Abstract
The capacity of mesenchymal stem cells (MSCs) to survive and engraft in the target tissue may lead to promising therapeutic effects. However, the fact that the majority of MSCs die during the first few days following transplantation complicates cell therapy. Hence, it is necessary to strengthen the stem cells to withstand the rigors of the microenvironment to improve the efficacy of cell therapy. In this study, we manipulated MSCs to express a cytoprotective factor, heme oxygenase-1 (HO-1), to address this issue. Full-length cDNA of human HO-1 was isolated and cloned into TOPO vector by TOPO cloning reaction. Then, the construct was ligated to gateway adapted adenovirus expression vector by LR recombination reaction. Afterwards, the recombinant virus expressing HO-1 was produced in appropriate mammalian cell line and used to infect MSCs. The HO-1 engineered MSCs were exposed to hypoxic and oxidative stress conditions followed by evaluation of the cells’ viability and apoptosis. Transient expression of HO-1 was detected within MSCs. It was observed that HO-1 expression could protect MSCs against cell death and the apoptosis triggered by hypoxic and oxidative stress conditions. The MSCs-HO-1 retained their ability to differentiate into adipogenic, chondrogenic, or osteogenic lineages. These findings could be applied as a strategy for prevention of graft cell death in MSCs-based cell therapy and is a good demonstration of how an understanding of cellular stress responses can be used for practical applications.
Keywords: Mesenchymal stem cells, HO-1, Adenovirus, Oxidative stress, Apoptosis
Introduction
The biological flexibility of mesenchymal stem cells (MSCs), which is their potential to differentiate into various lineages of connective tissue, including adipocytes, osteoblasts, chondrocytes, and myoblasts, makes them attractive target cells for the treatment of a variety of diseases such as hemophilia, bone disorders, acute myocardial infarction, and cancer (Wang et al. 2009). Furthermore, ease of harvest, ex vivo expansion, and high proliferative activity of MSCs are their other advantages for cell therapy and tissue repair (Chamberlain et al. 2007; Prockop et al. 2003).
However, the promising therapeutic effect of MSCs is affected by their capacity to survive and engraft in the target tissue. It has been shown that less than 0.44% of MSCs survive by day 4 after engraftment in experimental models (Toma et al. 2002; Zhu et al. 2006).
During isolation and processing of the MSCs from their natural niche, they are inevitably exposed to stress conditions such as oxidative stress, serum deprivation, and hypoxia (Zhu et al. 2006). On the other hand, the microenvironment of damaged tissue in the patients who receive MSCs as treatment does not favor the survival of MSCs. This could be due to inflammation, chemotherapy, radiotherapy, and expression of pro-apoptotic factors. Hence, it is necessary to reinforce the MSCs to withstand the rigors of the stresses.
Heme oxygenase-1 (HO-1) is one of the potent cytoprotective factors that represent an endogenous defensive system in organ transplantation and lead to prolonged graft survival (Tsubokawa et al. 2010). This inducible protein (HO-1) is a rate-limiting enzyme in heme catabolism that catalyzes the oxidation of heme to carbon monoxide and biliverdin, which plays a concerted action in critical cytoprotective mechanisms activated during cellular stress (Kikuchi et al. 2005). Several studies have showed that genetic modification of MSCs with cytoprotective factors prior to transplantation may result in their enhanced survival, better engraftment, and improved reconstruction in cell therapy. For instance, in an animal model, survival grade of grafted MSCs was improved by transfecting the MSCs with a hypoxia-regulated HO-1 vector (Tang et al. 2005). Although HO-1 is known as a cytoprotective enzyme that could adapt the MSCs to stressful conditions and protects them from injurious events, a possible disadvantage of transducing this gene is the risk for long-term expression of HO-1 that may be harmful to the cells (Abraham and Kappas 2008; Deramaudt et al. 1999).
To overcome this problem, we hypothesized that high level but short time expression of HO-1 may reinforce MSCs against oxidative stresses. To evaluate this hypothesis, we transiently overexpressed the human HO-1 gene in MSCs using the adenoviral expression system based on gateway technology and examined whether HO-1 producing MSCs had better anti-oxidative and anti-apoptotic capabilities over unmodified MSCs in vitro. Our results may provide important clues to increase the stability of MSCs for improving the efficacy of mesenchymal stem cell therapy.
