Abstract
The role of extracellular 70 kDa heat shock protein 70 (ehsp70) in central nervous system inflammation is vastly understudied, despite evidence supporting the ability to drive a pro-inflammatory state. We investigated the presence of ehsp70 in cerebrospinal fluid (CSF) and serum of dogs with Steroid Responsive Meningitis-Arteritis (SRMA), with the hypothesis that an ehsp70 response would occur, and might play a role in the pathogenesis of this disease. Samples from 30 dogs acutely affected with SRMA, and 30 dogs treated with corticosteroids and currently in clinical remission from SRMA were compared with normal dogs. Serum and CSF concentrations of ehsp70 were quantified using an enzyme-linked immunosorbent assay. An ehsp70 response occurred in the CSF of dogs with SRMA and this response was attenuated by corticosteroid treatment. There was no correlation between serum and CSF concentrations of ehsp70, supporting local production and release of ehsp70 and not simply leakage from serum. Dogs with SRMA thus represent a powerful spontaneous model by which to study the role of ehsp70 in CNS inflammation.
Keywords: Inflammation, Steroid Responsive Meningitis-Arteritis, Cerebrospinal fluid, Heat shock protein 70, Meningoencephalitis, Myelitis
1. Introduction
The role of the major inducible 70 kDa heat shock protein (hsp70) in the innate immune response to disease is an active area of study and there are potentially diverse roles. Intracellular hsp70 is a highly conserved molecular chaperone with well defined roles in cytoprotection; however, roles of extracellular hsp70 (ehsp70) are not clearly defined. When released into the extracellular space, ehsp70 serves as an immunoregulatory protein whose function may be context dependent (Awad el al. 2008). While anti-inflammatory roles exist (van Eden et al. 2005), ehsp70 may also serve as a damage-associated molecular pattern (DAMP) to stimulate toll-like receptors (Wheeler et al., 2009). In this context, ehsp70 may activate neutrophils, microglia, dendritic cells, T and B lymphocytes, and NK cells, and stimulate release of IL-1β and TNF-α (Kakimura et al., 2002; Vabulas et al., 2002,; Asea et al., 2005; Boros and Bromberg, 2006; Calderwood, 2007; Wheeler et al. 2009). These responses may be initially protective in nature, but if persistent, could perpetuate a detrimental inflammatory state. Increases in serum ehsp70 concentrations occur in various systemic inflammatory conditions such as Behςet’s disease and rheumatoid arthritis, but the role of ehsp70 in central nervous system (CNS) inflammation requires investigation. An ehsp70 response in autoimmune disease of the CNS has not been evaluated, although sustained ehsp70 release is described in the inflammatory sequel to ischemia-reperfusion injury of the spinal cord in dogs and humans (Awad et al., 2008; Hecker et al., 2008).
Steroid responsive meningitis-arteritis (SRMA) is a common inflammatory disorder of dogs (Tipold, 1995; Meric, 1998). Clinical features of this disease include cervical pain and rigidity occurring secondary to meningitis and fibrinous polyarteritis (Harcourt, 1978; Hayes et al., 1989; Tipold et al., 1995; Wrzosek et al., 2009). Affected dogs manifest a marked neutrophilic pleocytosis and increased protein concentrations in cerebrospinal fluid (CSF), as well as fever and an elevated white blood cell (WBC) count in peripheral blood. Treatment of affected dogs with corticosteroids suppresses the inflammatory response and yields a favorable prognosis. The inflammatory response in SRMA includes marked elevations in serum acute phase proteins such as C reactive protein (CRP), and infiltration of B-lymphocytes and increased IgA concentrations in CSF (Tipold et al., 1994; Tipold et al., 1995; Tipold et al., 1999; Bathen-Noethen et al., 2008; Schwartz et al., 2011). Like other immune-mediated diseases, an infectious or environmental trigger is suspected, but the molecules initiating and/or sustaining the inflammatory state have not been identified. Given the pro-inflammatory potential of ehsp70, particularly the association with neutrophil activation, and possibly in neutrophil recruitment (Wheeler et al., 2009), we hypothesized that ehsp70 may be a component of the inflammatory response in SRMA, and that SRMA may serve as an important model through which to study the immunomodulatory role of this protein in the disease state.
