Abstract
Background and Aims
Few epidemiological investigations characterize inflammatory bowel disease (IBD) in non-Caucasian children. Our study compared IBD characteristics between African-Americans and non-African-Americans enrolled in a multi-center pediatric IBD registry with endoscopic- and pathology-based diagnosis.
Methods
The study retrieved data entered from January 2000–October 2003 on children 1 to 17 years old, inclusive, followed by a consortium of academic and community U.S. pediatric gastroenterology practices. Analyses examined racial/ethnic differences by comparing the proportions of African-Americans and non-African-Americans in: each diagnostic disease classification (any IBD, Crohn's disease, ulcerative colitis, indeterminate colitis); age group (<6y, 6–12y or >12y) at diagnosis or symptom onset; presence of extraintestinal manifestations, Z-scores for height and weight, immunomodulatory therapy, anatomic disease location and abnormal hemoglobin, albumin or sedimentation rate at diagnosis.
Results
1,406 patients had complete data, 138 (10%) of whom were African-American. African-Americans more often: were >12y of age at diagnosis (52% vs. 37%, OR 1.82, 95% CI 1.28–2.59) and symptom onset (46% vs. 30%, OR 1.99, 95% CI 1.40–2.84); had Crohn's disease (78% vs. 59%, OR 2.36, 95% CI 1.56–3.58); had low hemoglobin at diagnosis (39% vs. 17%, OR 3.15, 95% CI 1.92–5.17).
Conclusions
IBD in African-American children and adolescents presents more commonly with CD and at older ages compared to non-African-Americans. Racial/ethnic differences in the epidemiology of IBD, particularly CD, among American youths require further investigation.
INTRODUCTION
Inflammatory bowel disease (IBD) encompasses the distinct disease classifications of Crohn's disease (CD), ulcerative colitis (UC), and indeterminate colitis (IC)1, 2. IBD can cause substantial morbidity among children in the United States (U.S.) and throughout the world3, 4. Recent epidemiologic studies describe incidence rates of IBD among African-American (AA) adults5–7 and African-Caribbean children8 approaching those in Caucasians. Few investigations have described the clinical characteristics of IBD in diverse pediatric populations9–11. A recent adult study reported significant differences of IBD in among different racial/ethnic groups12, while a recent pediatric investigation similar phenotypic features among African-American, Hispanic-American and White children13. One single-center study reported an equal distribution of IBD disease classifications among AA and non-African-American (non-AA) pediatric patients. However, when compared with non-AA children, AA children had longer symptom duration, and were older at IBD diagnosis than non-AA11. In a preliminary investigation of IBD in adults, Straus, et al.14 reported lower hemoglobin (Hgb) levels and more frequent prednisone use among AA compared to Caucasians. These observations suggest that disease phenotype might differ between racial/ethnic groups.
To fill gaps in understanding the epidemiology of pediatric IBD in the U.S., a national network of clinicians and researchers developed a large registry of pediatric IBD patients. Six large tertiary and community-based U.S. pediatric gastroenterology practices comprise the Pediatric IBD (PediIBD) Consortium (subsequently referred to as Consortium). The sites are geographically diverse, representing Western (San Francisco, CA), Midwestern (Chicago, IL), Northeastern (Boston, MA), Eastern (Philadelphia, PA), Southwestern (Houston, TX) and Southeastern (Atlanta, GA) regions of the continental U.S. Each practice follows pediatric patients from urban, suburban and rural areas, collecting clinical, laboratory and demographic data on every enrolled subject, and entering it into the database.
Using the Consortium patient registry, the present study examined the epidemiologic and clinical manifestations of IBD in AA children. We hypothesized that AA children with IBD have distinctly different clinical characteristics and disease patterns when compared with non-AA children diagnosed with IBD. To test this hypothesis, we compared age distributions, disease classifications and the clinical presentation of AA and non-AA IBD patients enrolled in the multi-center Consortium registry from 2000 to 2003.
METHODS
Data Extraction from the Consortium Registry
This study retrospectively retrieved and analyzed data recorded from January 2000 to July 2003 on children with IBD followed by the six academic referral and community-based pediatric gastroenterology practices of the Consortium: Atlanta, GA, Emory University – Children's Healthcare of Atlanta and Children's Center for Digestive Healthcare; Houston, TX, Baylor College of Medicine – Texas Children's Hospital; San Francisco, CA, University of California, San Francisco – Children's Hospital; Philadelphia, PA, University of Pennsylvania – Children's Hospital of Philadelphia; Chicago, IL, University of Chicago – The University of Chicago, Comer Children's Hospital; and Boston, MA, Harvard University – MassGeneral Hospital for Children. AA comprised varied proportions of the catchment populations of each consortium site: 28% in Atlanta; 17% in Houston; 6.5% in Boston; 19% in Chicago; 17% in Philadelphia; 8% in San Francisco15. The registry contains cross-sectional and longitudinal data on IBD patients aged 1 to 17 years, inclusive, abstracted from medical records and uniformly entered by each of the sites' investigators and study coordinators. Data on patients newly diagnosed or currently followed by a Consortium gastroenterologist include: medical, surgical and procedural histories; endoscopic and histologic evaluations of mucosal biopsies; patient clinical status (e.g., presence of extraintestinal manifestations (EIMC), perianal disease, skin tags, fissures and abscesses, abdominal pain/tenderness, stool pattern); laboratory results; radiographic studies; medication history; and use of nutritional supplements. At each site, the investigating physician or nurse researcher recorded data from the inpatient and outpatient clinic medical record. Over 80% of the patients in the data registry were prospectively enrolled, the remaining being retrospectively enrolled. All sites had approval from their respective Institutional Review Boards prior to data collection and entry. Informed consent and assent (when appropriate) was obtained from all participating parents and subjects prior to enrollment.
