Abstract
Background:
Primary care networks are a newer model of primary care that focuses on improved access to care and the use of multidisciplinary teams for patients with chronic disease. We sought to determine the association between enrolment in primary care networks and the care and outcomes of patients with diabetes.
Methods:
We used administrative health care data to study the care and outcomes of patients with incident and prevalent diabetes separately. For patients with prevalent diabetes, we compared those whose care was managed by physicians who were or were not in a primary care network using propensity score matching. For patients with incident diabetes, we studied a cohort before and after primary care networks were established. Each cohort was further divided based on whether or not patients were cared for by physicians enrolled in a network. Our primary outcome was admissions to hospital or visits to emergency departments for ambulatory care sensitive conditions specific to diabetes.
Results:
Compared with patients whose prevalent diabetes is managed outside of primary care networks, patients in primary care networks had a lower rate of diabetes-specific ambulatory care sensitive conditions (adjusted incidence rate ratio 0.81, 95% confidence interval [CI] 0.75 to 0.87), were more likely to see an ophthalmologist or optometrist (risk ratio 1.19, 95% CI 1.17 to 1.21) and had better glycemic control (adjusted mean difference −0.067, 95% CI −0.081 to −0.052).
Interpretation:
Patients whose diabetes was managed in primary care networks received better care and had better clinical outcomes than patients whose condition was not managed in a network, although the differences were very small.
Diabetes is a major cause of myocardial infarction, stroke, blindness and kidney failure, and accounts for nearly 15% of total health care expenditures.1,2 Its management is time-consuming and challenging, requires an individualized approach, and is usually coordinated by primary care physicians. Although safe, efficacious and cost-effective interventions for diabetes are available, studies suggest that patients are often not receiving these treatments.3,4 As such, programs that aim to improve the care of patients with chronic diseases, such as diabetes, must support providers of primary care, typically by coordinating teams of allied health providers. Although such comprehensive care can improve clinical outcomes,5 it is difficult to deliver and is optimized by programs that focus on the multidisciplinary management of chronic disease.6
Primary care networks were implemented in Alberta, Canada, in 2005 and are a potential strategy for improving care for patients with diabetes.7 A primary care network consists of primary care physicians and other health care providers working together to provide care to patients. In addition to typical physician services paid for on a fee-for-service basis, $50 per patient per year is provided to networks to support activities that fall outside of this model. Although networks may vary in size, the first 18 networks each provided care to nearly 90 000 patients and included an average of 75 primary care physicians (including groups of physicians from different practices). The objectives guiding all primary care networks are similar; they include increasing access to primary care, increasing emphasis on care for patients with chronic diseases and improving the coordination of primary health services with specialist care (Box 1). Caring for patients with diabetes was identified as a priority for 17 of the original 18 networks.7,8
Box 1: Objectives of Alberta’s primary care networks7.
Increasing the number of residents with access to primary care services
Managing access to appropriate around-the-clock primary care services
Emphasizing the promotion of health, prevention of disease and injury, and care of patients with medically complex problems or chronic disease
Improving the coordination of primary health services with hospitals and services providing long-term and specialty care
Fostering a team approach to providing primary health care
There is considerable flexibility in how networks may operate, and the additional funds may be used either to hire allied health care professionals or for other initiatives. The types of programs offered by primary care networks for patients with diabetes vary substantially across networks.8 Generally, most offer programs for the education of patients, and about one-third either offer case management or employ members of the multidisciplinary team, other than the primary physician, who have the authority to provide alternative prescriptions.
Although Alberta’s primary care networks have some unique features, they are similar to Ontario’s family health teams9,10 and patient-centered Medical homes in the United States.11 Each of these approaches seeks to improve access to and coordination of care.
We sought to determine the impact of Alberta’s primary care networks on measures of processes, including the provision of guideline-recommended laboratory testing, appropriate use of medications, glycemic control and outcomes relevant to patients with diabetes (admissions to hospital or visits to emergency departments for diabetes-specific ambulatory care sensitive conditions [hypoglycemia, hyperglycemia]12,13 that might be partially prevented by appropriate outpatient care).
Methods
Data sources
We obtained patients’ characteristics, vital status, admissions to hospital, visits to emergency departments, use of physician services and use of medications (for patients > 65 years of age) from Alberta Health and Wellness. We obtained the results of laboratory investigations (glycated hemoglobin levels, cholesterol panels, estimated glomerular filtration rate and measures of proteinuria) from a provincial repository (Appendix 1, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110755/-/DC1). The study was approved by the institutional review boards for the Universities of Alberta and Calgary.
