Abstract
Our objective was to determine screening practices of unaffected people in the general population at moderately increased and potentially high risk of colorectal cancer (CRC) due to their family history of the disease. 1627 participants in the Australasian Colorectal Cancer Family Registry (ACCFR) study were classified into two CRC risk categories according to the strength of their family history of the disease. We calculated the proportion of participants that adhered to national CRC screening guidelines by age-group and for each familial risk category. We performed a multinomial logistic regression analysis to evaluate the associations between screening and socio-demographic factors. Of the 1236 participants at moderately increased risk of CRC, 70 (6%) reported having undergone guideline-defined “appropriate” screening, 251 (20%) reported some, but less than appropriate screening and 915 (74%) reported never having had any CRC screening test. Of the 392 participants at potentially high risk of CRC, 3 (1%) reported appropriate screening, 140 (36%) reported some, but less than appropriate screening and 249 (64%) reported never having had any CRC screening test. On average, those of middle-age, higher education and who had resided in Australia longer were more likely to have had screening for CRC. The uptake of recommended screening by unaffected people at the highest familial risk of developing CRC is extremely low. Guidelines for CRC screening are not being implemented in the population. More research is needed to identify the reasons so as to enable development of strategies to improve participation in screening.
Keywords: colorectal cancer, family history, screening, guidelines
Introduction
Family history is one of the strongest and most consistently observed risk factors for colorectal cancer (CRC).[1-3] For example, the risk of developing the disease is about two and a half times higher for persons with a first degree relative (FDR) diagnosed with CRC. This risk is close to four times higher for FDRs of CRC cases diagnosed before the age of 45, and for those with more than one FDR affected.[3, 4] A recent study has estimated that a second- and third-degree family history is also associated with an increased risk of CRC especially if it is combined with a first-degree family history.[5] Excluding cases resulting from known inherited predispositions such as Lynch Syndrome and Familial Adenomatous Polyposis (FAP) it has been estimated that between 15% to 20% of all CRC cases are attributable to having family history of the disease.[6, 7] Therefore, persons with a family history of CRC represent a not insubstantial subset of the population that could benefit the most from screening and for which screening could be most cost effective.[8]
Many countries have specific CRC screening guidelines that recommend more aggressive screening strategies for persons with an established family history of the disease compared with those at average or ‘population’ risk.[7, 9, 10] However, there is limited information on the level of screening uptake, screening practices or the level of adherence to recommended screening guidelines. A recent literature review identified 14 studies on the screening participation of first degree relatives of persons with CRC.[11] The review noted that only a few studies had specifically investigated screening uptake by those at increased risk due to family history. It also found that many studies were unable to provide details of the family history and, therefore, could not determine if the screening undertaken was based on risk-appropriate recommended screening intervals. Only two studies made a clear distinction between diagnostic and screening tests by excluding from the analysis participants who underwent a diagnostic test.
Little is known about the factors influencing screening behavior of persons with a strong family history of CRC. The existing studies have reported inconsistent associations with age, and positive associations with healthcare provider recommendation, perceived risk and sibling “closeness” with screening compliance.[12-14] Most of these studies however included only persons aged 50 years or over and only used a non-specific definition of family history of CRC.
In this work we used a population-based family study to estimate the CRC screening practices of unaffected Australians at familial risk of CRC and to examine the association between self-reported screening behavior and socio-demographic factors.
Material and methods
Subjects
This study is based on the population-based families in the Australasian Colorectal Cancer Family Registry (ACCFR) a large family-based study that is part of an international consortium funded by the United States National Cancer Institute, designed to address specific research questions about CRC aetiology. Details of the ACCFR objectives and methodology have been reported elsewhere. [15] Briefly, population-based case-probands were incident first primary cases of adenocarcinoma of the colorectum (including those with Lynch syndrome) diagnosed in residents of the Melbourne metropolitan area, aged 18 to 59 years between 1997 and 2001 and identified from the Victorian Cancer Registry, who did not have a previous diagnosis of CRC or FAP. Attempts were made to recruit the adult first- and second-degree relatives (parents, siblings, offspring, aunts, uncles and grandparents) as well as the spouses/partners of all case-probands. In addition, all first-degree relatives of any relatives with a diagnosis of CRC were sequentially ascertained. Approaches to the relatives of case-probands were made after obtaining permission from the case-probands to contact these relatives.
Population control-probands were frequency matched by age and sex to the age at diagnosis and sex of the case-probands and identified from the federal electoral register as living in the Melbourne metropolitan area (adult voting is compulsory in Australia). As for the case-probands, control-probands were asked permission to contact their first-and second-degree relatives regarding participation in the ACCFR.
