A 49-year-old man was referred to our endocrine clinic because of rising thyroid-stimulating hormone (TSH) levels despite increasing doses of levothyroxine. The patient had a history of Graves disease, which had been successfully treated with radioiodine ablation 15 years earlier. Over the past several years, his serum TSH levels had risen to 31.5 (normal 0.4–4.5) mU/L, and the dose of levothyroxine he was prescribed had been increased to 225 μg per day, or 2.7 (usual recommended dose 1.6) μg/kg daily. The patient’s weight was 82 kg, and he did not report any change to his weight. The patient reported feeling well, and his physical exam was unremarkable. His level of free thyroxine was 15.8 (normal 10–25) pmol/L. The patient reported taking his antihypertensive medication (diltiazem) regularly as prescribed, and he was not taking any over-the-counter medications or herbal supplements.
To confirm our patient’s adherence to the drugs he had been prescribed, and to exclude impaired bioavailability of the medication, we performed a medically supervised test for the absorption of levothyroxine. The results of the test showed that only 30% of the medication administered was absorbed. We proceeded to rule out levothyroxine maldigestion related to gastric hypochlorhydria.
Laboratory investigations included a biochemistry panel and tests for serum levels of parathyroid hormone, 25-hydroxyvitamin D, ferritin, vitamin B12 and gastrin, all of which showed normal results. A serological test to determine the presence of Helicobacter pylori was negative, and the patient’s parietal cell antibody titers were normal. Given these results, it was unlikely that the patient’s treatment-refractory hypothyroidism was related to hypochlorhydria.
In our investigation of intestinal malabsorption, the screening serum test for gluten enteropathy was abnormal; the level of immunoglobulin A antibodies against transglutaminase was 75.4 (negative < 9.0, borderline 9–16, positive > 16.0) units/mL. A subsequent endoscopic biopsy of the patient’s bowel was consistent with a diagnosis of celiac disease. The patient was directed to follow a low-gluten diet. The patient’s histological abnormalities resolved, and his serum level of TSH normalized with his usual dose of thyroxine (225 μg daily).
Because of the patient’s previous Graves disease, we decided to investigate for an autoimmune polyglandular syndrome. Subsequent tests showed elevated antiadrenal and 21-hydroxylase antibodies, suggesting autoimmune adrenalitis. A short intravenous adrenocorticotropic hormone (ACTH) stimulation test was consistent with diminished adrenal cortisol reserve.
Discussion
Guidelines identify serum TSH as the best marker for assessing the appropriateness of thyroxine dosage.1 The mean treatment dosage of thyroxine is 1.6 μg/kg daily.2 Primary hypothyroidism is considered refractory to oral thyroxine substitution when there is biochemical or clinical evidence of hypothyroidism (serum level of TSH above the upper target level, usually 4.5 mU/L following a six-week interval after the dosage was last increased), despite increasing dosages of oral thyroxine beyond 2.5 μg/kg daily.3 In these circumstances, further increments in the dosage of thyroxine may not always be the most appropriate intervention. In such a situation, physicians need to search for causes of decreased absorption of thyroxine or increased demand for thyroxine (Table 1).4–7
Table 1:
| Cause of hypothyroidism | Suggested investigations |
|---|---|
| Decreased bioavalability | |
| Poor adherence to drug therapy | Patient report, clinical impression or frequency of prescription refills at pharmacy |
| Absorption of oral levothyroxine | |
| Maldigestion related to hypochlorhydria | |
| Proton-pump inhibitor therapy | Medication history |
| Autoimmune atrophic gastritis | Antiparietal cell antibodies |
| Gastric infection with Helicobacter pylori | Carbon-14 urea breath test, esophagogastroduodenoscopy |
| Intestinal malabsorption of l-thyroxine | |
| Luminal factors (e.g., food, coffee and medications) | Diet and medication history (including herbal and over-the-counter medications) |
| Intramural factors (e.g., short bowel syndrome, lactose intolerance, gluten enteropathy, inflammatory bowel disease, infiltrative enteropathy, infection with Giardia | Transglutaminase antibodies |
| Esophagogastroduodenoscopy with jejunal biopsy | |
| Hydrogen breath test for lactose intolerance | |
| Culture and microscopy of stool for ova and parasites | |
| Increased need for levothyroxine | |
| Weight gain | Increase > 5%–10% from baseline |
| Pregnancy | Serum β-hCG for women of reproductive age |
| Increased metabolism of thyroxine | Medication history |
| Other factors that can alter serum levels of TSH | |
| Addison disease | ACTH stimulation test |
| Altered regulation of the hypothalamic–pituitary–thyroid axis | Liothyronine TSH suppression test |
| TSH heterophile antibodies | Use a different immunoassay |
Note: ACTH = adrenocorticotropic hormone, hCG = human chorionic gonadotropin, TSH = thyroid-stimulating hormone.
