Abstract
Purpose of review
To understand mechanisms of muscle wasting and how inhibiting myostatin signaling affects them.
Recent findings
Myostatin signaling is critical for understanding the pathogenesis of muscle wasting since blocking it mitigates muscle losses in rodent models of catabolic diseases including cancer, chronic kidney or heart failure.
Summary
Muscle wasting increases the risks of morbidity and mortality. But, the reliability of estimates of the degree of muscle wasting is controversial as are definitions of terms like cachexia. Much has been learned about the pathophysiology of muscle wasting, including the major role of the ubiquitin-proteasome system (UPS) which along with other proteases degrades protein and limits protein synthesis. In contrast, few successful strategies for reversing muscle loss have been tested.
Several catabolic conditions are characterized by inflammation, increased glucocorticoid production and impaired intracellular signaling in response to insulin and IGF-1. These characteristics lead to activation of the UPS and other proteases producing muscle wasting. Another potential initiator of muscle wasting is myostatin and its expression is increased in muscles of animal models and patients with certain catabolic conditions. Myostatin is a member of the TGF-β family; it suppresses muscle growth and its absence stimulates muscle growth substantially. Recently, pharmacologic suppression of myostatin was found to counteract inflammation, increased glucocorticoids and impaired insulin/IGF-1 signaling and most importantly, prevents muscle wasting in rodent models of cancer and kidney failure. Myostatin antagonism as a therapy for patients with muscle wasting should become a topic of clinical investigation.
Keywords: Myostatin, activin A, ActRIIB, Smad, Foxo, muscle wasting, cancer, chronic kidney disease, heart failure, ubiquitin-proteasome system, muscle protein breakdown, protein degradation
Introduction
Epidemiologic and clinical reports document that muscle wasting increases the risk of mortality/morbidity in elderly patients as well as those with cancer, heart failure or kidney disease [1-4]. This association is believed to occur for two reasons: first, muscle loss leads to inactivity which by itself causes loss of muscle proteins. Secondly, a decrease in muscle mass reflects losses of proteins that regulate cellular metabolism and renewal. But a major problem in assigning cause-effect relationships for this problem is the confusion surrounding definitions of muscle wasting plus difficulties encountered in measuring protein stores reliably plus. For example, the International Society of Renal Nutrition and Metabolism proposed that low values of serum albumin, prealbumin and cholesterol and abnormalities in body weight and anthropometry identifies patients with chronic kidney disease (CKD) who have low protein stores. They suggested a new term, protein-energy wasting or PEW, be used to classify such patients [5]. This was not an idle exercise: a new term was needed because CKD patients are frequently categorized as being malnourished but this diagnosis is incorrect: malnutrition is defined as abnormalities due to an inadequate or unbalanced diet and hence, the abnormalities should be corrected by simply changing the diet [6]. But, dietary changes rarely accomplish so much. Other popular diagnoses were excluded for different reasons: the term sarcopenia was discarded because it generally describes the loss of muscle due to aging. Cachexia was discarded because it implies a more severe state of protein depletion: a separate consensus conference concluded that cachexia should be reserved to describe a complex metabolic syndrome due to illnesses causing loss of muscle with or without loss of fat mass [7]. They proposed that a 5% loss of edema-free body weight within 12 months plus anthropometric evidence of muscle wasting and evidence of inflammation and hypoalbuminemia can be used to diagnose cachexia. Obviously, there are elements of imprecision and confusion surrounding the different diagnoses of protein wasting. Another problem is the diagnostic difficulties in documenting changes in protein stores. One approach relies on measuring muscle mass by dual energy xray absorptiometry (DEXA) or magnetic resonance imaging (MRI) but distinguishing between tissue and fluid can be difficult with DEXA or bioelectrical impedance measurements while MRI assessments are expensive. Alternative methods would be to seek “signs” of protein wasting such as high levels of the 14 kDa actin fragment or myostatin in muscle biopsies (see below). More experience with these measurements is needed before they can be recommended.
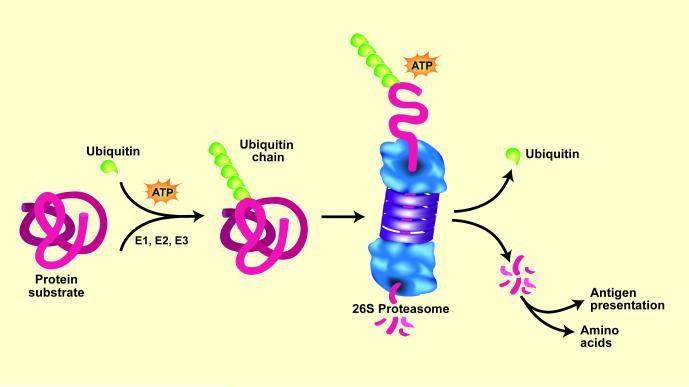
On a more positive note, a great deal has been learned about the pathophysiology of muscle wasting. In all organs, intracellular proteins are continually being degraded and replaced by new synthesis. These processes must be highly selective to avoid terminating the protein’s actions since this could interfere with critical cellular functions. For example, precise timing of the removal of proteins regulating transcription or metabolic pathways is required to avoid jeopardizing the organism’s survival. Thus, it is surprising that most intracellular proteins are degraded by the same ATP-dependent, ubiquitin-proteasome system (UPS) but it is now known that UPS-mediated degradation of specific proteins is regulated at several steps. First, proteins are marked by a conjugation to ubiquitin (Ub) via an ATP-dependent process requiring three enzymatic reactions (Figure 1) [8]. The first two reactions are catalyzed by a single, E1 Ub-activating enzyme and one of about 20 E2 Ub-carrier proteins. The third reaction is catalyzed by one of more than 1000 E3 ubiquitin ligases which can identify proteins to be degraded and then transfer the activated Ub to a lysine in the substrate protein (or to lysines present in Ub). These reactions form a Ub chain and the Ub-marked proteins are recognized and degraded by the very large complex of proteins known as the 26S proteasome [9*,10].