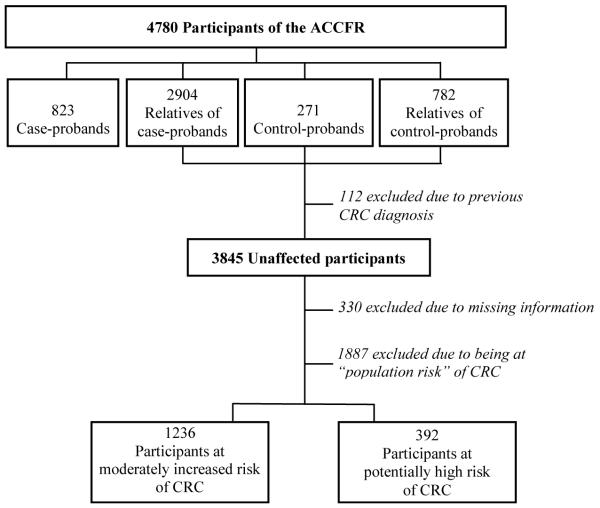
Figure 1 summarises the ACCFR subject recruitment and participants’ selection to the current study. To address our research question, we included all unaffected individuals available, regardless of their recruitment status in the ACCFR.
Figure 1.
Summary of participants’ selection
Data collection
A risk factor questionnaire and a family cancer history questionnaire were administered to all participants at baseline. Questionnaires were administered by face-to-face interviews with the probands and telephone interviews with their relatives. All participants were asked to provide information on:
demographics (age, sex, date of birth, marital status, education, relationship to proband, proband’s vital status and date of death if deceased),
personal medical history (history of diabetes, high blood cholesterol level, irritable bowel syndrome, FAP, history of cancer),
family history of cancer, and for CRC, age, sex, date of birth, type of cancer, age at diagnosis for each first- and second-degree relative,
history of faecal occult blood test (FOBT), sigmoidoscopy and colonoscopy including: reason for their first test (to investigate a new problem; family history of CRC; routine check up; follow-up of previous problem; other); their age when they first had a screening test; the number of separate tests they have had; and if more than one test their age when they last had a test.
Attempts were made to verify all reports of CRC in families against medical records, death certificates, pathology reports and cancer registry data. We identified a total of 150 CRC cases (multiple reports by some individuals) of which 95 (63.3%) have been verified and 55 (36.7%) remain unconfirmed.
Analysis
Risk categorisation
Participants without a personal history of CRC were classified into one of three CRC risk categories according to the Australian National Health and Medical Research Council (NHMRC) guidelines,[10] based on their family history of CRC and age at CRC diagnosis of their affected relatives.
At or slightly above average risk (no personal history of bowel cancer, advanced adenoma, or chronic ulcerative colitis and either no close relatives with bowel cancer or one first-degree or second-degree relative with bowel cancer diagnosed at age 55 years or older.)
Moderately increased risk (one first-degree relative with bowel cancer diagnosed before the age of 55 years or, two first- or one first- and one second-degree relative(s) on the same side of the family with bowel cancer diagnosed at any age);
Potentially high risk (three or more first-degree relatives or, a combination of first-and second-degree relatives on the same side of the family diagnosed with bowel cancer or, two or more first- or second-degree relatives on the same side of the family diagnosed with bowel cancer including any of the following features (first- or second-degree relative with multiple bowel cancer, first- or second-degree relative who has/had an Lynch Syndrome-related cancer (such as endometrial, ovarian, stomach, small bowel, renal pelvis or ureter, biliary tract or brain cancer[16]).
Participants with a previous diagnosis of cancer or who were classified as at or slightly above average risk were excluded from this study.
Screening participation
Screeners were defined as participants who reported having undergone an FOBT, and/or a sigmoidoscopy or a colonoscopy as a regular check-up or because of their family history of CRC. Non-screeners were defined as those participants who did not report any screening or who reported having undergone a procedure to investigate a new problem or as follow-up of a previous problem (i.e. tests for the later group were considered as diagnostic). Participants’ screening participation was assessed as appropriate or not, based on the NHMRC guidelines. For participants categorised as being at ‘moderately increased risk’, appropriate screening was defined as having at least one screening colonoscopy every 5 years from age 50, or from 10 years younger than the age of first diagnosis of CRC in the family. For participants categorised as being at ‘potentially high risk’, appropriate screening was defined as having at least one colonoscopy screening every 2 years from age 25, or 5 years younger than the age of first diagnosis of CRC in the family. For those identified as not having appropriate screening, screening participation by FOBT, sigmoidoscopy, colonoscopy or any of the three procedures was calculated. Radiological colonography was not considered as part of this study due to its very low use as a screening tool and that it is a non-rebated investigation for this indication in Australia.