Case Definitions10
The diagnosis of IBD is standardized across the Consortium based on a combination of clinical, laboratory, radiological, endoscopic, and histologic findings. CD was defined by (1) presence of granuloma in any one biopsy from upper and/or lower endoscopy; (2) in the absence of granuloma, evidence of skip lesions on colonoscopy and/or microscopic focal chronic inflammatory changes in upper endoscopy; (3) segmental small intestinal radiologic findings consistent with CD; (4) presence of perianal disease (i.e., abscesses, fistulae, large skin tags); and/or (5) evidence of transmural inflammation (i.e., strictures, fistulae). UC was defined as contiguous disease confined to the colon without evidence of small intestinal involvement (other than backwash ileitis) on biopsy or radiology. IC was defined as colitis that could not be definitely classified as either CD or UC, based on the above criteria. Patients with infectious, eosinophilic, or other underlying cause for enteritis or colitis were excluded.
Eligibility Requirements
All IBD patients followed by any Consortium gastroenterologist are eligible for enrollment in the registry. To minimize selection bias, the investigators attempted to enroll all newly diagnosed patients and those newly followed by the Consortium, regardless of race/ethnicity or IBD classification. Established patients were enrolled as time and research personnel permitted. The study cohort consisted of all patients enrolled in the registry during the study period (January 2001 through July 2003) that had complete data, as of October 2003, for: age at diagnosis, IBD classification, self- or parent-reported age of IBD symptom onset and race/ethnicity.
Definition of Terms and Disease Classification
For purposes of the study, patients (or their parents) identifying themselves as Black, non-Hispanic were considered AA. Non-AA encompassed all other children with a self- or parent-reported race/ethnicity of Caucasian, Asian-Pacific Islander, American Indian, Hispanic and non-AA, or race not specified. In this study, the most recently recorded IBD diagnosis as of the October 2003 data retrieval date represented the disease classification. The first diagnosis (if different) was not used because clinical disease can evolve over time, thereby changing the initial diagnosis,10 and because this information was not available for all those initially diagnosed outside the Consortium. For patients newly diagnosed by the Consortium, the date of the first definitive endoscopic and/or radiographic (small bowel barium contrast study) diagnosis of IBD by a Consortium pediatric gastroenterologist defined the date of diagnosis. For patients diagnosed outside of the Consortium but subsequently followed by a Consortium gastroenterologist and entered into the registry, the date of disease diagnosis recorded in the medical record and confirmed by subsequent Consortium endoscopy or radiographic evaluation denoted the date of diagnosis. Date of symptom onset was the approximate onset of symptoms consistent with IBD, as reported by the case child and/or the parent/legal guardian. IBD disease classifications were defined as CD, UC, IC or any IBD; any IBD refers to the sum or combination of all cohort patients diagnosed with CD, UC and IC. The following anatomic disease location categories were used to sub-analyze CD: isolated upper (esophagus and/or stomach); isolated small bowel (proximal, middle and/or distal small bowel); isolated colonic (any portion of the colon); isolated ileocolonic (any portion of the colon plus ileum); all other combinations. For ease of data analysis, the following anatomic disease locations were used to categorize UC: pancolonic (cecum to left colon inclusive, with/without rectum); extensive colonic (transverse colon to left colon inclusive, with/without rectum); left colonic (left colon with/without rectum); isolated rectum.
For this study, at or around the time of diagnosis was defined as the period extending from 90 days prior to 30 days after date of diagnosis. Perianal disease included fissures, tags, erythema, abscesses or fistulae in the perianal region. Fistulae were recorded as a separate variable in the data registry and thus could be analyzed separately when comparing ethnic/racial groups. The term fistulae defined one or more fistulae arising anywhere in the gastrointestinal tract, excluding the perianal region; by case definition, fistulae do not occur in UC. Early immunomodulatory therapy was defined as any combination of corticosteroids, 6-mercaptopurine (6-MP), azathioprine or Infliximab (Remicaid®) prescribed up to 30 days after diagnosis, including any patients begun on immunomodulatory therapy prior to diagnosis. Patients receiving methotrexate were not included in analyses due to small patient numbers.
The EIMC analyzed in this study were: fistulae, apthous stomatitis, gallstones, arthritis (peripheral, axial or both), growth failure, pyoderma gangrenosum, autoimmune hepatitis, vascular thrombosis, uveitis, renal calculi, keratoconjunctivitis, sclerosing cholangitis, erythema nodosum, osteopenia/osteoporosis, clubbing, and pancreatitis.
Erythrocyte sedimentation rate (ESR) >20mm/hr, hemoglobin (Hgb) <10gm/dL and albumin (Alb) <3gm/dL were considered abnormal. Z-scores for height and weight less than 1 standard deviation (SD) below the age appropriate mean were considered abnormal.
Comparisons to Evaluate Racial/Ethnic Differences
For each variable examined, all analyses compared only the proportion of AA to that of non-AA cohort subjects having information on that variable recorded in the database. Comparisons examined the differences in these proportions for all subjects diagnosed with CD, UC or IC, separately and combined (any IBD). In order to assess racial/ethnic differences in the age of IBD, CD, UC or IC diagnosis or symptom onset, subjects were stratified into three age groups that anecdotally may have different clinical behaviors10: <6 years old at diagnosis or symptom onset; 6–12 years old, inclusive, at diagnosis or symptom onset; and >12 years old at diagnosis or symptom onset.
Within each disease classification, we examined racial/ethnic differences (AA versus non-AA) in the presence of certain clinical parameters at the time of diagnosis: EIMC; Z-scores for height and weight; abnormal ESR, Hgb or Alb. Similar analyses evaluated racial/ethnic differences in anatomic disease location for CD and UC. Analyses of fistulae and strictures were restricted to combined IBD, CD and IC because, by convention, fistulae and strictures do not occur in UC. For each disease classification, additional analyses compared the proportions of AA and non-AA children prescribed early immunomodulatory therapy (up to 30 days post-diagnosis, including patients prescribed immunomodulatory therapy prior to diagnosis) and the type of therapy used—corticosteroids alone or any other immunomodulatory regimen.
Statistical Analyses
All analyses were conducted using SAS version 8.02 (SAS Institute, Cary, NC) odds ratios (OR) with 95% confidence intervals (95% CI) were computed. Proportions were compared using Chi-square or Fisher's Exact test when appropriate.