Study population
We identified a cohort of patients with diabetes using a validated algorithm.14,15 Patients were considered to have diabetes based on two or more physician claims for diabetes (International Classification of Diseases 9th revision [Clinical Modification] [ICD-9-CM], code 250) in two years, or one or more admissions to hospital with ICD-9-CM code 250, selected from all available diagnostic codes on the hospital discharge abstract between Apr. 1, 1994, and Mar. 31, 2002. After Mar. 31, 2002, we used the equivalent codes from the 10th revision of the ICD (E10–E14). Previous research comparing the use of this definition with chart-confirmed diagnoses in primary care has shown that it has 86% sensitivity and 97% specificity.15
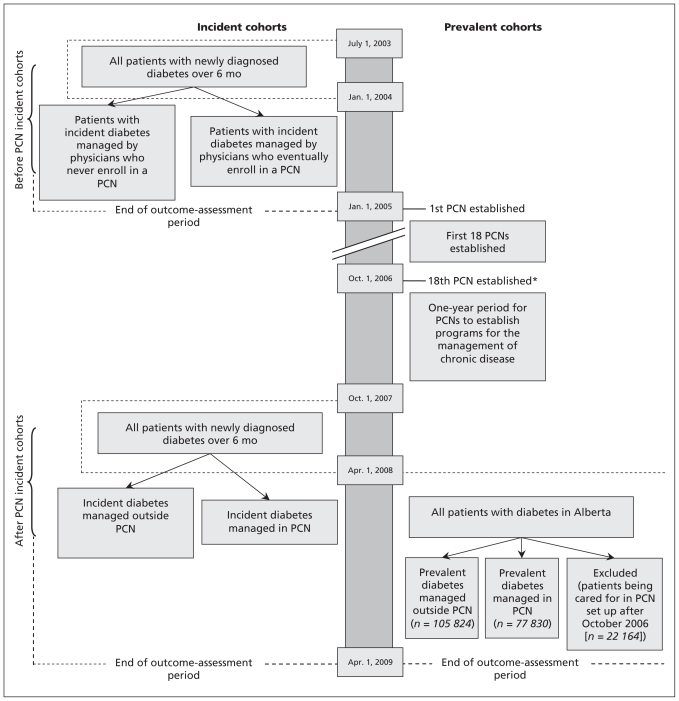
After primary care networks have been established, time is needed to enrol patients and measure their outcomes. For this reason, we assessed the first 18 primary care networks that were operational as of Oct. 1, 2006. We waited one year after primary care networks began before identifying the cohort of patients with incident diabetes. This delay gave each network an opportunity to reorganize care and establish functional programs for the management of chronic disease (Figure 1). We determined enrolment in a primary care network from administrative data.
Figure 1:
Description of patient cohorts (patients with incident or prevalent diabetes), before and after the start of primary care networks. PCN = primary care network.
Because the impact of a primary care network might differ for patients who have had diabetes for a long time, we created separate cohorts of patients with incident and prevalent diabetes. We studied patients with incident diabetes before and after primary care networks were established to control for possible improvements in diabetes care that were independent of the establishment of the networks (Appendix 1).
The cohort of patients with prevalent diabetes was defined as all patients who met our definition of diabetes by Apr. 1, 2008 (Figure 1), divided into two contemporary cohorts: those whose care was managed in a primary care network and those whose care was not managed in a network. We excluded patients with prevalent diabetes that was managed by primary care networks established after 2007. Patients were followed for one year to assess outcomes.
Outcomes
We considered a hierarchy of potential outcomes. We were unable to include some relevant outcomes, either because of the short-term nature of our study or because we did not have the data to inform the outcome (Appendix 2, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110755/-/DC1). Our primary outcome was admissions to hospital or visits to emergency departments for diabetes-specific ambulatory care sensitive conditions (i.e., hypoglycemia and hyperglycemia, which are partially preventable through appropriate outpatient care). This outcome is a validated proxy for the quality of primary care for diabetes16 and has been recommended by the Canadian Institute of Health Information (Appendix 3, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110755/-/DC1).13
Our secondary outcomes included the proportion of patients who underwent guideline-recommended laboratory investigations for glycated hemoglobin level, urine albumin–creatinine ratio and cholesterol panels; the use of indicated medications among patients aged 66 years and older (i.e., filling a prescription for statins,17 metformin [in patients for whom an oral hypoglycemic agent is prescribed] and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers [for patients with proteinuria]18); and outpatient visits to primary care physicians or specialists in internal medicine or endocrinology. For patients for whom a measurement of glycated hemoglobin was available, we also assessed glycemic control (last measurement of glycated hemoglobin during the assessment period for patients with incident diabetes; mean level of glycated hemoglobin for patients with prevalent diabetes). Finally, we assessed the proportion of patients who saw an ophthalmologist or optometrist (a proxy for retinal screening).
