Abstract
Alopecia areata (AA) is an autoimmune non-scarring hair loss disorder. AA can be acute, recurrent, or chronic. Current therapeutic options for AA are limited, and there is no effective prevention for recurrent AA. We have previously shown a correlation between the expression of HSP70 (HSPA1A/B), a heat shock protein involved in the inflammatory response, and the onset of AA in the C3H/HeJ mouse model. In this study, we tested the effects of quercetin, a bioflavonoid with anti-inflammatory properties, on AA development and HSP70 expression in the C3H/HeJ model. Mice with spontaneous AA were treated with subcutaneous quercetin or sham injections. Hair regrowth was observed in lesional areas in all the quercetin-treated mice, but in none of the sham-treated mice. In addition, non-alopecic C3H/HeJ mice were heat-treated to induce alopecia, along with quercetin or sham injections. Whereas 24% of the heat-treated mice with sham injections developed alopecia, none of the mice receiving quercetin injections did. As expected, the level of HSP70 expression in quercetin-treated areas was comparable to control. Furthermore, we showed that systemic delivery of quercetin by intraperitoneal injections prevented/reduced spontaneous onset of AA. Our results demonstrated that quercetin provided effective treatment for AA as well as prevention of onset of AA in the C3H/HeJ model, and warrant further clinical studies to determine whether quercetin may provide both treatment for preexisting AA and prevention of recurrent AA. The ready availability of quercetin as a dietary supplement may lead to increased patient compliance and positive outcomes for AA.
Keywords: Alopecia areata, C3H/HeJ, HSP70, Heat shock, Quercetin
Introduction
Alopecia areata (AA) is a non-scarring, autoimmune hair loss disorder (Alkhalifah et al. 2010a). AA often has a sudden onset (Gilhar and Kalish 2006) and may be acute, recurrent, or chronic (Gilhar and Kalish 2006; Garg and Messenger 2009). AA is a T-cell-mediated inflammatory disorder specific for the hair follicle (HF); affected skin appears normal, with no apparent signs of inflammation (Gilhar et al. 2007). Clinical presentation varies considerably (McElwee et al. 2003), but a well-defined, circumscribed patch is most common initially (Dudda-Subramanya et al. 2007). AA can occur on any region of hair-bearing skin, but is found on the scalp 90% of the time (Wasserman et al. 2007). AA has an incidence of 0.1–0.2% in the general population with a lifetime risk of 1.7% (Safavi et al. 1995). Likelihood of onset is irrespective of sex or age (Safavi et al. 1995); however, initial patches present before age 20 in most cases (Price 1991). Scalp hair loss may impose a high psychosocial burden on patients, especially in severe and chronic situations (Safavi et al. 1995; Dudda-Subramanya et al. 2007). AA can occasionally progress to alopecia totalis (AT), loss of all scalp hair; or alopecia universalis (AU), loss of all body hair (Kos and Conlon 2009).
Histologically, AA is characterized by peri- and intra-follicular mononuclear cell infiltration of the HF (Gilhar et al. 2007). HFs susceptible to the infiltrate seem to be in the growth phase (anagen) and undergoing active melanogenesis (Gilhar et al. 2007). The mononuclear infiltrate is composed of both CD4+ and CD8+ T lymphocytes (McElwee et al. 2003; Wasserman et al. 2007). CD4+ T cells predominate around the HF, while CD8+ T cells predominate within the HF (Gilhar et al. 2007; Cetin et al. 2009).
AA is thought to develop as a result of a loss of the immune privilege (IP) of the HF (Paus et al. 2003; Arck et al. 2008; Gilhar 2010). Normally, the lower portion of the HF is immunoprivileged due to (1) the absence of major histocompatibility complex (MHC) class II expression, (2) very low levels of MHC class I expression, (3) active suppression of natural killer (NK) cells, and (4) a decreased density of antigen-presenting cells (APCs) such as Langerhans cells (Gilhar and Kalish 2006; Gilhar et al. 2007). However, as the APCs present the antigens, responsive lymphocytes are activated and therefore migrate to and infiltrate the HFs. These inflammatory infiltrates are deleterious to the HFs (McElwee et al. 2003; Arck et al. 2008).
Currently, corticosteroids are the most commonly used anti-inflammatory therapy for acute AA; they can be administered topically, intralesionally, or systemically (Wasserman et al. 2007). Other therapies in use include topical sensitizers, dithranol (anthralin), photochemotherapy, minoxidil, and immunomodulators including some biologics (Wasserman et al. 2007; Garg and Messenger 2009). In addition, some alternative and complementary therapies have been evaluated (van den Biggelaar et al. 2010). However, no existing therapy for AA is known to alter the course of disease or prevent recurrence (Garg and Messenger 2009). High relapse rates, varying levels of efficacy, and concerns about adverse effects warrant the search for alternative treatments and preventative therapies for recurrent AA.
