Abstract
The signaling pathways utilized by naïve and experienced effector CD4 T cells during activation and proliferation were evaluated. While inhibition of either mTOR or MAPK alone was able to inhibit naïve T cell proliferation, both mTOR and MAPK (ERK) pathway inhibition was required to efficiently block experienced, effector CD4 T cell proliferation. This was demonstrated both in vitro, and in vivo by treating mice with collagen-induced arthritis using mTOR and/or ERK inhibitors. The combination of mTOR and ERK inhibition prevented or treated disease more efficiently than either agent alone. These data illustrate the different requirements of naïve and experienced effector CD4 T cells in the use of the mTOR and MAPK pathways in proliferation, and suggest that therapies targeting both the mTOR and MAPK pathways may be more effective than targeting either pathway alone in the treatment of CD4 T cell-mediated autoimmunity.
Keywords: CIA, CD4 T cells, mTOR, MAPK, activation pathways, combination therapy
1. Introduction
The immune system is imbued with the ability to recognize and respond against harmful pathogens encountered in the environment. These responses are balanced by mechanisms that control the activation and modulation of inflammation by the innate and adaptive immune responses [1]. Normal tolerance mechanisms prevent the immune system from mounting a response against self while maintaining the vast diversity of recognition against foreign pathogens [2]. When tolerance mechanisms are compromised, autoimmune disease arise wherein the immune system mounts a response against one’s own tissues. CD4 T cells have been demonstrated to be a critical component of autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, and Type 1 diabetes [3–4]. Correspondingly, therapeutics have been developed that target CD4 T cells and their activation pathways as a strategy for treating autoimmune diseases [5].
Naïve CD4 T cells have not previously been activated by antigen in the periphery and reside primarily in lymph nodes where they are poised for activation and proliferation following encounter with antigen presented by professional antigen-presenting cells (APCs) when accompanied by co-stimulatory signals [6, 7]. The activation and proliferation of naïve CD4 T cells coincides with their differentiation to effector states where they acquire the capacity to secrete cytokines, express activation and inhibitory receptors, induce B cell class-switching, and mediate cytotoxicity and apoptosis. A portion of the generated effector T cell population persists as a pool of memory cells capable of mounting secondary immune responses [8–10]. The requirements to activate naïve CD4 T cells have been shown to be more stringent than those required for activating memory T cells. These include the number of peptide-MHC molecules required for activation induced TCR engagement, co-stimulatory receptor signaling, and the requirement for certain cytokines such as IL-2 [11–14]. This system of “checks and balances” is thought to maintain tolerance in naïve T cells in the absence of inflammation, while allowing previously activated memory cells to mount a vigorous response to antigen encounter.
2. Materials and Methods
2.1 Mice
BALB/c female mice were purchased from The Jackson Laboratory. All procedures involving mice were conducted in accordance with Institutional Animal Care and Use Committee policies as set forth by Stanford University’s Administrative Panel on Laboratory Animal Care, as accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
2.2 Isolation and stimulation of mouse CD4 T cells
Spleen and lymph nodes were harvested from 8–12 week old female BALB/c mice and homogenized through a strainer. Red blood cells were lysed using Red Blood Cell Lysing Buffer (Sigma). Lymphocytes were isolated by density centrifugation using Lympholyte-M (Cedarlane Laboratories). CD4 T cells were sorted via negative selection using an AutoMACS sorter (Miltenyi Biotec). 24-well flat-bottom culture plates were first coated with 30 ug/ml of goat anti-hamster IgG antibodies (Vector Labs) in 50 mM sodium carbonate buffer for 1–2 hours at 37° C. Plates were washed twice with PBS then coated with 500 ng/ml of hamster anti-mouse CD3 antibody (145-2C11, eBioscience) in PBS for 1–2 hours at 37° C. Plates were washed twice with PBS before addition of cells. 1 × 106 CD4 T cells were costimulated in anti-CD3 coated 24-well flat-bottom culture plates with 500 ng/ml of soluble anti-CD28 (37.51, eBiosciene). Cells were cultured in RPMI media (Gibco) supplemented with 10% heat-inactivated FCS (Cellgro), 100 nM sodium pyruvate (Gibco), 2 mM L-glutamine (Gibco), 100 nM non-essential amino acids (Gibco), 100 U/ml penicillin/streptomycin (Gibco), and 50 uM β-mercaptoethanol (Sigma).
2.3 Isolation and stimulation of human naïve CD4 CD45RA+ T cells
Human peripheral blood mononuclear cells from buffy coats of different donors were obtained from the Stanford Blood Center under Stanford University IRB approval. Buffy coats were separated into leukocytes using Ficoll-Paque Plus (GE Health Sciences). T cells were prepared using a RosetteSep Human CD4 T Cell Enrichment (Stem Cell Technologies) followed by a Naïve CD4 T cell Isolation Kit II along with LS MACS columns (Miltenyi Biotec) to yield negatively selected CD4 CD45RA+ CD45RO- CD25T cells. 24-well flat-bottom culture plates were first coated with 30 ug/ml of goat anti-mouse IgG antibodies (Vector Labs) in 50 mM sodium carbonate buffer for 1–2 hours at 37° C. Plates were washed twice with PBS then coated with 100 ng/ml of mouse antihuman CD3 antibody (OKT3, eBioscience) in PBS for 1–2 hours at 37° C. Plates were washed twice with PBS before addition of cells. 1 × 106 CD4 T cells were stimulated in anti-CD3 coated 24-well flat-bottom culture plates with 500 ng/ml of soluble anti-CD28 (CD28.2, eBiosciene). Cells were cultured in X-Vivo 15 media (Lonza) supplemented with 10% heat-inactivated FCS (Cellgro), 2 mM L-glutamine (Gibco), 100 U/ml penicillin/streptomycin (Gibco), and 50 uM β-mercaptoethanol (Sigma).
2.4 Cell culture reagents
Rapamycin (Eton Bioscience) was used at a concentration of 100 nM. PD0325901 (Stemgent) was used at a concentration of 10 nM. LY294002 (Sigma) was used at a concentration of 10 uM, SL0101 (Tocris Bioscience) was used at a concentration of 10 uM. Anti-IL-2 antibody (JES6-1A12, eBioscience) was used at a concentration of 10 ug/ml.