Levothyroxine sodium is the most commonly used preparation of thyroid hormone for the treatment of hypothyroidism. Most adults with this condition take 100–125 μg of levothyroxine per day.2 About 60%–80% of an oral dose of thyroxine is absorbed, both in patients with normal thyroid function and in those with hypothyroidism.3 Absorption occurs within three to four hours of ingestion and is localized mainly to the jejunum and ileum.3,8 Adequate gastric acidity is required to dissolve the salt-based tablet, allowing for intestinal absorption.4
Approach to treatment-refractory hypothyroidism
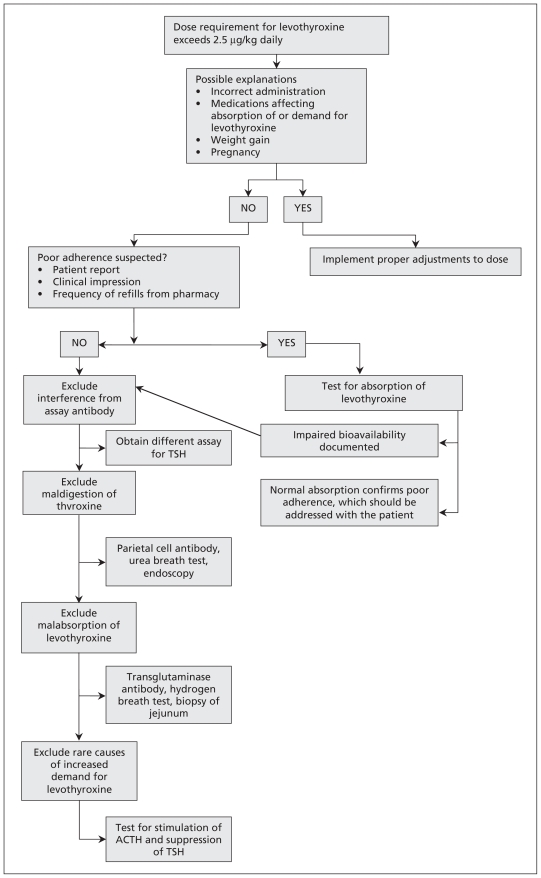
An approach to treating hypothyroidism refractory to supernormal doses of thyroxine is summarized in Figure 1. Common causes for treatment-refractory hypothyroidism include poor adherence to therapy and interactions between thyroxine and medication or food.
Figure 1:
Suggested approach to treatment-resistant hypothyroidism. ACTH = adrenocorticotropic hormone, TSH = thyroid-stimulating hormone.
The absorption of levothyroxine can be affected by the ingestion of certain foods and the timing of meals.9 Fibre-enriched diets and espresso coffee have been shown to interfere with levothyroxine’s absorption.10,11 Waiting to eat for at least 60 minutes after taking the tablet may improve absorption of the drug.
Several foods and medications have been shown to alter the bioavailability of levothyroxine.2,11–15 Some of these substances may interfere with absorption, whereas others may result in accelerated metabolism of levothyroxine via the increased activity of cytochrome P450 enzymes and clearance of the drug, leading to higher dose requirements (Box 1).