One potential therapy is the natural compound quercetin, which is known for its anti-inflammatory properties (Boots et al. 2008). Quercetin (3,3′,4′,5,7-pentahydroxyflavone, also called quercetine, sophretin, or meletin) is a bioflavonoid. As an anti-inflammatory, quercetin has been observed to inhibit the activation of nuclear factor kappa-B (NF-κB), a transcription factor that stimulates the production of pro-inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and the interleukins IL-1, IL-2, and IL-6 (Nam 2006; Bhaskar et al. 2011).
Heat shock proteins (HSPs) are known to play a role in the inflammatory response (Pockley et al. 2008). HSPs can act as intercellular signaling molecules promoting the production of inflammatory cytokines and adhesins; they can also deliver maturation signals and present peptides to APCs (Roberts et al. 2010). Heat shock protein 70 (HSP70 or HSPA1A/B), when found outside the cell, can serve as a “danger signal” triggering inflammation (Pockley et al. 2008). Indeed, we have previously demonstrated a correlation between AA and HSP70 expression in a mouse model; increased HSP70 expression was observed in lesional skin as compared to control (Wikramanayake et al. 2010). Furthermore, HSP70 has been shown to interact with immunoregulatory T cell populations (Pockley et al. 2008).
Thus, in this study, we tested the effects of quercetin, an anti-inflammatory and known HSP70 inhibitor (Wang et al. 2009), in the C3H/HeJ mouse model for AA.
Materials and methods
Mice and treatment
Animal care and use procedures were approved by the University of Miami Institutional Animal Care and Use Committee. Retired female C3H/HeJ breeders (approximately 6 months old) were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Mice were fed ad libitum.
To test the effects of quercetin on preexisting alopecia areata (AA), 16 mice with spontaneous AA were randomly assigned to two groups: Eight mice were injected subcutaneously with 100 μL of 10 μM quercetin (Sigma-Aldrich, St. Louis, MO, USA) in 10% dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS) once daily for 8 days, and eight mice were injected with vehicle only (10% DMSO in PBS; sham treatment). Mice were monitored for 6 weeks for hair regrowth.
To test whether quercetin has protective effects for heat-induced AA, 100 retired female C3H/HeJ breeders were randomly assigned to two groups: 50 mice received daily heat treatment for 12 consecutive days along with daily subcutaneous injections of quercetin as described above, and the other 50 mice received heat treatment along with vehicle injections. To apply heat, petroleum jelly was applied to the haired dorsal skin to improve conductance. Heat was then applied with a copper cylinder connected to a precision water bath (48.5°C) for 20 min. The heat-treated area was immediately cooled with a small icepack for 5 min (Jimenez et al. 2008). Mice were monitored for 6 weeks for hair loss.
To determine the effects of systemic quercetin on the onset of spontaneous AA, we divided 100 old retired female C3H/HeJ breeders randomly into two groups: 50 mice received daily intraperitoneal injections of quercetin (100 μL of 10 μM, in 10% DMSO) for 8 days, and 50 mice received vehicle injections. Mice were monitored for hair loss. For all the comparisons between quercetin- and sham-treated mice, Fisher’s exact test was carried out to calculate statistical significance.
Histological analysis
Skin samples from corresponding dorsal area of quercetin- and sham-treated mice, as well as from control mice and alopecic areas in mice with spontaneous AA, were collected and fixed in 10% formalin. Paraffin-embedded sections (5 μm) were stained with hematoxylin and eosin. The stained slides were subsequently evaluated using an Axio Observer D1 microscope (Carl Zeiss Microimaging, Thornwood, NY, USA).
Western blot analysis
Protein extraction and Western blot analysis were carried out as previously described (Wikramanayake et al. 2010). Briefly, skin biopsies (approximately 1 cm2) were homogenized in lysis buffer for 1 min after subcutaneous fat was removed. Tissue lysates were cleared by centrifugation and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot. Inducible HSP-70 (HSP-70i, HSPA1A/B) was detected using a mouse monoclonal antibody (Assay Designs, Ann Arbor, MI, USA) and secondary antibodies conjugated with alkaline phosphatase and color reaction.