The present study sought to determine if ehsp70 is a component of the inflammatory process in SRMA and if so, if there is evidence for CNS-specific production. Such findings would support the more broad relevance of ehsp70 to CNS inflammation and would establish a model system in which to study its role in inflammation.
2. Materials and methods
2.1 Sample collection
All samples evaluated in this study were collected by two of the investigators (AM, AT) under approved animal care and use protocols in accordance with the investigator’s institution. Previously established clinical criteria (Bathen-Noethen at al., 2009; Maiolini et al., 2011) were used to identify clinical cases of SRMA, including results of serum and CSF analyses. Serum and CSF samples were collected from dogs acutely affected with SRMA (n=30), a population of SRMA affected dogs treated with anti-inflammatory corticosteroids (n=30), and healthy control dogs (n=8). The time frame for remission in dogs in the treated group was variable, however all dogs included in this group were currently received 0.5–1.0 mg/kg/day of prednisolone and were in clinical remission from signs associated with SRMA at the time of sample collection. Cerebrospinal fluid samples were collected from anesthetized dogs via sub-occipital puncture. Samples were processed routinely and the cell- free supernatant was collected and stored at −80°C until analysis was performed. Serum samples from each patient were also simultaneously collected and processed identically.
2.2 hsp70 ELISA
The concentration of ehsp70 in each serum and CSF sample was determined using an hsp70 high sensitivity ELISA (Assay Designs, Ann Arbor, MI; sensitivity 90pg/ml), an assay previously validated by our laboratory for use in dogs (Awad et al., 2008). Each sample (100 µl) was diluted 1:5 in phosphate buffered saline and analyzed in duplicate. Samples below the limit of detection of the assay were reported as “zero”.
2.3 IgA measurement
Concentrations of CSF and serum IgA for 27 of the acutely affected dogs had been previously measured and published (Bathen-Noethen, 2008; Maiolini et al., 2011). For samples which this information was available, these values were correlated with ehsp70 concentrations in CSF and serum.