Methods and materials
MSCs isolation, expansion, and identification
Heparinized human bone marrow was obtained by aspiration from the posterior iliac of three healthy 10–20-year-old volunteers. MSCs were successfully isolated as described previously (Halabian et al. 2010). Briefly, bone mononuclear cells were prepared by density gradient centrifugation with ficoll (below 1.073 g/ml, GE Healthcare, Sweden). Then, the cells were washed, counted, and distributed in a 6-well plate at the density of 1 × 106 cells/ml in Dulbecco’s modified Eagle’s medium-low glucose (MSCs medium, PAA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco, USA). Non-adherent cells were removed by changing the medium at 72 h and every 3 days thereafter. The average number of nucleated cells isolated from BM was 1 × 106 cells/cm and the yield of adherent cells was 2 × 105 cells/cm. After two passages, homogeneous MSCs devoid of hematopoietic cells were used. In order to verify the ex vivo-expanded MSCs, they were analyzed by flow cytometry for the expression of surface markers as described previously (Halabian et al. 2010).
Isolation and cloning of HO-1 coding sequence
The human lung carcinoma cell line, A549, was obtained from the National Cell Bank of Iran (Pasteur Institute of Iran) and used as a source of HO-1 gene. The cells were cultured in 25-cm2 cell culture flasks and were grown in RPMI 1640 medium (Gibco-BRL, Germany) containing 10% heat-inactivated fetal bovine serum (Gibco-BRL, Germany) and 1% penicillin streptomycin solution (10,000 units of penicillin and 10 mg of streptomycin) in a humidified atmosphere of 5% carbon dioxide (CO2) and 95% air at 37°C. In order to induce expression of HO-1, the A549 cells were UV irradiated for 1 h, then, they were subjected to RNA extraction by a commercially available kit according to the manufacturer’s protocols (Invitrogen, Carlsbad, CA, USA). The cDNA was synthesized using the cDNA synthesis kit (Invitrogen) by using random hexamers. Afterwards, full-length human HO-1 cDNA was amplified using specific forward:
5′-CACCATGGAGCGTCCGCAACCCGAC-3′ and reverse
5′-CATGGCATAAAGCCCTACAGCAACTG-3′ primers. The forward primer included four nucleotides (underlined) for performing Topo cloning reaction and Kozak sequence site. The blunt-end polymerase chain reaction (PCR) products were then TOPO cloned into pENTR TOPO/D vector according to the manufacturer’s protocol (Invitrogen, USA) and used for transformation of competent Escherichia coli cells. Then, the recombinant bacteria were screened using LB agar medium containing 50 μg/ml kanamycin. The presence of the insert was confirmed by PCR and finally, the fidelity of the cloned sequence was confirmed by DNA sequencing. This construct is called the entry clone (p ENTR TOPO/D-HO-1).
Construction of recombinant Ad vector DNA containing human HO-1
The Adeno pAd/CMV/V5-DEST adenovirus vector was purchased from Invitrogen. The LR recombination reaction was carried out between the entry clone (PENTR TOPO/D-HO-1) and destination vector (pAd/CMV/V5-DEST) according to the manufacturer’s protocols (Invitrogen).
Producing viral stocks and transducing MSCs
The 293A cells (a subclone of the 293 cell line) were transfected with PacI linearized recombinant adenoviruses. In brief, the recombinant adenoviruses containing the entire coding sequence of hHO-1 (pAd/CMV/V5-HO-1) were digested with PacI to expose the ITRs. Then, the 293A cells were cultured in a 60-mm plate and transfected with the linear pAd/CMV/V5-HO-1 at a confluency of 70%. The transfection reaction was carried out using the lipofectamin reagent as described by the manufacturer (Invitrogen). In order to harvest the viruses, the cells were lysed with three consecutive freeze–thaw cycles, and then the viruses were collected from supernatant. Next, the adenoviruses were amplified by infecting additional 293A cells with the crude viral lysate, and then subjected to viral titer determination by plaque assay on 293A cells.
Subsequently, for transduction of MSCs with pAd/CMV/V5-HO-1, 8 × 105 cells/well were seeded in 6-well plates in growth medium containing DMEM-LG (Gibco) and 10% FBS and incubated for 12 h. Virus stock was diluted with serum-free low glucose DMED and serial dilution of viral stock was added to the cells. Following determination of the multiplicity of infection (MOI), MSCs were infected with pAd/CMV/V5-HO-1 at the appropriate MOI. The HO-1 transduced MSCs were analyzed by reverse transcription polymerase chain reaction (RT-PCR) and western blotting for overexpression of HO-1.
Analysis of HO-1 expression by RT-PCR and western blotting
To assess the expression of HO-1 by RT-PCR, total RNA was extracted from cells and used at the concentration of 500 ng/μl to construct cDNA using the First Strand cDNA Synthesis Superscript kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Then, the cDNA was PCR amplified by using specific HO-1 primers. PCR condition included a primary denaturation time of 5 min at 94°C, followed by 33 cycles of 30 s at 94°C, 30 s at 55°C and 45 s at 72°C, and a final extension time of 5 min at 72°C. Finally, 7 μl of each PCR product were analyzed by 2% agarose gel electrophoresis. β-actin expression was evaluated for normalization.