Results
Amelioration of spontaneous AA with subcutaneous quercetin injections
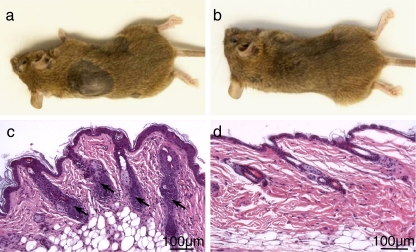
The C3H/HeJ mice develop AA spontaneously with age, and have been the most extensively characterized and most widely utilized mouse model for AA (McElwee et al. 2003). To test the effects of quercetin on preexisting AA, mice with spontaneous AA were randomly assigned for subcutaneous injections of quercetin (100 μL of 10 μM quercetin in 10% DMSO) or vehicle (sham treatment), once daily for 8 days. Within 6 weeks, all eight mice receiving quercetin showed hair regrowth in the lesional areas, while none of the sham-treated mice did (Fig. 1a, b) (p ≪ 0.01). Histology showed massive lymphocyte infiltrates within or around the HFs in the alopecic skin of sham-treated mice (Fig. 1c), which were absent in quercetin-treated mouse skin (Fig. 1d). These results demonstrated that quercetin treatment ameliorated AA in the C3H/HeJ model.
Fig. 1.
Gross phenotype (a, b) and histology (c, d) of sham-treated (a, c) or quercetin-treated (b, d) C3H/HeJ mice with spontaneous alopecia areata. Arrows point to lymphocyte infiltrates (c)
Prevention of the onset of heat-induced AA with subcutaneous quercetin injections
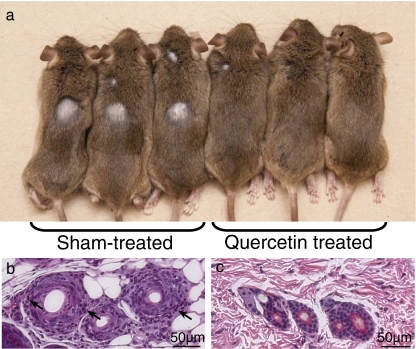
We have previously established a heat treatment scheme to induce the onset of AA in younger C3H/HeJ mice; approximately 20% of heat-treated mice developed AA in 6 weeks after 12 days of daily heat treatment (Wikramanayake et al. 2010). To determine whether quercetin can prevent the onset of heat-induced AA, we treated C3H/HeJ mice with heat along with quercetin or sham injections. We randomly assigned 100 C3H/HeJ mice to two groups: 50 mice received heat treatment along with subcutaneous injections of quercetin, and 50 mice received heat treatment and vehicle injections. Six weeks later, 12 of the 50 mice (24%) treated with heat and sham injections developed alopecia on the dorsal area, as expected. However, none of the quercetin-treated mice showed any signs of hair loss (Fig. 2a) (p ≪ 0.01). Histology detected lymphocyte infiltrates within or around the HFs in the alopecic skin of sham-treated mice (Fig. 2b), similar to that observed in mouse skin with spontaneous AA. Conversely, lymphocyte infiltrates were absent in quercetin-treated mouse skin (Fig. 2c). These results indicated that quercetin treatment at the time of induction prevented the onset of heat-induced AA in the C3H/HeJ mouse model.
Fig. 2.
Gross phenotype (a) and histology (b, c) of sham-treated (a, b) or quercetin-treated (a, c) C3H/HeJ mice after heat treatment to induce alopecia areata. None of the quercetin-treated mice developed alopecia (a). Arrows point to lymphocyte infiltrates (b)
We have previously detected elevated HSP70 levels in lesional skin from C3H/HeJ mice with spontaneous or heat-induced AA (Wikramanayake et al. 2010). When we examined skin biopsies from mice treated with heat and quercetin with Western blot analysis, we detected a reduced HSP70 level, now comparable to that of normal C3H/HeJ mouse skin (data not shown). These results suggest that quercetin prevented the induction of HSP70 and AA by heat treatment.