2.4 WBC quantification in Cerebrospinal fluid
WBC concentrations in the CSF had been previously measured for 27 acutely affected dogs during routine clinicopathologic examination at one of the authors’ institutions (AT). These values were correlated with ehsp70 concentrations in CSF.
2.5 Statistical analysis
Statistical significance between groups was analyzed using the Kruskal-Wallis ANOVA followed by Dunn’s multiple comparisons tests with the StatView software (SAS Institute Inc., Cary, NC). For samples where IgA, and WBC concentrations were available, these values were correlated with hsp70 concentrations using Pearson’s correlation coefficient and a two tailed test for significance. P ≤ 0.05 was considered significant for all analyses.
3. Results
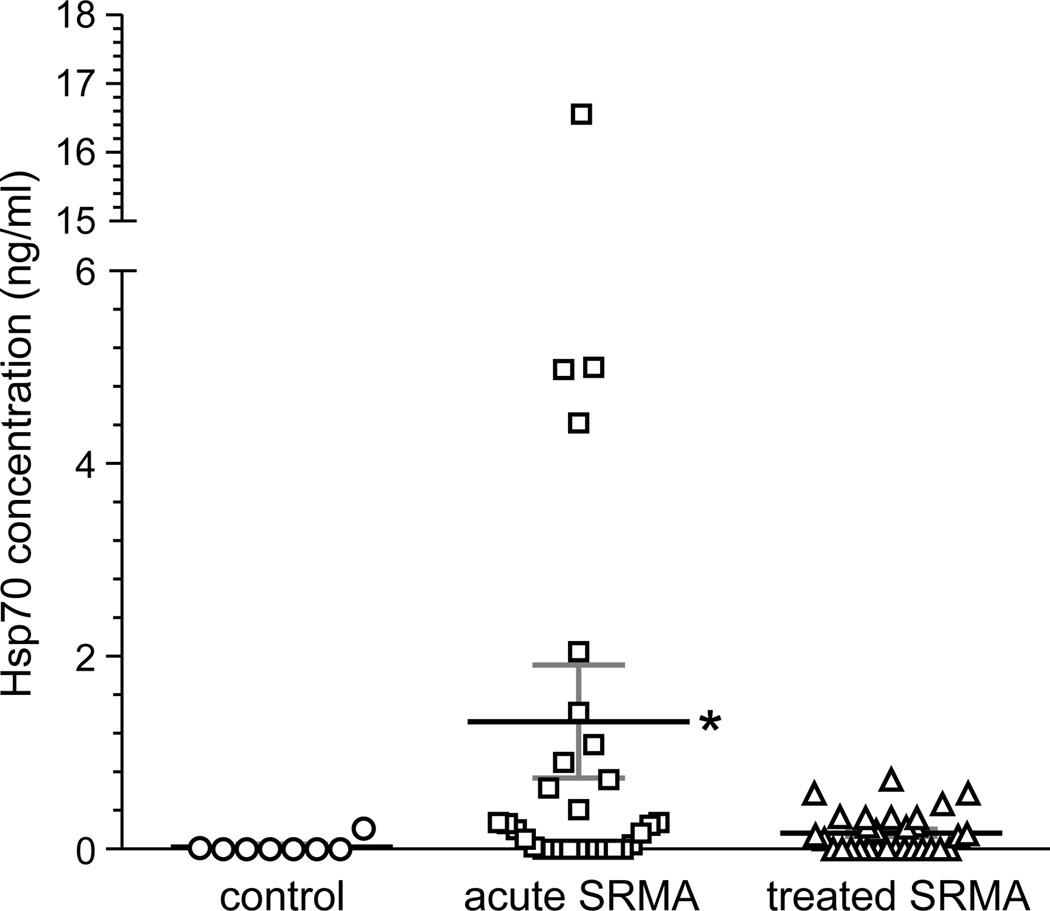
Extracellular hsp70 was not detected in CSF for 7 of 8 control dogs, and the concentration was less than 1 ng/ml in the 8th dog (Fig. 1). Concentrations were significantly elevated in dogs presenting with acute SRMA (p = 0.03). Substantial individual variability in CSF ehsp70 concentration was exhibited by this group, achieving a high of 16.6 ng/ml in one animal. To provide assurance that this one value did not unduly influence our statistical interpretation, comparisons were made by both including and excluding this value. Results showed that the significance in the elevation of the mean were not dependent on this one particular value. Corticosteroid treatment reduced mean ehsp70 concentration to levels that were not statistically different from the control group. (Fig. 1).
Figure 1.
Hsp70 concentrations in CSF of dogs acutely affected with SRMA, those treated with corticosteroids, and healthy controls. Shown are individual values and the mean ± SEM (horizontal bars). Values were established using an hsp70 ELISA, in which concentration was defined by a standard curve based upon serial dilutions of purified human recombinant hsp70. The asterisk indicates statistically significance differences in the mean by ANOVA.