Western blot analysis was performed to evaluate the expression of HO-1 at protein level. For detection of HO-1 protein, total cell proteins were released by using Complete Lysis M reagent (Roche, Germany) according to the manufacturer’s instructions. Then, the sample proteins were boiled in loading buffer containing 4% sodium dodecyl sulfate (SDS), 20% glycerol, and bromophenol blue for 5 min. Proteins were resolved on 12% SDS-PAGE and transblotted onto polyvinylidene fluoride membrane (Roche, Germany). The membranes were incubated with specific primary antibodies, i.e., β-actin (Sigma, USA; catalog number, A5441) or polyclonal anti-HO-1 antibodies (Stressgen Biotechnologies, Victoria, BC, Canada; catalog number, SPA-895F). Following an overnight incubation at 4°C with the primary antibody, the membranes were washed with tris buffered saline containing 0.1% Tween 20, then incubated with secondary horse radish peroxidase-conjugated antibodies (Sigma). Finally, the membranes were developed by diaminobenzidin (DAB) solution (Sigma).
In order to detect any residual viral DNA, the differentiated cells were subjected to viral DNA extraction using QIAamp DNA Mini Kit (Qiagen, Germany) according to the manufacturer’s recommendations. Then, it was evaluated via PCR using specific pAd/CMV/V5 primers.
In vitro treatment of MSCs with different stresses followed by cell survival assay
For oxidative experiments, 2 × 104 cells/well were seeded in a 96-well plate and after 12 h, the cells were exposed to 1–10 mM hydrogen peroxide (H2O2; Sigma, Dusseldorf, Germany), hypoxia (95% N2, 5% CO2) or serum deprivation for different time lengths. At appropriate time points, independently, the cytotoxic effects of H2O2, hypoxia or serum deprivation administration were determined by trypan blue dye exclusion and 3-(4,5-dimethlthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays.
To perform MTT assay, cells were incubated with 10 μl of a 5 mg/ml solution of MTT (Sigma, Dusseldorf, Germany) at 37°C in a 5% CO2 atmosphere for 4 h. Finally, the reaction was stopped by addition of 10% SDS and 0.01 M HCl. When the insoluble crystals dissolved completely, the absorbance of mixture was measured using a microplate reader at 570 nm.
Assessment of cell apoptosis during H2O2 toxicity
Apoptotic and necrotic cells were quantified by flow cytometry after double staining of cells with annexin-V and propidium iodide (PI) using Annexin-V-FLOUS staining kit in accordance with the manufacturer’s protocol (Roche, Mannheim, Germany).
Briefly, appropriate numbers of cells were incubated with different concentrations of H2O2 and different time lengths. Afterwards, cells were detached with trypsin (0.25%) and EDTA (0.5 mM). Finally, the cells were incubated with annexin-V-GFP and PI, and analyzed by flow cytometry. Annexin-V and PI-positive cells were considered to be apoptotic and necrotic cells, respectively.
Differentiation studies
Following infection of MSCs with pAd/CMV/V5-HO-1, the differentiation potential of MSCs was examined. All reagents used for induction were purchased from Gibco-BRL (Germany). For osteogenic and adipogenic differentiation, cells were plated in 6-well plates at a density of 1 × 104 cells/well. The cells were incubated in growth medium at 37°C in a humidified atmosphere of 5% CO2 for a minimum of 24 h. The medium was replaced with prewarmed osteogenic or adipogenesis differentiation medium 24 h later and incubation continued. The cultures were re-fed every 4 days. After 15 days of cultivation, adipogenic cultures were processed for LipidTOX staining and following 18 days of incubation, the cells were stained using the Alizarin Red S staining to detect the presence of calcium deposition in osteocytes. Subsequently, for induction of chondrogenesis differentiation, 5 μl of a 1 × 106 cells/well solution was seeded in the center of multiwell plate wells. After 2 h, warmed chondrogenesis media was added to culture vessels and incubated at 37°C in the presence of 5% CO2. Cultures were replaced every 2–3 days. Following certain periods of cultivation, chondrogenic pellets were processed for Alcian Blue. The levels of osteocalcin and accumulation of triglycerides and glycosaminoglycan, the determinants of osteogenic, adipogenic, and chondrogenenic differentiation, respectively, were evaluated to determine whether HO-1 expression affects the ability of the cells to differentiate to the different cell types. The osteocalcin level was determined by using a specific ELISA test according to the manufacturer’s protocol (BioSource International, Inc., Camarillo, CA, USA).
Accumulation of triglycerides was measured using BioVision’s adipogenesis assay kit as instructed by the manufacturer (BioVision Research Products, Mountain View, CA, USA). The glycosaminoglycan (GAG) was also measured by the GAG assay kit (Kamiya Biomedical, Seattle, WA, USA) according to the manufacturer’s protocol.