Box 1: Substances that affect thyroid function in patients taking levothyroxine2,11–15.
Interference with absorption
Iron
Calcium
Aluminum hydroxide
Chromium picolinate
Cholestyramine
Colestipol
Grapefruit juice
Sevelamer hydrochloride
Sucralfate
Raloxifene
Increased hepatic metabolism by cytochrome P450 enzymes
Phenobarbitol
Phenytoin
Carbamazepine
Rifampin
Tyrosine kinase inhibitors
Rexinoid compounds
Poor adherence is the most common cause of failure of thyroxine therapy.3 Adherence to therapy may be investigated by direct patient report, clinical impression or frequency of refills at the pharmacy. If poor adherence to treatment is suspected, a supervised test for the absorption of orally administered levothyroxine may be helpful5,16 (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110994/-/DC1). Although complications with this test are rare, patients with substantial coronary artery disease should probably be excluded. For patients who have difficulty maintaining regular intake of their thyroid medication, supervised weekly thyroxine replacement (the total weekly dose given once per week) appears to be a safe and well-tolerated option for treatment.17 Parenteral administration (intramuscular or intravenous) of levothyroxine has also been shown to be useful among patients with poor adherence.17
Reduced bioavailability
A deficient supply of levothyroxine may be related to impaired dissolution of the tablet (maldigestion). A recent study linked reduced intestinal absorption to conditions associated with hypochlorhydria such as proton pump inhibitor therapy, infection with Helicobacter pylori and atrophic gastritis.7 It is thought that, unlike native lipophilic thyroxine, the levothyroxine sodium salt preparation does not undergo adequate dissolution with gastric hypochlorhydria, remains hydrophilic and is thus unavailable for intestinal absorption.4 Patients with hypochlorhydria may describe dyspepsia and present with iron or vitamin B12 deficiency. Diagnostic investigations include tests for serum levels of gastrin and antiparietal cell antibodies, a carbon-14 urea breath test and gastroscopy with biopsy (Table 1).
Alternatively, inadequate delivery of levothyroxine may be related to intestinal malabsorption. Treatment-refractory hypothyroidism may be the only presenting symptom of a gastrointestinal disorder. Stone and colleagues noted reduced peak serum levels of levothyroxine among patients with shortened bowel, although no consistent relationship was found between the degree of absorption and bowel length.18 Pre-existing malabsorption conditions such as celiac disease,5 inflammatory bowel disease and lactose intolerance can reduce bioavailability of levothyroxine. This association appears to be strongest with celiac disease, which is frequently associated with autoimmune thyroid disease. Histologically proven celiac disease affects 3.2%–4.8% of people with autoimmune thyroid disease, compared with 0.4% of the general population.5 Because the symptoms of celiac disease are often subtle, some authors suggest screening patients who require higher than expected doses of levothyroxine to treat their hypothyroidism with tissue transglutaminase antibodies.4 The patient’s serum TSH levels usually improve after instituting a gluten-free diet for celiac disease or a lactose-restricted diet and lactose-free levothyroxine formulation for patients with lactose intolerance.5
Increased demand for thyroxine
Sometimes, despite normal absorption of levothyroxine and lack of interactions between medications, a higher dose is needed to normalize a patient’s TSH level. One cause for this increased demand is weight gain. Although the total daily dose of levothyroxine will be higher for people who are obese, the dose per kilogram tends to be lower.19 A case series of 75 consecutive patients post-thyroidectomy reported an inverse relation between the dose of levothyroxine required to normalize TSH levels and body weight (e.g., for a person with a body mass index [BMI] of 18–28, the required dosage was 2.10 ± 0.31 μg/kg daily; for a person with a BMI > 30, the required dosage was 1.63 μg/kg daily). In a prospective study involving 100 patients post-thyroidectomy, the optimal dose of levothyroxine (in micrograms) could be predicted using a simple formula: dose = weight − age + 125, where weight is measured in kilograms and age is measured in years.20
In a prospective study involving 20 pregnant women with treated hypothyroidism, an increased levothyroxine requirement of up to 50% started early in the first trimester, peaked midway through pregnancy and remained constant until delivery.21 Accordingly, women with treated hypothyroidism may need to increase the dose of levothyroxine they are taking to prevent hypothyroidism and its associated adverse outcomes for pregnancy.22 A pregnancy test should thus be part of the workup for every woman of reproductive age with treatment-refractory hypothyroidism.
