dependent apoptosis, was used as a positive control. In AN3 cells, oxamflatin and HDAC-I1 were as effective at inducing apoptosis as the positive control. In Ishikawa cells, these agents induced apoptosis at approximately twice the efficiency as thapsigargin. As observed previously in Fig. 3, oxamflatin appears to be particularly effective for inducing apoptosis in Ark2 cells. Over 25% of Ark2 cells became apoptotic after oxamflatin administration as compared to 6% and 10% with thapsigargin and HDAC-I1, respectively.
Figure 5.
Loss of mitochondria membrane potentials. Cells were treated with oxamflatin (0.25 μM), HDAC-I1 (0.5 μM), or Thapsigargin (0.75 μM) as a positive control for 2 days. The loss of mitochondrial potential was detected with MitoTrack red (CMXRos). A significant increase (p<0.05, marked by asterisks) in the percentage of cells with dissipated mitochondria membrane potentials were observed following treatment with both HDAC inhibitors.
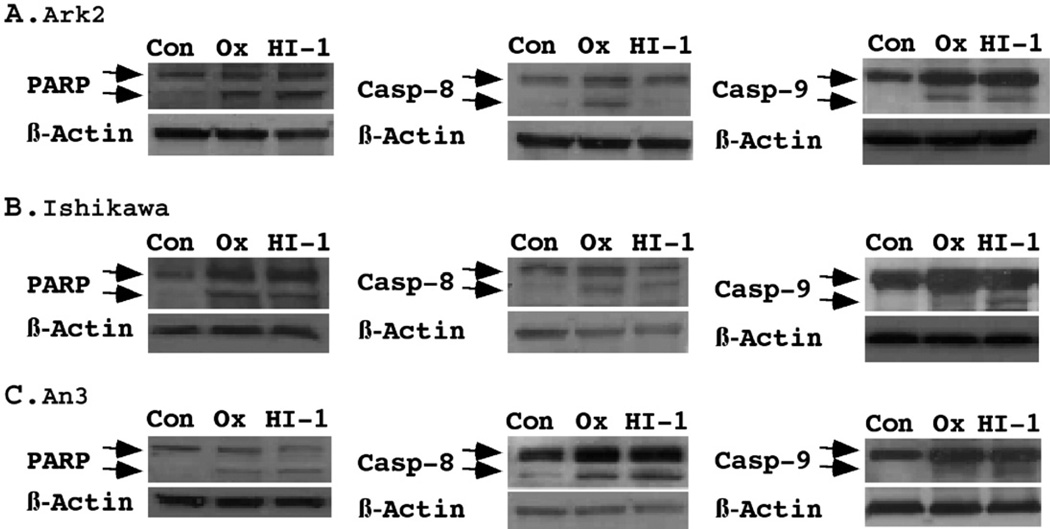
To further characterize the specific apoptotic pathways activated by these agents, we performed Western blot analysis on PARP cleavage, as well as capsase-8 and caspase-9 activation (Fig. 6). PARP cleavage was observed in all cell lines following treatment with either HDAC inhibitor, confirming the apoptotic effects of HDAC inhibitors. Caspase-9 activation has been known as an early event following mitochondria alterations. Cleavage of caspase-9 confirmed the involvement of intrinsic apoptotic pathway. Since cleavage of caspase-8 could be a downstream event of death receptor oligomerization, and/or caspase-3 activation, our results on cleavage of caspase-8 also raised the possibility for HDAC inhibitor-mediated activation of extrinsic pathway. The two different HADC inhibitors showed diverged activation pattern in Type I and II cell lines. In Ishikawa and AN3 cells, both caspase-8 and caspase-9 were activated by oxamflatin and HDAC-I1. In Ark2 cells, however, caspase-8 activation was observed with oxamflatin, but not HDAC-I1. Both agents appeared to be equally effective in activating caspase-9. The possible induction of both apoptotic pathways by oxamflatin may contribute to its increased efficacy in inhibiting the growth of serous endometrial cancer cells as compared to HDAC-I1 in Ark2 cells (Fig. 1).
Figure 6.