Results
MSCs express specific MSC-related cell surface markers
To determine whether human bone marrow-derived MSCs express typical MSC-related cell surface antigens, flow cytometry analysis was performed. These cells revealed the typical antigenic profile of MSCs and were positive for CD166, CD105, CD90, and CD73 antigens as reported previously. In contrast, these cells were negative for CD45, CD34, and CD14 (Halabian et al. 2010).
Transient expression of HO-1 in MSCs by using adenovirus vector
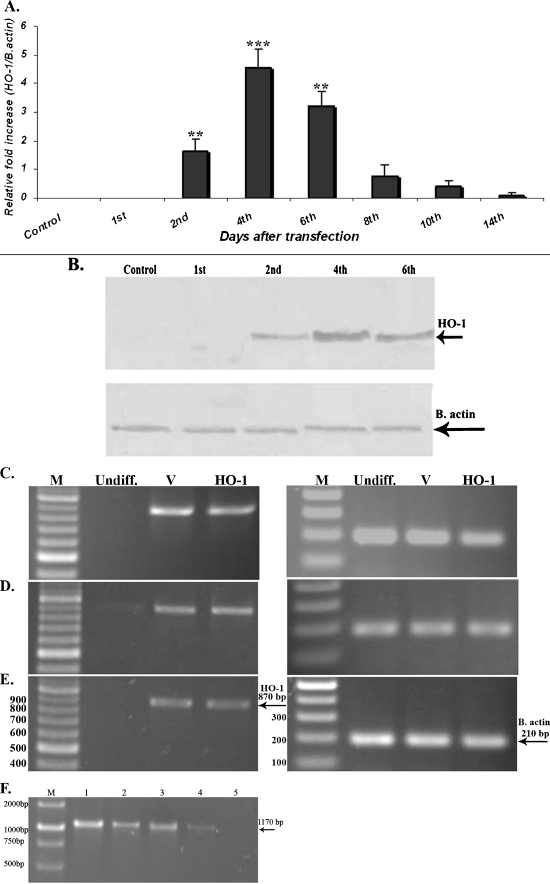
The HO-1 cDNA was amplified by using specific primers and cloned into the pENTR/D-TOPO vector. The fidelity of the cloned sequence was confirmed by DNA sequencing (GenBank accession number JF323038). Amplification of a fragment of about 870 bp length (data not shown) via PCR using HO-1 specific primers confirmed the translocation of the HO-1 coding sequence into the adenovirus destination vector by LR recombination reaction. After four passages, MSCs were infected with adenovirus vectors harboring the HO-1 gene or control viral vector with the appropriate MOI. Real-time PCR was performed on cell samples at various time points (1, 2, 4, 8, 10, and 14 days) post-infection to evaluate the expression of HO-1. Initial expression of HO-1 was observed on day 2 and reached the highest level on day 4, then decreased after 6 days and no expression was observed on day 14 (Fig. 1a). Expression of HO-1 in protein level was also confirmed by western blot analysis (Fig. 1b). These results indicate that the upregulation of HO-1 is transient. During these time periods, most MSC infected with HO-1 (MSC-HO-1s) appeared to adhere to tissue culture surfaces and no morphological changes were observed. Next, the expression of HO-1 following differentiation of the cells was evaluated. Interestingly, HO-1 was expressed in both MSC-V and MSC-HO-1 cells following differentiation of the cells, which indicated the basal expression level of HO-1 in these cells, but no expression was observed in the undifferentiated MSCs (Fig. 1c–e). However, attempts to detect HO-1 in protein level in the differentiated cells were unsuccessful via western blotting by DAB staining method. To determine whether the viral DNA can be detected in the differentiated cells, the cells were subjected to extraction of viral DNA followed by PCR analysis, but no residual viral DNA was detected in this experiment (Fig. 1f).
Fig. 1.