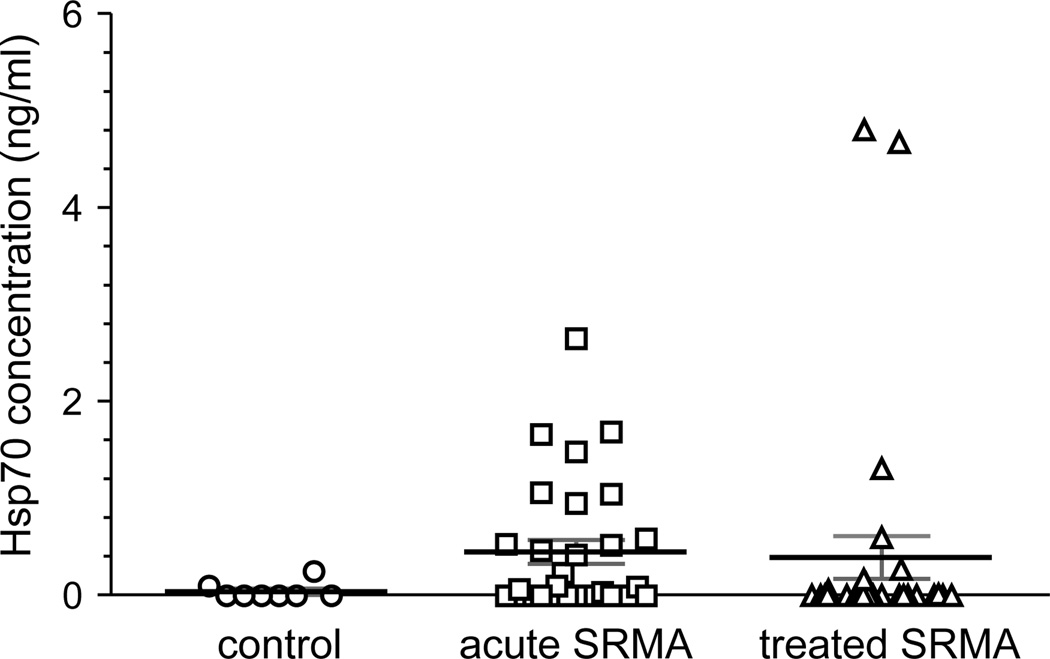
Extracellular hsp70 concentrations were higher in the serum of dogs with both acute and treated SRMA than in healthy controls (Fig. 2), with a high degree of variability in ehsp70 levels in both former groups. Differences in mean ehsp70 concentration between groups was not statistically significant, serum ehsp70 concentrations did not achieve levels observed in CSF in the acute phase of SRMA, and no correlation existed between serum and CSF concentrations of ehsp70 in each group of dogs (r= −0.124, p= 0.550).
Figure 2.
Hsp70 concentrations in serum of dog acutely affected with SRMA, those treated with corticosteroids, and healthy controls. Shown are individual values and the mean ± SEM (horizontal bars). Values were established using an hsp70 ELISA, in which concentration was defined by a standard curve based upon serial dilutions of purified human recombinant hsp70. There was no statistical difference in mean serum ehsp70 concentrations between groups.
For dogs acutely affected with SRMA, the mean CSF concentration of WBC was 1658/µL (range 140 to 9720) and the mean CSF IgA concentration was 5.2 µg/mL (range 0.39 to 42.6). There was not significant correlation between CSF ehsp70 concentrations and CSF IgA or WBC concentrations in acutely affected SRMA dogs (r=0.318, p=0.106; r=0.134, p=0.511 respectively). The mean serum concentration of IgA was 400.2 µg/mL (range 73–2390). There was not significant correlation between serum hsp70 and IgA concentrations (r=−0.027; p=0.447) in these dogs.
4. Discussion
Our results show that elevated ehsp70 is a CNS-specific feature of the inflammatory response of acute SRMA and that concentrations are reduced following steroid treatment in concert with remission of clinical signs. A lack of correlation between serum and CSF ehsp70 concentrations supports local production and release of ehsp70 and not simply leakage from serum. Results support segregation of CSF and serum pools of ehsp70, a phenomenon that has been previously observed in humans and in experimental canine models (Oglesbee et al., 1999; Steensberg et al., 2006; Awad et al., 2008). Heat shock protein 70 release can occur passively from necrotic cells or by active secretory release (Basu et al., 2000; Bausero et al., 2005). Sources of secretory release of hsp70 into the CSF include ependymal cells (Awad et al., 2008; Vydra et al., 2009) or vascular endothelial cells associated with the meninges or choroid plexus (Zhan et al., 2009). Vascular necrosis is a feature of SRMA, such that necrosis of vascular endothelial cells could contribute to ehsp70 concentrations in CSF; however, the role of choroid plexus and ependymal cells in secretory release of ehsp70 during CNS inflammation is intriguing and warrants further investigation.
While understanding of the pathogenesis of SRMA is limited, primary dysregulation of the immune system is suspected. The disease is associated with a polarization of the immune response (IL-4 dominant, Th2 skewed) and increased IL-4 mRNA expression in peripheral blood mononuclear cells (PBMNCs) and CSF WBCs, implicating environmental antigens as likely initiators of disease (Schwartz et al., 2011). IL-4 has been implicated as a physiologic trigger for hsp70 production and release from monocyte derived dendritic cells, and ehsp70 appears to act as an “immunoadjuvant” promoting dendritic cell maturation in vivo, possibly implicating it in the adaptive immune responses associated with autoimmunity (Martin et al., 2009).The effect of IL-4 on ehsp70 release thus warrants further investigation.