Covariables and other variables
Demographic data were determined from the Alberta Health and Wellness registry file. We used validated algorithms to define hypertension;19 other comorbid conditions were defined using validated coding algorithms.20 Chronic kidney disease was defined using the most recent outpatient assessment of estimated rate of glomerular filtration (< 60 mL/min per 1.73 m2).21
Statistical analysis
For patients with prevalent diabetes, we used propensity scores to adjust for potential confounders.22 We first calculated the propensity score for each patient, defined as the conditional probability of assignment to a primary care network given the observed confounders.22,23 We then used the propensity scores to construct a one-to-one matched sample, using nearest-neighbour matching without replacement by applying a Greedy matching algorithm22 on the logit of the propensity score within a caliper width of 0.25 times the pooled standard deviation. After confirming balance between the groups, we determined the association between enrolment in a primary care network and each outcome using population-averaged negative binomial models with robust variance adjusted for clustering on matching.
For continuous and binary outcomes, we used population-averaged linear models and logit models to determine the association between the intervention (primary care network) and each of the outcomes, separately.22,24
When esimating the incidence rate difference (IRD), we used an approach based on weighted least-squares regression.25
The available data determined the sample size, thus we did not calculate an a priori sample size.
Results
In this paper, we report the results for the cohort of patients with prevalent diabetes. The results for the cohort of patients with incident diabetes are presented in Appendix 1.
As of Apr. 1, 2008, there were 77 830 patients with prevalent diabetes whose condition was managed in one of the 18 primary care networks of interest; 105 824 patients with prevalent diabetes were not enrolled in one of the networks. We excluded 22 164 patients with prevalent diabetes whose conditions were managed by other primary care networks established after 2007 (Figure 1). Patients with prevalent diabetes that was managed in primary care networks were similar to those whose care was managed outside of a network, although patients in networks were slightly older and had a higher number of comorbidities (Table 1).
Table 1:
Characteristics of patients with prevalent diabetes at baseline, stratified by whether or not the patient’s care was managed in a primary care network, before and after matching by propensity score
| Characteristics | Before matching, %* | After matching, %* | ||||
|---|---|---|---|---|---|---|
| Not managed in a PCN n = 105 824 |
Managed in a PCN n = 77 830 |
Standardized difference† | Not managed in a PCN n = 77 464 |
Managed in a PCN n = 77 464 |
Standardized difference† | |
| Age, mean, yr (SD) | 60.7 (15.2) | 62.1 (15.1) | 9.9 | 61.8 (15.0) | 62.1 (15.0) | 1.8 |
| Female sex | 47.3 | 47.6 | 0.6 | 47.8 | 47.6 | −0.4 |
| First Nations status | 5.0 | 3.5 | −7.5 | 3.5 | 3.5 | 0.1 |
| Receiving income support | 5.1 | 4.2 | −4.4 | 4.1 | 4.2 | 0.2 |
| Comorbidities | ||||||
| Hypertension | 63.9 | 66.5 | 5.4 | 66.3 | 66.4 | 0.3 |
| Cancer | 7.4 | 9.2 | 6.3 | 8.8 | 8.9 | 0.3 |