Expression of HO-1 gene in MSCs. a Expression of HO-1 mRNA until various time points after infection with adenovirus vectors harboring the HO-1 gene (Ad-hHO-1 data represents mean ± SD; number of replicates = 3, **p < 0.01, ***p < 0.001). b Western blot analysis of the HO-1 protein expression following infection of the MSCs with the recombinant virus. c–e Evaluation of HO-1 expression following MSCs differentiation. HO-1 was expressed in both MSC-V and MSC-HO-1 cells following cell differentiation indicating that HO-1 is expressed in a basal level in these cells, but no expression was detected in the undifferentiated MSCs. c Osteocytes, d adipocytes, e chondrocyte, f extraction of viral DNA and PCR analysis. Following infection of MSCs with the Ad-hHO-1 and induction of osteocyte differentiation, viral DNA was extracted and PCR was performed using pAd/CMV/V5 primers in different time intervals. A strong (1) and a faint band (4) of about 1,170 bp was detected 5 and 12 days post-infection, respectively. However, no bands were observed on day 20. In the two other differentiated cells, adipocytes and chondrocytes, the same results were observed; 2 (8th day), 3 (10th day)
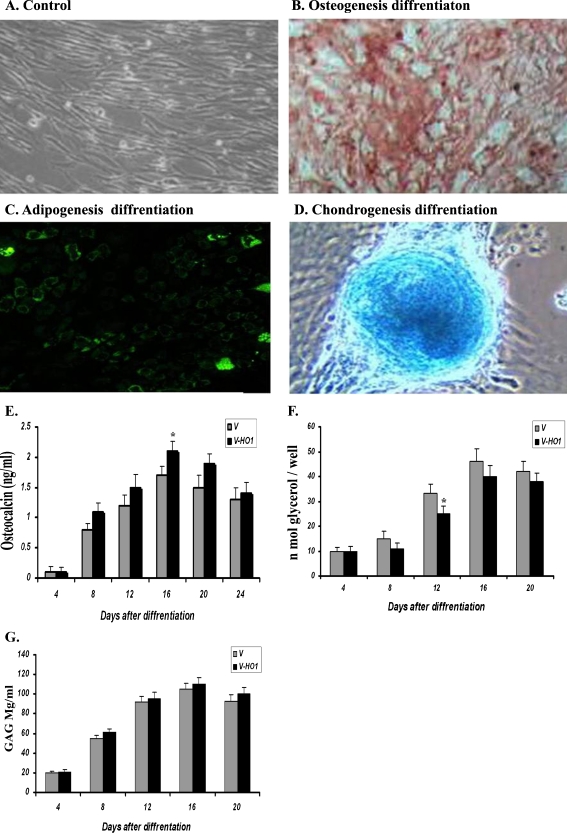
HO-1 transduced MSCs maintained their multidifferentiation capacity
Differentiation of MSCs into multiple lineages (such as bone, adipose tissues, and chondrocyte) is one of the well-known properties of MSCs. This ability is considered a functional criterion for MSC precursor cells, and therefore, to determine whether the HO-1 expression affected the differentiation capacity of the modified MSCs, HO-1-MSCs underwent adipogenic, chondrogenic, and osteogenic differentiation 5 days post-infection. After 21 days, there was positive reaction for Alizarin red S staining (Fig. 1b) confirming the ability of the cells to differentiate into osteocytes. Also, after 21 days, a characteristic morphological change with accumulation of lipid vacuoles was observed following induction toward an adipogenic lineage (Fig. 2c). After 15 days of induction of MSCs toward chondrogenic lineage, blue staining reaction indicated the synthesis of proteoglycans by chondrocytes (Fig. 2d). Next, to determine whether HO-1 expression has any effect on the efficiency of cell differentiation to various cell types, the level of osteocalcin, and accumulation of triglycerides and glycosaminoglycan were measured. The ability of the MSCs to differentiate into osteocytes was enhanced rather than inhibited by the expression of HO-1 (Fig. 2e). HO-1 expression slightly decreased the differentiation of MSCs to adipocytes (Fig. 2f) but did not affect the differentiation to chondrocytes (Fig. 2g). Taken together, these results showed that the HO-1-MScs retained their multi-differentiation potential into bone, adipogenic, and chondrogenic lineages.
Fig. 2.
Differentiation capacity of MSC-HO-1s. MSCs infected with HO-1 were cultured in osteogenic, adipogenic and chondrogenic mediums. MSC-HO-1s maintained their multi differentiation capacity. a Control MSCs cultivated under normal medium (DMEM low glucose), b osteogenic differentiation, c adipogenic differentiation, and d chondrogenic differentiation. e The osteocalcin levels; HO-1 expression promoted rather than inhibited the ability of the MSCs to differentiate to osteocytes but also promoted it. f Accumulation of triglycerides; HO-1 expression slightly decreased the differentiation of the MSCs to adipocytes. g Measurement of glycosaminoglycan showed no inhibitory effect of the expressed HO-1 on chondrocyte differentiation
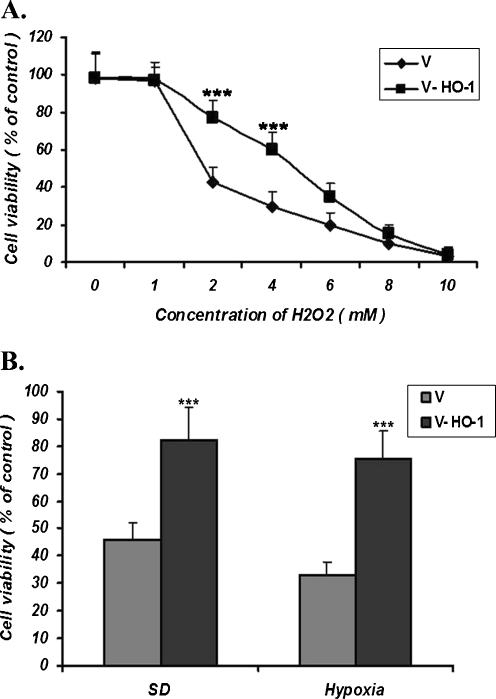
HO-1 protects MSCs against H2O2, hypoxia, and serum deprivation-induced toxicities
To determine the cytoprotective effect of HO-1 in MSC-HO-1 cells against H2O2, hypoxia, and serum deprivation toxicities, apoptotic cells infected with empty vector (V-MSCs) and MSC-HO-1s were exposed to the stresses for different time lengths, and then subjected to cytotoxicity and proliferation assays. The viability of the cells was not changed following their exposure to 1 mM H2O2 for 2 h. However, high concentrations, i.e., more than 4 mM, caused a dramatic decrease in cell viability in both V-MSCs and MSC-HO-1s. Nevertheless, MSC-HO-1s were more resistant to cell death than the V-MSCs following exposure to H2O2 at concentrations of 2–4 mM (Fig. 3a). This confirmed the cytoprotective effect of the HO-1 overexpression against H2O2 cytotoxicity.