2.5 Immunoblots
Whole cell lysates were made using lysis buffer consisting of 0.5% NP-40, 100 mM sodium chloride, 0.5 mM EDTA, 20 mM Tris (pH 7.6–8.0), with protease inhibitor cocktail (Pierce) and phosphatase inhibitor cocktail (Pierce). Protein samples were loaded on 4–15% Tris-HCl gels (Bio-Rad) and separated by SDS-PAGE. Protein was transferred from gel to Immobolin-P PVDF membrane (Millipore) using Trans-Blot SD Semi-dry Transfer Apparatus (Bio-Rad) following manufacturer’s instructions. StartingBlock (Tris Buffered Saline with 0.05% Tween-20) (Pierce) was used to block membranes, and was also used during primary and secondary antibody staining. Secondary antibodies were all Horse Radish Peroxidase conjugated (Zymed). ECL Plus Western Blotting Reagents (GE Healthcare) were used for chemiluminescent detection of protein. Chemiluminescene signal was exposed onto Amersham Hyperfilm ECL (GE Healthcare). Membranes were stripped using Restore Western Blot Stripping Buffer (Pierce). Primary antibodies used were anti-β-actin (ab8226, Abcam), anti-phospho-Akt (Ser473) (44-623G, Invitrogen), anti-Akt (9272, Cell Signaling Technology), anti-GRAIL (H11-744, Becton Dickenson), anti-Kip1/p27 (57, Becton Dickenson), anti-phospho-S6K1 (Thr421/Ser424) (9204, Cell Signaling Technology), anti-S6K1 (9202, Cell Signaling Technology), anti-phosphoSTAT5 (Tyr694/699) (8-5-2, Upstate Biotechnology), and anti-STAT5 (9363, Cell Signaling Technology).
2.6 CFSE cell division
Cells were labeled with 1 uM CFDA-SE (Sigma) in serum-free RPMI for 10 minutes and washed twice before culturing. Flow cytometry was conducted on a LSR I flow cytometer (Becton Dickenson).
2.7 Phospho-flow cytometry
Phospho-flow cytometry was conducted as described [15]. Briefly, cells were fixed in 1.5% formaldehyde (Polysciences) and permeabilized with 100% cold methanol (Fisher Scientific) prior to staining. Antibodies used were anti-phospho-S6 ribosomal protein (S235/236) Alexa-488 (D57.2.2E, Cell Signaling Technology), Ki-67 Alexa-647 (B56, Becton Dickenson).
2.8 Collagen-induced arthritis mouse model treatment
CIA was induced in 7–9 week old male DBA/1 mice (The Jackson Laboratory) as described previously [16]. Briefly, DBA/1 mice received intradermal tail immunizations with 100 ug/mouse bovine Type II collagen (Chondrex) emulsified in CFA containing 250 ug/mouse heat-killed Mycobacterium tuberculosis H37Ra (Becton Dickenson). Twenty-one days following immunization, mice were randomized into groups and boosted by subcutaneous injection at the base of the tail with 100 ug/mouse bovine Type II collagen emulsified in IFA. Doses of 15 mg/kg/day PD0325901, 1.0 mg/kg/day Rapamycin, or vehicle control were administered by intraperitoneal injection in 100 ul of total volume. Mice were scored for arthritis using the following visual scoring system: grade 0, no swelling or erythema; grade 1, mild swelling and erythema or digit inflammation; grade 2, moderate swelling and erythema confined to distal and mid-paw; grade 3, more pronounced swelling and erythema with extension to the ankle; grade 4, severe swelling, erythema, and joint rigidity of the ankle, foot, and digits. Each limb was graded with a score of 0–4, with a maximum possible score of 16 for each individual mouse. Paw thickness was determined by measuring the thickness of each hind-paw using 0 to 10-mm calipers.
2.9 Histopathology
Hind limbs were fixed and decalcified in Cal-Ex II (Fischer Scientific) for 3 days prior to embedding in paraffin. Sections were stained with H&E and evaluated by an investigator blinded to treatment status for synovitis, pannus formation, and bone and/or cartilage destruction based on a previously described scoring system: grade 0, normal; grade 1, mild inflammation, mild hyperplasia of the synovial lining layer, mild cartilage destruction without bone erosion; grades 2–4, increasing degrees of inflammatory cell infiltrates, synovial lining hyperplasia, and pannus formation and cartilage and bone destruction [17].
2.10 Ex vivo stimulation of CIA mouse T cells
Spleens from CIA mice with arthritic score > 6 were harvested and lymphocytes separated using Lympholyte-M. Antibodies used for surface staining were CD4 (GK1.5, BioLegend), CD8 (53-6.7, BioLegend), CD62L (MEL-14, BioLegend), and CD44 (IM7, BioLegend). Lymphocytes were stimulated in vitro with 10 ug/ml bovine Type II collagen with or without PD0325901, Rapamycin, or both drugs using previously stated concentrations. Phospho-flow staining was conducted using antibodies to phospho-S6 and Ki-67.
2.11 Proliferation assay
Cells cultured in 96-well U-bottom plate wells were pulsed with 1 uCi of methyl-3 H thymidine (Amersham) for 6 hours during the last 72 hours of stimulation and harvested onto filters (Wallac). Filters were wetted with Betaplate Scintillation fluid (Perkin Elmer) and counts per minute (cpm) read on a 1205 Betaplate Liquid Scintillation Counter (Wallac).
3. Theory
Activation of CD4 T cells results in protein signaling cascades that are mediated by kinase phosphorylation that allow the cells to undergo cell division. A major component of this activation is the promotion of protein synthesis; a function of integrating environmental cues allowing proliferation through multiple cell divisions as well as the acquisition of new effector functions [18]. TCR engagement, co-stimulatory receptor ligand binding, and growth factor availability have all been shown to signal to T cells through the mTOR pathway [19]. Activation of the kinase mTOR results in the phosphorylation of its targets 4E-BP1 and S6K1, subsequently leading to phosphorylation of S6 promoting ribosomal protein translation [20]. The mTOR pathway and phosphorylation of S6 have been demonstrated to be important for T cell down-modulation of genes mediating CD4 T cell unresponsiveness and cell cycle inhibitors via CD28 and IL-2 receptor signaling [11, 19]. TCR activation of naïve T cells absent CD28 or IL-2 receptor signaling, or inhibition of mTOR by rapamycin, inhibits proliferation and leads to an anergic state.