Western Blot analysis of apoptotic pathways. The cleavage of PARP, caspase-8, and caspase-9, was determined using specific antibodies as described under Materials and methods. The pro-apoptotic proteins and their cleaved products were indicated by arrows on the top and bottom, respectively. For each blot, β-actin levels were measured as a protein loading control. Oxamflatin (0.25 μM) and HDAC-I1 (0.5 μM) treatments resulted in significant activation of PARP in Ark2, Ishikawa and AN3 cells. Oxamflatin displayed preferential effects on caspase-9 cleavage in Ark2 in comparison to Ishikawa and AN3 cells. While oxamflatin induced caspase-8 cleavage in Ark2 cells, HDAC-I1 has little effect in this cell line.
Discussion
Recent interests in epigenetic modification reagents for cancer treatment have generated a wealth of information. It has been shown that HDAC inhibitors can induce apoptosis by several mechanisms in a variety of cancer cells. In an acute T-cell leukemia cell line, HDAC inhibitors induced mitochondrial membrane damage with concomitant cytochrome C release and apoptosis [26]. Caspase-2 activation, but not caspase-3 activation was required for this effect. Furthermore, HDAC inhibitor administration was shown to activate the proapoptotic protein, Bid, an upstream mediator of mitochondrial membrane disruption. These authors also showed that apoptosis could be abrogated by overexpression of antiapoptotic Bcl-2, known to be down-regulated by HDAC inhibitors [27]. A cowpox virus protein that inhibits caspase-8 and -10 was used to show that apoptosis in response to oxamflatin was mediated by the intrinsic pathway in a T-cell leukemia cell line. In contrast, other HDAC inhibitors such as apicidin have been shown to activate the death receptor pathway in leukemia cell lines [28]. Others have shown that administration of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), known to activate the death receptor pathway, potentiates the apoptotic response in combination with HDAC inhibitors [29].
Although far less data exist, we and others have also investigated the effects of these inhibitors and other epigenetic modification reagents on endometrial cancer cells [30,31]. Takai showed that the inhibitors suberoylanilide hydroxamic acid (SAHA), valproic acid, trichostatin-A (TSA), and sodium butyrate (NaB) induced apoptosis and decreased Bcl-2 protein expression in six endometrioid adenocarcinoma cell lines [32]. Terao demonstrated growth inhibition of both endometrial and ovarian cancer cell lines with NaB administration [33]. In this report we show that the HDAC inhibitors oxamflatin and HDAC-I1 profoundly inhibit the growth of endometrial cancer cells and results in morphologic changes consistent with apoptosis. Sensitivity to individual agents appears to be celltype- specific, with oxamflatin having a more significant growth inhibitory effect than HDAC-I1 in the Ark2 cell line, while the reverse is true in the AN3 cell line (Figs. 1–3). These effects increased dramatically with escalating doses of either agent. With respect to the specific apoptotic pathways involved, our data show that both caspase-8 and caspase-9 are activated by oxamflatin in the Ark2 cell line. Furthermore, loss of mitochondrial membrane potentials occurs after treatment. These results suggest that intrinsic pathway may play an important role in the induction of apoptosis by oxamflatin. These results differ from findings in leukemia cell lines in which only death receptor pathway was shown to be important. The reason for this discrepancy may be both cell line- and HDAC inhibitorspecific. For example, while HDAC-I1 activated caspase-8 in the endometrioid cell lines, this effect was not seen in Ark2 cells (Fig. 6).
For the first time, we show that HDAC inhibitors are efficacious for suppressing the growth of Type II endometrial cancers. This cell type displays distinct genetic aberrations and a uniquely aggressive phenotype. While representing only 5% of all cases, it accounts for 20% of deaths due to endometrial cancer [34]. The fact that nearly two thirds of patients diagnosed with serous endometrial cancer will ultimately die of the disease attests to the poor response rates of current chemotherapeutic agents. Given this information, HDAC inhibitors could potentially have an important impact on the treatment of the most aggressive subset of endometrial cancers. However, the effects of HDAC inhibitors on normal endometrial cells have not been examined and clinical trials are required to evaluate the in vivo toxicity and side effects of these agents.