| Cerebrovascular disease | 5.8 | 6.3 | 1.8 | 5.9 | 6.2 | 1.3 |
| Congestive heart failure | 8.0 | 8.7 | 2.4 | 8.2 | 8.6 | 1.5 |
| COPD | 19.2 | 18.8 | −1.0 | 19.0 | 18.8 | −0.7 |
| Dementia | 3.6 | 4.3 | 3.2 | 3.9 | 4.1 | 0.8 |
| Myocardial infarction | 6.5 | 6.6 | 0.2 | 6.3 | 6.6 | 1.1 |
| Diabetes with complications | 11.7 | 12.8 | 3.4 | 12.4 | 12.6 | 0.5 |
| Diabetes without complications | 74.6 | 76.3 | 3.9 | 77.1 | 76.5 | −1.5 |
| AIDS/HIV | 0.1 | 0.1 | −1.0 | 0.1 | 0.1 | 0.1 |
| Metastatic cancer | 1.0 | 1.2 | 1.8 | 1.1 | 1.1 | 0.6 |
| Mild liver disease | 1.9 | 1.6 | −2.0 | 1.6 | 1.6 | −0.1 |
| Moderate/severe liver disease | 0.4 | 0.3 | −0.5 | 0.3 | 0.3 | 1.2 |
| Paralysis | 0.9 | 0.8 | −0.8 | 0.7 | 0.8 | 1.3 |
| Peptic ulcer disease | 3.0 | 2.1 | −5.4 | 2.0 | 2.2 | 1.2 |
| Peripheral vascular disease | 4.1 | 5.0 | 4.3 | 4.6 | 4.7 | 0.7 |
| Renal disease | 6.0 | 6.5 | 2.1 | 6.1 | 6.4 | 1.2 |
| Rheumatic disease | 2.0 | 2.3 | 2.0 | 2.2 | 2.2 | 0.6 |
| eGFR, mL/min per 1.73 m2, mean (SD) | 79.0 (25.8) | 77.1 (25.1) | −7.3 | 78.1 (25.5) | 77.2 (25.1) | −3.5 |
| ≥ 60 | 66.2 | 69.9 | 7.8 | 70.1 | 70.0 | −0.3 |
| 30–59.9 | 15.3 | 17.7 | 6.4 | 17.3 | 17.5 | 0.4 |
| < 30 | 2.4 | 2.6 | 1.5 | 2.5 | 2.6 | 0.5 |
| Not measured | 16.1 | 9.9 | −18.7 | 10.0 | 9.9 | −0.4 |
| Diabetes duration, yr, mean (SD) | 6.6 (4.4) | 6.7 (4.4) | 1.2 | 6.7 (4.4) | 6.7 (4.4) | −0.2 |
| Baseline glycated hemoglobin, mean (SD) | 7.3 (1.6) | 7.2 (1.5) | −7.9 | 7.2 (1.6) | 7.2 (1.5) | −3.1 |
| ≥ 7.0% | 40.6 | 39.9 | −1.5 | 40.3 | 40.0 | −0.5 |
| < 7.0% | 45.4 | 50.5 | 10.2 | 50.3 | 50.3 | −0.1 |
| Not measured | 14.0 | 9.7 | −13.5 | 9.4 | 9.7 | 1.0 |
Note: COPD = chronic obstructive pulmonary disease, eGFR = estimated glomerular filtration rate, PCN = primary care network, SD = standard deviation.
Unless otherwise indicated.
Standardized difference between patients enrolled in a primary care network and patients who were not enrolled in a network for each baseline variable.
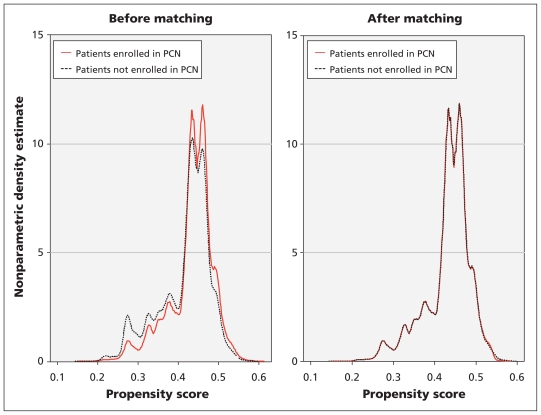
Matching by propensity score resulted in a well-balanced matched sample (Figure 2), which was confirmed using a two-sample Kolmogorov–Smirnov test for equality of distribution (p = 0.80) (Table 1).
Figure 2:
Distribution of propensity scores for patients whose care was managed in a primary are network versus those whose care was not managed in a network, before and after matching by propensity score. PCN = primary care network.
Admissions to hospital and visits to emergency departments
Our analysis of the sample of patients matched by propensity scores showed that patients with prevalent diabetes who received care in primary care networks were less likely to be admitted to hospital or to visit an emergency department for a diabetes-specific ambulatory care sensitive condition (adjusted incidence risk ratio [IRR] 0.81, 95% confidence interal [CI] 0.75 to 0.87) than those whose care was not managed in a network. However, the size of the difference was small (IRD −0.67, 95% CI −0.92 to −0.42, per 1000 patient-months) (Table 2 and Appendix 4, available at www.cmaj.ca/lookup/suppl/doi:10.1503/cmaj.110755/-/DC1). When the components of this outcome were analyzed separately, the adjusted IRRs were 0.75 (95% CI 0.64 to 0.87) for admissions to hospital and 0.82 (95% CI 0.76 to 0.88) for visits to an emergency department.