Figure 1.
The ubiquitin-proteasome system (UPS) of protein degradation. Proteins destined for degradation are conjugated to Ub by an ATP-dependent process that involves three enzymes: E1, E2, and E3. Selectivity of the protein substrates for degradation principally depends on recognition of the protein by specific E3 ligases. When a chain of five Ub proteins is attached to the protein substrate, the complex can be recognized by the 26S proteasome. This leads to release of the Ub chain, which is cleaved to individual Ubs, and unfolding of the protein substrate for injection into the proteasome and digestion to peptides. Peptidases in the cytoplasm degrade peptides into amino acids. In catabolic states, a similar process degrades muscle proteins. (Ub = ubiquitin). From Lecker, SH, Goldberg, AL and Mitch, WE; reproduced with permission from Journal of the American Society of Nephrology volume 17, pages 1807-1819, 2006.
In muscle, the UPS is the major protease that degrades muscle proteins. It is activated when intracellular insulin/IGF-1 signaling is impaired leading to low levels of phosphorylated-Akt (p-Akt) [11,12]. The activation of the UPS generally requires glucocorticoids because they mediate a non-genomic pathway that stimulates muscle protein degradation [13]. The UPS is also activated by inflammation, angiotensin II or cancer and in each case, the mechanism involves depression of insulin and IGF-1 intracellular signaling [13-18].
Muscle wasting in catabolic conditions depends on increased expression of specific two E3 ligases, Atrogin-1 (also called MAFbx) and MuRF1 (for muscle ring finger type) because they recognize specific muscle proteins [19-21]. Not surprisingly, therefore, there is an increase in Atrogin-1/MAFbx and MuRF1 expression in many catabolic conditions including cancer, diabetes, chronic kidney disease, starvation and denervation [22,23]. Consequently, increased expression of these E3 ligases is often considered synonymous with activation of the UPS.
Other proteases involved in muscle protein degradation include caspase-3. It exerts two functions. First, it catalyzes an initial cleavage of the complex structure of muscle proteins to provide substrates for the UPS. Second, activated caspase-3 cleaves specific subunits of the proteasome which increases its proteolytic activity. This response provides a “feed-forward” augmentation of muscle protein degradation. Notably, there is evidence for such responses including activation of the UPS and caspase-3 in humans with catabolic conditions, including kidney failure or cancer [24,25].
Another factor influencing the loss of muscle protein is impaired activation and proliferation of muscle progenitor or satellite cells. These cells respond to muscle injury and participate in its repair and are involved in the maintenance of muscle protein stores. There is evidence that satellite cell function is impaired by CKD and cancer [26,27]. These reports document major hormonal and metabolic responses in catabolic conditions that activate muscle wasting. They also raise the question: can such a variety of abnormalities be blocked or suppressed by a single treatment strategy? Recent reports indicate a positive response: experimentally, inhibition of myostatin signaling blocks muscle protein losses and interferes with inflammatory processes while improving intracellular insulin/IGF-1 signaling.
Therapeutic rationale for targeting myostatin pathway
Myostatin Signaling
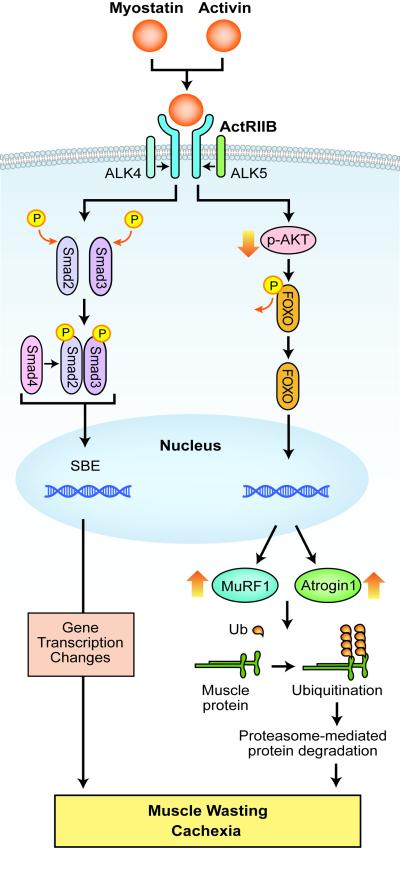
Myostatin is a member of the TGF-β family of secreted proteins but unlike TGF-β, it is predominantly expressed in skeletal muscle (cardiac muscle and adipose tissue have low levels of myostatin). In skeletal muscle, myostatin is produced as a prepromyostatin and is processed to promyostatin, consisting of a propeptide and myostatin. The propeptide binds to myostatin to produce an inactive, “latent complex”. This complex is activated by proteolysis or by the action of free radicals or a decrease in pH yielding “free” myostatin which binds to ActRIIB, the high-affinity type-2 activin receptor, on muscle membranes [28]. Myostatin-ActRIIB binding results in the activation of type-1 activin receptor serine kinases, ALK4 or ALK5, which phosphorylate Smads 2/3 to exert changes in gene transcription (Figure 2). Notably, other TGF-β family members (e.g., activin A and GDF11) can bind to ActRIIB and stimulate the same intracellular signaling pathway.