Statistical analysis
Proportions of participants that reported appropriate, less than appropriate, and never screening were calculated by age-group and for each CRC risk category. For those who reported less than appropriate screening according to recommendations, we computed screening by frequency and screening modality. Participants in each risk category were compared using the Pearson χ2 test of independence.
A multinomial logistic regression analysis was performed on the entire sample to evaluate the association, as odds ratios (OR) and their 95 percent confidence intervals (CI), between the outcome: screening (three mutually exclusive levels: never screened (baseline), less than appropriate screening, appropriate screening); and the explanatory variables: age, education (tertiary vs. other), marital status (married or living as married vs. other), degree of relatedness to the proband (first-degree vs. other), regular physical activity (at least 30 minutes a week for a minimum of 3 consecutive months in the most recent decade of life vs. less activity), body mass index (<25 kg/m2 vs. ≥25 kg/m2), cigarette smoking (ex-smoker vs. current smoker vs. never smoked), number of years lived in Australia (>20 years vs. ≤ 20 years), diabetes (affected vs. non-affected), high cholesterol (affected vs. non-affected), and personal history of any cancer apart from CRC (yes vs. no). These variables were selected based on prior studies suggesting an association with either CRC risk or screening practices.[12-14-17]
To determine our best-fitting statistical model we followed the strategy described by Homsher and Lemeshow.[18] Variable selection was performed by fitting two separate binary logistic models for two outcomes: less than appropriate screening vs. never screened; and appropriate screening vs. never screened. Variables significant at p<0.05 in the parsimonious binary logistic models were then tested for significance in the multinomial model. For variables significantly associated with only one of the outcomes, we estimated that association in the multinomial model by fixing the coefficient of the association with the other outcome to zero (i.e. no association). We used clustered robust standard errors to account for intra-class correlation due to non-independence of family members. Any participant that did not provide data for any of the explanatory variables, or did not provide sufficient information on screening practices to generate the outcome variables, were excluded from all analyses. All statistics were calculated using STATA version 10.
Results
A total of 1,627 participants met the inclusion criteria for being at moderately increased or potentially high CRC risk. Table 1 shows participants’ characteristics for each CRC risk category.
Table 1.
Participants’ characteristics
| Participants at Moderately increased-risk of CRC (n=1236) |
Participants at Potentially high-risk of CRC (n=391) |
p value* |
Total (n=1627) |
||||
|---|---|---|---|---|---|---|---|
| Category | n | % | n | % | n | % | |
| Age group | |||||||
| Mean age, years (SD) | 45.5 (18.2) | 48.5 (16.3) | 0.01 | 46.2 (17.8) | |||
| 18-24 | 195 | 15.7 | 33 | 8.4 | 228 | 14.0 | |
| 25-29 | 124 | 10.0 | 26 | 6.6 | 150 | 9.2 | |
| 30-34 | 95 | 7.7 | 29 | 7.4 | 124 | 7.6 | |
| 35-39 | 90 | 7.3 | 32 | 8.1 | 122 | 7.5 | |
| 40-44 | 111 | 8.9 | 33 | 8.4 | 144 | 8.9 | |
| 45-49 | 121 | 9.8 | 51 | 13.0 | 172 | 10.5 | |
| 50-54 | 107 | 8.6 | 52 | 13.3 | 159 | 9.8 | |
| 55-59 | 94 | 7.6 | 41 | 10.5 | 135 | 8.3 | |
| 60-64 | 76 | 6.1 | 22 | 5.6 | 98 | 6.0 | |
| 65-70 | 77 | 6.2 | 29 | 7.4 | 106 | 6.5 | |
| >70 | 146 | 11.8 | 43 | 11.0 | 189 | 11.6 | |
| Sex | |||||||
| Female | 655 | 53.0 | 224 | 57.1 | 0.1 | 879 | 53.9 |
| Male | 581 | 47.0 | 167 | 42.7 | 748 | 45.9 | |
| Tertiary education | |||||||
| No | 861 | 69.6 | 293 | 74.9 | 0.04 | 1154 | 70.9 |