Table 2:
Outcomes of patients with prevalent diabetes, stratified by whether or not the patient was cared for in a primary care network, based on the sample matched by propensity scores
| Outcome | Patients with physicians who never enrolled in a PCN n = 77 464 |
Patients with physicians who enrolled in PCNs of interest n = 77 464 |
Comparison of patients with prevalent diabetes cared for in PCNs with patients cared for outside PCNs (reference group) | |||
|---|---|---|---|---|---|---|
| Crude | Adjusted* | Crude | Adjusted* | Adjusted* measure (95% CI) | p value | |
| Rate of admissions to hospital or visits to emergency departments per 1000 patient months (95%CI) | 3.67 (3.49 to 3.86) | 1.96 (1.85 to 2.09) | 2.99 (2.83 to 3.17) | 1.58 (1.49 to 1.68) | IRR 0.81 (0.75 to 0.87) | < 0.001 |
| Rate of admissions to hospital per 1000 patient months (95% CI) | 0.69 (0.62 to 0.77) | 0.30 (0.26 to 0.35) | 0.52 (0.46 to 0.58) | 0.23 (0.20 to 0.26 | IRR 0.75 (0.64 to 0.87) | < 0.001 |
| Rate of visits to emergency departments per 1000 patient months (95% CI) | 2.97 (2.82 to 3.13) | 1.62 (1.52 to 1.73) | 2.47 (2.33 to 2.62) | 1.33 (1.25 to 1.42) | IRR 0.82 (0.76 to 0.88) | < 0.001 |
| Glycated hemoglobin, mean (95% CI) |
n = 51 565 7.31 (7.30 to 7.33) |
n = 51 565 7.26 (7.25 to 7.28) |
n = 54 228 7.24 (7.23 to 7.26) |
n = 54 228 7.20 (7.18 to 7.21) |
MD −0.067 (−0.081 to −0.052) | < 0.001 |
| Mean glycated hemoglobin < 7.0, % (95% CI) |
n = 51 565 51.8 (51.4 to 52.2) |
n = 51 565 54.9 (54.4 to 55.5) |
n = 54 228 53.2 (52.8 to 53.6) |
n = 54 228 57.0 (56.4 to 57.6) |
RR 1.02 (1.01 to 1.03) | < 0.001 |
| No. of measurements of glycated hemoglobin, mean (95% CI) |
n = 51 565 1.99 (1.98 to 2.00) |
n = 51 565 1.87 (1.86 to 1.88) |
n = 54 228 2.15 (2.14 to 2.16) |
n = 54 228 2.02 (2.01 to 2.03) |
MD 0.078 (0.070 to 0.085) | < 0.001 |
| Patients who saw an ophthalmologist/optometrist, % (95% CI) | 27.5 (27.2 to 27.9) | 25.7 (25.4 to 26.0) | 32.7 (32.4 to 33.0) | 31.1 (30.8 to 31.5) | RR 1.19 (1.17 to 1.21) | < 0.001 |
| Patients who had albumin–creatinine ratio measured, % (95% CI) | 41.0 (40.6 to 41.3) | 36.7 (36.3 to 37.1) | 44.7 (44.4 to 45.1) | 41.2 (40.8 to 41.6) | RR 1.10 (1.09 to 1.11) | < 0.001 |
| Patients who had LDL measured, % (95% CI) | 61.8 (61.4 to 62.1) | 61.5 (61.2 to 61.9) | 63.5 (63.1 to 63.8) | 63.8 (63.4 to 64.1) | RR 1.03 (1.02 to 1.04) | < 0.001 |
| Patients who had glycated hemoglobin measured, % (95% CI) | 66.6 (66.2 to 66.9) | 67.3 (66.9 to 67.6) | 70.0 (69.7 to 70.3) | 72.1 (71.7 to 72.5) | RR 1.05 (1.04 to 1.06) | < 0.001 |
| Patients taking a statin,† % (95% CI) |
n = 31 776 54.1 (53.6 to 54.7) |
n = 31 776 53.7 (53.2 to 54.3) |
n = 32 209 55.5 (55.0 to 56.1) |
n = 32 209 55.8 (55.2 to 56.4) |
RR 1.03 (1.02 to 1.05) | < 0.001 |
| Patients with proteinuria taking an ACE/ARB,† % (95% CI) |
n = 6 979 77.6 (76.7 to 78.6) |
n = 6 979 80.3 (79.3 to 81.2) |
n = 6 273 79.3 (78.2 to 80.3) |
n = 6 27382.3 (81.3 to 83.3) | RR 1.02 (1.01 to 1.04) | 0.007 |
| Patients taking metformin† who received any oral hypoglycemic drug during the assessment period but were not receiving insulin, % (95% CI) |
n = 4 206 85.3 (84.2 to 86.4) |
n = 4 206 87.2 (86.1 to 88.2) |
n = 3 575 84.3 (83.1 to 85.5) |
n = 3 575 86.4 (85.2 to 87.5) |
RR 0.99 (0.97 to 1.01) | 0.24 |
| Rate of outpatient visits to primary care physicians per 1000 patient-months (95%CI) | 627.7 (623.5 to 631.9) | 596.5 (592.8 to 600.3) | 631.1 (626.8 to 635.5) | 591.8 (588.2 to 595.4) | IRR 0.99 (0.98 to 1.00) | 0.07 |
| Rate of outpatient visits to specialists in internal medicine/endocrinology per 1000 patient-months (95%CI) | 51.8 (50.9 to 52.7) | 44.6 (43.8 to 45.3) | 52.7 (51.7 to 53.7) | 45.1 (44.3 to 45.9) | IRR 1.01 (0.99 to 1.04) | 0.34 |