As patients with autoimmune diseases present in the clinic with signs and symptoms indicative of an active autoimmune disease often driven by activated effector T cells as well as ongoing activation of naïve T cells (Supplementary Figure 1), we focused on identifying and inhibiting pathways responsible for T cell activation and proliferation in naïve and experienced effector CD4 T cells. Our results suggest that experienced effector CD4 T cells, unlike naïve CD4 T cells, do not require IL-2 receptor signaling for proliferation nor does mTOR inhibition alone suffice to inhibit these cells. Further studies revealed that the ERK pathway is also utilized in conjunction with the mTOR pathway to promote phosphorylation of S6. While inhibition of either pathway was sufficient to inhibit naïve CD4 T cells, inhibition of both pathways was required for optimal inhibition of experienced effector CD4 T cells. Proof of concept studies in vivo were conducted using a collagen-induced arthritis mouse model where the combination of mTOR and ERK inhibitors ameliorated disease to a greater extent than either drug alone. Activation of T cells from arthritic mice ex vivo showed that proliferation was optimally inhibited by the combination of mTOR and ERK inhibitors and correlated with inhibition of S6 phosphorylation. Altogether, our results demonstrate different requirements for naïve and experienced effector CD4 T cells in their utilization of the mTOR and ERK pathways for S6 phosphorylation and resultant proliferation. As both pathogenic naïve and experienced effector CD4 T cells are present in patients with autoimmune diseases, combinatorial targeting of mTOR by rapamycin, an approved drug, and ERK inhibitors currently in clinical trials, may be a strategy for more effective treatment of these diseases.
4. Results and Discussion
4.1 IL-2 is not required for experienced effector CD4 T cell mTOR activation and proliferation
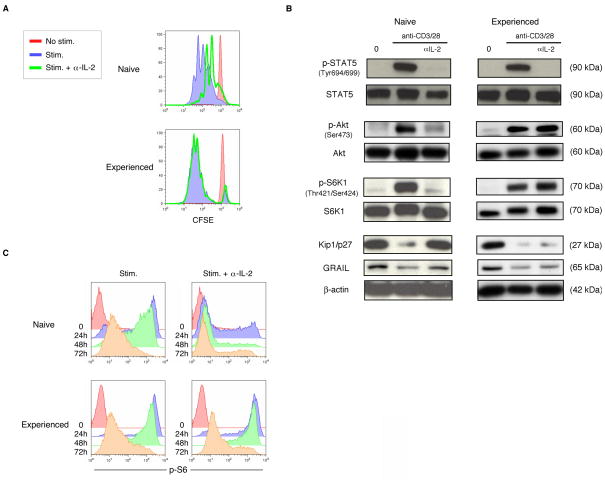
Previously it has been reported that IL-2 binding and signaling through the IL-2R is required for T cell activation of Akt and mTOR [19]. In conjunction with TCR/CD3 and CD28 signaling, the activation of mTOR drives protein synthesis and results in cell division [11]. These requirements have been demonstrated for naïve T cells but the involvement of IL-2 in experienced effector CD4 T cells has not been fully elucidated. To ask whether IL-2 was required for proliferation of effector CD4 T cells, we collected resting naïve CD4 T cells and generated resting experienced effector CD4 T cells in vitro (Supplementary Fig. 2). Naïve and experienced effector cells were stimulated with anti-CD3 and anti-CD28 antibodies in the presence or absence of anti-IL-2 neutralizing antibody and proliferation was measured by CFSE dilution (Fig. 1A). In naïve CD4 T cells, the addition of anti-IL-2 antibody inhibited proliferation. However in experienced effector CD4 T cells the addition of anti-IL-2 antibody did not lead to an observable inhibition of proliferation. Protein lysates were collected in order to examine signaling events in naïve and experienced effector CD4 T cells in the presence or absence of anti-IL-2 antibody (Fig. 1B). In naïve CD4 T cells, engagement of the IL-2R induced phosphorylation of STAT5 and Akt. Phosphorylation of Akt leads to mTOR activation and resultant S6K1 phosphorylation, in turn downregulating the cell cycle inhibitor Kip1/p27 [11, 19] and the T cell unresponsiveness factor GRAIL [21]. When IL-2R signaling was abrogated by anti-IL-2 antibody there was a lack of STAT5, Akt, and S6K1 phopshorylation while Kip1/p27 and GRAIL expression were maintained. In experienced effector CD4 T cells, activation similarly results in phosphorylation of STAT5, Akt, and S6K1 and the downregulation of Kip1/p27 and GRAIL. The presence of anti-IL-2 antibody prevented IL-2 binding and signaling through the IL-2R as demonstrated by the lack of STAT5 phosphorylation, yet absent IL-2R signaling, Akt and S6K1 were phosphorylated in the activated experienced CD4 T cells. Consistent with proliferation, even when IL-2 was neutralized by anti-IL-2 antibody, Kip1/p27 and GRAIL were downregulated in the experienced effector CD4 T cells. An important function of mTOR activation is promotion of protein translation, a requisite process during T cell proliferation. As a surrogate marker of protein translational activity, we measured ribosomal S6 phosphorylation, a component of the protein translational complex [22] by phospho-flow cytometric measurement. Naïve CD4 T cell activation resulted in abundant S6 phosphorylation that required IL-2R signaling (Fig. 1C). Experienced effector CD4 T cell activation also displayed abundant S6 phosphorylation, but phosphorylation of Akt and resultant S6 phosphorylation was independent of IL-2R signaling. These experiments were conducted on naïve and experienced human and mouse effector CD4 T cells using the same stimulation conditions with similar results in both systems. Importantly, the experienced effector CD4 T cells were documented to be in a quiescent state similar to resting naïve cells, as evidenced by lack of proliferation, baseline protein phosphorylation levels (including S6 phospohrylation) and expression of cell cycle inhibitor proteins. Our results differentiate naïve from experienced effector CD4 T cells on the basis of their different requirements for IL-2 to drive the phosphorylation of mTOR and S6, and resultant protein translation and proliferation.
Figure 1. Experienced effector CD4 T cells do not require IL-2 for mTOR activation and proliferation.