Although uncommon, the possibility of an analytical error related to interference by heterophilic antibodies during an assay should be considered before proceeding to an exhaustive workup of impaired bioavailability. Case reports describe interference from heterophilic antibodies reacting with monoclonal antibodies in an assay, yielding falsely increased TSH values.6 Physicians may suspect the presence of these antibodies when escalating doses of levothyroxine raise serum thyroxine levels into the hyperthyroxemic range (i.e., serum free thyroxine > 25 pmol/L) despite elevated levels of TSH. A repeat test for TSH using a different immunoassay will usually clarify this issue.6
Other causes of treatment-refractory hypothyroidism are more difficult to identify, such as dysfunction in the hypothalamic–pituitary–thyroid axis (e.g., resistance to thyroid hormone) and Addison disease. Glucocorticoid agents normally depress the secretion of TSH, mainly acting at the hypothalamic level; with adrenal insufficiency in Addison disease, secretion of TSH would be disinhibited. Thus, patients with these conditions should be referred to an endocrinologist.
Case revisited
Obvious reasons for our patient’s inappropriate elevation of TSH were easily excluded in his history and clinical examination. Following our initial history, we could have proceeded directly to investigating for maldigestion or malabsorption of levothyroxine. Instead, we confirmed poor bioavailability with a test for the absorption of the drug. Although our patient did not complain of dyspepsia, abdominal bloating, diarrhea or weight loss, a search for gastrointestinal disorders yielded elevated antibodies for transglutaminase, and esophagogastroduodenoscopy confirmed gluten-sensitive enteropathy (celiac disease). Because two autoimmune disorders had already been diagnosed in our patient over a span of 15 years, we looked for other components of an autoimmune polyglandular syndrome, leading to a further diagnosis of autoimmune adrenalitis with partial adrenal insufficiency.
Our patient’s case shows the importance of a systematic approach to people with treated primary hypothyroidism and persistently elevated serum TSH levels despite adequate levothyroxine replacement.
Key points
When levothyroxine requirements exceed 2.5 μg/kg daily, treatment-refractory hypothyroidism is a possibility.
A supervised test for the absorption of oral levothyroxine can exclude patient nonadherence to the medication.
The results of investigations for causes of decreased absorption of or increased demand for levothyroxine will guide treatment.
Supplementary Material
See also practice article by Clemens and Van Uum on page 210 and at www.cmaj.ca/lookup/doi/10.1503/cmaj.111883
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Both authors contributed to the planning of the manuscript, the review of the literature, and the writing of the manuscript. Both authors approved of the version submitted for publication.