Although p53 is one of the most commonly mutated genes in cancer, it is mutated in only 10% of Type I endometrial cancers [35]. In contrast, this is a common finding in serous endometrial cancers (65–70%), raising the possibility that this cell type would be more resistant to the pro-apoptotic effects of HDAC inhibitors [36,37]. Previous investigations have provided limited evidence to support this assertion, showing that the presence of intact p53 protein is essential for an efficient HDAC inhibitor-induced apoptotic response [38]. This dependence appears to vary with the agent used and may be due to differences in potency. Furthermore, acetylation of p53 occurs following HDAC inhibitor administration and may increase its activity and reduce targeting of p53 for degradation [22,39,40]. However, others have shown HDAC inhibitors to have apoptotic effects independent from p53 [41]. More experiments are required to define the expression, mutation, and role of p53 in HDAC inhibitor-mediated apoptosis of Ark2 cells.
In conclusion, we show that HDAC inhibitors effectively induce death receptor and mitochondria-mediated apoptotic pathways in endometrial cancer cells. This results in growth inhibition of both endometrioid and serous endometrial carcinomas. Serous endometrial carcinomas represent a major cause of endometrial cancer-related death. The use of these inhibitors may result in significant improvements in treatment given the recalcitrant nature of this cell type to current chemotherapeutic regimens.
Acknowledgments
The authors thank Ying Zhao for her technical assistance. This work was supported by NIH grant R01 HD41577 (S.-W. Jiang), University of Texas M.D. Anderson Cancer Center Uterine Cancer SPORE Development Award (S.-W. Jiang, S.C. Dowdy, and K.C. Podratz), and Mayo Clinic and Foundation Eagles Funds for Cancer Research (S.-W. Jiang and K.C. Podratz).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lax SF. Molecular genetic pathways in various types of endometrial carcinoma: from a phenotypical to a molecular-based classification. Virchows Arch. 2004 Mar;444(3):213–223. doi: 10.1007/s00428-003-0947-3. [DOI] [PubMed] [Google Scholar]
- 2.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JP, Lees JA, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J Natl Cancer Inst. 2000 Jun 7;92(11):924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 3.Caduff RF, Johnston CM, Frank TS. Mutations of the Ki-ras oncogene in carcinoma of the endometrium. Am J Pathol. 1995 Jan;146(1):182–188. [PMC free article] [PubMed] [Google Scholar]
- 4.Saegusa M, Hashimura M, Yoshida T, Okayasu I. beta-Catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer. 2001 Jan;84(2):209–217. doi: 10.1054/bjoc.2000.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacDonald ND, Salvesen HB, Ryan A, Iversen OE, Akslen LA, Jacobs IJ. Frequency and prognostic impact of microsatellite instability in a large population-based study of endometrial carcinomas. Cancer Res. 2000 Mar 15;60(6):1750–1752. [PubMed] [Google Scholar]
- 6.Tashiro H, Isacson C, Levine R, Kurman RJ, Cho KR, Hedrick L. p53 gene mutations are common in uterine serous carcinoma and occur early in their pathogenesis. Am J Pathol. 1997 Jan;150(1):177–185. [PMC free article] [PubMed] [Google Scholar]
- 7.Rolitsky CD, Theil KS, McGaughy VR, Copeland LJ, Niemann TH. HER-2/neu amplification and overexpression in endometrial carcinoma. Int J Gynecol Pathol. 1999 Apr;18(2):138–143. doi: 10.1097/00004347-199904000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Barrett RJ, Blessing JA, Homesley HD, Twiggs L, Webster KD. Circadian-timed combination doxorubicin-cisplatin chemotherapy for advanced endometrial carcinoma. A phase II study of the Gynecologic Oncology Group. Am J Clin Oncol. 1993 Dec;16(6):494–496. doi: 10.1097/00000421-199312000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Burke TW, Heller PB, Woodward JE, Davidson SA, Hoskins WJ, Park RC. Treatment failure in endometrial carcinoma. Obstet Gynecol. 1990 Jan;75(1):96–101. [PubMed] [Google Scholar]