RESULTS
As of the data accession October 1, 2003, 1,406 cohort patients—138 (10%) AA and 1,268 (90%) non-AA (83% Caucasian; 3% Hispanic; 2% Asian/Pacific Islander; and 2% Non-AA, race not specified)—had complete data for IBD classification, age at diagnosis, self- or parent-reported age of IBD symptom onset and self- or parent-reported race/ethnicity. Of these, 51% (N=719), 44% (N=624) and 38% (N=529) had recorded Hgb, ESR and Alb, respectively, at the time of diagnosis. Z-scores for height and weight were available for 59% (N=835) and 63% (N=886) of patients, respectively. . Forty-two percent (N=593) of the cohort had received some type of immunomodulatory therapy by 30 days after diagnosis and the entire cohort had data on the presence or absence of EIMC at the time of diagnosis. Due to the manner of data extraction from the registry, absolute numbers and mean values for Hgb, ESR and Alb were not available for analyses.
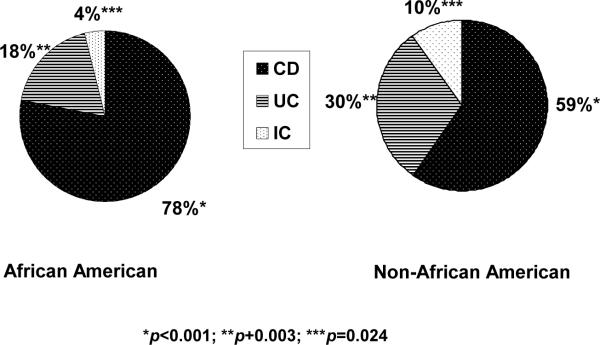
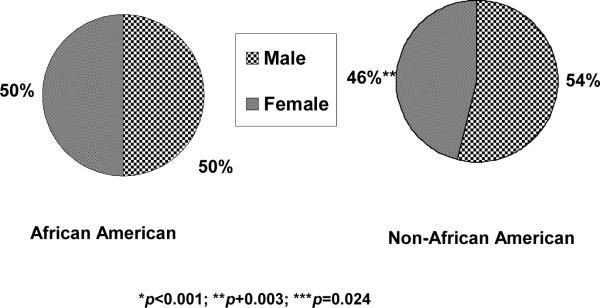
Overall, 860 (61%) of the study cohort children were diagnosed with CD, 409 (29%) with UC and 137 (10%) with IC. Of these, 107 AA and 753 non-AA had CD. Proportionally more AA (78% of the cohort AA), compared to non-AA (59% of the cohort non-AA), had CD (OR 2.36, 95% CI 1.56–3.58). Conversely, proportionally fewer AA (18%; N=25) versus non-AA (30%; N=384) had UC (OR 0.51, 95% CI 0.32–0.80). Only, 6 AA (4% of the cohort AA) and 131 non-AA (10% of the cohort non-AA) had IC (OR 0.39, 95% CI 0.17–0.91) (Figure 1). The proportions of male African-Americans (N=69, 50%) and non-African-Americans (N=686, 54%) with IBD were similar (Figure 2).
Figure 1.
Disease classification of the pediatric IBD patient cohort we analyzed comparing those African American patients to non-African American. Statistically significant differences were observed between African American children with Crohn's compared to non-African American (78% v. 59%); i.e., proportionally more African American children had Crohn's. Differences were also significant when comparing African American to non-African American children with UC (18% v. 30%) and IC (4% v. 10%); whereas more non-African American children proportionally had UC or IC than did their African American counter parts.
Figure 2.
Gender classification of our pediatric IBD cohort comparing males to females whether African American or non-African American. There were no statistically significant differences in the gender distribution in either main racial/ethnic categories.
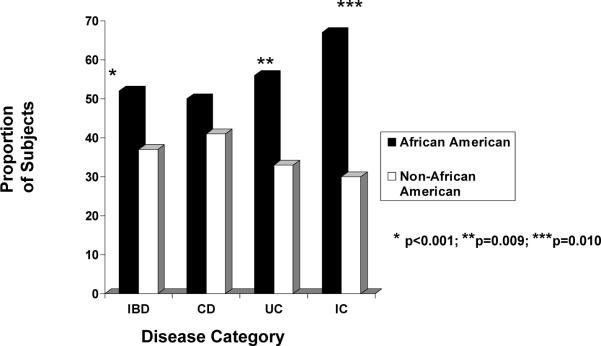
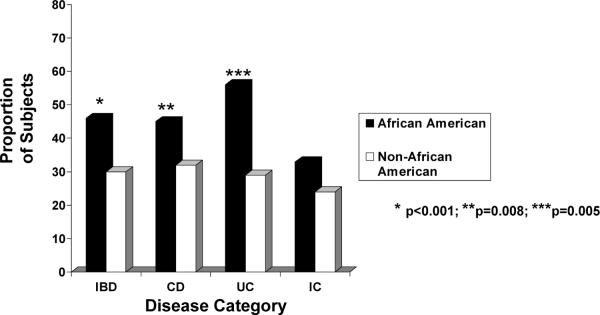
The distributions of age at IBD diagnosis and age at symptom onset, by race/ethnicity, are depicted for each disease classification in Tables 1 and 2, respectively. Of the entire cohort, 547 (39%) and 448 (32%) children were >12 years old at the time of diagnosis and at symptom onset, respectively. A significantly greater proportion of AA, compared to non-AA, were >12 years old when diagnosed with any IBD (52% vs. 37%, OR 1.82, 95% CI 1.28–2.59) as well as with UC (56% vs. 33%, OR 2.58, 95% CI 1.14–5.83) (Figure 3). A greater proportion of AA with any type of IBD also had symptom onset after 12 years of age compared to non-AA patients (46% vs. 30%, OR 1.99, CI 1.40–2.84). Significant racial-ethnic differences were observed in age at symptom onset >12 years for CD (45% AA vs. 32% non-AA; OR 1.74, CI 1.15–2.62) and UC (56% AA vs. 29% non-AA; OR 3.05, CI 1.34–6.93 (Figure 4).
Table 1.
