Imagine a world where prices have gone completely haywire, spiking more than, say, 600%. A cup of coffee for $13? Gas at $7 a litre?
Such is the plight of many hospitals and other health care providers in the United States as they grapple with extraordinary markups for vital drugs they can’t get any other way. In one stark example, a generic beta blocker for high blood pressure, normally US$25.90 a dose, went for US$1200.
With the US facing a pernicious shortage of key meds (www.cmaj.ca/lookup/doi/10.1503/cmaj.109-4018), middlemen have rushed into the breach, snapping up supplies where they can find them and selling them for many times their usual worth. Now the aggressive spread of this pharmaceutical grey market has rung alarm bells in Congress and cast light on outdated drug-supply laws.
At its best, the grey market has been a lifeline for patients who need drugs that traditional channels can’t always deliver. But the seat-of-the-pants nature of the business means that pharmacy managers are paying exorbitant costs for drugs that may be of dubious quality. The source of the drug’s production, its handling, refrigeration and expiration dates may be unknown or misreported. Sometimes the drugs are stolen, diluted or counterfeit.
In July 2011, the US Food and Drug Administration proposed rules that only require wholesalers to document a drug’s pedigree back to its last authorized distributor, not all the way back to the manufacturer. That’s just one of the information gaps that providers are left to deal with as they scramble to meet pharmaceutical demands.
Two recent studies laid bare the dilemma facing providers and helped prod legislators in Washington to open investigations into the grey market.
Premier Healthcare Alliance, a large purchasing network for providers, collected more than 1700 drug-sale offers from grey market vendors to 42 acute care hospitals, all covering meds that were unavailable or back-ordered from wholesalers. The main finding: Prices were marked up by an average of 650%, sensationally higher in many cases, especially for drugs needed by the critically ill. The cardiology drug labetalol was marked up 4533%; the cancer drug cytarabine, 3980% (www.premierinc.com/about/news/11-aug/Gray-Market/Gray-Market-Analysis-08152011.pdf)
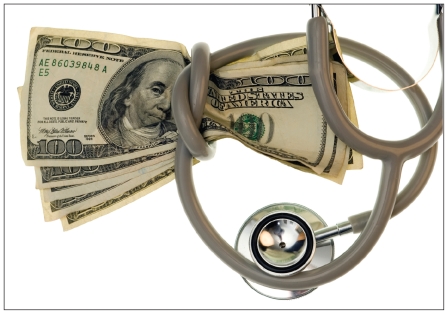
Hospitals and health care providers say they are being strangled by extraordinary markups for vital drugs.
Image courtesy of ©2012 Thinkstock
Meanwhile a survey of more than 500 hospitals by the Institute for Safe Medication, a patient safety group, found that more than half had bought at least one drug from a grey market vendor in the last two years. One-third reported being charged at least 10 times the usual contract price (www.ismp.org/newsletters/acutecare/showarticle.asp?id=3).
Survey respondents were quick to vent their anger at having to deal with “scalpers” in matters of life and death. One reported paying US$325 for a 25-count box of electrolytes, normally US$17. Another paid US$750 instead of the usual US$50 for calcium gluconate, and US$25 000 instead of $1500 for propofol. “You are hesitant to tell gray market vendors what you need because they will buy it all up if they find it,” one respondent said, “then harass you (to buy it) for months afterwards.”
Dr. Adam J. Fein, a consultant on pharmaceutical economics, says the Wild West has come to drug distribution and “the results aren’t pretty.”
It’s not clear, though, that the market is being driven only by sketchy operators. There is no federal law against drug price gouging, although Democratic Senator Charles Schumer of New York has plans to introduce such legislation, and Fein counts 20 states as having no licensing or drug-pedigree regulations. “It’s scarily plausible that some secondary vendors are technically playing by the rules,” he says on www.drugchannels.net, his website.
Dr. Scott Gottlieb, a resident fellow at the conservative American Enterprise Institute, says small distributors have higher costs than wholesalers, rush far a field to fill urgent needs and have their place. “Cracking down on inappropriate profiteering, while an important endeavor, won’t solve the shortages,” he says, “and will only add to our challenges if it ends up also impacting the legitimate activity of small distributors that help plug gaps in the existing supply chain.”
Even so, costs have gotten so out of whack that Washington may take action, however tentatively.
President Barack Obama has ordered the FDA to notify the Justice Department of instances of possible collusion or gouging, to lean on companies to report impending drug shortages and to expedite applications to develop new sources for those drugs. The powerful Senate Finance Committee is examining the use of makeshift drugs in emergency rooms and supply disruptions.
In the House of Representatives, Democratic congressman Elijah Cummings from Maryland is leading an investigation by the Committee on Oversight and Government Reform, so far pressing five grey-market companies to turn over documents on their operations. “We want to know where these companies are getting these drugs, and how much they are making in profits,” he said. “Obtaining this information will help us develop concrete solutions.”
One of the companies apparently has a lot more on the go than drugs. It peddled cytarabine for more than US$990 per vial, instead of the typical US$12, working from an address that also promotes “Passive Income for Life” and “NO MONEY DOWN APARTMENT BUILDINGS.”