(A) Proliferation as measured by CFSE dilution in naïve or experienced effector mouse CD4 T cells absent stimulation (red), or with anti-CD3 and anti-CD28 antibody stimulation without (blue) or with anti-IL-2 neutralizing antibody (green). Data are representative of three experiments with similar results. (B) Phospho-STAT5, total STAT5, phospho-Akt, total Akt, phospho-S6K1, total S6K1, Kip1/p27, GRAIL, and β-actin immunoblots from protein lysates collected from naïve or experienced effector mouse CD4 T cells absent stimulation (0) or stimulated using antiCD3 and anti-CD28 antibodies for 24 hours, without or with anti-IL-2 neutralizing antibody. Data are representative of two experiments with similar results. (C) S6 phosphorylation measured by flow cytometry in naïve or experienced effector mouse CD4 T cells absent stimulation (0) or stimulated using anti-CD3 and anti-CD28 antibodies for the indicated number of hours (h), without or with anti-IL-2 neutralizing antibody. Data are representative of two experiments with similar results.
4.2 Inhibition of mTOR is insufficient to prevent S6 phosphorylation or proliferation in experienced effector CD4 T cells
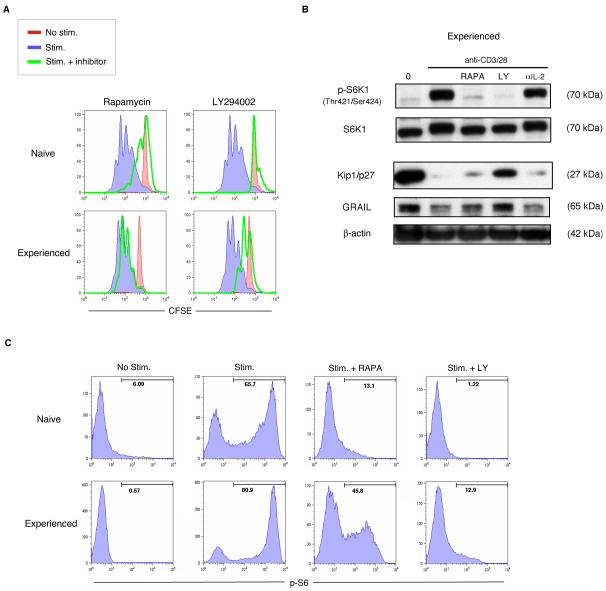
Having observed that mTOR activation and Akt phosphorylation were intact in activated experienced effector CD4 T cells without IL-2R signaling, we asked whether small molecule inhibitors of the mTOR pathway would inhibit S6 phosphorylation and proliferation in these cells. We utilized the small molecule inhibitors rapamycin and LY294002, to target mTOR and PI3K respectively [23] in human and mouse CD4 T cells. In naïve human or mouse CD4 T cells, either rapamycin or to an even greater extent, LY294002 could inhibit proliferation (Fig. 2A). Experienced effector CD4 T cells were only modestly inhibited by rapamycin but could be effectively inhibited by the PI3K inhibitor LY294002. Both rapamycin and LY294002 blocked mTOR activation in experienced effectors, as seen by the lack of S6K1 phosphorylation (Fig. 2B), but only LY294002 sustained the quiescent factors, Kip1/p27 and GRAIL. Protein translation in naïve T cells could be inhibited by rapamycin or LY294002 as measured by downregulation of S6 phosphorylation, but in experienced effector T cells, rapamycin had only a partial inhibitory effect while LY294002 showed effective inhibition and an absence of proliferation (Fig. 2C) in these cells. Unexpectedly rapamycin, while effective in its inhibition of mTOR, did not inhibit proliferation of activated experienced effector T cells while LY294002 was effective in both contexts. The partial inhibition of S6 phosphorylation by rapamycin suggested the possibility that the mTOR pathway might not be the only pathway involved in driving protein translation in experienced effector CD4 T cells.
Figure 2. Inhibition of PI3K but not inhibition of mTOR alone blocks experienced effector CD4 T cell proliferation.
(A) Proliferation as measured by CFSE dilution in naïve or experienced effector human CD4 T cells absent stimulation (red), or with antiCD3 and anti-CD28 antibody stimulation without (blue) or with mTOR inhibitor Rapamycin or PI3K inhibitor LY294002 (green). Data are representative of three experiments with similar results. Similar results were observed in naïve and experienced effector mouse CD4 T cells. (B) Phospho-S6K1, total S6K1, Kip1/p27, GRAIL, and βactin immunoblots from protein lysates collected from experienced effector human CD4 T cells absent stimulation (0) or stimulated using anti-CD3 and anti-CD28 antibodies for 24 hours, without or with Rapamycin (RAPA), LY294002 (LY), or anti-IL-2 neutralizing antibody (αIL-2). Data are representative of two experiments with similar results. Similar results were observed in experienced effector mouse CD4 T cells. (C) S6 phosphorylation measured by flow cytometry in naïve or experienced effector human CD4 T cells absent stimulation (No stim.) or stimulated (Stim.) using anti-CD3 and antiCD28 antibodies for 24 hours, without or with Rapamycin (RAPA) or LY294002 (LY). Marker number indicates percent positive for phospho-S6. Data are representative of two experiments with similar results. Similar results were observed in naïve and experienced effector mouse CD4 T cells.