| Yes | 375 | 30.3 | 98 | 25.1 | 473 | 29.0 | |
| Marital status | |||||||
| Married/living as | 376 | 30.4 | 85 | 21.7 | 0.01 | 1166 | 71.7 |
| Not married | 860 | 69.6 | 306 | 78.2 | 461 | 28.3 | |
| Physical activity | |||||||
| No | 858 | 69.4 | 296 | 75.7 | 0.01 | 1154 | 70.9 |
| Yes | 378 | 30.6 | 95 | 24.2 | 473 | 29.0 | |
| BMI | |||||||
| ≥ 25 | 618 | 50.0 | 203 | 51.8 | 0.5 | 806 | 49.5 |
| < 25 | 618 | 50.0 | 188 | 48.0 | 821 | 50.4 | |
| Smoking status | |||||||
| Never smoke | 523 | 42.3 | 167 | 42.6 | 0.9 | 690 | 42.4 |
| Ex-smoker | 409 | 33.1 | 128 | 32.7 | 537 | 33.0 | |
| Current smoker | 304 | 24.6 | 96 | 24.5 | 400 | 24.6 | |
| Years in Australia | |||||||
| ≤20 years | 203 | 12.5 | 54 | 13.8 | 0.3 | 203 | 12.5 |
| > 20 years | 1425 | 87.5 | 337 | 86.2 | 1424 | 87.5 | |
| Irritable bowel syndrome | |||||||
| No | 1176 | 95.2 | 370 | 94.6 | 0.6 | 1546 | 95.0 |
| Yes | 60 | 4.8 | 21 | 5.4 | 81 | 4.9 | |
| Diabetes | |||||||
| No | 1189 | 96.2 | 369 | 94.3 | 0.1 | 1558 | 95.7 |
| Yes | 47 | 3.8 | 22 | 5.6 | 69 | 4.2 | |
| High cholesterol | |||||||
| No | 1014 | 82.1 | 308 | 78.8 | 0.1 | 1322 | 81.2 |
| Yes | 222 | 17.9 | 83 | 21.1 | 305 | 18.7 | |
| History of other cancers | |||||||
| No | 1172 | 94.8 | 370 | 94.6 | 0.8 | 1542 | 94.7 |
| Yes | 64 | 5.2 | 83 | 5.4 | 85 | 5.22 | |
Pearson χ2 test of independence between the two risk groups
Participants at ‘Moderately increased risk’
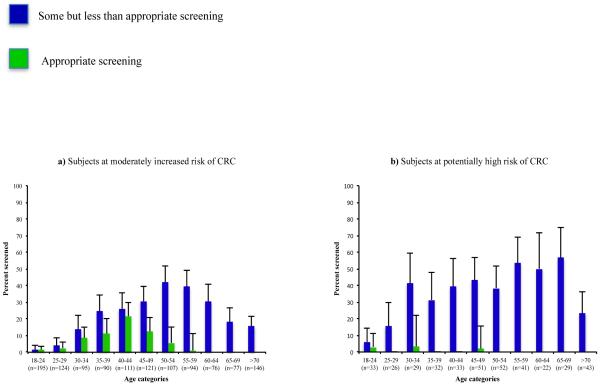
Based on family cancer history, 1236 participants were categorised as being at moderately increased CRC risk. Of these, 70 (6%) reported a modality and frequency of screening consistent with appropriate screening. The age group with the highest level of appropriate screening (15%) was the 35 to 49 year-old group. This was 10 times the level for participants aged 50 years or older for whom only 1% reported appropriate screening. Of the 1166 participants who reported less than appropriate screening, 915 (78%) reported never having had a CRC screening test (this included any endoscopy and FOBT). All of the remaining 251 (22%) who reported some, but inappropriate, screening reported at least one colonoscopy, with the majority (237 of 251, 94%) having undergone the most recent procedure more than 10 years prior to interview (Fig. 2a). Of these 251, 16 (1%) and 14 (1%) screeners reported FOBT and sigmoidoscopy screening, respectively, all of which were performed more than 10 years prior to interview.
Figure 2.
CRC screening participation by age category and type of screening
Participants at ‘Potentially high risk’
Based on their family cancer history, 392 participants were categorised as being at potentially high risk of CRC. Three of them (1%) reported screening practices consistent with the national guidelines. Of the 389 participants who reported less than appropriate screening, 249 (64%) reported never having had a CRC screening test. Of the remaining 140 screeners, colonoscopy was the most commonly reported test (36%), with the highest uptake levels (>50%) observed for participants aged between 55 and 69 years (Fig. 2b). The second most common test was FOBT (6%) followed by sigmoidoscopy (2%). The vast majority of these procedures (92%) were last performed more than 10 years before interview.
Factors associated with CRC screening
Middle-aged participants, those with higher education and those who had resided longer in Australia reported higher CRC screening participation (Table 2). Participants who had lived for more than 20 years in Australia were 80% more likely to report having undergone at least one CRC screening procedure compared with those who had lived in the country for a shorter period of time. Ex-smokers and current smokers were less likely to report having ever been screened compared with never smokers.
Table 2.