Figure 2.
Myostatin and activin signaling in muscle. Myostatin or activin binds to type IIB activin receptor (ActRIIB) on muscle membrane to cause its dimerization, which leads to recruitment and activation of type I activin receptor transmembrane kinase ALK4 or ALK5. This in turn causes phosphorylation of Smad2 and Smad3 and the recruitment of Smad4 into a Smad complex. The Smad complex translocates into the nucleus to elicit transcription changes of downstream genes, which result in muscle wasting. Myostatin/activin binding to the receptor also reduces AKT activity and consequently diminishes FOXO phosphorylation. Dephosphrylated FOXO enters the nucleus to activate transcription of atrophy-specific E3 ligases MuRF1 and Atrogin1, which cause muscle protein uniquitination and degradation by the proteasome. (SBE = S mad binding element; Ub = Ubiquitin).
Myostatin genetics
Myostatin plays a pivotal role in regulating skeletal muscle mass and function. Genetic evidence demonstrates this conclusively since deletion or knockout of the myostatin gene produces mice with a phenotype exhibiting a dramatic increase in the size and number of skeletal muscle fibers [29]. Transgenic mice overexpressing a dominant negative myostatin receptor, a propeptide or other proteins that sequester myostatin yield phenotyes with a dramatic increase in muscle mass [30]. Besides mouse models, cattle, sheep, dogs and a human with a loss-of-function myostatin mutation exhibit enormous muscles [31-34]. Mosher et al (2007) quantitatively linked such myostatin mutations to athletic performance: in competitive racing events, whippet dogs bearing a single copy of the myostatin mutation are among the fastest. But, whippets with two copies of the same mutation are overmuscled and win no races Myostatin polymorphism in elite thoroughbred horses also reveal a strong association with racing outcomes in [35,36]. Clearly, myostatin deficiency can produce muscle hypertrophy and improved physical performance.
Myostatin pathway activation in catabolic states
Myostatin protein and the myostatin/activin signaling pathway are upregulated in disease states causing muscle wasting. Increased levels of myostatin in muscle are found in aging subjects [37] or in response to prolonged bed rest [38,39]. Myostatin is also increased in muscles of patients with AIDS [40], renal failure [41] or heart failure [42, 43*, 44]. Similar results occur in rodent models of cancer cachexia [45,46**], chronic kidney disease [47**], glucocorticoid administration [48,49], burn injury [48], mechanical unloading and space flight [50-52]. Serum levels of Activin A, which shares myostatin receptor, rise in response to cancer [46**,53-55], kidney failure [56] and heart failure [57,58]. Experimentally, mice given either myostatin [59] or activin A [46**] experienced ~30% decrease in muscle mass. These responses document that both myostatin and activin A influence muscle size. The question is: can their influence be overcome by an acceptable treatment strategy?
Techniques for inhibiting myostatin
Antibodies, including a peptibody (a genetically engineered myostatin-neutralizing peptide fused to Fc), or administration of the myostatin propeptide, follistatin or soluble ActRIIB receptors have been evaluated for their ability to increase muscle mass in animals. These myostatin inhibitor strategies increase muscle mass in normal animals: cynomolgus monkeys were treated using an adenovirus strategy to express follistatin-isoform FS344, a myostatin-sequestering protein; the treatment increased muscle mass and strength [60]. We find that the muscle wasting induced by different forms of cancer or CKD is blocked by inhibiting myostatin [46**,47**].
Beneficial responses to blocking myostatin pathway in cancer models
Cancer is a “poster child” of muscle wasting since as many as 80% of patients with advanced cancers develop muscle wasting and 25% of cancer-related deaths are ascribed to cachexia. The main mechanism causing loss of muscle mass is acceleration of protein degradation by the UPS which is initiated by suppression of intracellular p-Akt signaling, presumably related to hormonal abnormalities or cytokines. When p-Akt is low, forkhead transcription factors stimulate the expression of the muscle-specific ubiquitin E3 ligases, Atrogin-1/MAFbx and MuRF1. The low p-Akt also activates the UPS and caspase-3 to increase muscle protein breakdown (see above). On the other hand, cancers that activate NF-κB or Smads could also contribute to muscle wasting by raising autophagy and the breakdown of muscle proteins [20,46**,61*]. At least in mouse models of cancer, the myostatin/activin signaling pathway also plays a major role in the cancer-induced pathogenesis of muscle wasting [46**,62*]. For example, blocking the ActRIIB signaling pathway protects against muscle wasting in various tumor-bearing mice [46**,62*,63*].