- 10.Pliskow S, Penalver M, Averette HE. Stage III and stage IV endometrial carcinoma: a review of 41 cases. Gynecol Oncol. 1990 Aug;38(2):210–215. doi: 10.1016/0090-8258(90)90043-k. [DOI] [PubMed] [Google Scholar]
- 11.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev, Genet. 2002 Jun;3(6):415–428. doi: 10.1038/nrg816. [DOI] [PubMed] [Google Scholar]
- 12.Esteller M, Catasus L, Matias-Guiu X, Mutter GL, Prat J, Baylin SB, et al. hMLH1 promoter hypermethylation is an early event in human endometrial tumorigenesis. Am J Pathol. 1999 Nov;155(5):1767–1772. doi: 10.1016/S0002-9440(10)65492-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horowitz N, Pinto K, Mutch DG, Herzog TJ, Rader JS, Gibb R, et al. Microsatellite instability, MLH1 promoter methylation, and loss of mismatch repair in endometrial cancer and concomitant atypical hyperplasia. Gynecol Oncol. 2002 Jul;86(1):62–68. doi: 10.1006/gyno.2002.6724. [DOI] [PubMed] [Google Scholar]
- 14.Baak JP, Van Diermen B, Steinbakk A, Janssen E, Skaland I, Mutter GL, et al. Lack of PTEN expression in endometrial intraepithelial neoplasia is correlated with cancer progression. Hum Pathol. 2005 May;36(5):555–561. doi: 10.1016/j.humpath.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi H, Fujimoto J, Hong BL, Nakagawa Y, Tamaya T. Drastic decrease of progesterone receptor form B but not A mRNA reflects poor patient prognosis in endometrial cancers. Gynecol Oncol. 2004 May;93(2):394–399. doi: 10.1016/j.ygyno.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Podratz KC. Hormonal therapy in endometrial carcinoma. Recent Results Cancer Res. 1990;118:242–251. doi: 10.1007/978-3-642-83816-3_23. [DOI] [PubMed] [Google Scholar]
- 17.Kelly WK, O’Connor OA, Krug LM, Chiao JH, Heaney M, Curley T, et al. Phase I study of an oral histone deacetylase inhibitor, suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol. 2005 Jun 10;23(17):3923–3931. doi: 10.1200/JCO.2005.14.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Saunthararajah Y, Redner RL, Liu JM. Inhibitors of histone deacetylase relieve ETO-mediated repression and induce differentiation of AML1-ETO leukemia cells. Cancer Res. 1999 Jun 15;59(12):2766–2769. [PubMed] [Google Scholar]
- 19.Marks P, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev, Cancer. 2001 Dec;1(3):194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 20.Johnstone RW. Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev, Drug Discov. 2002 Apr;1(4):287–299. doi: 10.1038/nrd772. [DOI] [PubMed] [Google Scholar]
- 21.Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res. 2000 Sep 15;60(18):5165–5170. [PubMed] [Google Scholar]
- 22.Juan LJ, Shia WJ, Chen MH, Yang WM, Seto E, Lin YS, et al. Histone deacetylases specifically down-regulate p53-dependent gene activation. J Biol Chem. 2000 Jul 7;275(27):20436–20443. doi: 10.1074/jbc.M000202200. [DOI] [PubMed] [Google Scholar]
- 23.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998 Dec 10;396(6711):594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 24.Lowe SW, Cepero E, Evan G. Intrinsic tumour suppression. Nature. 2004 Nov 18;432(7015):307–315. doi: 10.1038/nature03098. [DOI] [PubMed] [Google Scholar]
- 25.Soengas MS, Capodieci P, Polsky D, Mora J, Esteller M, Opitz-Araya X, et al. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature. 2001 Jan 11;409(6817):207–211. doi: 10.1038/35051606. [DOI] [PubMed] [Google Scholar]
- 26.Peart MJ, Tainton KM, Ruefli AA, Dear AE, Sedelies KA, O’Reilly LA, et al. Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res. 2003 Aug 1;63(15):4460–4471. [PubMed] [Google Scholar]