Other previously documented immunologic mechanisms in SRMA may contribute to ehsp70 release (Maiolini et al., 2011). No significant correlations with other known inflammatory mediators of SRMA were identified in this study. However, a positive correlation between CSF IgA and ehsp70 concentrations approached significance (p= 0.1). A positive correlation between hsp70 expression and IgA serum titers has been documented in various disease processes including Epstein-Barr virus associated nasopharyngeal carcinoma and diabetes mellitus (Figueredo et al., 1996; Chen et al., 2008). Excessive IgA concentrations in CSF are a unique feature of canine SRMA, and this finding is not observed in other inflammatory disorders of the canine CNS (Maiolini et al., 2011). The trigger for excessive IgA production in SRMA is unclear, and whether these represent immunoglobulins directed against host proteins such as hsp70, or whether IgA and hsp70 are both increased secondary to a yet unidentified environmental or infectious trigger warrants further investigation.
5. Conclusion
Collectively, our results support the potential utility of dogs with SRMA as a model system in which to examine the contribution of ehsp70 to CNS inflammation. Future studies will examine larger cohorts in an attempt to correlate CSF ehsp70 and IgA levels. The relationship between ehsp70 and the duration of clinical signs, presence or absence of fever and other markers of disease severity will also be examined, such data being unavailable for the present cohort. Cerebrospinal fluid samples can be readily collected and analyzed over time, with inhibition of hsp70 secretory release being the ultimate test of ehsp70’s role as a mediator versus marker of SRMA-associated inflammation. If hsp70 ultimately proves to be a mediator of inflammation in SRMA, then the genetic basis for variability in hsp70 expression demonstrated in humans (Maugeri et al., 2010) may contribute to breed predilections in development of SRMA (Tipold and Jaggy, 1994; Behr and Cauzinille, 2006; Wilbe et al., 2009), further substantiating the proposed role of hsp70 in other autoimmune conditions (Furnrohr et al., 2010).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asea A. Stress proteins and initiation of immune response: Chaperokine activity of hsp72. Exerc. Immunol. Rev. 2005;11:34–45. [PMC free article] [PubMed] [Google Scholar]
- Awad H, Suntres Z, Heijmans J, Smeak D, Bergdall-Costell D, Christofi FL, Magro C, Oglesbee M. Intracellular and extracellular expression of the major inducible 70kDa heat shock protein in experimental ischemia-reperfusion injury of the spinal cord. Exper. Neurol. 2008;212:275–284. doi: 10.1016/j.expneurol.2008.03.024. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Intern. Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Bathen-Noethen, Carlson AR, Menzel D, Mischke R, Tipold A. Concentrations of acute-phase proteins in dogs with steroid responsive meningitis-arteritis. J. Vet. Intern. Med. 2008;22:1149–1156. doi: 10.1111/j.1939-1676.2008.0164.x. [DOI] [PubMed] [Google Scholar]
- Bausero MA, Gastpar R, Multhoff C, Asea A. Alternative mechanism by which IFN-gamma enhances tumor recognition: Active release of heat shock protein 72. J. Immunol. 2005;175:2900–2912. doi: 10.4049/jimmunol.175.5.2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr S, Cauzinille L. Aseptic suppurative meningitis in juvenile boxer dogs: retrospective study of 12 cases. J Am Vet Med Assoc. 2006;42:277–282. doi: 10.5326/0420277. [DOI] [PubMed] [Google Scholar]
- Boros P, Bromberg JS. New cellular and molecular immune pathways in ischemia/reperfusion injury. Am J Transplant. 2006;6:652–658. doi: 10.1111/j.1600-6143.2005.01228.x. [DOI] [PubMed] [Google Scholar]