Note: ACE = angiotensin-converting enzyme, ARB = angiotensin receptor blocker, CI = confidence interval, IRR = incidence rate ratio, LDL = low-density lipoprotein, MD = mean difference, PCN = primary care network, RR = risk ratio.
Adjusted for the demographic factors including age, sex, socioeconomic status, and score on the Charlson comorbidity index.
Among patients aged ≥ 66 yrs.
Secondary outcomes
Patients whose care was managed in primary care networks were more likely than patients whose care was not managed in a network to see an ophthalmologist or optometrist (risk ratio [RR] 1.19, 95% CI 1.17 to 1.21), and to undergo guideline-recommended laboratory investigations (Table 2). Mean glycated hemoglobin during the assessment period was slightly lower in the matched cohort of patients whose care was managed in a primary care network compared with those whose care was managed outside of a network (adjusted mean difference −0.067, 95% CI −0.081 to −0.052). Among patients with prevalent diabetes 66 years of age and older, those whose conditions were managed within primary care networks were slightly more likely to receive statins than those who were not enrolled in a network (adjusted probability 55.8% v. 53.7%; RR 1.03, 95% CI 1.02 to 1.05) (Table 2). There was no significant difference in the rate of visits to primary care physicians or to specialists in internal medicine/endocrinology between patients enrolled in a primary care network and those who were not (Table 2).
Interpretation
Main findings
Management of diabetes in a primary care network was associated with a 19.4% relative reduction in admissions to hospital or visits to emergency departments for ambulatory care sensitive conditions among patients with prevalent diabetes, although this relative change represents a small absolute change (IRD −0.67 per 1000 patient-months). In addition, receiving care in primary care networks was associated with better glycemic control for patients with incident and prevalent diabetes, although the absolute mean differences in glycated hemoglobin in the cohorts were small (−0.20 for the incident cohorts and −0.067 for the prevalent cohorts). We noted more use of guideline-recommended laboratory and retinal screening, as well as some potentially beneficial medications, among patients cared for in primary care networks, although the differences we saw were small.
Enrolment in primary care networks was not associated with a significant reduction in the rate of diabetes-specific ambulatory care sensitive conditions among the patients with incident diabetes, possibly because these patients had not had diabetes long enough for complications to have arisen.
Comparison with other studies
Most of the research on the impact of reforms to primary care has come from the National Health Service in the United Kingdom, which experimented with primary care groups and fundholding by general practitioners (subsequently primary care trusts),26 each of whom controlled partial (or full) budgets to support patient care.27 There is little evidence as to the impact that these initiatives have had on patient care and outcomes.28
More closely related to the primary care network is the concept of the patient-centred medical home, which includes an ongoing relationship between provider and patient, and a comprehensive and coordinated approach to care involving physicians, allied health providers and community services.29,30 The National Demonstration Project tested two methods of implementing a patient-centred medical home in the US,11,30 noting only a very small improvement in outcomes for processes of care for chronic diseases and no improvement in patient-rated outcomes.30 These results may have been seen because the initiative did not include reforms to payment. Given the lack of a control group, it is unclear if the very small improvements were due to implementation of certain elements of the medical home.
