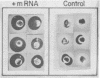
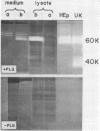
Abstract
Induction of synthesis of the protease plasminogen activator (PA) by hormones, oncogenic viruses and tumor promoters occurs at the transcription level. A novel bioassay for PA messenger RNA was developed to study the regulation of PA synthesis and the genetic elements involved in it. Poly(A)-containing RNA from HEp-3, a PA-rich tumor of human origin, was found to direct the synthesis of a new proteolytic activity when microinjected into Xenopus oocytes. Newly synthesized protease can be detected within a few hours after microinjection of minute quantities of unfractionated mRNA. The new enzymatic activity is indistinguishable from human PA: it is absolutely dependent on human plasminogen; it is neutralized by serum raised against urokinase, the human urinary PA; and it comigrates with urokinase and HEp-3 PA in gel electrophoresis, exhibiting a molecular weight of 60,000.
Full text
PDF


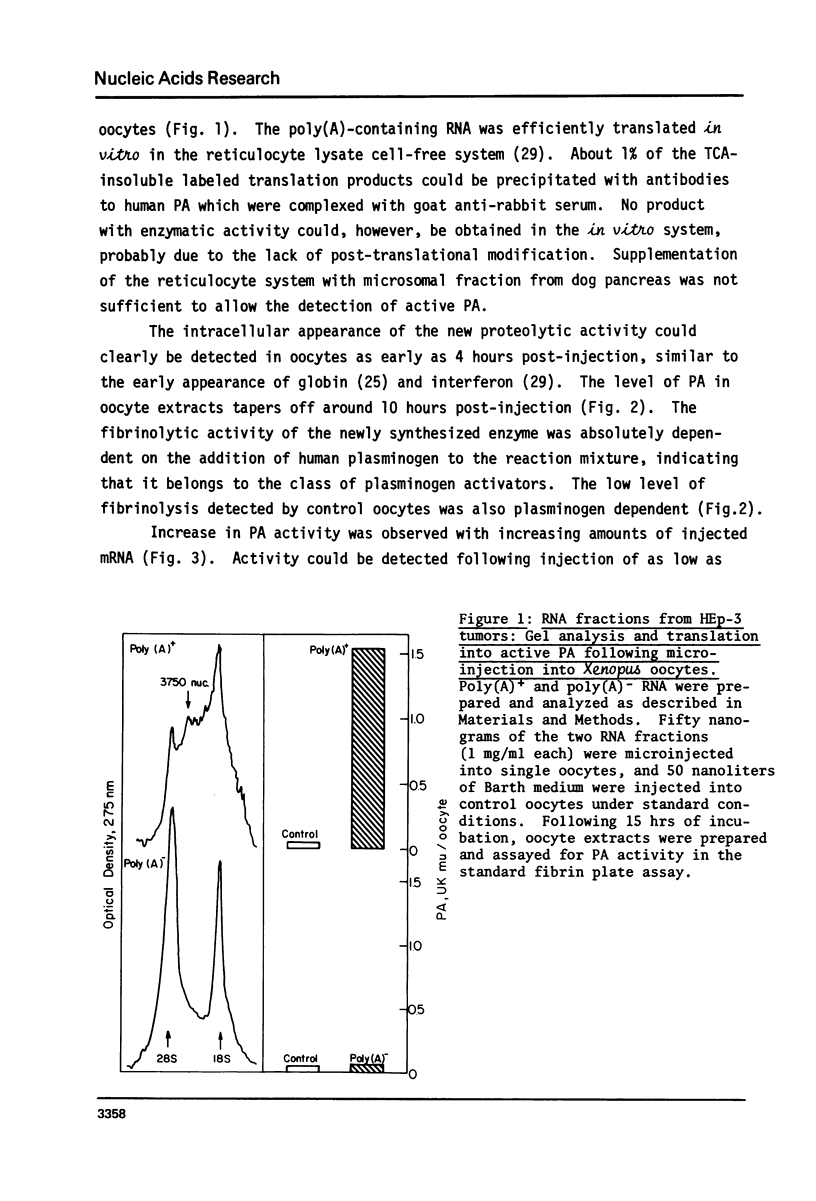
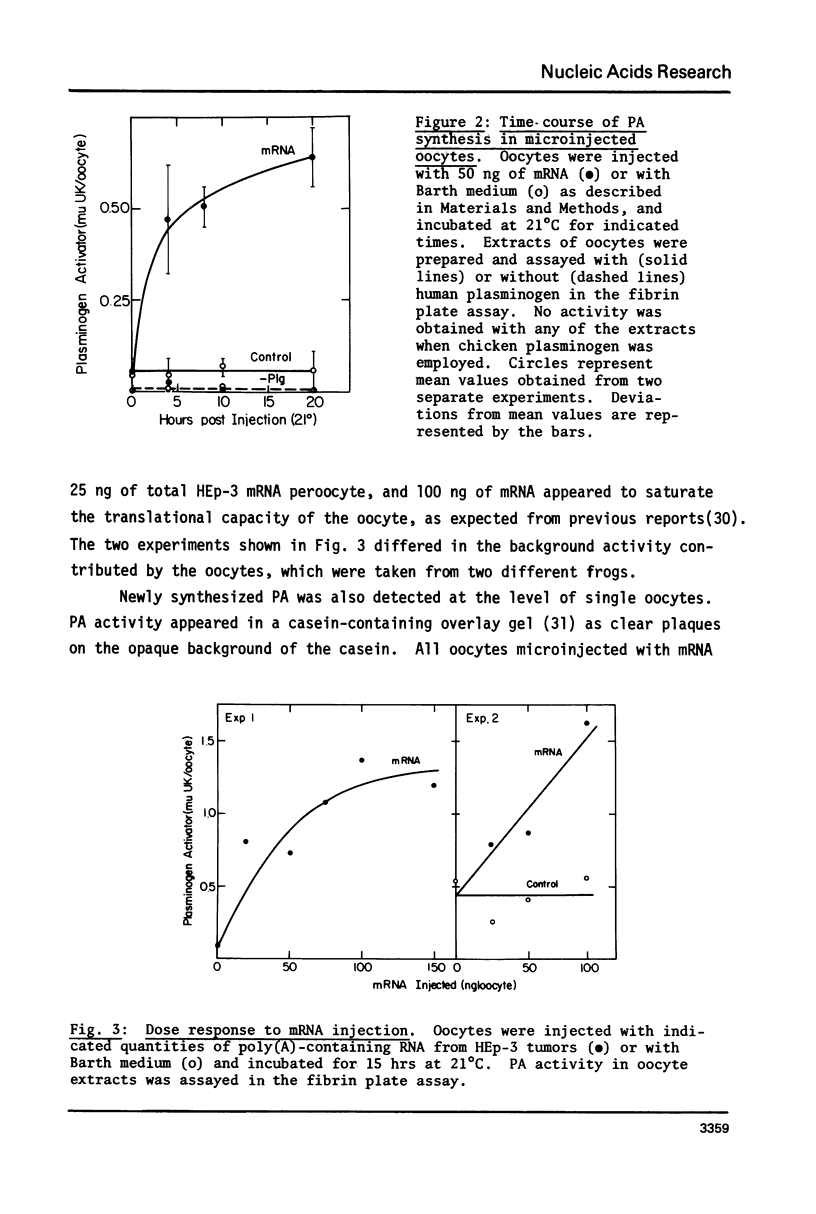



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colman A., Morser J. Export of proteins from oocytes of Xenopus laevis. Cell. 1979 Jul;17(3):517–526. doi: 10.1016/0092-8674(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R. Increased protease levels in transformed cells: a casein overlay assay for the detection of plasminogen activator production. Cell. 1974 Jun;2(2):95–102. doi: 10.1016/0092-8674(74)90097-x. [DOI] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. MRNA-directed synthesis of catalytically active mouse beta-glucuronidase in Xenopus oocytes. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4462–4465. doi: 10.1073/pnas.74.10.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane C. D., Marbaix G., Gurdon J. B. Rabbit haemoglobin synthesis in frog cells: the translation of reticulocyte 9 s RNA in frog oocytes. J Mol Biol. 1971 Oct 14;61(1):73–91. doi: 10.1016/0022-2836(71)90207-5. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D., Gurdon J. B., Partington G. A. Protein synthesis in oocytes of Xenopus laevis is not regulated by the supply of messenger RNA. Cell. 1977 Jun;11(2):345–351. doi: 10.1016/0092-8674(77)90051-4. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Hubert E., Content J., De Wit L., Braude I. A., De Clercq E. Translation of mouse interferon mRNA in Xenopus laevis oocytes and in rabbit reticulocyte lysates. Biochem Biophys Res Commun. 1978 May 30;82(2):665–673. doi: 10.1016/0006-291x(78)90926-9. [DOI] [PubMed] [Google Scholar]
- Miskin R., Easton T. G., Reich E. Plasminogen activator in chick embryo muscle cells: induction of enzyme by RSV, PMA and retinoic acid. Cell. 1978 Dec;15(4):1301–1312. doi: 10.1016/0092-8674(78)90055-7. [DOI] [PubMed] [Google Scholar]
- Miskin R., Reich E. Plasminogen activator: induction of synthesis by DNA damage. Cell. 1980 Jan;19(1):217–224. doi: 10.1016/0092-8674(80)90403-1. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Biegel D., Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979 Apr;16(4):929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Reich E. Experimental model for quantitative study of metastasis. Cancer Res. 1980 Jul;40(7):2300–2309. [PubMed] [Google Scholar]
- Ossowski L., Reich E. Loss of malignancy during serial passage of human carcinoma in culture and discordance between malignancy and transformation parameters. Cancer Res. 1980 Jul;40(7):2310–2315. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Premkumar E., Pitha P. M. Interferon activity produced by translation of human interferon messenger RNA in cell-free ribosomal systems and in Xenopus oöcytes. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4881–4885. doi: 10.1073/pnas.72.12.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman L., Revel M. Interferon-dependent induction of mRNA activity for (2'-5')oligo-isoadenylate synthetase. Nature. 1980 Nov 6;288(5786):98–100. doi: 10.1038/288098a0. [DOI] [PubMed] [Google Scholar]