- Burns JC, Felsburg PJ, Wilson H, Rosen FS, Glickman LT. Canine pain syndrome is a model for the study of kawasaki disease. Persp. Biol. and Med. 1991;35:68–73. doi: 10.1353/pbm.1991.0040. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ, Theriault JR. Extracellular heat shock proteins in cell signaling. Fed Euro Biochem Soc. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Chen k, Wang h, Zhang Z, Lu X, Ouyang D. Correlation of heat shock protein expression in nasopharyngeal carcinoma to immunoglobulin A against viral capsid antigen of Epstein-Barr virus in sera and its clinical relevance. Ai Zheng. 2008;27:650–653. [PubMed] [Google Scholar]
- Fang HY, Ko WJ, Lin CY. Inducible heat shock protein 70, interleukin-18, and tumor necrosis factor alpha correlate with outcomes in spontaneous intracerebral hemorrhage. J. Clin. Neurosci. 2007;14:435–441. doi: 10.1016/j.jocn.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Felsburg PJ, Hogenesch H, Somberg RL, Snyder PW, Glickman LT. Immunologic abnormalities in canine juvenile polyarteritis syndrome: A naturally occurring animal model of kawasaki disease. Clin. Immunol. Immunopath. 1992;65:110–118. doi: 10.1016/0090-1229(92)90213-8. [DOI] [PubMed] [Google Scholar]
- Figueredo A, Ibarra J, Rodriguez A, Molino A, Gomez-de La Concha E, Fernandez-Cruz A, Patino R. Increased serum levels of IgA antibodies to hsp70 protein in patients with diabetes mellitus: Their relationship to vascular complications. Clin Immuno Immunopath. 1996;79:252–255. doi: 10.1006/clin.1996.0076. [DOI] [PubMed] [Google Scholar]
- Furnrohr BG, Wach S, Kelly JA, Haslbeck M, Weber CK, Stach CM, Hueber AJ, Graef D, Spriewald BM, Manger K, Herrman M, Kaufman KM, Frank SG, Goodman E, James JA, Schett G, Winkler TH, Harley JB, Voll RE. Polymorphisms in the Hsp70 gene locus are genetically associated with systemic lupus erythematosus. Ann Rheum Dis. 2010;69:1983–1989. doi: 10.1136/ard.2009.122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcourt RA. Polyarteritis in a colony of beagles. Vet Rec. 1978;102:519–522. doi: 10.1136/vr.102.24.519. [DOI] [PubMed] [Google Scholar]
- Hayes TJ, Roberts GK, Halliwell WH. An idiopathic febrile necrotizing arteritis syndrome in the dog: beagle pain syndrome. Toxicol Pathol. 1989;17:129–137. doi: 10.1177/019262338901700109. [DOI] [PubMed] [Google Scholar]
- Hecker JG, Sundram H, Zou S, Praestgaard A, Bavaria JE, Ramchandren S, McGarvey M. Heat shock proteins HSP70 and HSP27 in the cerebral spinal fluid of patients undergoing thoracic aneurysm repair correlate with the probability of postoperative paralysis. Cell Stress Chaperones. 2008;13:435–446. doi: 10.1007/s12192-008-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakimura JI, Kitamura Y, Takata K, Umeki M, Suzuki S, Shibagaki K, Taniguchi T, Nomura Y, Gebicke-Haerter PJ, Smith MA, Perry G, Shimohma S. Microglial activation and amyloid-β clearance induced by exogenous heat-shock proteins. FASEB J. 2002;16:601–603. doi: 10.1096/fj.01-0530fje. [DOI] [PubMed] [Google Scholar]
- Martin CA, Kurkowski DL, Valentino AM, Santiago-Schwarz F. Increased intracellular, cell surface, and secreted inducible heat shock protein 70 responses are triggered during the monocyte to dendritic cell (DC) transition by cytokines independently of heat stress and infection and may positively regulate DC growth. J Immunol. 2006;183:388–399. doi: 10.4049/jimmunol.0802688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiolini A, Carlson R, Schwartz M, Gandini G, Tipold A. Determination of immunoglobulin A concentrations in the serum and cerebrospinal fluid of dogs: An estimation of its diagnostic value in canine steroid-responsive meningitis-arteritis. Vet J. 2011 doi: 10.1016/j.tvjl.2010.12.018. [DOI] [PubMed] [Google Scholar]