Of more relevance is the model used in Ontario, the family health team. This model is designed to expand the capacity of primary care by developing interdisciplinary teams and to improve the breadth and quality of care through incentives provided by a blended model of physician payment.9,10 Physicians are responsible for a defined number of patients and are assisted by nurse practitioners, psychologists, pharmacists, social workers and health educators, whose salaries are provided by the provincial ministry. A full evaluation of the impact of family health teams in Ontario is ongoing. It is worth noting, however, that family health teams differ from primary care networks because they incorporate a different model of funding for physicians.
Limitations
Assessing the effectiveness of interventions is best done using randomized controlled trials. Although some of the elements that were included in many of the primary care networks we studied, such as case management by nurses, have been tested in such trials,31 our study was not randomized.
We tried to enhance the comparability of patients and reduce potential confounding by including four cohorts of patients with incident diabetes over time, and two cohorts of patients with prevalent diabetes matched by propensity score. However, even after adjustment, residual confounding is possible.
Because we were unable to identify which physicians not enrolled in a primary care network worked together in a practice, we were unable to include clustering by practice in our analysis. As a result, we may see lower standard errors and smaller p values.
As with all studies that assess outcomes using administrative data, our study is subject to the general shortcomings of research using such databases. These limitations include the possibility of missing data, a lack of data on the elements of care that patients received, or a lack of information on patient-level outcomes including body weight, blood pressure or patient satisfaction.
We defined diabetes using administrative data. It is thus possible that a small proportion of patients in our cohorts did not have diabetes, which would dilute the associations seen.
The funds provided to primary care networks were not designated solely for the care of patients with diabetes; some or all of the funds may have been used to pay for other programs. It is unclear whether funding was adequate to achieve all of the potential objectives for each primary care network.
Our study focused on the first wave of primary care networks, allowing the networks only a short time to establish new programs for the management of chronic disease. Although a recent study shows that many primary care networks in Alberta offer such programs for diabetes8 (including strategies that have been shown to improve glycemic control),31 the types of programs offered vary substantially between networks.8 Acknowledging limited resources, it will be critical for payers to provide guidance and support to enhance uptake of the most effective practices across primary care networks.
Despite these limitations, our population-based study reflects real-world experience with primary care networks in a large area served by a universal health care system, with careful assessment of both evidence-and process-based outcomes.
Conclusion
The care received by patients with diabetes in the primary care networks we studied was associated with more use of guideline-recommended screening and (for patients with prevalent diabetes) a lower rate of admissions to hospital or visits to emergency departments for diabetes-specific ambulatory care sensitive conditions. However, the absolute changes we saw were small, and the observational nature of our data does not permit us to establish causality. Future studies should aim to determine how primary care networks can best implement equitable access to the best programs for the management of diabetes.
Supplementary Material
Acknowledgements
Dr. Tonelli and Dr. Manns are supported by Alberta Innovates — Health Solutions. Dr. Hemmelgarn was supported by a Population Health Investigator award from Alberta Innovates — Health Solutions. Dr. Tonelli was also supported by a Government of Canada Research Chair in the optimal care of people with chronic kidney disease. Dr. Johnson is a Senior Scholar with Alberta Innovates — Health Solutions and holds a Government of Canada Research Chair in Diabetes Health Outcomes. Dr. Tonelli, Dr. Hemmelgarn and Dr. Manns were supported by an alternative funding plan from the Government of Alberta and the Universities of Alberta and Calgary.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: All of the authors contributed to the conception and design of the study. Braden Manns, Marcello Tonelli, Brenda Hemmelgarn and Jianguo Zhang collected and analyzed the data, and drafted the manuscript. All of the authors contributed to interpreting the data, critically revised the manuscript for important intellectual content and approved the final version submitted for publication.
Funding: This research was supported by an interdisciplinary team grant from Alberta Innovates — Health Solutions (formerly Alberta Heritage Foundation for Medical Research Health Scholar Awards), the Interdisciplinary Chronic Disease Collaboration and the Canadian Institutes of Health Research (application number 238543).