Fig. 3.
a Cytotoxic effects of different concentrations of H2O2 on the MSCs infected with the recombinant construct or empty vector were determined by MTT assay after 2 h of exposure. The viability of the MSCs infected with the Ad-HO-1(V-HO-1) was higher than the cells infected with empty virus (V) at the H2O2 concentrations of 2 and 4 mM, indicating that HO-1 protects cell against H2O2 toxicity. b Cytotoxic effects of serum deprivation (SD) and hypoxia on cells infected with the construct or empty vector determined by MTT assay. After 24 h of incubation of the cells under SD, the number of viable cells in MSC-HO-1s was higher than those of MSCs infected with the empty virus. Also, following 12 h of incubation of the cells under hypoxia conditions, HO-1 protected MSCs (mean ± SD; ***p < 0.001; three independent experiments were carried out)
Following exposure to conditions of serum deprivation or hypoxia for different time lengths, V-MSCs or MSC-HO1s were subjected to cell viability assay. This revealed a higher number of surviving MSC-HO1 cells than the V-MSCs indicating that HO-1 protects MSCs against hypoxia/serum deprivation induced toxicities (Fig. 3b).
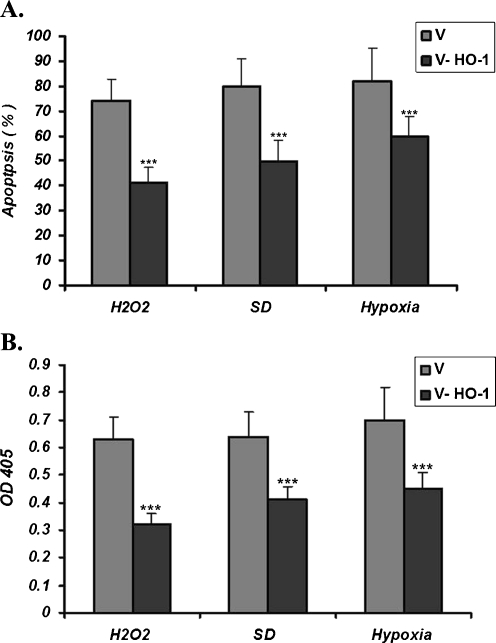
HO-1 expression protects against MSC apoptosis in vitro
Apoptosis is one of the well-known causes of MSC death. To test the inhibitory effects of HO-1 on MSCs apoptosis, V-MSCs and MSC-HO-1s were exposed to serum deprivation, H2O2 or hypoxia condition for different time periods. Subsequently, the evaluation of apoptosis was performed using Anexin V and caspase assay kit.
Following incubation of the cells with 100 μM H2O2 for 12 h, the number of V-MSCs was higher than those infected with HO-1 (MSC-HO-1; Fig. 4a). V-MSCs and MSCs-HO-1s were also treated under hypoxic and serum deprivation conditions. Again, the number of apoptotic V-MSCs was higher than the MSC-HO-1s (Fig. 4a).
Fig. 4.
Apoptotic effects of H2O2, serum deprivation (SD) and hypoxia on the MSCs. a Detection of apoptosis using Annexin-V. After treatment of the cells with 100 μM H2O2, 6 h of hypoxia or 12 h of serum depriviation, the number of apoptotic cells of those infected with the empty virus (V) was higher than those infected with the Ad-HO-1virus. b Detection of apoptotic activity using caspase-3 assay kit. Under the conditions described above, the lower level of the activated caspase 3 was observed in MSCs-HO-1 cells compared to the control cells (mean ± SD of three independent experiments; ***p < 0.001)
Induction of apoptosis was also studied by the assessment of caspase-3 activity. As shown in Fig. 4b, under the oxidative conditions, higher levels of activated caspase-3 were observed in V-MSCs. In fact, under stress conditions, i.e., H2O2 treatment, serum deprivation, and hypoxia, lower levels of activated caspase-3 were observed in MSC-HO-1s. Taken together, these findings indicated that HO-1 can protect MSCs against H2O2, serum deprivation, and hypoxia-induced apoptosis.