Distribution of Age at Disease Diagnosis, by Racial/Ethnic Group (African-American, non-African-American)
| All IBD N=1406 (100%) | CD N=860 (61%) | UC N=409 (29%) | IC N=137 (10%) | |||||
|---|---|---|---|---|---|---|---|---|
| Age Group | AA N=138 (10%) | Non-AA N=1268 (90%) | AA N=107 (12%) | Non-AA N=753 (88%) | AA N=25 (6%) | Non-AA N=384 (94%) | AA N=6 (4%) | Non-AA N=131 (96%) |
| <6 years N=254 (18%) | 10 (7%) | 206 (16%) | 8 (7%) | 83 (11%) | 2 (8%) | 84 (22%) | 0 (0%) | 39 (30%) |
| 6–12 years N=704 (50%) | 56 (41%) | 587 (46%) | 45 (42%) | 361 (48%) | 8 (36%) | 173 (45%) | 2 (33%) | 53 (40%) |
| >12 years N=448 (32%) | 72 (52%) | 475 (37%) | 54 (50%) | 309 (41%) | 14 (56%) | 127 (33%) | 4 (67%) | 39 (30%) |
|
| ||||||||
| Total N=1406 (100%) | 138 (100%) | 1268 (100%) | 107 (100%) | 753 (100%) | 25 (100%) | 384 (100%) | 6 (100%) | 131 (100%) |
Table 2.
Distribution of Age at Disease Symptom Onset, by Racial/Ethnic group (African-American, non-African-American)
| All IBD N=1406 (100%) | CD N=860 (61%) | UC N=409 (29%) | IC N=137 (10%) | |||||
|---|---|---|---|---|---|---|---|---|
| Age Group | AA N=138 (10%) | Non-AA N=1268 (90%) | AA N=107 (12%) | Non-AA N=753 (88%) | AA N=25 (6%) | Non-AA N=384 (94%) | AA N=6 (4%) | Non-AA N=131 (96%) |
| <6 years N=472 (30%) | 14 (10%) | 240 (19%) | 10 (9%) | 103 (14%) | 3 (12%) | 92 (24%) | 1 (17%) | 45 (34%) |
| 6–12 years N=695 (44%) | 60 (43%) | 644 (51%) | 49 (46%) | 410 (54%) | 8 (32%) | 179 (47%) | 3 (50%) | 55 (42%) |
| >12 years N=420 (26%) | 64 (46%) | 384 (30%) | 48 (45%) | 240 (32%) | 14 (56%) | 113 (29%) | 2 (33%) | 31 (24%) |
|
| ||||||||
| Total N=1587 (100%) | 138 (100%) | 1268 (100%) | 107 (100%) | 753 (100%) | 25 (100%) | 384 (100%) | 6 (100%) | 131 (100%) |
Figure 3.
The proportion of African American children versus non-African American children diagnosed after the age of 12 years by disease category (all IBD, CD, UC or IC). Interestingly, for reasons yet to be explained, statistically more African American children were diagnosed with all IBD, UC or IC compared to non-African American children.
Figure 4.
The proportion of African American children versus non-African American children with symptom onset after the age of 12 years by disease category (all IBD, CD, UC or IC). In our cohort, statistically more African American children had symptom onset whether they had all IBD, CD or UC compared to non-African American children.
Irrespective of race, 254 children (18% of the entire study cohort) had symptom onset and 216 (15% of the entire study cohort) were diagnosed with IBD before 6 years of age (Tables 1 and 2). Of these, a significantly smaller proportion of AA (7%, N=10) when compared with non-AA (16%, N=206) were diagnosed at <6 years old (OR 0.40 95% CI 0.21–0.78) (Table 3). Of the 1,406 cohort IBD subjects, significantly lower proportions of AA (10%; N=14) than non-AA (19%; N=240) reported symptom onset by 6 years of age (OR 0.48, 95% CI 0.27–0.86) (Table 3). Among the 1,406 cohort IBD patients, 643 (46%) were between 6 and 12 years old at IBD diagnosis (Table 1) while 704 (50%) were between 6 and 12 years old at the onset of IBD symptoms (Table 2). The proportions of AA and non-AA diagnosed or with symptom onset between 6 and 12 years old did not differ statistically for all IBD, CD, UC or IC (Tables 3–6).
Table 3.
IBD – Parameters at Diagnosis
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=138 | % | N=1268 | % | |||
| Age at Diagnosis | ||||||
| <6y | 10 | 7 | 206 | 16 | 0.40 | 0.21–0.78 |
| 6–12Y | 56 | 41 | 587 | 46 | 0.79 | 0.55–1.13 |
| >12y | 72 | 52 | 475 | 37 | 1.82 | 1.28–2.59 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=138 | % | N=1268 | % | |||
| Age at Symptom Onset | ||||||
| <6y | 14 | 10 | 240 | 19 | 0.48 | 0.27–0.86 |
| 6–12y | 60 | 43 | 644 | 51 | 0.75 | 0.52–1.06 |
| >12y | 64 | 46 | 384 | 30 | 1.99 | 1.40–2.84 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=79 | % | N=640 | % | |||
| Hgb <10mg/dL | 31 | 39 | 109 | 17 | 3.15 | 1.92–5.17 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=69 | % | N=555 | % | |||
| ESR >20 mm/hr | 55 | 80 | 304 | 55 | 3.24 | 1.76–5.97 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=55 | % | N=474 | % | |||
| Alb <3mg/dL | 15 | 27 | 93 | 20 | 1.54 | 0.81–2.90 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=138 | % | N=1268 | % | |||
| EIMC present | 17 | 12 | 113 | 9 | 1.44 | 0.83–2.47 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=138 | % | N=1268 | % | |||
| Received Any Immunomodulators | 78 | 57 | 515 | 41 | 1.90 | 1.33–2.71 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=78 | % | N=515 | % | |||
| Received Steroids | 58 | 74 | 401 | 78 | 0.82 | 0.48–1.43 |
| Alone | ||||||
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=94 | % | N=741 | % | |||
| Height - abnormal z-score | 21 | 22 | 194 | 26 | 0.81 | 0.49–1.35 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=99 | % | N=787 | % | |||
| Weight - abnormal z-score | 35 | 35 | 241 | 31 | 1.24 | 0.80–1.92 |
Table 6.