Results of multinomial logistic regression* of factors associated with CRC screening
| Less than appropriate screening vs. No screening |
Appropriate screening vs. No screening |
|||||
|---|---|---|---|---|---|---|
| Predictors | OR | 95% CI | P value | OR | 95% CI | P value |
| Age group | ||||||
| 18-29 | 0.06 | [0.03-0.11] | <0.001 | 0.08 | [0.03-0.21] | <0.001 |
| 30-39 | 0.5 | [0.31-0.82] | 0.006 | 0.49 | [0.27-0.88] | 0.02 |
| 40-49 | 1 | reference | 1 | reference | ||
| ≥50 | 0.70 | [0.50-0.96] | 0.03 | 0.07 | [0.03-0.17] | <0.001 |
| Education | ||||||
| No Tertiary education | 1 | reference | 1 | reference | ||
| Tertiary education | 1.30 | [0.97-1.74] | 0.07 | 2.72 | [1.65-4.48] | <0.001 |
| Smoking status | ||||||
| Never smoke | 1 | reference | – | |||
| Ex smoker | 0.87 | [0.66-1.13] | 0.3 | – | ||
| Current smoker | 0.59 | [0.42-0.83] | 0.003 | – | ||
| Number of years in Australia | ||||||
| Between 10&20 years | 1 | reference | – | |||
| More than 20 years | 1.81 | [0.95-3.46] | 0.07 | – | ||
Parsimonious model only
Discussion
This study reports the first population-based and risk-category-specific estimates of CRC screening by persons at moderately increased risk or at potentially high risk of CRC due to having a family history of the disease. For both risk categories examined, the level of screening uptake was low. Of 1236 participants considered at increased risk of CRC, only about one in four reported ever having a screening colonoscopy and only one in 15 screened according to the published guidelines. Participation in colonoscopy screening was slightly higher for participants at potentially high risk of CRC for whom one in three had some screening but only about one in 130 had appropriate screening.
The reasons for such low screening could not be addressed by this study. There are several plausible explanations including, but not limited to: insufficient level of risk awareness among CRC family members;[19-20] patients’ under reporting of family history of CRC to clinicians resulting in management strategies relevant to lower risk persons;[21] and low level of clinician awareness of the current screening guidelines. A recent study has shown that information on family history and other CRC risk factors was poorly reported in patients’ medical records.[22] Issues related to privacy and the dissemination of information to relatives at risk might also represent a barrier to providing accurate family history thus resulting in inappropriate screening.[23, 24] Harris et al.[25] and Cockburn et al.[26] have previously reported screening participation by first-degree relatives of CRC patients to be 50% and 42% respectively. Although these studies were conducted in Australia, they are not comparable to our population-based findings as they were both small cross-sectional surveys, and only one of them excluded from analysis participants who had had a diagnostic colonoscopy.[26] Several other studies have investigated the screening practices of CRC patients’ relatives, with reported participation estimates ranging from 16% to 69%.[11, 16, 27, 28] Most of these studies, however, were community surveys with modest levels of response and prone to several shortcomings. For example, many studies investigated the three main screening procedures (FOBT, sigmoidoscopy, colonoscopy) but failed to discriminate between screening and diagnostic tests or to report details on the participants’ family history such as age at diagnosis and number of affected family members.[11]
One of the main strengths of the current study is our ability to present screening participation with respect to specific CRC risk levels defined by family history of cancer. This was possible because of our systematic data collection from all participants and systematic attempts to validate information provided by relatives.
Australia has one of the highest incidence of CRC in the world with more than 13 500 cases diagnosed each year[29] and an age-standardised incidence rate of 38.7 per 100,000 persons.[30] Studies have reported that 15% to 20% of all CRC cases can be attributed to a family history of the disease,[6, 7] i.e. between 2025 and 2700 cases annually in Australia. Given that screening is known to reduce CRC risk for persons with a family history of the disease[31] and, as we have demonstrated here, the majority of persons with a family history undergo inappropriate or no screening, we can infer that hundreds of predictable and potentially preventable CRC cases occur in Australia each year.
Evidence-based guidelines for the prevention, early detection and management of CRC were first introduced in Australia in 1999 (and updated in 2002 and 2005),[10] two years after the beginning of the ACCFR recruitment phase and three years before the final recruitment of participants for this analysis. This time lag might account for a proportion of the inappropriate level of screening. Another limitation of our analysis is that it is based on a one-time survey of participants so we do not know whether personal screening practices have changed over time. A longitudinal study is needed to determine the true screening history of persons at increased risk of CRC and to help understand the impact, if any, of existing guidelines. The ACCFR is currently conducting follow-up surveys of participants and these data will be available for analysis in due course.