Discussion
Despite their several advantages, the low survival rate of MSCs following cell therapy is a major challenge that limits their efficacy in clinical settings (Wang et al. 2010). Oxidative stresses and pro-apoptotic factors are well-known causes of MSCs death (Zhu et al. 2006). Therefore, equipping MSCs with cytoprotective factors to withstand these cytotoxic conditions could be one of the reasonable strategies to overcome the problem. Previous experimental studies demonstrated that genetic modulation of the donor mesenchymal stem cells with cell survival-promoting genes has favorable effects in the process of transplantation (Abdel-Mageed et al. 2009; Cao et al. 2008; Shu et al. 2010; Tsubokawa et al. 2010; Wang et al. 2009). Since HO-1 has been shown to act as an antioxidant, anti-apoptotic, and anti-inflammatory agent following exposure to stress-related conditions (Dulak 2007), in the present study we exploited these cytoprotective properties of HO-1 to augment MSCs against the arduous stress conditions that MSCs inevitably face in vitro. Oxidative stress, serum deprivation, and hypoxia are known stresses that MSCs face them primarily in vitro, and eventually they may affect the viability of MSCs in vivo.
We treated MSC-HO-1 cells with H2O2 and under serum deprivation or hypoxia conditions to test the survival rate of MSCs. The results revealed that HO-1 gene transfer into mesenchymal stem cells improved their survival and resistance to oxidative stresses. These in vitro results suggest that introduction of the HO-1 gene to the MSCs may enhance an endogenous defensive system that is capable of attenuating oxidative stress injuries.
Supporting our study, in an in vivo study, Abdel-Mageed et al. (2009) showed that intravenous administration of MSCs following their genetic modification with extracellular superoxide dismutase improves survival in irradiated mice. Additionally, Tang et al. (2005) demonstrated that modification of MSCs by a hypoxia-regulatable HO-1 vector increases the tolerance of engrafted MSCs to hypoxia-re-oxygen injury in vitro and improves their viability in ischemic hearts.
Apoptosis is a well-known factor affecting the viability of MSCs. On the other hand, one of the well-known functions of HO-1 is its anti-apoptotic effect. Taking this into consideration, we proposed that the expression of HO-1 by the MSCs might inhibit the activation of programmed cell death within the sensitized cells. Our results showed that the number of apoptotic MSC-HO-1 cells were lower than the control cells. Supporting our study, it has been shown recently that the MSC-HO-1s had better anti-oxidative and anti-apoptotic properties than unmodified MSCs (Tsubokawa et al. 2010). It should be noted that in the Tsubokawa study, a nonviral vector was used for expression of HO-1 while in the present study we transiently over-expressed the human HO-1 gene in the MSCs using an adenoviral expression system. Tsubokawa et al. showed that the modified MSCs were resistant to oxidative stresses caused by different concentrations (100 μM to 1.5 mM) of H2O2. But in the present study, the MSC-HO-1s were more resistant to cell death caused by higher concentrations (2–4 mM) of H2O2 compared to the V-MSCs. Additionally, they examined the protective capabilities of HO-1 in an in vivo study on injured heart. In accordance with our findings, the protective effects of MSC-HO-1s against hypoxia and serum deprivation stresses were also studied by Tsubokawa et al.
There are several advantages to the adenoviral expression system such as transient and high expression levels, and the simplicity of infection conditions (Conget and Minguell 2000). It is noteworthy that in most cell types, HO-1 is induced in response to oxidative stresses and rapidly returns to the basal levels. Meanwhile, continuous expression of HO-1 may be harmful to the cells (Deramaudt et al. 1999). Therefore, considering the fact that a majority of MSCs die a few days after transplantations, it would be reasonable to use the adenoviral expression system to take advantage of the short-term expression of HO-1 in MSCs.
In the present study, we showed that the transduction of MSCs with the HO-1 harboring adenoviral vector did not alter the differentiation capability of the MSCs, and this suggests the potential application of HO-1 engineered MSCs for further in vivo studies. Also, the efficient gene delivery with adenoviral vectors in conjunction with the use of gateway technology adds to the advantages of our study.
In conclusion, enhanced anti-apoptotic and anti oxidative capabilities of MSCs following HO-1 infection via adenoviral vectors could be a graft cell death prevention strategy in transplantation and may emerge as an alternative plan for stem cell therapy.