IC – Parameters at Diagnosis
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=6 | % | N=131 | % | |||
| Age at Diagnosis | ||||||
| <6y | 0 | 0 | 39 | 30 | - | - |
| 6–12y | 2 | 33 | 53 | 40 | 0.74 | 0.13–4.16 |
| >12y | 4 | 67 | 39 | 30 | 4.72 | 0.83–26.83 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=6 | % | N=131 | % | |||
| Age at Symptom Onset | ||||||
| <6y | 1 | 17 | 45 | 34 | 0.38 | 0.04–3.37 |
| 6–12y | 3 | 50 | 55 | 42 | 1.38 | 0.27–7.11 |
| >12y | 2 | 33 | 31 | 24 | 1.61 | 0.28–9.23 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=5 | % | N=64 | % | |||
| Hgb <10mg/dL | 1 | 20 | 3 | 5 | 5.08 | 0.43–60.64 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=5 | % | N=49 | % | |||
| ESR >20 mm/hr | 3 | 60 | 14 | 29 | 3.75 | 0.56–24.91 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=2 | % | N=43 | % | |||
| Alb <3mg/dL | 0 | 0 | 0 | 0 | - | - |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=6 | % | N=131 | % | |||
| EIMC present | 0 | 0 | 6 | 5 | - | - |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=6 | % | N=131 | % | |||
| Received Any Immunomodulators | 3 | 50 | 40 | 31 | 2.28 | 0.44–11.76 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=3 | % | N=40 | % | |||
| Received Steroids Alone | 2 | 67 | 35 | 88 | 0.29 | 0.02–3.76 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=5 | % | N=92 | % | |||
| Height - abnormal z-score | 1 | 20 | 19 | 21 | 0.96 | 0.10–9.10 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=5 | % | N=93 | % | |||
| Weight - abnormal z-score | 0 | 0 | 17 | 18 | - | - |
Seven hundred nineteen (719) IBD cohort children had a recorded Hgb at time of diagnosis, 448 with CD, 202 with UC and 69 with IC; 79 (11%) were AA and 640 (89%) were non-AA. In the entire cohort, a significantly greater proportion of AA (39%; N=31) than non-AA (17%; N=109) had abnormally low Hgb levels (OR 3.15, 95% CI 1.92–5.17) (Table 3). Of the 61 AA and 387 non-AA with CD and recorded Hgb data, a significantly greater proportion of AA had abnormally low Hgb levels (43% vs. 19%; OR 3.20, 95% CI 1.81–5.64) (Table 4). Only 13 AA and 189 non-AA with recorded Hgb had UC, while 5 AA and 64 non-AA had IC. Although the proportions were higher for AA, they did not met statistical significance (Tables 5, 6).
Table 4.
CD – Parameters at Diagnosis
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=107 | % | N=753 | % | |||
| Age at Diagnosis | ||||||
| <6y | 8 | 7 | 83 | 11 | 0.65 | 0.31–1.39 |
| 6–12y | 45 | 42 | 361 | 48 | 0.79 | 0.52–1.19 |
| >12y | 54 | 50 | 309 | 41 | 1.46 | 0.98–2.20 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=107 | % | N=753 | % | |||
| Age at Symptom Onset | ||||||
| <6y | 10 | 9 | 103 | 14 | 0.65 | 0.33–1.29 |
| 6–12y | 49 | 46 | 410 | 54 | 0.71 | 0.47–1.06 |
| >12y | 48 | 45 | 240 | 32 | 1.74 | 1.15–2.62 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=61 | % | N=387 | % | |||
| Hgb <10mg/dL | 26 | 43 | 73 | 19 | 3.20 | 1.81–5.64 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=51 | % | N=344 | % | |||
| ESR >20 mm/hr | 40 | 78 | 220 | 64 | 2.05 | 1.02–4.14 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=44 | % | N=284 | % | |||
| Alb <3mg/dL | 13 | 30 | 75 | 26 | 1.17 | 0.58–2.35 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=107 | % | N=753 | % | |||
| EIMC present | 15 | 14 | 94 | 12 | 1.14 | 0.64–2.06 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=107 | % | N=753 | % | |||
| Received Any Immunomodulators | 60 | 56 | 342 | 45 | 1.53 | 1.02–2.31 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=60 | % | N=342 | % | |||
| Received Steroids Alone | 42 | 70 | 251 | 73 | 0.85 | 0.46–1.54 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=69 | % | N=446 | % | |||
| Height - abnormal z-score | 18 | 26 | 132 | 30 | 0.84 | 0.47–1.49 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=73 | % | N=475 | % | |||
| Weight - abnormal z-score | 30 | 41 | 174 | 37 | 1.21 | 0.73–1.99 |
Table 5.
UC – Parameters at Diagnosis
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=25 | % | N=384 | % | |||
| Age at Diagnosis | ||||||
| <6y | 2 | 8 | 84 | 22 | 0.31 | 0.07–1.34 |
| 6–12y | 9 | 36 | 173 | 45 | 0.69 | 0.30–1.59 |
| >12y | 14 | 56 | 127 | 33 | 2.58 | 1.14–5.83 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=25 | % | N=384 | % | |||
| Age at Symptom Onset | ||||||
| <6y | 3 | 12 | 92 | 24 | 0.43 | 0.13–1.48 |
| 6–12y | 8 | 32 | 179 | 47 | 0.54 | 0.23–1.28 |
| >12y | 14 | 56 | 113 | 29 | 3.05 | 1.34–6.93 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=13 | % | N=189 | % | |||
| Hgb <10mg/dL | 4 | 31 | 33 | 17 | 2.10 | 0.61–7.23 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=13 | % | N=162 | % | |||
| ESR >20 mm/hr | 12 | 92 | 70 | 43 | 15.77 | 2.00–124.2 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=9 | % | N=147 | % | |||
| Alb <3mg/dL | 2 | 22 | 18 | 12 | 2.05 | 0.39–10.63 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=25 | % | N=384 | % | |||
| EIMC present | 2 | 8 | 13 | 3 | 2.48 | 0.53–11.66 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=25 | % | N=384 | % | |||
| Received Any Immunomodulators | 15 | 60 | 133 | 35 | 2.83 | 1.24–6.47 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=15 | % | N=133 | % | |||
| Received Steroids Alone | 14 | 93 | 115 | 86 | 2.19 | 0.27–17.69 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=20 | % | N=203 | % | |||
| Height - abnormal z-score | 2 | 10 | 43 | 21 | 0.41 | 0.09–1.85 |
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=21 | % | N=219 | % | |||
| Weight - abnormal z-score | 5 | 24 | 50 | 23 | 1.06 | 0.37–3.03 |
Of the 624 children with ESR recorded at diagnosis (395 CD, 175 UC, 54 IC), the proportion of AA with abnormally elevated ESR was significantly greater than non-AA for combined IBD diagnoses (80% vs. 55%; OR 3.24, 95% CI 1.76–5.97), and separately for CD (78% vs. 64%; OR 2.05, 95% CI 1.02 – 4.14) and UC (92% vs. 43%; OR 15.77, 95% CI 2.00–124.2) (Tables 3, 4 and 5). For the 529 children with a recorded Alb at diagnosis (328 CD, 156 UC, 45 IC), no racial differences existed in the proportions with abnormally low levels, for any IBD disease classification (Tables 3–6).