- Maugeri N, Radhakrishnan J, Knight J. Genetic determinants of hsp70 gene expression following heat shock. Human Mol Gen. 2010;19:4939–4947. doi: 10.1093/hmg/ddq418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meric SM. Canine meningitis. A changing emphasis. J Vet Intern Med. 1998;2:26–35. doi: 10.1111/j.1939-1676.1988.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Oglesbee MJ, Diehl K, Crawford E, Kearns R, Krakowka S. Whole body hyperthermia: effects upon canine immune and hemostatic function. Vet Immunol Immunopathol. 1999;69:185–199. doi: 10.1016/s0165-2427(99)00053-7. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Puff C, Stein VM, Baumgartner W, Tipold A. Pathogenetic factors for excessive IgA production: Th2-dominated immune response in canine steroid-responsive meningitis-arteritis. Vet. J. 2011;187:260–266. doi: 10.1016/j.tvjl.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Steensberg S, Dalsgaard MK, Secher NH, Pedersen BK. Cerebrospinal fluid IL-6, hsp72, and TNF-alpha in exercising humans. Brain Behav Immun. 2006;20:585–589. doi: 10.1016/j.bbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Tipold A, Pfister H, Zurbriggen A, Vandevelde M. Intrathecal synthesis of major immunoglobulin classes in inflammatory diseases of the canine CNS. Vet. Immunol. Immunopath. 1994;42:149–159. doi: 10.1016/0165-2427(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Tipold A, Jaggy A. Steroid responsive meningitis-arteritis in dogs: long -term study of 32 cases. J Small Anim Prac. 1994;35:311–316. [Google Scholar]
- Tipold A. Diagnosis of inflammatory and infectious diseases of the central nervous system in dogs: a retrospective study. J Vet Intern Med. 1995;9:304–314. doi: 10.1111/j.1939-1676.1995.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Tipold A, Vandevelde M, Zurbriggen A. Neuroimmunological studies in steroid-responsive meningitis-arteritis in dogs. Res. Vet. Sci. 1995;58:103–108. doi: 10.1016/0034-5288(95)90060-8. [DOI] [PubMed] [Google Scholar]
- Tipold A, Moore P, Zurbriggen A, Vandevelde M. Lymphocyte subset distribution in steroid responsive meningitis-arteriitis in comparison to different canine encephalitides. Zentralblatt Fur Veterinarmedizin.Reihe A. 1999;46:75–85. doi: 10.1046/j.1439-0442.1999.00193.x. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, hmad-Nejad P, Ghose S, Kirsching CJ, Issels RD, Wagner H. HSP70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J. Biol. Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Vydra N, Winiarski B, Rak-Raszewska A, Piglowski W, Mazurek A, Scieglinska D, Widlak W. The expression pattern of the 70-kDa heat shock protein Hspa2 in mouse tissues. Histochem. Cell Biol. 2009;132:319–330. doi: 10.1007/s00418-009-0605-1. [DOI] [PubMed] [Google Scholar]
- Wheeler D, Chase M, Senft A, Poyneter S, Wong H, Page K. Extracellular Hsp72, an endogenous DAMP, is released by virally infected airway epithelial cells and activates neutrophils via Toll-like receptor (TLR)-4. Resp Res. 2009;10 doi: 10.1186/1465-9921-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbe M, Jokinen P, Hermanrud C, Kennedy LJ, Strandberg E, Hansson-Hamlin H, Lohi H, Andersson H. MHC class II polymorphism is associated with a canine SLE-related disease complex. Immunogen. 2009;61:557–564. doi: 10.1007/s00251-009-0387-6. [DOI] [PubMed] [Google Scholar]
- Wrzosek M, Konar M, Vandevelde M, Oevermann A. Cerebral extension of steroid responsive meningitis-arteritis in a boxer. J Small Anim Prac. 2009;50:35–37. doi: 10.1111/j.1748-5827.2008.00653.x. [DOI] [PubMed] [Google Scholar]