- 27.Duan H, Heckman CA, Boxer LM. Histone deacetylase inhibitors down-regulate bcl-2 expression and induce apoptosis in t(14;18) lymphomas. Mol Cell Biol. 2005 Mar;25(5):1608–1619. doi: 10.1128/MCB.25.5.1608-1619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon SH, Ahn SH, Kim YK, Bae GU, Yoon JW, Hong S, et al. Apicidin, a histone deacetylase inhibitor, induces apoptosis and Fas/Fas ligand expression in human acute promyelocytic leukemia cells. J Biol Chem. 2002 Jan 18;277(3):2073–2080. doi: 10.1074/jbc.M106699200. [DOI] [PubMed] [Google Scholar]
- 29.Shankar S, Singh TR, Fandy TE, Luetrakul T, Ross DD, Srivastava RK. Interactive effects of histone deacetylase inhibitors and TRAIL on apoptosis in human leukemia cells: involvement of both death receptor and mitochondrial pathways. Int J Mol Med. 2005 Dec;16(6):1125–1138. [PubMed] [Google Scholar]
- 30.Xiong Y, Dowdy SC, Podratz KC, Jin F, Attewell JR, Eberhardt NL, et al. Histone deacetylase inhibitors decrease DNA methyltransferase-3B messenger RNA stability and down-regulate de novo DNA methyltransferase activity in human endometrial cells. Cancer Res. 2005 Apr 1;65(7):2684–2689. doi: 10.1158/0008-5472.CAN-04-2843. [DOI] [PubMed] [Google Scholar]
- 31.Xiong Y, Dowdy SC, Xue A, Shujuan J, Eberhardt NL, Podratz KC, et al. Opposite alterations of DNA methyltransferase gene expression in endometrioid and serous endometrial cancers. Gynecol Oncol. 2005 Mar;96(3):601–609. doi: 10.1016/j.ygyno.2004.11.047. [DOI] [PubMed] [Google Scholar]
- 32.Takai N, Desmond JC, Kumagai T, Gui D, Said JW, Whittaker S, et al. Histone deacetylase inhibitors have a profound antigrowth activity in endometrial cancer cells. Clin Cancer Res. 2004 Feb 1;10(3):1141–1149. doi: 10.1158/1078-0432.ccr-03-0100. [DOI] [PubMed] [Google Scholar]
- 33.Terao Y, Nishida J, Horiuchi S, Rong F, Ueoka Y, Matsuda T, et al. Sodium butyrate induces growth arrest and senescence-like phenotypes in gynecologic cancer cells. Int J Cancer. 2001 Oct 15;94(2):257–267. doi: 10.1002/ijc.1448. [DOI] [PubMed] [Google Scholar]
- 34.Podratz KC, Mariani A. Uterine papillary serous carcinomas: the exigency for clinical trials. Gynecol Oncol. 2003 Dec;91(3):461–462. doi: 10.1016/j.ygyno.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 35.Hamel NW, Sebo TJ, Wilson TO, Keeney GL, Roche PC, Suman VJ, et al. Prognostic value of p53 and proliferating cell nuclear antigen expression in endometrial carcinoma. Gynecol Oncol. 1996 Aug;62(2):192–198. doi: 10.1006/gyno.1996.0214. [DOI] [PubMed] [Google Scholar]
- 36.Miller B, Umpierre S, Tornos C, Burke T. Histologic characterization of uterine papillary serous adenocarcinoma. Gynecol Oncol. 1995 Mar;56(3):425–429. doi: 10.1006/gyno.1995.1075. [DOI] [PubMed] [Google Scholar]
- 37.Zheng W, Cao P, Zheng M, Kramer EE, Godwin TA. p53 overexpression and bcl-2 persistence in endometrial carcinoma: comparison of papillary serous and endometrioid subtypes. Gynecol Oncol. 1996 May;61(2):167–174. doi: 10.1006/gyno.1996.0120. [DOI] [PubMed] [Google Scholar]
- 38.Henderson C, Mizzau M, Paroni G, Maestro R, Schneider C, Brancolini C. Role of caspases, Bid, and p53 in the apoptotic response triggered by histone deacetylase inhibitors trichostatin-A (TSA) and suberoylanilide hydroxamic acid (SAHA) J Biol Chem. 2003 Apr 4;278(14):12579–12589. doi: 10.1074/jbc.M213093200. [DOI] [PubMed] [Google Scholar]
- 39.Kim MS, Kwon HJ, Lee YM, Baek JH, Jang JE, Lee SW, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001 Apr;7(4):437–443. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 40.Ito A, Kawaguchi Y, Lai CH, Kovacs JJ, Higashimoto Y, Appella E, et al. MDM2-HDAC1-mediated deacetylation of p53 is required for its degradation. Embo J. 2002 Nov 15;21(22):6236–6245. doi: 10.1093/emboj/cdf616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Tan J, Zhuang L, Jiang X, Liu ET, Yu Q. Inhibitors of histone deacetylases target the Rb-E2F1 pathway for apoptosis induction through activation of proapoptotic protein Bim. Proc Natl Acad Sci U S A. 2005 Nov 4;102(44):16090–16095. doi: 10.1073/pnas.0505585102. [DOI] [PMC free article] [PubMed] [Google Scholar]