These findings have been extended yielding evidence of improved survival of mice despite the presence of cancer. Zhou et al (2010) administered the ActRIIB decoy receptor to mice with different cancers including CDF1 mice implanted with colon-26 adenocarcinoma, nude mice transplanted with human TOV-21G ovarian carcinoma or human G361 melanoma and inhibin-deficient mice that spontaneously develop gonadal tumors. In each case, the muscle wasting was prevented and the protection was associated with a significant increase in survival. In mice bearing the colon-26 adenocarcinoma, muscle wasting was prevented even though the tumor continued to grow.
Other benefits of these remarkable responses to myostatin/activin pathway blockade include virtual elimination of cancer-induced cardiac atrophy [46**]. Secondly, there was an increase in muscle levels of p-Akt and p-FoxO3a that would explain the suppressed transcription of Atrogin-1/MAFbx and MuRF1 genes and hence, the reduced ubiquitin conjugation to muscle proteins (see above; also see Figure 2). Thirdly, there was an improvement in the function of muscle progenitor or satellite cells, including an increase in their ability to proliferate (see above). Together, these results in several models of cancer could contribute to the rapid reversal of muscle atrophy following inhibition of ActRIIB signaling. Finally, reversal of muscle atrophy occurred even though circulating levels of TNF-α, IL-1β and IL-6 in mice bearing colon-26 cancer were not suppressed suggesting that inflammation would not interfere with the ability of the ActRIIB decoy receptor to block myostatin/activin signaling in muscle.
Beneficial responses to blocking myostatin in chronic kidney disease
CKD is among a group of disorders (e.g., diabetes, starvation and some forms of cancer) characterized by increased levels of circulating markers of inflammation, increased glucocorticoid production, impaired insulin/IGF-1 signaling and muscle protein wasting. These disorders also exhibit similar patterns of gene expression in muscles (see above). Notably, these characteristics can be detected even when CKD is relatively mild (i.e., serum creatinine < 3 mg/dL). The pathophysiology of CKD-induced muscle wasting includes a decrease in p-Akt leading to increased expression of Atrogin-1/MAFbx and accelerated muscle proteolysis via the UPS and caspase-3 (see above). In addition, CKD impairs satellite cell function and myostatin is activated in muscle [47**,64*]. Reliable treatments to block these abnormalities are not available.
To evaluate whether myostatin stimulates muscle wasting in CKD, Zhang et al (2011) studied a mouse model of CKD (subtotal nephrectomy plus a high protein diet). Mice with BUN values >80 mg/dL were paired for BUN and age and pair-fed for 4 weeks [47**]. In each pair, one mouse with CKD received subcutaneous injection of an anti-myostatin peptibody every other day while the paired mouse was injected with the diluent. With peptibody treatment, myostatin was suppressed in muscle and the loss of body weight was reversed while weights of individual muscles increased. Muscle atrophy was prevented by both an increase in the rate of protein synthesis and a decrease in protein degradation; there also were improvements in satellite cell function.
Unlike responses to administering the decoy ActRIIB receptor to mouse models of cancer, myostatin inhibition with the peptibody decreased the circulating levels of inflammatory cytokines and especially, IL-6. Determining how the inhibition of myostatin suppresses inflammatory cytokines was a priority because earlier studies had demonstrated that inflammation can lead to an increase in circulating IL-6 and serum amyloid A. This cytokine and acute phase protein combined to suppress intracellular IGF-1 signaling resulting in a decrease in the muscle level of p-Akt. The result was activation of muscle protein degradation in the UPS (see above). These studies also identified the link between myostatin and IL-6. Cultured muscle cells treated with TNF-α produced more myostatin. Secondly, treatment of muscle cells with myostatin produced more IL-6. Thus the high TNF-α commonly present in CKD patients acts to increase myostatin production in muscle cells and this response stimulates muscle to produce IL-6 [47**]. This is relevant because an increase in IL-6 reduces p-Akt in muscle leading to activation of the UPS and caspase-3 and ultimately, muscle atrophy.
Emerging role of myostatin in heart failure-induced muscle wasting
Patients with congestive heart failure (CHF) develop loss of body weight and muscle mass plus extreme fatigue and weakness and shortened survival, a complication known as cardiac cachexia [65,66]. The etiology of cardiac cachexia may involve neurohormonal abnormalities, inflammation and metabolic disturbances but as with cancer or CKD, myostatin signaling could be involved in the pathogenesis of cardiac cachexia. In animal models of heart failure it has been reported that myostatin expression occurs in the peri-infarct zone and in the ventricular muscle in response to cardiac overload [67,68]. In a mouse model of aortic pressure overload, heart failure is accompanied by muscle wasting and this response is attenuated by administration of an anti-myostatin antibody [69**]. Remarkably, Heineke et al (2010) demonstrated that a cardiac muscle-specific knockout of myostatin prevented skeletal muscle atrophy in this model of heart failure The interpretation of these results in mice is that myostatin actions in cardiomyocytes might extend to influencing skeletal muscle mass. On the other hand, CHF (or inactivity) by itself can induce myostatin expression in skeletal muscles and this can produce muscle wasting [70,71]. Which interpretation is correct is unsettled but CHF like cancer or kidney failure could be another muscle wasting condition ultimately related to responses induced by myostatin.