The database recorded whether or not the child received corticosteroids, 6-mercaptopurine, azathioprine or Infliximab, alone or in any combination, around the time of diagnosis for all of the 1,406 cohort children. Early treatment with immunomodulatory agents (within 30 days of diagnosis) was given to 593 patients, including a significantly greater proportion of AA: 57% (N=78) compared to 41% (N=515) of non-AA (OR 1.90, 95% CI 1.33–2.71) (Table 3). This racial/ethnic difference in early therapy was also noted among those with CD: 56% (N=60) of AA compared to 45% (N= 342) of non-AA (OR 1.53, 95% CI 1.02–2.31) and those with UC: 60% (N=15) of AA compared to 35% (N=133) of non-AA (OR 2.83, 95% CI 1.24–6.47) (Tables 4 and 5). Analyses found no statistically significant difference in race/ethnicity among the IC patients receiving early immunomodulatory therapy (Table 6). No significant difference existed in the proportions of AA and non-AA with IBD or any IBD subtype who received corticosteroid monotherapy (Tables 3–6).
The entire cohort had information recorded for the presence or absence of EIMC. The frequencies of EIMC did not differ with regard to race/ethnicity (Tables 3, 4, 5, and 6). Subanalyses of each EIMC were not performed.
Examining data on all CD discerned no racial/ethnic differences in the proportions of patients with perianal disease, strictures or fistulae (Table 7). The proportions of AA and non-AA with abnormally low z-scores for height and weight were similar for the 835 and 886 patients, respectively, with recorded data. Distributions of anatomic disease location were similar among AA and non-AA for both CD and UC (Tables 3–6).
Table 7.
CD Disease Location and Complications at Diagnosis
| AA | Non-AA | OR | CI | |||
|---|---|---|---|---|---|---|
| N=107 | % | N=753 | % | |||
| Isolated Colon | 13 | 12 | 118 | 16 | 0.74 | 0.40–1.37 |
| Ileocolonic Alone | 15 | 14 | 82 | 11 | 1.33 | 0.74–2.41 |
| Small bowel Alone | 1 | 1 | 14 | 2 | 0.50 | 0.06–3.83 |
| Upper GI Alone | 0 | 0 | 9 | 1 | - | - |
| All Other Combinations | 78 | 73 | 530 | 70 | 1.13 | 0.72–1.78 |
| Perianal Disease | 3 | 3 | 36 | 5 | 0.57 | 0.17–1.90 |
| Fistulae | 6 | 6 | 34 | 5 | 1.26 | 0.51–3.07 |
| Strictures | 4 | 4 | 33 | 4 | 0.85 | 0.29–2.44 |
DISCUSSION
Our study describes the characteristics of IBD among AA in the largest cohort of affected children reported in the literature to date. The majority of previous investigations describing the characteristics of IBD in minority populations evaluated much smaller cohorts. In fact, the sample size for the only other published description of IBD in AA children was <10% of this study's sample size11.
Consistent with two previous reports that describe CD as the most prevalent IBD disease classification in children, regardless of race9, 11, CD also predominates in this investigation. The present study also showed that AA children were more likely to have CD than their non-AA counterparts but less likely to have UC. This contrasts with two earlier but smaller studies from Atlanta11 and Britain8 where the distributions of IBD classifications did not differ by race. The increased statistical power of our sample size may have permitted small but statistically significant and clinically relevant differences to be discerned.
Previous studies reported mean ages at diagnosis ranging from 9 to 13 years old for children with CD and UC, whereas children with IC were slightly younger (7 to 12 years old) when diagnosed9, 16. In our cohort, AA children were more likely to be >12 years old when diagnosed with IBD than the non-AA children. This remained true for the subsets diagnosed with UC or IC. Interestingly, the age distribution of CD patients did not vary by race/ethnicity, despite the increased likelihood that AA would be diagnosed with CD. We speculate that the older age of IBD diagnosis observed among the AA in our cohort may be due in part to a low index of suspicion for IBD in minority children among medical providers because it has traditionally been viewed as a disease of Caucasians and adults. This perception could delay appropriate diagnostic investigations, leading to an older age at the definitive diagnosis of IBD. Secondly, as observed in other chronic medical conditions, under-represented minorities may have decreased access to medical care17 or different patterns of health care seeking behavior14, 17 that could lead to an older age at diagnosis. The present retrospective study could not assess access to healthcare or the physician and patient knowledge, attitudes and practices that might influence the speed of diagnosis. Moreover, socioeconomic status (SES) data on individual patients was not ascertained in this data registry, another limitation of this study. Although potentially an important piece of information to include in a prospective study, we are unable to speculate about the influence of SES on healthcare access and speed of diagnosis after symptom recognition in AA compared to non-AA children. Finally, IBD in AA children may represent a phenotype distinct from that of non-AA children. Prospective, population-based, case:control studies are needed to better understand the reasons underlying the older age at diagnosis, as observed in our study cohort.