Medical practitioners are often not familiar with CRC screening guidelines or not proactive in implementing them.[32] Given that patients’ compliance with guidelines is unlikely without their doctor’s influence and encouragement,[33, 34] we speculate that our findings remain relevant to the current Australian context, as no major or specific initiative to increase screening participation by people above average-risk of CRC has been implemented during the last decade. This situation is not specific to Australia. Several countries have introduced CRC screening guidelines based on family history. These often refer to the same evidence and provide very similar screening recommendations. For example, a brief comparison of the NHMRC criteria applied in our study with those used in guidelines published in the UK[35] and the United States[36, 37] shows a strong concordance in terms of familial-risk categorisation, test frequency and recommended screening modality. To date, no country has introduced a national screening programme targeting persons at increased risk of CRC, the results presented in this study are, therefore, likely to reflect trends in other countries. A recent Italian study has reported that only 8% of first-degree relatives of patients with CRC reported having undergone colonoscopy screening despite having been exposed to an extensive health promotion campaign as part of a regional CRC screening programme for persons at increased risk.[38] This suggest that, rather than using generic campaigns designed for the whole population, alternative, targeted methods such as educating health care providers about the specific cancer risk incurred by relatives of CRC patients are needed to increase screening.
A number of predictors of CRC screening for relatives of CRC patients have been reported previously.[12-14, 17] Our analysis found that older age was the strongest predictor of both having ever had a CRC screening test and of having a risk-category specific and timely screening procedure. However, once persons reached age 50 years, screening decreased with age – despite their risk of CRC increasing with age. Participants who were university educated had a higher level of appropriate screening suggesting that patient related factors and an understanding of risk and its implications may contribute to screening uptake.
In summary, this study reports the most accurate estimates of CRC screening participation by persons at moderately increased risk of CRC, and the first estimates for those at potentially high risk of CRC. It also provides the first detailed assessment of existing CRC screening recommendations for these familial risk populations, against the actual practices in the community. The results clearly suggest that screening practice does not meet the level recommended by current guidelines and, overall, expose a significant shortfall in terms of CRC prevention for those at highest risk of developing CRC. We hypothesise that the very low levels of appropriate screening observed result from the absence of an organised approach to CRC screening for increased risk categories in the population. In 2006, Australia was one of the first countries in the world to implement an organised, population-based CRC screening programme for average risk persons. Since then increasing resources and efforts have been dedicated to the fight again CRC.[39, 40] Our results suggests that screening strategies to reduce the risk of CRC focused on those with a family history of the disease might be a fruitful avenue to reduce the burden of CRC for those most at risk.
Acknowledgments
The authors thank Kelly Aujard for her assistance with variable design and ascertainment.
Grant Support:
This study was supported by the NIH (National Cancer Institute grants RFA CA-95-011, UO1 CA097735). Driss Ait Ouakrim is supported by a Commonwealth Scientific and Industrial Research Organisation PhD scholarship (CSIRO, Preventative Health Flagship). The study was conducted independently of funding agencies.
Financial support:
This study was supported by the NIH (National Cancer Institute grants RFA CA-95-011, UO1 CA097735). Driss Ait Ouakrim is supported by a Commonwealth Scientific and Industrial Research Organisation PhD scholarship (CSIRO, Preventative Health Flagship).
The study was conducted independently of funding agencies.
Footnotes
Conflict of interest:
None declared.
References