- Soreq H., Harpold M., Wilson M., Darnell J. E., Jr Rate of synthesis and concentration of specific mRNA sequences in cultured Chinese hamster ovary cells compared to liver cells. Biochem Biophys Res Commun. 1980 Jan 29;92(2):485–491. doi: 10.1016/0006-291x(80)90359-9. [DOI] [PubMed] [Google Scholar]
- Soreq H., Sagar A. D., Sehgal P. B. Translational activity and functional stability of human fibroblast beta 1 and beta 2 interferon mRNAs lacking 3'-terminal RNA sequences. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1741–1745. doi: 10.1073/pnas.78.3.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOOLAN H. W. Transplantable human neoplasms maintained in cortisone-treated laboratory animals: H.S. No. 1; H.Ep. No. 1; H.Ep. No. 2; H.Ep. No. 3; and H.Emb.Rh. No. 1. Cancer Res. 1954 Oct;14(9):660–666. [PubMed] [Google Scholar]
- Unkeless J. C., Tobia A., Ossowski L., Quigley J. P., Rifkin D. B., Reich E. An enzymatic function associated with transformation of fibroblasts by oncogenic viruses. I. Chick embryo fibroblast cultures transformed by avian RNA tumor viruses. J Exp Med. 1973 Jan 1;137(1):85–111. doi: 10.1084/jem.137.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Hamilton J., Reich E. Macrophage plasminogen activator: modulation of enzyme production by anti-inflammatory steroids, mitotic inhibitors, and cyclic nucleotides. Cell. 1976 Jun;8(2):271–281. doi: 10.1016/0092-8674(76)90011-8. [DOI] [PubMed] [Google Scholar]
- Wigler M., DeFeo D., Weinstein I. B. Induction of plasminogen activator in cultured cells by macrocyclic plant diterpene esters and other agents related to tumor promotion. Cancer Res. 1978 May;38(5):1434–1437. [PubMed] [Google Scholar]
- Wilson E. L., Reich E. Modulation of plasminogen activator synthesis in chick embryo fibroblasts by cyclic nucleotides and phorobol myristate acetate. Cancer Res. 1979 May;39(5):1579–1586. [PubMed] [Google Scholar]
- Wilson E. L., Reich E. Plasminogen activator in chick fibroblasts: induction of synthesis by retinoic acid; synergism with viral transformation and phorbol ester. Cell. 1978 Oct;15(2):385–392. doi: 10.1016/0092-8674(78)90007-7. [DOI] [PubMed] [Google Scholar]