4.3 Inhibition of both the ERK and mTOR pathways is effective in preventing S6 phosphorylation and proliferation in experienced effector CD4 T cells
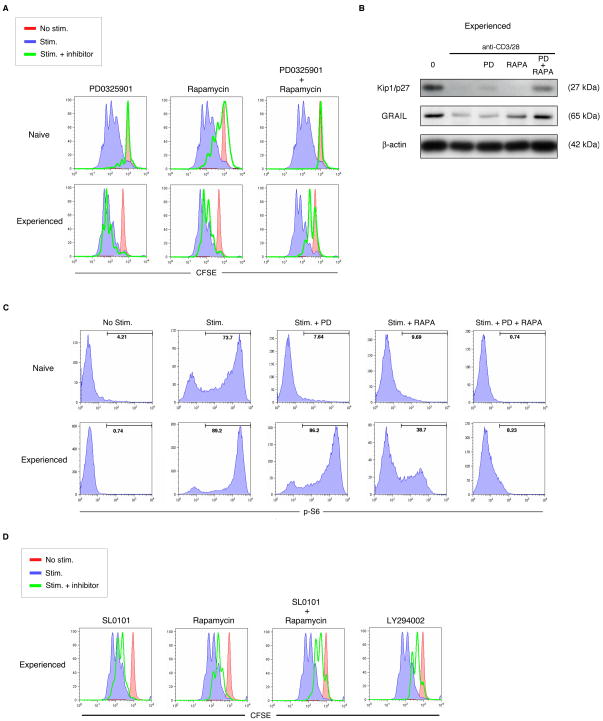
The effectiveness of the PI3K inhibitor, LY294002, on experienced effector CD4 T cell proliferation led us to investigate potential signaling pathways downstream of PI3K activation. PI3K signaling is upstream of many pathways involved in cell activation. Our analysis of these pathways eventually revealed the importance of the ERK, MAPK pathway. The ERK pathway is one of three major MAPK pathways and has been specifically implicated in having a role in promoting protein translation [24]. The ERK and mTOR pathways converge in controlling protein translation through S6 as can be measured by S6 phosphorylation [25–26]. We utilized the MEK1/2 inhibitor PD0325901 [23] to inhibit the ERK pathway (Supplementary Fig. 3). Naïve CD4 T cell proliferation was inhibited by PD0325901 or rapamycin alone (Fig. 3A). By contrast, neither PD0325901 nor rapamycin alone was effective in inhibiting experienced effector CD4 T cell proliferation unless both inhibitors were used in combination. PD0325901 or rapamycin alone did not sustain the expression of factors, Kip1/p27 or GRAIL, that control CD4 T cell unresponsiveness, unless both inhibitors were used in combination (Fig. 3B). Naïve T cell S6 phosphorylation was inhibited by either PD0325901 or rapamycin alone, but experienced effector CD4 T cell S6 phosphorylation was not inhibited by PD0325901 alone, and was only partially inhibited by rapamycin alone, however S6 phosphorylation was potently inhibited by the combination (Fig. 3C). To further solidify the importance of the ERK pathway in combination with the mTOR pathway during experienced effector CD4 T cell proliferation, we utilized SL0101, an inhibitor of RSK [23]. RSK is a kinase downstream of MEK1/2 and ERK and is analogous to S6K1 in the mTOR pathway, as both can phosphorylate S6 [27]; Supplementary Fig. 3]. Similar to the MEK1/2 inhibitor PD0325901, SL0101 was only marginally effective at decreasing proliferation in experienced effector T cells (Fig. 3D). The combination of SL0101 and ramapycin was effective in decreasing proliferation in a manner similar to LY294002. These results suggest that the ERK and mTOR pathways both contribute to S6 phosphorylation and proliferation. While inhibition of either pathway was sufficient to prevent naïve CD4 T cell proliferation, inhibition of both pathways was required to inhibit experienced effector T cell proliferation. As both ERK and mTOR pathways converge at the level of S6 phosphorylation we believe their mechanism of action is through regulation of protein translation.
Figure 3. Inhibition of both the MEK/ERK and mTOR pathways is necessary to block experienced effector CD4 T cell proliferation.
(A) Proliferation as measured by CFSE dilution in naïve or experienced effector human CD4 T cells absent stimulation (red), or with anti-CD3 and anti-CD28 antibody stimulation without (blue) or with MEK inhibitor PD0325901, mTOR inhibitor Rapamycin, or both drugs (green). Data are representative of four experiments with similar results. Similar results were observed in naïve and experienced effector mouse CD4 T cells. (B) Kip1/p27, GRAIL, and β-actin immunoblots from protein lysates collected from experienced effector human CD4 T cells absent stimulation (0) or stimulated using anti-CD3 and anti-CD28 antibodies for 24 hours, without or with PD0325901 (PD), Rapamycin (RAPA), or both drugs. Data are representative of two experiments with similar results. Similar results were observed in experienced effector mouse CD4 T cells. (C) S6 phosphorylation measured by flow cytometry in naïve or experienced effector human CD4 T cells absent stimulation (No stim.) or stimulated (Stim.) using anti-CD3 and anti-CD28 antibodies for 24 hours, without or with PD0325901 (PD), Rapamycin (RAPA), or both drugs. Marker number indicates percent positive for phospho-S6. Data are representative of two experiments with similar results. Similar results were observed in naïve and experienced effector mouse CD4 T cells. (D) Proliferation as measured by CFSE dilution in experienced effector human CD4 T cells absent stimulation (red), or with anti-CD3 and anti-CD28 antibody stimulation without (blue) or with RSK inhibitor SL0101, mTOR inhibitor Rapamycin, both drugs, or PI3K inhibitor LY294002 (green). Similar results were observed in experienced effector mouse CD4 T cells.
4.4 Combinatorial inhibition of ERK and mTOR pathways is effective in vivo in treating collagen-induced arthritis
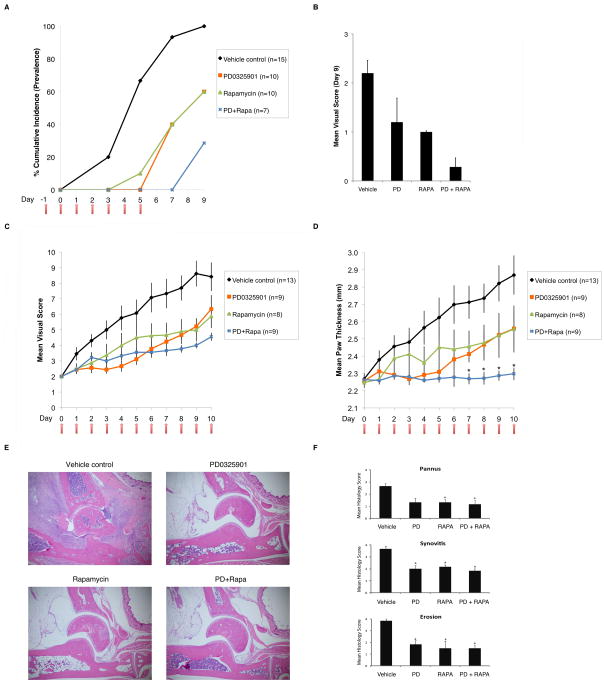
In order to examine the efficacy of ERK and mTOR inhibition in an autoimmune setting we utilized the collagen-induced arthritis (CIA) model as a mouse model of human rheumatoid arthritis. CIA involves a priming challenge followed by a boost challenge where collagen is administered along with adjuvant at two time points, day zero and day 21 after priming. When treatment is administered at day 20, just before the booster challenge, a proportion of the reactive pathogenic T cells are no longer naïve but rather have become experienced effectors from the priming challenge. Vehicle control, PD0325901 alone, rapamycin alone, or PD0325901 and rapamycin together were administered prophylactically daily for seven days, and the cumulative incidence of mice developing signs of arthritic disease was tracked for each group (Fig. 4A). PD0325901 or rapamycin alone were effective and the combination of the two was even more effective at delaying disease onset. Continuous daily administration of drug yielded similar results as assessed by disease incidence (Fig. 4B). We next tested the use of these inhibitors as therapeutics in CIA mice that already exhibited signs of arthritis. CIA mice were enrolled when they developed a mean visual score of 2 and were then continuously administered daily doses of vehicle control, PD0325901 alone, rapamycin alone, or PD0325901 and rapamycin together in a blinded fashion for seven days (Fig. 4C). Mean visual scores indicated that PD0325901 or rapamycin alone decreased the severity of arthritic disease but that the combination of the two drugs decreased disease to an even greater extent. The efficacy of the treatments was shown quantitatively through measurement of mean paw thickness for the treatment groups (Fig. 4D). Again, the combination of the two drugs was superior in effect to either drug given alone. Histopathologic analysis of sections of joints from the various treatment groups harvested at day 10 demonstrated massive lymphocyte infiltration and morphologic changes indicative of arthritis in the vehicle control treated mice but not in the drug treated groups (Fig. 4E) as is also indicated by blinded scores for pannus formation, synovitis, and bone erosion (Fig. 4F). Our results using ERK and mTOR inhibitors in vivo to treat CIA recapitulate the stimulation experiments in vitro. As autoimmune arthritis encompasses both naïve and experienced effector pathogenic T cells our results suggest that the combination of ERK and mTOR inhibition could have a greater therapeutic effect than targeting either pathway alone.