References
- 1.Simpson SH, Corabian P, Jacobs P, et al. The cost of major comorbidity in people with diabetes mellitus. CMAJ 2003;168:1661–7 [PMC free article] [PubMed] [Google Scholar]
- 2.Johnson JA. Alberta diabetes atlas. Edmonton (AB): Institute of Health Economics; 2009 [Google Scholar]
- 3.Tonelli M, Bohm C, Pandeya S, et al. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am J Kidney Dis 2001;37:484–9 [PubMed] [Google Scholar]
- 4.Tonelli M, Gill J, Pandeya S, et al. Barriers to blood pressure control and angiotensin enzyme inhibitor use in Canadian patients with chronic renal insufficiency. Nephrol Dial Transplant 2002;17:1426–33 [DOI] [PubMed] [Google Scholar]
- 5.Gaede P, Vedel P, Larsen N, et al. Multifactorial interventions and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 2003;348:383–93 [DOI] [PubMed] [Google Scholar]
- 6.Von Korff M, Gruman J, Schaefer J, et al. Collaborative management of chronic illness. Ann Intern Med 1997;127:1097–102 [DOI] [PubMed] [Google Scholar]
- 7.Alberta Primary Care Initiative [homepage]. Edmonton (AB): The Initiative; 2011. Available: www.albertapci.ca/Pages/default.aspx [accessed 2009 Dec. 30]. [Google Scholar]
- 8.Campbell D, Sargious P, Lewanczuk R, et al. The use of chronic disease management programs for diabetes by Alberta’s primary care networks. Can Fam Phys. In press. [PMC free article] [PubMed] [Google Scholar]
- 9.Rosser WW, Colwill JM, Kasperski J, et al. Patient-centered medical homes in Ontario. N Engl J Med 2010;362:e7. [DOI] [PubMed] [Google Scholar]
- 10.Rosser WW, Colwill JM, Kasperski J, et al. Progress of Ontario’s family health team model: a patient-centered medical home. Ann Fam Med 2011;9:165–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stange KC, Miller WL, Nutting PA, et al. , Context for understanding the National Demonstration Project and the patient-centered medical home. Ann Fam Med, 2010; 8 Suppl 1: S2–8; S92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth GL, Hux JE. Relationship between avoidable hospitalizations for diabetes mellitus and income level. Arch Intern Med 2003;163:101–6 [DOI] [PubMed] [Google Scholar]
- 13.Canadian Institute for Health Information Technical note: ambulatory care sensitive conditions (ACSC). Ottawa (ON): The Institute; 2010; Available: www.cihi.ca/CIHI-ext-portal/pdf/internet/DEFINITIONS_052010_EN (accessed 2011 Nov. 15). [Google Scholar]
- 14.Blanchard JF, Ludwig S, Wajda A, et al. Incidence and prevalence of diabetes in Manitoba, 1986–1991. Diabetes Care 1996; 19:807–11 [DOI] [PubMed] [Google Scholar]
- 15.Hux JE, Ivis F, Flintoft V, et al. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care 2002;25:512–6 [DOI] [PubMed] [Google Scholar]
- 16.Majumdar S, Johnson JA, Bowker SL, et al. A Canadian consensus for the standardized evaluation of quality improvement interventions in type 2 diabetes. Can J Diabetes 2005;29:220–9 [Google Scholar]
- 17.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96 [DOI] [PubMed] [Google Scholar]
- 18.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861–9 [DOI] [PubMed] [Google Scholar]
- 19.Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension 2009;54:1423–8 [DOI] [PubMed] [Google Scholar]
- 20.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39(Suppl 1):S1–266 [PubMed] [Google Scholar]
- 22.Rubin DB. On principles for modeling propensity scores in medical research. Pharmacoepidemiol Drug Saf 2004;13:855–7 [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997;127:757–63 [DOI] [PubMed] [Google Scholar]
- 24.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38:963–74 [PubMed] [Google Scholar]
- 25.Xu Y, Cheung YB, Lam KF, et al. A simple approach to the estimation of incidence rate difference. Am J Epidemiol 2010;172:334–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith J, Mays N. Primary-care trusts: Do they have a future? BMJ 2005;331:1156–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gillam S, Lewis RQ. Practice based commissioning in the UK. BMJ 2009;338:b832. [DOI] [PubMed] [Google Scholar]
- 28.Higgins J. A new look at NHS commissioning. BMJ 2007;334: 22–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Association of American Medical Colleges The medical home: position statement. Washington (DC): The Association; 2008 [Google Scholar]
- 30.Jaén CR, Ferrer RL, Miller WL, et al. Patient outcomes at 26 months in the patient-centered medical home National Demonstration Project. Ann Fam Med, 2010;8 Suppl 1:S57–67; S92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shojania KG, Ranji SR, McDonald KM, et al. Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006;296:427–40 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.