- [1].Carstensen B, Soll-Johanning H, Villadsen E, Sondergaard JO, Lynge E. Familial aggregation of colorectal cancer in the general population. Int J Cancer. 1996;68(4):428–35. doi: 10.1002/(SICI)1097-0215(19961115)68:4<428::AID-IJC5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- [2].Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138(6):2044–58. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].John DJ, McDermott FT, Hopper JL, Debney EA, Johnson WR, Hughes ES. Cancer risk in relatives of patients with common colorectal cancer. Ann Intern Med. 1993;118(10):785–90. doi: 10.7326/0003-4819-118-10-199305150-00005. [DOI] [PubMed] [Google Scholar]
- [4].Johns LE, Houlston RS. A systematic review and meta-analysis of familial colorectal cancer risk. Am J Gastroenterol. 2001;96(10):2992–3003. doi: 10.1111/j.1572-0241.2001.04677.x. [DOI] [PubMed] [Google Scholar]
- [5].Taylor DP, Burt RW, Williams MS, Haug PJ, Cannon-Albright LA. Population-based family history-specific risks for colorectal cancer: a constellation approach. Gastroenterology. 2010;138(3):877–85. doi: 10.1053/j.gastro.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Burt RW, Bishop DT, Lynch HT, Rozen P, Winawer SJ, WHO Collaborating Centre for the Prevention of Colorectal Cancer Risk and surveillance of individuals with heritable factors for colorectal cancer. Bull World Health Organ. 1990;68(5):655–65. [PMC free article] [PubMed] [Google Scholar]
- [7].Winawer SJ, Fletcher RH, Miller L, Godlee F, Stolar MH, Mulrow CD, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology. 1997;112(2):594–642. doi: 10.1053/gast.1997.v112.agast970594. [DOI] [PubMed] [Google Scholar]
- [8].Ladabaum U, Ferrandez A, Lanas A. Cost-effectiveness of colorectal cancer screening in high-risk Spanish patients: use of a validated model to inform public policy. Cancer Epidemiol Biomarkers Prev. 2010;19(11):2765–76. doi: 10.1158/1055-9965.EPI-10-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups. Gut. 2010;59(5):666–89. doi: 10.1136/gut.2009.179804. update from 2002. [DOI] [PubMed] [Google Scholar]
- [10].NHMRC . Clinical practice guidelines for the prevention, early detection and management of colorectal cancer. National Health and Medical Research Council; 2005. [Google Scholar]
- [11].Rees G, Martin PR, Macrae FA. Screening participation in individuals with a family history of colorectal cancer: a review. Eur J Cancer Care (Engl) 2008;17(3):221–32. doi: 10.1111/j.1365-2354.2007.00834.x. [DOI] [PubMed] [Google Scholar]
- [12].Mack LA, Cook LS, Temple WJ, Carlson LE, Hilsden RJ, Paolucci EO. Colorectal cancer screening among first-degree relatives of colorectal cancer patients: benefits and barriers. Ann Surg Oncol. 2009;16(8):2092–100. doi: 10.1245/s10434-009-0528-z. [DOI] [PubMed] [Google Scholar]
- [13].Madlensky L, Esplen MJ, Gallinger S, McLaughlin JR, Goel V. Relatives of colorectal cancer patients: factors associated with screening behavior. Am J Prev Med. 2003;25(3):187–94. doi: 10.1016/s0749-3797(03)00202-2. [DOI] [PubMed] [Google Scholar]
- [14].Manne S, Markowitz A, Winawer S, Meropol NJ, Haller D, Rakowski W, et al. Correlates of colorectal cancer screening compliance and stage of adoption among siblings of individuals with early onset colorectal cancer. Health psychology. 2002;21(1):3–15. [PubMed] [Google Scholar]
- [15].Newcomb PA, Baron J, Cotterchio M, Gallinger S, Grove J, Haile R, et al. Colon Cancer Family Registry: an international resource for studies of the genetic epidemiology of colon cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2331–43. doi: 10.1158/1055-9965.EPI-07-0648. [DOI] [PubMed] [Google Scholar]
- [16].Vasen HF, Watson P, Mecklin JP, Lynch HT. New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC, Lynch syndrome) proposed by the International Collaborative group on HNPCC. Gastroenterology. 1999;116(6):1453–6. doi: 10.1016/s0016-5085(99)70510-x. [DOI] [PubMed] [Google Scholar]
- [17].Griffith KA, McGuire DB, Royak-Schaler R, Plowden KO, Steinberger EK. Influence of family history and preventive health behaviors on colorectal cancer screening in African Americans. Cancer. 2008;113(2):276–85. doi: 10.1002/cncr.23550. [DOI] [PubMed] [Google Scholar]
- [18].Hosmer DWLS. Applied Logistic Regression. 2nd ed. New York: 1980. [Google Scholar]
- [19].Harris MA, Treloar CJ, Byles JE. Colorectal cancer screening: discussions with first degree relatives. Aust N Z J Public Health. 1998;22(7):826–8. doi: 10.1111/j.1467-842x.1998.tb01502.x. [DOI] [PubMed] [Google Scholar]
- [20].Rubin DT, Gandhi RK, Hetzel JT, Kinnear SH, Aronsohn A, Wood G, et al. Do colorectal cancer patients understand that their family is at risk? Dig Dis Sci. 2009;54(11):2473–83. doi: 10.1007/s10620-009-0940-z. [DOI] [PubMed] [Google Scholar]