Figure 4. Combination MEK/ERK and mTOR inhibition demonstrates additive in vivo preventive and therapeutic efficacy in a collagen-induced arthritis mouse model.
(A) Percent cumulative incidence (prevalence) of arthritis disease development was measured in a prevention study in a collagen-induced arthritis mouse model. Mice were previously primed and then boosted on the indicated Day 0. Seven daily injections of vehicle control (black), PD0325901 (orange), Rapamycin (green), or both drugs (blue) were administered starting from Day -1 and ending on Day 5. Number of mice in each group is indicated (n). (B) Percent cumulative incidence (prevalence) of arthritis disease development was measured as in (A). Fourteen daily injections of vehicle control (black), PD0325901 (orange), Rapamycin (green), or both drugs (blue) were administered starting from Day -1 and ending on Day 12. Number of mice in each group is indicated. (C) Mean visual score was measured in a treatment study in a collagen-induced arthritis mouse model. Mice were primed and then boosted and subsequently monitored daily for enrollment in the treatment study upon reaching a visual disease score of 2. Daily injections of vehicle control (black), PD0325901 (orange), Rapamycin (green), or both drugs (blue) were administered. Error bars indicate standard error of mean for (n) number of mice in each group. (D) Mean paw thickness in millimeters (mm) was measured as in (C). Error bars indicate standard error of mean for (n) number of mice in each group. Mann-Whitney U-test of scores in PD+Rapa group marked by (*) indicates (p < 0.05) compared with vehicle control and single drug groups. (E) Representative hemotoxylin and eosin stained joint tissue sections of three mice per group on Day 10 from CIA treatment study as in (C). (F) Histopathological scores of inflammation, pannus formation, and bone and cartilage erosions from three mice per group on Day 10 from CIA treatment study as in (C). Error bars indicate standard error of 1 mean for three mice in each group. Mann-Whitney U-test of scores marked by (*) indicates (p < 0.006) compared with vehicle control group.
4.5 Activation of experienced effector T cells from CIA mice can be prevented by combinatorial ERK and mTOR inhibition ex vivo
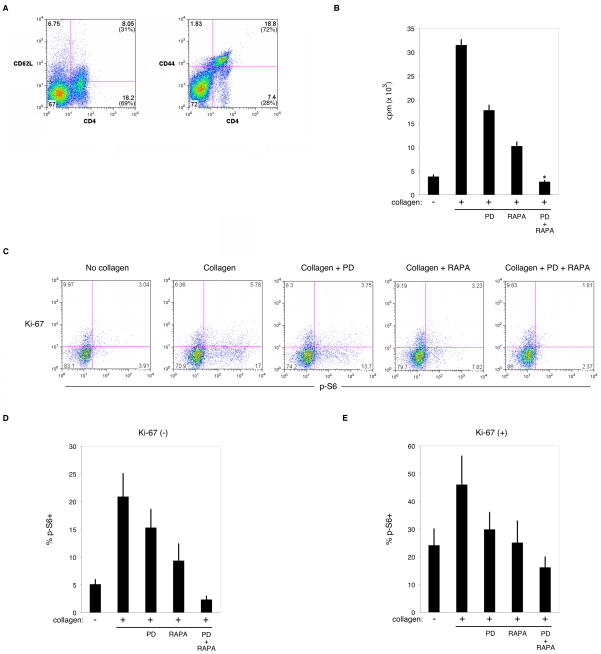
We asked whether the mechanism of action of treatment of CIA mice was coincident with T cell inhibition. CD4 T cells from arthritic CIA mice showed an activated phenotype, with 69% of the CD4 T cell population expressing low CD62L and 72% expressing high CD44 (Fig. 5A) similar to activation markers expressed on the CD8 T cell populations (data not shown). Stimulation of CD4 T cells from arthritic CIA mice with type II collagen in vitro led to proliferation that could be inhibited by PD0325901 or rapamycin alone, and to a greater extent with both drugs (Fig. 5B). We also measured S6 phosphorylation and expression of Ki-67, a marker of dividing cells [28], in T cells from arthritic CIA mice stimulated with collagen in vitro (Fig. 5C). The percent phospho-S6 positive cells was enumerated for non-dividing Ki-67(−) (Fig. 5D) and dividing Ki-67(+) (Fig. 5E) cells. Treatment with PD0325901 or rapamycin alone diminished the percentage of phospho-S6 positive CD4 T cells in both Ki-67(−) and Ki67(+) populations, while the combination of both inhibitors was even more effective. Similar inhibition was seen in the CD8 T cell population (data not shown). Inhibition of S6 phosphorylation in the non-dividing and dividing T cell populations suggests that protein translation in newly activated naïve and experienced effector CD4 T cells was inhibited in both populations, halting further activation and/or proliferation. These data support a mechanism of action by ERK and mTOR inhibition in vivo that inhibits both naïve and experienced effector T cell activation and proliferation in CIA mice.