Using dates of diagnosis in conjunction with duration of symptoms, estimated ages of IBD symptom onset in recent pediatric investigations ranged from 6 to 12 years old, irrespective of race/ethnicity16, 18. Among AA in our study population, symptoms of CD and UC, as well as all IBD classifications combined, appeared to begin more frequently in the teenage years (>12 years old). The reasons underlying the older age of symptom onset in AA children with IBD remain unexplained. However, they could parallel a lower index of suspicion, less frequent perceptions of poor health among certain cultures and socioeconomic groups or recall bias.
Anemia, hypoalbuminemia and elevated ESR are common features of IBD among both adult and pediatric patients18–19. However, lower Hgb levels were recently reported in adult AA when compared to Caucasians14. Similarly, AA children in our study were more often anemic at the time of diagnosis, regardless of their IBD classification, than the other racial/ethnic groups in the cohort. A recent pediatric study that did not take race/ethnicity into account reported significantly higher ESR values between CD than UC patients,20 whereas another pediatric study described similar ESR values between CD and UC patients9. In our cohort, a greater proportion of AA with UC and IC had abnormally high ESR, whereas among CD patients, the proportions with elevated ESR did not differ between racial/ethnic groups. Since only designations of normal or abnormal Hgb or ESR were extracted from the registry, the severity and clinical significance of these parameters remain unclear. Possible explanations include longer duration of inflammation prior to diagnosis (consistent with but not conclusive for an older age at diagnosis), poorer underlying health or nutrition unrelated to the IBD or even different GI microbial environments related to the onset of or secondary to the presence of IBD.
EIMC are reported in 3 to 50% of children with IBD3. One adult study reported that a larger percentage of AA IBD patients had sclerosing cholangitis, toxic megacolon, arthritis, erythema nodosum and pyoderma gangrenosum compared to Caucasians with IBD21. The numbers of children in our cohort with EIMC at diagnosis were small so the findings must be interpreted with caution despite statistical significance. Nevertheless, although proportionally fewer AA children had UC, those with UC were four times more likely to have EIMC at diagnosis than their non-AA counterparts. These observations could suggest that a different disease phenotype exists or predominates among AA children with UC. Of note, this study compared IBD among AA children only to the broad group of non-AA children, comprised mostly of Caucasians (by self-report). Therefore we cannot relate this hypothesis to disease phenotype in individual racial/ethnic groups. Further studies are needed to explain this observation and determine if it translates into a higher cancer risk among AA with UC.
Our report is unique among epidemiologic descriptions of pediatric IBD in that it characterizes the largest cohort of children with IBD to date, using standardized case definitions and evaluation protocols. However, retrospective analysis of registry data presents certain limitations. The PediIBD Consortium patient registry represents a convenience sampling of all IBD patients followed by Consortium gastroenterologists. Even though each participating site draws patients from varied demographic and geographic populations, we cannot be certain that the Consortium cohort fully represents pediatric IBD in the general population—regional, national or even global. This study could not examine the true prevalence of IBD among AA children in the U.S.; AA comprised 9% of the patients in the study cohort, compared with 14% of the U.S. pediatric population22. Additionally, because at least five of the contributing sites are tertiary care and referral centers with outreach clinical practices (one is exclusively a large community private practice), the cohort may have a selection bias for more severe IBD cases. It is also conceivable that older (e.g., teenage) minority groups are more often referred to academic centers that care for underinsured patients, while Caucasian adolescents may be more often evaluated and followed in private practice settings.
For purposes of this study, the most recent diagnosis recorded at the date of data accession was considered the IBD disease classification, a cross-sectional view. Clinically, disease could also have evolved after the data accession date. Additionally, we cannot exclude disease misdiagnosis and misclassification. Unknown medical record accuracy and completeness of information, particularly for those children diagnosed elsewhere prior to inclusion in the Consortium, may contribute to misclassification. Moreover, the varying completeness of Z-scores for height and weight, ESR, Hgb, and Alb, for example, could miss significant relationships or over-interpret the study results. Similar to other retrospective investigations, subjective recall of symptom onset by patients and their parents (recall bias) impact the accurate and specific ascertainment of IBD symptom onset in our registry. Finally, due to small numbers, meaningful analyses could not be made for non-AA, non-Caucasian populations such as Asians or Hispanics. Nevertheless, the findings are noteworthy and suggest racial/ethnic differences in pediatric IBD that may have substantial implications for diagnosis, treatment and disease prognosis. Clearly, prospective investigations which test specific hypotheses regarding pathobiology of IBD in AA as compared to non-AA children, and that employ such disease predictors as serology (e.g., ANCA, ASCA, Omp-C) and/or specific genotype presence absence (e.g., NOD2/CARD15) are needed.
We believe our observations provide a framework for further investigations that characterize IBD in racially diverse pediatric populations. Potential causes for the noted older age at symptom onset and diagnosis in AA children should be examined. The duration of time between symptom onset and disease diagnosis can be important in determining IBD severity, phenotype, and management options, and should be further studied with regard to race/ethnicity. Cross-sectional and longitudinal analyzes with increased power and more representation of diverse racial/ethnic groups are clearly needed in order to further characterize the immunology, genetic and socio-economic influences on IBD phenotype in racial/ethnic diverse populations.
Grant Support and Acknowledgments
The authors want to thank Daphne Pierce-Smith, MSN, FNP for her guidance and critical review, Joel Cutler for his vision and leadership in raising awareness and support for issues relevant to children with inflammatory bowel disease, and Karen Cowgill, PhD, RN for her guidance during the preparation of this manuscript. Initial data gathering was supported by the Crohn's and Colitis Foundation of America (CCFA). Benjamin D. Gold, MD is supported in part by grants from the NIH (DK53708-07 and DK06544-01). Melvin B. Heyman, MD is supported in part by NIH grant (DK060617). Barbara S. Kirschner, MD is supported in part by the Nathan Cummings Foundation, Chicago, IL. Harland Winter, MD is supported in part by the Wallace Family.