- [21].Glanz K, Grove J, Lerman C, Gotay C, Le Marchand L. Correlates of intentions to obtain genetic counseling and colorectal cancer gene testing among at-risk relatives from three ethnic groups. Cancer Epidemiol Biomarkers Prev. 1999;8(4 Pt 2):329–36. [PubMed] [Google Scholar]
- [22].Trano G, Wasmuth HH, Sjursen W, Hofsli E, Vatten LJ. Awareness of heredity in colorectal cancer patients is insufficient among clinicians: a Norwegian population-based study. Colorectal Dis. 2009;11(5):456–61. doi: 10.1111/j.1463-1318.2009.01830.x. [DOI] [PubMed] [Google Scholar]
- [23].Leggett BA. Family-based screening for colorectal cancer: The Australian perspective. J Gastroenterol Hepatol. 2009;24(Suppl 3):S29–32. doi: 10.1111/j.1440-1746.2009.06068.x. [DOI] [PubMed] [Google Scholar]
- [24].Suthers G. Information management in familial cancer. Cancer Forum. 2007;31:138–41. [Google Scholar]
- [25].Harris MA, Byles JE. A survey of screening compliance among first degree relatives of people with colon cancer in New South Wales. J Med Screen. 1997;4(1):29–34. doi: 10.1177/096914139700400110. [DOI] [PubMed] [Google Scholar]
- [26].Cockburn J, Paul C, Tzelepis F, McElduff P, Byles J. Screening for bowel cancer among NSW adults with varying levels of risk: a community survey. Aust N Z J Public Health. 2002;26(3):236–41. doi: 10.1111/j.1467-842x.2002.tb00680.x. [DOI] [PubMed] [Google Scholar]
- [27].Longacre A. V. CLD, Gross C. P. Screening Colonoscopy Use Among Individuals at Higher Colorectal Cancer Risk. J Clin Gastroenterol. 2006;40(6):490–96. doi: 10.1097/00004836-200607000-00006. [DOI] [PubMed] [Google Scholar]
- [28].Ruthotto F, Papendorf F, Wegener G, Unger G, Dlugosch B, Korangy F, et al. Participation in screening colonoscopy in first-degree relatives from patients with colorectal cancer. Ann Oncol. 2007;18(9):1518–22. doi: 10.1093/annonc/mdm200. [DOI] [PubMed] [Google Scholar]
- [29].AIHW. Australian Institute of Health and Welfare . Cancer - Australian cancer statistics. 2010. update May 2010. [Google Scholar]
- [30].Ferlay JSH, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, cancer incidence and mortality worldewide: IARC CancerBase no. 10. International Agency for Research on Cancer; Lyon, France: 2010. [Google Scholar]
- [31].Dove-Edwin SP, Sasieni P, Adams J, Thomas HJ. Prevention of colorectal cancer by colonoscopic surveillance in individuals with a family history of colorectal cancer: 16 year, prospective, follow-up study. BMJ. 2005;331:1047–49. doi: 10.1136/bmj.38606.794560.EB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schattner A, Gilad A. Primary care physicians’ awareness and implementation of screening guidelines for colorectal cancer. Prev Med. 2002;35(5):447–52. doi: 10.1006/pmed.2002.1101. [DOI] [PubMed] [Google Scholar]
- [33].Giveon S, Kahan E. Patient adherence to family practitioners’ recommendations for breast cancer screening: a historical cohort study. Fam Pract. 2000;17(1):42–5. doi: 10.1093/fampra/17.1.42. [DOI] [PubMed] [Google Scholar]
- [34].Snell JL, Buck EL. Increasing cancer screening: a meta-analysis. Prev Med. 1996;25(6):702–7. doi: 10.1006/pmed.1996.0109. [DOI] [PubMed] [Google Scholar]
- [35].Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups. Gut. 2010;59(5):666–689. doi: 10.1136/gut.2009.179804. update from 2002. [DOI] [PubMed] [Google Scholar]
- [36].US Preventative Services Task Force Screening for Colorectal Cancer, Recommendation Statement. 2008 www.giejournal.org http://www.ahrq.gov/CLINIC/USPSTF/uspscolo.htm
- [37].American Cancer Society Cancer screening guidelines in the United States, 2008. CA Cancer J Clin. 2008;58:161–179. doi: 10.3322/CA.2007.0017. Available at: http://caonline.amcancersoc.org/cgi.content/full/58/3/161. [DOI] [PubMed] [Google Scholar]
- [38].Armelao F, Orlandi PG, Tasini E, Franceschini G, Franch R, Paternolli C, et al. High uptake of colonoscopy in first-degree relatives of patients with colorectal cancer in a healthcare region: a population-based, prospective study. Endoscopy. 2010;42(1):15–21. doi: 10.1055/s-0029-1215324. [DOI] [PubMed] [Google Scholar]
- [39].Flitcroft KL, Salkeld GP, Gillespie JA, Trevena LJ, Irwig LM. Fifteen years of bowel cancer screening policy in Australia: putting evidence into practice? Med J Aust. 2010;193(1):37–42. doi: 10.5694/j.1326-5377.2010.tb03739.x. [DOI] [PubMed] [Google Scholar]
- [40].Young GP. Population-based screening for colorectal cancer: Australian research and implementation. J Gastroenterol Hepatol. 2009;24(Suppl 3):S33–42. doi: 10.1111/j.1440-1746.2009.06069.x. [DOI] [PubMed] [Google Scholar]