Conclusion
Cancer and disorders like CKD that are characterized by inflammation, impaired insulin/IGF-1 signaling, increased glucocorticoid production and muscle protein wasting are associated with an increased risk of morbidity and mortality. The physiologic and molecular mechanisms that cause muscle protein wasting have been uncovered but despite these advances, there are no reliable therapies that prevent or reverse the muscle atrophy in these disorders. Recent reports suggest we are entering a new therapeutic era because blocking myostatin signaling can not only prevent a loss of muscle mass but also can improve survival from catabolic conditions. The potential for developing a treatment strategy is exciting in part, because it is not necessary to manipulate genes to block myostatin signaling. As noted, myostatin signaling in muscle can be blocked pharmacologically. Although much information is needed before embarking on therapy for patients, we are entering an era of treatment and no longer stuck at devising new definitions for the degree of muscle wasting.
Acknowledgements
H.Q.H. is currently a full time employee of Amgen Inc. This work is supported in part by NIH Grants R37 DK-37175, NIH MERIT Award and R01 DK-80306 (W.E.M.).
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue.
- 1.Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb-Peploe KM, Harrington D, Kox WJ, Poole-Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–1053. doi: 10.1016/S0140-6736(96)07015-8. [DOI] [PubMed] [Google Scholar]
- 2.Griffiths RD. Muscle mass, survival, and the elderly ICU patient. Nutrition. 1996;12(6):456–458. doi: 10.1016/s0899-9007(96)00141-4. [DOI] [PubMed] [Google Scholar]
- 3.Huang CX, Tighiouart H, Beddhu S, Cheung AK, Dwyer JT, Eknoyan G, Beck GJ, Levey AS, Sarnak MJ. Both low muscle mass and low fat are associated with higher all-cause mortality in hemodialysis patients. Kidney International. 2010;77(7):624–629. doi: 10.1038/ki.2009.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inagaki J, Rodriguez V, Bodey GP. Proceedings: Causes of death in cancer patients. Cancer. 1974;33(2):568–573. doi: 10.1002/1097-0142(197402)33:2<568::aid-cncr2820330236>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 5.Fouque D, Kalantar-Zadeh K, Kopple J, Cano N, Chauveau P, Cuppari L, Franch H, Guarnieri G, Ikizler TA, Kaysen G, Lindholm B, et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int. 2008;73(4):391–398. doi: 10.1038/sj.ki.5002585. [DOI] [PubMed] [Google Scholar]
- 6.Mitch WE. Malnutrition: a frequent misdiagnosis for hemodialysis patients. J Clin Invest. 2002;110(4):437–439. doi: 10.1172/JCI16494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans WJ, Morley JE, Argiles J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi: 10.1016/j.clnu.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17(7):1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 9.Lecker SH, Mitch WE. Proteolysis by the ubiquitin-proteasome system and kidney disease. J Am Soc Nephrol. 2011;22(5):821–824. doi: 10.1681/ASN.2010090958. * This article reviews recent evidence for uniquitin-proteasome pathway activation in catabolic states with special reference to kidney disease.
- 10.Mitch WE, Goldberg AL. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N Engl J Med. 1996;335(25):1897–1905. doi: 10.1056/NEJM199612193352507. [DOI] [PubMed] [Google Scholar]
- 11.Bailey JL, Zheng B, Hu Z, Price SR, Mitch WE. Chronic kidney disease causes defects in signaling through the insulin receptor substrate/phosphatidylinositol 3-kinase/Akt pathway: implications for muscle atrophy. J Am Soc Nephrol. 2006;17(5):1388–1394. doi: 10.1681/ASN.2004100842. [DOI] [PubMed] [Google Scholar]
- 12.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297(16):1772–1774. doi: 10.1001/jama.297.16.1772-b. [DOI] [PubMed] [Google Scholar]
- 13.Hu Z, Wang H, Lee IH, Du J, Mitch WE. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J Clin Invest. 2009;119(10):3059–3069. doi: 10.1172/JCI38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.NITROGEN balance studies after tube feeding in cancer cachexia. Nutr Rev. 1956;14(2):43–45. doi: 10.1111/j.1753-4887.1956.tb01468.x. [DOI] [PubMed] [Google Scholar]
- 15.Cai D, Frantz JD, Tawa NE, Jr., Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 16.Kwak KS, Zhou X, Solomon V, Baracos VE, Davis J, Bannon AW, Boyle WJ, Lacey DL, Han HQ. Regulation of protein catabolism by muscle-specific and cytokine-inducible ubiquitin ligase E3alpha-II during cancer cachexia. Cancer Res. 2004;64(22):8193–8198. doi: 10.1158/0008-5472.CAN-04-2102. [DOI] [PubMed] [Google Scholar]
- 17.Song YH, Li Y, Du J, Mitch WE, Rosenthal N, Delafontaine P. Muscle-specific expression of IGF-1 blocks angiotensin II-induced skeletal muscle wasting. J Clin Invest. 2005;115(2):451–458. doi: 10.1172/JCI22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Du J, Hu Z, Han G, Delafontaine P, Garcia G, Mitch WE. IL-6 and serum amyloid A synergy mediates angiotensin II-induced muscle wasting. J Am Soc Nephrol. 2009;20(3):604–612. doi: 10.1681/ASN.2008060628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, Latres E, Goldberg AL. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol. 2009;185(6):1083–1095. doi: 10.1083/jcb.200901052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98(25):14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickering WP, Price SR, Bircher G, Marinovic AC, Mitch WE, Walls J. Nutrition in CAPD: serum bicarbonate and the ubiquitin-proteasome system in muscle. Kidney Int. 2002;61(4):1286–1292. doi: 10.1046/j.1523-1755.2002.00276.x. [DOI] [PubMed] [Google Scholar]