References
- 1.Baloch Z, Carayon P, Conte-Devolx B, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid 2003;13:3–126 [DOI] [PubMed] [Google Scholar]
- 2.Baskin HJ, Cobin RH, Duick DS, et al. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the evaluation and treatment of hyperthyroidism and hypothyroidism. Endocr Pract. 2002;8:457–69 [PubMed] [Google Scholar]
- 3.Lips DJ, van Reisen MT, Voigt V, et al. Diagnosis and treatment of levothyroxine pseudomalabsorption. Neth J Med 2004;62:114–8 [PubMed] [Google Scholar]
- 4.Benvenga S, Bartolone L, Squadrito S, et al. Delayed intestinal absorption of levothyroxine. Thyroid 1995;5:249–53 [DOI] [PubMed] [Google Scholar]
- 5.d’Estève-Bonetti L, Bennet AP, Malet D, et al. Gluten-induced enteropathy (coeliac disease) revealed by resistance to treatment with levothyroxine and alfacalcidol in a sixty-eight-year-old patient: a case report. Thyroid 2002;12:633–6 [DOI] [PubMed] [Google Scholar]
- 6.Santhana Krishnan SG, Pathalapati R, Kaplan L, et al. Falsely raised TSH levels due to human anti-mouse antibody interfering with thyrotropin assay. Postgrad Med J 2006;82:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centanni M, Gargano L, Canettieri G, et al. Thyroxine in goiter, Helicobacter pylori infection, and chronic gastritis. N Engl J Med 2006;354:1787–95 [DOI] [PubMed] [Google Scholar]
- 8.Fish LH, Schwartz HL, Cavanaugh J, et al. Replacement dose, metabolism, and bioavailability of levothyroxine in the treatment of hypothyroidism. Role of triiodothyronine in pituitary feedback in humans. N Engl J Med 1987;316:764–70 [DOI] [PubMed] [Google Scholar]
- 9.Bach-Huynh TG, Nayak B, Loh J, et al. Timing of levothyroxine administration affects serum thyrotropin concentration. J Clin Endocrinol Metab 2009;94:3905–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu AC, Sherman SI. Effects of pharmacological fiber supplements on levothyroxine absorption. Thyroid 1998;8:667–71 [DOI] [PubMed] [Google Scholar]
- 11.Benvenga S, Bartolone L, Pappalardo MA, et al. Altered intestinal absorption of l-thyroxine caused by coffee. Thyroid 2008; 18:293–301 [DOI] [PubMed] [Google Scholar]
- 12.Liwanpo L, Hershman JM. Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab 2009; 23:781–92 [DOI] [PubMed] [Google Scholar]
- 13.Leghait J, Gayrard V, Picard-Hagen N, et al. Fipronil-induced disruption of thyroid function in rats is mediated by increased total and free thyroxine clearances concomitantly to increased activity of hepatic enzymes. Toxicology 2009;255:38–44 [DOI] [PubMed] [Google Scholar]
- 14.John-Kalarickal J, Pearlman G, Carlson HE. New medications which decrease levothyroxine absorption Thyroid 2007;17:763–5 [DOI] [PubMed] [Google Scholar]
- 15.David DG. Bailey. Fruit juice inhibition of uptake transport. A new type of food–drug interaction. Br J Clin Pharmacol 2010; 70:645–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa D, Otsuka F, Mimra Y, et al. Pseudomalabsorption of levothyroxine: a case report. Endocr J 2000;47:45–50 [DOI] [PubMed] [Google Scholar]
- 17.Grebe SK, Cooke RR, Ford HC, et al. Treatment of hypothyroidism with once weekly thyroxine. J Clin Endocrinol Metab 1997;82:870–5 [DOI] [PubMed] [Google Scholar]
- 18.Stone E, Leiter LA, Lambert JR, et al. l-Thyroxine absorption in patients with short bowel. J Clin Endocrinol Metab 1984;59: 139–41 [DOI] [PubMed] [Google Scholar]
- 19.Santini F, Pinchera A, Marsili A, et al. Lean body mass is a major determinant of levothyroxine dosage in the treatment of thyroid diseases. J Clin Endocrinol Metab 2005;90:124–7 [DOI] [PubMed] [Google Scholar]
- 20.Mistry D, Atkin S, Atkinson H, et al. Predicting thyroxine requirements following total thyroidectomy. Clin Endocrinol (Oxf) 2011;74:384–7 [DOI] [PubMed] [Google Scholar]
- 21.Alexander EK, Marqusee E, Lawrence J, et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism. N Engl J Med 2004;351:241–9 [DOI] [PubMed] [Google Scholar]
- 22.Soldin OP, Soldin SJ, Vinks AA, et al. Longitudinal comparison of thyroxine pharmacokinetics between pregnant and nonpregnant women: a stable isotope study. Ther Drug Monit 2010;32:767–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.