Figure 5. Combination MEK/ERK and mTOR inhibition demonstrates additive efficacy against pathogenic T cell activation and S6 phospohrylation ex vivo.
(A) Flow cytometric measurement of CD62L and CD44 expression on CD4 T cells from spleens of arthritic mice. Quadrant numbers indicate percentage of total population, number in parentheses indicate percentage among CD4 T cells. Similar results were seen among the CD8 T cell population. Data are representative of three individually measured mice with similar results. (B) Proliferation assay of spleen leukocytes from mice as in (A) absent (−) or with (+) collagen protein, without or with PD0325901 (PD), Rapamycin (RAPA), or both drugs (PD + RAPA). Measured as mean counts per minute (cpm) of triplicates per group. Error bars indicate standard deviation. Mann-Whitney U-test in PD+RAPA group marked by (*) indicates (p < 0.05) compared to vehicle control and single drug groups. Data are representative of three individually measured mice with similar results. (C) S6 phosphorylation and proliferating cell marker Ki-67 expression measured by flow cytometry on spleen leukocyte CD4 T cells from mice as in (A) absent (No collagen) or with (Collagen) collagen protein for 24 hours, without or with PD0325901 (PD), Rapamycin (RAPA), or both drugs (PD + RAPA). Quadrant numbers indicate percentage among CD4 T cells. Similar results were seen among the CD8 T cell population. Data are representative of three individually measured mice with similar results. (D) Mean percent phospho-S6 positive CD4 T cells that are Ki-67 (−) (non-proliferating) stimulated and treated as in (C) from three individually measured mice. Similar results were seen among the CD8 T cell population. Error bars indicate standard error of means. (E) Mean percent phospho-S6 positive CD4 T cells that are Ki-67 (+) (proliferating) stimulated and treated as in (C) from three individually measured mice. Similar results were seen among the CD8 T cell population. Error bars indicate standard error of means.
5. Conclusions
In summary, we found that naïve and experienced effector CD4 T cells differentially require ERK and mTOR pathways for robust activation of protein translation and proliferation. While inhibition of either ERK or mTOR pathway could inhibit naïve CD4 T cells, inhibition of both ERK and mTOR pathways was required for robust inhibition of experienced effector CD4 T cells. CD4 T cells activated from a resting state undergo multiple rounds of division as well as acquire new functional capabilities requiring vigorous protein synthesis. The ERK and mTOR pathways converge upon the important process of protein translation as measured by S6 phosphorylation. This serves as an important checkpoint ensuring that appropriate activation signals and environmental cues are permissible for the highly bioenergetic requirements of cellular proliferation [18, 20]. While naïve T cells have more safeguards to deter autoimmune activation, experienced effectors will necessarily have fewer safeguards as they transit to sites of inflammation to expand and activate effector functions. The nature of autoimmune diseases such as rheumatoid arthritis include recurrent waves of naïve T cells being activated as well as experienced effector T cells being re-activated at the pathological site. By treating both naïve and experienced effector T cell populations through concomitant ERK and mTOR inhibition we demonstrate greater efficacy in treating arthritis in CIA mice. It should be noted however that while similarities exist between various autoimmune indications each has its own unique underlying disease mechanisms that may alter the efficacy of targeting one or more lymphocyte signaling pathways. Indeed, it has been suggested that rapamycin alone is efficacious in ameliorating murine experimental autoimmune encephalomyelitis (EAE) model of multiple sclerosis and a murine model of type one diabetes, the non-obese diabetic mouse [29–31]. Our own studies in a C57BL/6 murine EAE model corroborate the finding that rapamycin mTOR inhibition alone shows strong efficacy in treating EAE (unpublished observations). However, ERK inhibition alone showed no efficacy and the combination of mTOR and ERK inhibition unexpectedly resulted in a worse therapeutic index than mTOR inhibition alone (unpublished observations). We speculate that the regulatory T cell promoting capability of rapamycin may have been negated by ERK inhibition in EAE, but in CIA the mTOR and ERK combinatorial inhibition effect is net beneficial by preventing activation of pathogenic effector and naive T cells. As rapamycin is a clinically approved drug and small molecule inhibitors of ERK are in clinical trials, our results suggest that this particular combination of inhibitors may provide greater clinical benefit than either alone through immunomodulation of T cell protein translation and proliferation. Further development of small and large molecule drugs that target these pathways and delineation of their efficacy in various indications could be useful in the context of treating rheumatoid arthritis and other T cell driven autoimmune diseases.
Supplementary Material
Highlights.
Naïve CD4 T cells can be inhibited through either the MAPK or the mTOR pathway
Silencing memory effector CD4 T cells requires inhibition of both pathways
CIA treatment requires inhibition of both the MAPK and mTOR pathways
Acknowledgments
We are grateful to Ms. Cariel Taylor for animal husbandry and laboratory management, and Ms. Carol Fernandez provided administrative assistance.
Funding
This work was supported by grants from the National Institutes of Health to C.G.F. (CA065237), C.G.F. and P.J.U. (AI082719), and J.T.L. (AR050942), and a Mason Case Fellowship to M.T.W.