- 23.Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21(1):140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 24.Bossola M, Muscaritoli M, Costelli P, Bellantone R, Pacelli F, Busquets S, Argiles J, Lopez-Soriano FJ, Civello IM, Baccino FM, Fanelli F Rossi, et al. Increased muscle ubiquitin mRNA levels in gastric cancer patients. Am J Physiol Regul Integr Comp Physiol. 2001;280(5):R1518–1523. doi: 10.1152/ajpregu.2001.280.5.R1518. [DOI] [PubMed] [Google Scholar]
- 25.Workeneh BT, Rondon-Berrios H, Zhang L, Hu Z, Ayehu G, Ferrando A, Kopple JD, Wang H, Storer T, Fournier M, Lee SW, et al. Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J Am Soc Nephrol. 2006;17(11):3233–3239. doi: 10.1681/ASN.2006020131. [DOI] [PubMed] [Google Scholar]
- 26.Wang H, Garzon R, Sun H, Ladner KJ, Singh R, Dahlman J, Cheng A, Hall BM, Qualman SJ, Chandler DS, Croce CM, et al. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell. 2008;14(5):369–381. doi: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarzkopf M, Coletti D, Sassoon D, Marazzi G. Muscle cachexia is regulated by a p53-PW1/Peg3-dependent pathway. Genes Dev. 2006;20(24):3440–3452. doi: 10.1101/gad.412606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SJ. Genetic analysis of the role of proteolysis in the activation of latent myostatin. PLoS One. 2008;3(2):e1628. doi: 10.1371/journal.pone.0001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci U S A. 2001;98(16):9306–9311. doi: 10.1073/pnas.151270098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clop A, Marcq F, Takeda H, Pirottin D, Tordoir X, Bibe B, Bouix J, Caiment F, Elsen JM, Eychenne F, Larzul C, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38(7):813–818. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- 32.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94(23):12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosher DS, Quignon P, Bustamante CD, Sutter NB, Mellersh CS, Parker HG, Ostrander EA. A mutation in the myostatin gene increases muscle mass and enhances racing performance in heterozygote dogs. PLoS Genet. 2007;3(5):e79. doi: 10.1371/journal.pgen.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuelke M, Wagner KR, Stolz LE, Hubner C, Riebel T, Komen W, Braun T, Tobin JF, Lee SJ. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350(26):2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 35.Binns MM, Boehler DA, Lambert DH. Identification of the myostatin locus (MSTN) as having a major effect on optimum racing distance in the Thoroughbred horse in the USA. Anim Genet. 2010;41(Suppl 2):154–158. doi: 10.1111/j.1365-2052.2010.02126.x. [DOI] [PubMed] [Google Scholar]
- 36.Hill EW, Gu J, Eivers SS, Fonseca RG, McGivney BA, Govindarajan P, Orr N, Katz LM, MacHugh DE. A sequence polymorphism in MSTN predicts sprinting ability and racing stamina in thoroughbred horses. PLoS One. 2010;5(1):e8645. doi: 10.1371/journal.pone.0008645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60-92 year old women and men with muscle wasting. J Nutr Health Aging. 2002;6(5):343–348. [PubMed] [Google Scholar]
- 38.Reardon KA, Davis J, Kapsa RM, Choong P, Byrne E. Myostatin, insulin-like growth factor-1, and leukemia inhibitory factor mRNAs are upregulated in chronic human disuse muscle atrophy. Muscle Nerve. 2001;24(7):893–899. doi: 10.1002/mus.1086. [DOI] [PubMed] [Google Scholar]
- 39.Zachwieja JJ, Smith SR, Sinha-Hikim I, Gonzalez-Cadavid N, Bhasin S. Plasma myostatin-immunoreactive protein is increased after prolonged bed rest with low-dose T3 administration. J Gravit Physiol. 1999;6(2):11–15. [PubMed] [Google Scholar]
- 40.Gonzalez-Cadavid NF, Taylor WE, Yarasheski K, Sinha-Hikim I, Ma K, Ezzat S, Shen R, Lalani R, Asa S, Mamita M, Nair G, et al. Organization of the human myostatin gene and expression in healthy men and HIV-infected men with muscle wasting. Proc Natl Acad Sci U S A. 1998;95(25):14938–14943. doi: 10.1073/pnas.95.25.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun DF, Chen Y, Rabkin R. Work-induced changes in skeletal muscle IGF-1 and myostatin gene expression in uremia. Kidney Int. 2006;70(3):453–459. doi: 10.1038/sj.ki.5001532. [DOI] [PubMed] [Google Scholar]
- 42.Breitbart A, Auger-Messier M, Molkentin JD, Heineke J. Myostatin from the heart: local and systemic actions in cardiac failure and muscle wasting. Am J Physiol Heart Circ Physiol. 2011;300(6):H1973–1982. doi: 10.1152/ajpheart.00200.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, Sweeney HL, Maybaum S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail. 2010;12(5):444–453. doi: 10.1093/eurjhf/hfq039. * This article documents that myostatin expression levels were elevated in patients with advanced heart failure.