Abbreviations
- (AA)
African-Americans
- (Alb)
Albumin
- (CD)
Crohn's Disease
- (ESR)
Erythrocyte sedimentation rate
- (EIMC)
Extraintestinal manifestations and complications
- (Hgb)
Hemoglobin
- (IC)
Indeterminate colitis
- (IBD)
Inflammatory bowel disease
- (UC)
Ulcerative colitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Burakoff R. Indeterminate colitis: clinical spectrum of disease. J Clin Gastroenterol. 2004;38:S41–3. doi: 10.1097/01.mcg.0000123991.13937.7e. [DOI] [PubMed] [Google Scholar]
- 2.Odze RD. Pathology of indeterminate colitis. J Clin Gastroenterol. 2004;38:S36–40. doi: 10.1097/01.mcg.0000127686.69276.5b. [DOI] [PubMed] [Google Scholar]
- 3.Hyams JS. Extraintestinal manifestations of inflammatory bowel disease in children. J Pediatr Gastroenterol Nutr. 1994;19:7–21. doi: 10.1097/00005176-199407000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Markowitz J, Grancher K, Rosa J, Simpser E, Aiges H, Daum F. Highly destructive perianal disease in children with Crohn's disease. J Pediatr Gastroenterol Nutr. 1995;21:149–53. doi: 10.1097/00005176-199508000-00005. [DOI] [PubMed] [Google Scholar]
- 5.Loftus EV. Clinical Epidemiology of inflammatory bowel disease: incidence, prevalence, and environmental influences. Gastroenterology. 2004;126:1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 6.Kurata JH, Kantor-Fish S, Frankl H, Godby P, Vadheim CM. Crohn's disease among ethnic groups in a large heath maintenance organization. Gastroenterology. 1992;102:1940–8. doi: 10.1016/0016-5085(92)90317-r. [DOI] [PubMed] [Google Scholar]
- 7.Reddy SI, Burakoff R. Inflammatory bowel disease in African Americans. Inflamm Bowel Dis. 2003;9:380–5. doi: 10.1097/00054725-200311000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Sawczenko A, Sandhu BK, Logan RF, Jenkins H, Taylor CJ, Mian S, Lynn R. Prospective survey of childhood inflammatory bowel disease in the British Isles. Lancet. 2001;357:1093–4. doi: 10.1016/s0140-6736(00)04309-9. [DOI] [PubMed] [Google Scholar]
- 9.Kugathasan S, Judd RH, Hoffmann RG, Heikenen J, Telega G, Khan F, Weisdorf-Schindele S, San Pablo W, Jr., Perrault J, Park R, Yaffe M, Brown C, Rivera-Bennett MT, Halabi I, Martinez A, Blank E, Werlin SL, Rudolph CD, Binion DG. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J Pediatr. 2003;143:525–31. doi: 10.1067/s0022-3476(03)00444-x. [DOI] [PubMed] [Google Scholar]
- 10.Heyman M, Kirschner B, Gold B, Ferry G, Baldassano R, Cohen SA, Winter HS, Fain P, Smith T, El-Serag HB. Children with Early Onset Inflammatory Bowel Disease: Findings of a Pediatric IBD Consortium Registry. J Pediatr. 2005;146:35–40. doi: 10.1016/j.jpeds.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 11.Ogunbi SO, Ransom JA, Sullivan K, Schoen BT, Gold BD. Inflammatory bowel disease in African-American children living in Georgia. J Pediatr. 1998;133:103–7. doi: 10.1016/s0022-3476(98)70187-8. [DOI] [PubMed] [Google Scholar]
- 12.Basu D, Lopez I, Kulkarni A, Sellin J. Impact of race and ethnicity on inflammatory bowel disease. Am J Gastroenterol. 2005;100:2254–61. doi: 10.1111/j.1572-0241.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 13.Kugathasan S, Loizides A, Babusukumar U, McGuire E, Wang T, Hooper P, Nebel J, Kofman G, Noel R, Broeckel U, Tolia V. Comparative phenotypic and CARD15 mutational analysis among African American, Hispanic, and White children with Crohn's disease. Inflamm Bowel Dis. 2005;11:631–8. doi: 10.1097/01.mib.0000171279.05471.21. [DOI] [PubMed] [Google Scholar]
- 14.Straus WL, Eisen GM, Sandler RS, Murray SC, Sessions JT. Crohn's disease: does race matter? The Mid-Atlantic Crohn's Disease Study Group. Am J Gastroenterol. 2000;95:479–83. doi: 10.1111/j.1572-0241.2000.t01-1-01531.x. [DOI] [PubMed] [Google Scholar]
- 15.U.S. Census Bureau; Census 2000, Summary File 2; generated by Jolanda White; using American Fact Finder;; (22 December 2004)
- 16.Heikenen J, Werlin S, Brown C, Balint J. Presenting symptoms and diagnostic lag in children with inflammatory bowel disease. Inflamm Bowel Dis. 1998;5:158–160. doi: 10.1097/00054725-199908000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Smedley B, Stith A, Nelson A. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Institute of Medicine; Washington: 2005. [PubMed] [Google Scholar]
- 18.Langholz E, Munkholm P, Krasilnikoff PA, Binder V. Inflammatory bowel diseases with onset in childhood. Clinical features, morbidity, and mortality in a regional cohort. Scand J Gastroenterol. 1997;32:139–47. doi: 10.3109/00365529709000184. [DOI] [PubMed] [Google Scholar]
- 19.Lindberg E, Lindquist B, Holmquist L, Hildebrand H. Inflammatory bowel disease in children and adolescents in Sweden, 1984-1995. J Pediatr Gastroenterol Nutr. 2000;30:259–64. doi: 10.1097/00005176-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Weinstein T, Levine M, Pettei M, Gold D, Kessler B, Levine J. Age and family history at presentation of pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2003;37:609–613. doi: 10.1097/00005176-200311000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Simsek H, Schuman BM. Inflammatory bowel disease in 64 black patients: analysis of course, complications, and surgery. J Clin Gastroenterol. 1989;11:294–8. 18. doi: 10.1097/00004836-198906000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Characteristics of Children under 18 years of Age for the United States, Regions, States and Puerto Rico: 2000. Census 2000 PHC-T-30. [Google Scholar]