Abbreviations used in this paper
- MHC
Major Histocompatability Complex
- TCR
T Cell Receptor
- mTOR
Mammalian Target of Rapamycin
- IL-2
interleukin-2
- 4E-BP1
Eukaryotic Translation Initiation Factor 4E-binding Protein 1
- S6K1
p70 Ribosomal Protein S6 Kinase 1
- S6
Ribosomal Protein S6
- PI3K
Phosphatidylinositol 3-kinases
- ERK
Extracellular-signal-regulated Kinases
- MAPK
Mitogen-activated Protein Kinase
- MEK
Mitogen-activated Protein Kinase Kinase
- RSK
p90 Ribosomal Protein S6 Kinase
- GRAIL
Gene Related to Anergy in Lymphocytes
6. Appendix
Figure S1 is a diagram depicting the presence of both naïve and experienced effector T cells in ongoing autoimmune disease. Figure S2 shows the culture conditions for generation of experienced CD4 T cells from naïve CD4 T cells. Figure S3 depicts the mTOR and ERK signaling cascade leading to S6 phosphorylation and the targets along this pathway by the various employed drugs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pancer Z, Cooper MD. The evolution of adaptive immunity. Annu Rev Immunol. 2006;24:497–518. doi: 10.1146/annurev.immunol.24.021605.090542. [DOI] [PubMed] [Google Scholar]
- 2.St Clair EW, Turka LA, Saxon A, Matthews JB, Sayegh MH, Eisenbarth GS, Bluestone J. New reagents on the horizon for immune tolerance. Annu Rev Med. 2007;58:329–46. doi: 10.1146/annurev.med.58.061705.145449. [DOI] [PubMed] [Google Scholar]
- 3.Jager A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–84. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lohr J, Knoechel B, Nagabhushanam V, Abbas AK. T-cell tolerance and autoimmunity to systemic and tissue-restricted self-antigens. Immunol Rev. 2005;204:116–27. doi: 10.1111/j.0105-2896.2005.00241.x. [DOI] [PubMed] [Google Scholar]
- 5.Podojil JR, Miller SD. Molecular mechanisms of T-cell receptor and costimulatory molecule ligation/blockade in autoimmune disease therapy. Immunol Rev. 2009;229:337–55. doi: 10.1111/j.1600-065X.2009.00773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riley JL, June CH. The CD28 family: a T-cell rheostat for therapeutic control of T-cell activation. Blood. 2005;105:13–21. doi: 10.1182/blood-2004-04-1596. [DOI] [PubMed] [Google Scholar]
- 7.Watts TH. Staying alive: T cell costimulation, CD28, and Bcl-xL. J Immunol. 2010;185:3785–7. doi: 10.4049/jimmunol.1090085. [DOI] [PubMed] [Google Scholar]
- 8.Kallies A. Distinct regulation of effector and memory T-cell differentiation. Immunol Cell Biol. 2008;86:325–32. doi: 10.1038/icb.2008.16. [DOI] [PubMed] [Google Scholar]
- 9.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 10.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–42. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 11.Colombetti S, Basso V, Mueller DL, Mondino A. Prolonged TCR/CD28 engagement drives IL-2-independent T cell clonal expansion through signaling mediated by the mammalian target of rapamycin. J Immunol. 2006;176:2730–8. doi: 10.4049/jimmunol.176.5.2730. [DOI] [PubMed] [Google Scholar]
- 12.Dienz O, Eaton SM, Krahl TJ, Diehl S, Charland C, Dodge J, Swain SL, Budd RC, Haynes L, Rincon M. Accumulation of NFAT mediates IL-2 expression in memory, but not naive, CD4+ T cells. Proc Natl Acad Sci U S A. 2007;104:7175–80. doi: 10.1073/pnas.0610442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol. 2001;19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 14.Malek TR. The biology of interleukin-2. Annu Rev Immunol. 2008;26:453–79. doi: 10.1146/annurev.immunol.26.021607.090357. [DOI] [PubMed] [Google Scholar]
- 15.Schulz KR, Danna EA, Krutzik PO, Nolan GP. Single-cell phospho-protein analysis by flow cytometry. Curr Protoc Immunol. 2007;Chapter 8(Unit 8):17. doi: 10.1002/0471142735.im0817s78. [DOI] [PubMed] [Google Scholar]
- 16.Coligan JE. Current protocols in immunology. John Wiley and Sons; New York: 1991. [Google Scholar]
- 17.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 18.Powell JD, Delgoffe GM. The mammalian target of rapamycin: linking T cell differentiation, function, and metabolism. Immunity. 2010;33:301–11. doi: 10.1016/j.immuni.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng Y, Collins SL, Lutz MA, Allen AN, Kole TP, Zarek PE, Powell JD. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–70. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 20.Thomson AW, Turnquist HR, Raimondi G. Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol. 2009;9:324–37. doi: 10.1038/nri2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin JT, Lineberry NB, Kattah MG, Su LL, Utz PJ, Fathman CG, Wu L. Naive CD4 t cell proliferation is controlled by mammalian target of rapamycin regulation of GRAIL expression. J Immunol. 2009;182:5919–28. doi: 10.4049/jimmunol.0803986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proud CG. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J. 2007;403:217–34. doi: 10.1042/BJ20070024. [DOI] [PubMed] [Google Scholar]
- 23.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, Klevernic I, Arthur JS, Alessi DR, Cohen P. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–58. doi: 10.1038/nrm2509. [DOI] [PubMed] [Google Scholar]
- 25.Salmond RJ, Emery J, Okkenhaug K, Zamoyska R. MAPK, phosphatidylinositol 3-kinase, and mammalian target of rapamycin pathways converge at the level of ribosomal protein S6 phosphorylation to control metabolic signaling in CD8 T cells. J Immunol. 2009;183:7388–97. doi: 10.4049/jimmunol.0902294. [DOI] [PubMed] [Google Scholar]
- 26.Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, Hershey JW, Blenis J, Pende M, Sonenberg N. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J. 2006;25:2781–91. doi: 10.1038/sj.emboj.7601166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux PP, Shahbazian D, Vu H, Holz MK, Cohen MS, Taunton J, Sonenberg N, Blenis J. RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J Biol Chem. 2007;282:14056–64. doi: 10.1074/jbc.M700906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soares A, Govender L, Hughes J, Mavakla W, de Kock M, Barnard C, Pienaar B, Janse van Rensburg E, Jacobs G, Khomba G, Stone L, Abel B, Scriba TJ, Hanekom WA. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods. 2010;362:43–50. doi: 10.1016/j.jim.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito M, Ruffini F, Bellone M, Gagliani N, Battaglia M, Martino G, Furlan R. Rapamycin inhibits relapsing experimental autoimmune encephalomyelitis by both effector and regulatory T cells modulation. J Neuroimmunol. 2010;220:52–63. doi: 10.1016/j.jneuroim.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Donia M, Mangano K, Amoroso A, Mazzarino M, Imbesi R, Castrogiovanni P, Coco M, Meroni P, Nicoletti F. Treatment with rapamycin ameliorates clinical and histological signs of protracted relapsing eperimental allergic encephalomyelitis in Dark Agouti rats and induces expansion of peripheral CD4+CD25+Foxp3+ regulatory T cells. J Autoimmunol. 2009;33:135–140. doi: 10.1016/j.jaut.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Baeder WL, Sredy J, Sehgal SN, Chang JY, Adams LM. Rapamycin prevents the onset of insulin-dependent diabetes mellitus (IDDM) in NOD mice. Clin Exp Immunol. 1992;89:174–178. doi: 10.1111/j.1365-2249.1992.tb06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.