- 44.Gruson D, Ahn SA, Ketelslegers JM, Rousseau MF. Increased plasma myostatin in heart failure. Eur J Heart Fail. 2011 doi: 10.1093/eurjhf/hfr024. [DOI] [PubMed] [Google Scholar]
- 45.Costelli P, Muscaritoli M, Bonetto A, Penna F, Reffo P, Bossola M, Bonelli G, Doglietto GB, Baccino FM, Fanelli F Rossi. Muscle myostatin signalling is enhanced in experimental cancer cachexia. Eur J Clin Invest. 2008;38(7):531–538. doi: 10.1111/j.1365-2362.2008.01970.x. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, Wang JL, Lu J, Song Y, Kwak KS, Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, Lacey DL, et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell. 2010;142(4):531–543. doi: 10.1016/j.cell.2010.07.011. ** This article reveals that in several models of cancer cachexia, pharmacological blockade of myostatin/activin-ActRIIB signaling pathway not only prevents muscle further wasting but also reverses pre-existent loss of skeletal muscle and cardiac atrophy, thereby dramatically prolonging survival, even of animals in which tumor growth is not inhibited. Blocking this pathway is found to completely abolish the induction of atrophy-specific ubiquitin ligases in muscles and markedly stimulates muscle stem cell growth.
- 47.Zhang L, Rajan V, Lin E, Hu Z, Han HQ, Zhou X, Song Y, Min H, Wang X, Du J, Mitch WE. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 2011;25(5):1653–1663. doi: 10.1096/fj.10-176917. ** This study demonstrates that in mice with CKD, pharmacological inhibition of myostatin is able to reverse muscle wasting and suppress circulating inflammatory cytokines. Myostatin antagonism is found to decrease the rate of protein degradation, increase protein synthesis in muscles, enhance satellite cell function, and improve IGF-1 intracellular signaling in CKD mice.
- 48.Lang CH, Silvis C, Nystrom G, Frost RA. Regulation of myostatin by glucocorticoids after thermal injury. FASEB J. 2001;15(10):1807–1809. doi: 10.1096/fj.00-0849fje. [DOI] [PubMed] [Google Scholar]
- 49.Ma K, Mallidis C, Bhasin S, Mahabadi V, Artaza J, Gonzalez-Cadavid N, Arias J, Salehian B. Glucocorticoid-induced skeletal muscle atrophy is associated with upregulation of myostatin gene expression. Am J Physiol Endocrinol Metab. 2003;285(2):E363–371. doi: 10.1152/ajpendo.00487.2002. [DOI] [PubMed] [Google Scholar]
- 50.Carlson CJ, Booth FW, Gordon SE. Skeletal muscle myostatin mRNA expression is fiber-type specific and increases during hindlimb unloading. Am J Physiol. 1999;277(2 Pt 2):R601–606. doi: 10.1152/ajpregu.1999.277.2.r601. [DOI] [PubMed] [Google Scholar]
- 51.Lalani R, Bhasin S, Byhower F, Tarnuzzer R, Grant M, Shen R, Asa S, Ezzat S, Gonzalez-Cadavid NF. Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J Endocrinol. 2000;167(3):417–428. doi: 10.1677/joe.0.1670417. [DOI] [PubMed] [Google Scholar]
- 52.Wehling M, Cai B, Tidball JG. Modulation of myostatin expression during modified muscle use. FASEB J. 2000;14(1):103–110. doi: 10.1096/fasebj.14.1.103. [DOI] [PubMed] [Google Scholar]
- 53.Otani T, Minami S, Yamoto M, Umesaki N. Production of activin A in hyperplasia and adenocarcinoma of the human endometrium. Gynecol Oncol. 2001;83(1):31–38. doi: 10.1006/gyno.2001.6350. [DOI] [PubMed] [Google Scholar]
- 54.Seder CW, Hartojo W, Lin L, Silvers AL, Wang Z, Thomas DG, Giordano TJ, Chen G, Chang AC, Orringer MB, Beer DG. INHBA overexpression promotes cell proliferation and may be epigenetically regulated in esophageal adenocarcinoma. J Thorac Oncol. 2009;4(4):455–462. doi: 10.1097/JTO.0b013e31819c791a. [DOI] [PubMed] [Google Scholar]
- 55.Wildi S, Kleeff J, Maruyama H, Maurer CA, Buchler MW, Korc M. Overexpression of activin A in stage IV colorectal cancer. Gut. 2001;49(3):409–417. doi: 10.1136/gut.49.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rydzewska-Rosolowska A, Borawski J, Mysliwiec M. Hepatocyte growth factor/activin A/follistatin system activation during hemodialysis with different low molecular weight heparins. Ren Fail. 2009;31(9):791–797. doi: 10.3109/08860220903180608. [DOI] [PubMed] [Google Scholar]