However, a possible disadvantage of the HO-1 gene transduction is the risk of long-term expression of HO-1, nevertheless, when short-term therapeutic or diagnostic gene therapies are considered, transient adenovirus-mediated gene expression would be advantageous. Thus, we propose the adenoviral-mediated transduction of the HO-1 gene into the MSCs for further studies to evaluate the safety and efficacy of the MSC-HO-1s in vivo.
References
- Abdel-Mageed AS, Senagore AJ, Pietryga DW, Connors RH, Giambernardi TA, Hay RV, Deng W. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood. 2009;113(5):1201–1203. doi: 10.1182/blood-2008-07-170936. [DOI] [PubMed] [Google Scholar]
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Cao Y-A, Wagers AJ, Karsunky H, Zhao H, Reeves R, Wong RJ, Stevenson DK, Weissman IL, Contag CH. Heme oxygenase-1 deficiency leads to disrupted response to acute stress in stem cells and progenitors. Blood. 2008;112(12):4494–4502. doi: 10.1182/blood-2007-12-127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25(11):2739–2749. doi: 10.1634/stemcells.2007-0197. [DOI] [PubMed] [Google Scholar]
- Conget PA, Minguell JJ. Adenoviral-mediated gene transfer into ex vivo expanded human bone marrow mesenchymal progenitor cells. Exp Hematol. 2000;28(4):382–390. doi: 10.1016/S0301-472X(00)00134-X. [DOI] [PubMed] [Google Scholar]
- Deramaudt TB, Silva J-L, Remy P, Kappas A, Abraham NG. Negative regulation of human heme oxygenase in microvessel endothelial cells by dexamethasone. Proc Soc Exp Biol Med. 1999;222(2):185–193. doi: 10.1046/j.1525-1373.1999.d01-130.x. [DOI] [PubMed] [Google Scholar]
- Dulak J. Changing faces of heme oxygenases. Antioxid Redox Signal. 2007;9(12):2043–2047. doi: 10.1089/ars.2007.1833. [DOI] [PubMed] [Google Scholar]
- Halabian R, Mohammadi MH, Salimi M, Amani M, Roushande AM, Aghaipoor M, Amirizadeh N, Ebrahimi M, Najafabadi AJ, Roudkenar MH. Genetically engineered mesenchymal stem cells stably expressing green fluorescent protein. IJBMS. 2010;13(2):24–30. [Google Scholar]
- Kikuchi G, Yoshida T, Noguchi M. Heme oxygenase and heme degradation. Biochem Biophys Res Commun. 2005;338:558–567. doi: 10.1016/j.bbrc.2005.08.020. [DOI] [PubMed] [Google Scholar]
- Prockop DJ, Gregory CA, Spees JL. One strategy for cell and gene therapy: harnessing the power of adult stem cells to repair tissues. Proc Natl Acad Sci USA. 2003;100(Suppl 1):11917–11923. doi: 10.1073/pnas.1834138100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Zeng B, Ren X, Li Y. HO-1 modified mesenchymal stem cells modulate MMPs/TIMPs system and adverse remodeling in infarcted myocardium. Tissue and Cell. 2010;42(4):217–222. doi: 10.1016/j.tice.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Tang YL, Tang Y, Zhang YC, Qian K, Shen L, Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46(7):1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Toma C, Pittenger M, Cahill K, Byrne B, Kessler P. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- Tsubokawa T, Yagi K, Nakanishi C, Zuka M, Nohara A, Ino H, Fujino N, Konno T, M-A K, Ishibashi-Ueda H, Nagaya N, Yamagishi M. Impact of anti-apoptotic and anti-oxidative effects of bone marrow mesenchymal stem cells with transient overexpression of heme oxygenase-1 on myocardial ischemia. Am J Physiol Heart Circ Physiol. 2010;298(5):H1320–H1329. doi: 10.1152/ajpheart.01330.2008. [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao T, Huang W, Wang T, Qian J, Xu M, Kranias EG, Wang Y, Fan G-C. Hsp20-engineered mesenchymal stem cells are resistant to oxidative stress via enhanced activation of Akt and increased secretion of growth factors. Stem Cells. 2009;27(12):3021–3031. doi: 10.1002/stem.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Li W, Ou L, Flick E, Mark P, Nesselmann C, Lux CA, Gatzen H-H, Kaminski A, Liebold A, Lützow K, Lendlein A, Li R-K, Steinhoff G, Ma N (2010) Polyethylenimine-mediated gene delivery into human bone marrow mesenchymal stem cells from patients. J Cell Mol Med. doi:10.1111/j.1582-4934.2010.01130.x [DOI] [PMC free article] [PubMed]
- Zhu W, Chen J, Cong X, Hu S, Chen X. Hypoxia and serum deprivation-induced apoptosis in mesenchymal stem cells. Stem Cells. 2006;24(2):416–425. doi: 10.1634/stemcells.2005-0121. [DOI] [PubMed] [Google Scholar]