- 57.Bjornstad JL, Neverdal NO, Vengen OA, Knudsen CW, Husebye T, Pepper J, Lie M, Christensen G, Tonnessen T. Alterations in circulating activin A, GDF-15, TGF-beta3 and MMP-2, -3, and -9 during one year of left ventricular reverse remodelling in patients operated for severe aortic stenosis. Eur J Heart Fail. 2008;10(12):1201–1207. doi: 10.1016/j.ejheart.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 58.Yndestad A, Ueland T, Oie E, Florholmen G, Halvorsen B, Attramadal H, Simonsen S, Froland SS, Gullestad L, Christensen G, Damas JK, et al. Elevated levels of activin A in heart failure: potential role in myocardial remodeling. Circulation. 2004;109(11):1379–1385. doi: 10.1161/01.CIR.0000120704.97934.41. [DOI] [PubMed] [Google Scholar]
- 59.Zimmers TA, Davies MV, Koniaris LG, Haynes P, Esquela AF, Tomkinson KN, McPherron AC, Wolfman NM, Lee SJ. Induction of cachexia in mice by systemically administered myostatin. Science. 2002;296(5572):1486–1488. doi: 10.1126/science.1069525. [DOI] [PubMed] [Google Scholar]
- 60.Kota J, Handy CR, Haidet AM, Montgomery CL, Eagle A, Rodino-Klapac LR, Tucker D, Shilling CJ, Therlfall WR, Walker CM, Weisbrode SE, et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci Transl Med. 2009;1(6):6ra15. doi: 10.1126/scitranslmed.3000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care. 2010;13(3):225–229. doi: 10.1097/mco.0b013e32833862df. * This review describes the intracellular signaling mechanisms underlying muscle loss with special reference to the ubiquitin-proteasome pathway and the phosphatidylinositol-3 kinase/Akt/Foxo pathway.
- 62.Klimek ME Benny, Aydogdu T, Link MJ, Pons M, Koniaris LG, Zimmers TA. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem Biophys Res Commun. 2010;391(3):1548–1554. doi: 10.1016/j.bbrc.2009.12.123. * This study shows that blocking the ActRIIB (ACVR2B) signaling pathway with a decoy receptor prevents muscle atrophy and fat loss in mice bearing colon-26 adenocarcinoma and Lewis lung carcinoma.
- 63.Murphy KT, Chee A, Gleeson BG, Naim T, Swiderski K, Koopman R, Lynch GS. Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am J Physiol Regul Integr Comp Physiol. 2011 doi: 10.1152/ajpregu.00121.2011. *This study demonstrates that administration of an anti-myostatin antibody attenuated muscle atrophy and loss of muscle force-producing capacity in mice bearing Lewis Lung carcinoma,.
- 64.Zhang L, Wang XH, Wang H, Du J, Mitch WE. Satellite cell dysfunction and impaired IGF-1 signaling cause CKD-induced muscle atrophy. J Am Soc Nephrol. 2010;21(3):419–427. doi: 10.1681/ASN.2009060571. * This paper shows that in mice with CKD, there is an impairment in IGF1/AKT signaling, which leads to protein catabolism, satellite cell dysfunction and fibrosis in muscle.
- 65.Anker SD, Steinborn W, Strassburg S. Cardiac cachexia. Ann Med. 2004;36(7):518–529. doi: 10.1080/07853890410017467. [DOI] [PubMed] [Google Scholar]
- 66.Coats AJ. Origin of symptoms in patients with cachexia with special reference to weakness and shortness of breath. Int J Cardiol. 2002;85(1):133–139. doi: 10.1016/s0167-5273(02)00242-5. [DOI] [PubMed] [Google Scholar]
- 67.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180(1):1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 68.Shyu KG, Lu MJ, Wang BW, Sun HY, Chang H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur J Clin Invest. 2006;36(10):713–719. doi: 10.1111/j.1365-2362.2006.01718.x. [DOI] [PubMed] [Google Scholar]
- 69.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121(3):419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. ** This study demonstrates that heart-specific deletion of the myostatin gene in mice does not alter baseline heart size or secondarily affect skeletal muscle size, but prevents atrophy of skeletal muscle during pressure overload-induced heart failur, suggesting an endocrine role of myostatin released from myocardium in cardiac cachexia.
- 70.Lenk K, Erbs S, Hollriege R, Beck E, Linke A, Gielen S, Winkler S Mobius, Sandri M, Hambrecht R, Schuler G, Adams V. Exercise training leads to a reduction of elevated myostatin levels in patients with chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2011 doi: 10.1177/1741826711402735. [DOI] [PubMed] [Google Scholar]
- 71.Lenk K, Schur R, Linke A, Erbs S, Matsumoto Y, Adams V, Schuler G. Impact of exercise training on myostatin expression in the myocardium and skeletal muscle in a chronic heart failure model. Eur J Heart Fail. 2009;11(4):342–348. doi: 10.1093/eurjhf/hfp020. [DOI] [PubMed] [Google Scholar]