Prevention of the onset of spontaneous AA with systemic quercetin treatment
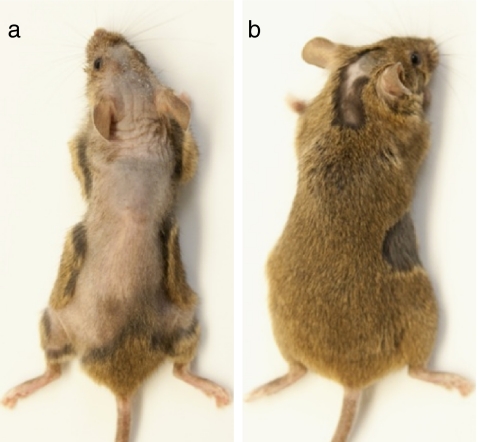
Quercetin is found in many foods and is available as a dietary supplement. To explore the possibility of using systemic quercetin to treat or prevent the recurrence of AA, we gave C3H/HeJ mice eight daily intraperitoneal injections of quercetin (100 μL of 10 μM, in 10% DMSO) or vehicle. After 6 weeks, nine of 50 (18%) vehicle-treated mice developed severe alopecia on the dorsal skin (Fig. 3), three (6%) developed focal alopecia, and 38 (76%) showed no apparent hair loss (Table 1). However, among the 50 quercetin-treated mice, none developed severe alopecia, two (4%) developed focal alopecia (Fig. 3), and 48 (96%) showed no apparent hair loss (Table 1). There is statistical significance in the numbers of mice with severe or focal alopecia between quercetin- and sham-treated mice (p < 0.01). These results showed that systemic quercetin delivered via intraperitoneal injections could prevent/reduce the onset of spontaneous AA in C3H/HeJ mice.
Fig. 3.
Gross phenotype of C3H/HeJ mice that received daily intraperitoneal injections of vehicle or quercetin. a A vehicle-injected mouse with severe alopecia; b a quercetin-injected mouse with focal alopecia
Table 1.
The effects of intraperitoneally injected quercetin on the development of spontaneous AA in C3H/HeJ mice
| Severe alopecia | Focal alopecia | No alopecia | Total | |
|---|---|---|---|---|
| Sham treated | 9 | 3 | 38 | 50 |
| Quercetin treated | 0 | 2 | 48 | 50 |
Discussion
In this study, we demonstrated that quercetin treatment could improve AA in the C3H/HeJ mouse model. All of the mice with spontaneous AA receiving subcutaneous quercetin injections showed hair regrowth within 6 weeks after cessation of treatment, but none of the mice receiving sham injections did. We also found that subcutaneous quercetin injections could prevent the onset of heat-induced AA. Furthermore, we showed that systemic quercetin could prevent/reduce the onset of spontaneous AA. Our results suggest that quercetin may provide both therapeutic treatment for acute AA and preventive benefits for recurrent AA.
We have previously shown increased HSP70 expression in lesional C3H/HeJ skin in both induced AA and spontaneous AA as compared to control (Wikramanayake et al. 2010). In this study, we showed that HSP70 levels were reduced in the skin of mice treated with both heat and quercetin as compared to mice treated with heat and sham injections. In fact, HSP70 levels in the skin of quercetin-treated mice were comparable to control.
Pathogenesis of AA
Normally, the immune privilege (IP) in the lower portion of the HFs protects them from immune-mediated inflammation. Local immunosuppressants, such as transforming growth factor beta 1 (TGF-β1), melanocyte-stimulating hormone (α-MSH), interleukin 10 (IL-10), and insulin-like growth factor 1 (IGF-1), have also been shown to help maintain IP (Paus et al. 2003).
The loss of IP in AA is not fully understood. It is not known whether the antigens presented are endogenous or exogenous, or whether the epitope expression is normal or aberrant (McElwee et al. 2003). AA is characterized by infiltration of T lymphocytes within and around the anagen HF in active melanogenesis (Gilhar et al. 2007). The majority of lymphocytes within the HF are CD8+ T cells while the majority of lymphocytes around the HF are CD4+ (Gilhar et al. 2007; Cetin et al. 2009). It has been documented that active AA is associated with increased expression of MHC classes I and II and greater numbers of APCs such as macrophages and Langherhans cells (McElwee et al. 2003). Significantly more CD8+, CD3+, and CD57+ lymphocytes and mast cells were found to be present in lesional skin of AA patients than in nonlesional skin (Cetin et al. 2009).
Cytokines known to be aberrantly expressed in AA include interferon-gamma (IFN-γ) and TNF-α (Gregoriou et al. 2010). Production of IFN-γ by peri-follicular and follicular antigen-presenting cells is CD4+ T helper 1 (Th1) mediated (Gregoriou et al. 2010). IL-1, which recruits inflammatory cells including T cells, is also potentially relevant to AA pathogenesis (Dudda-Subramanya et al. 2007). In addition, studies in mice have demonstrated that AA-affected skin contained more pro-inflammatory Th1 and T helper 2 (Th2) cytokines, as well as an upregulation of costimulatory molecules CD28, CD40L, and their ligands (McElwee et al. 2002). Moreover, neuropeptides may also be involved in AA pathogenesis (Cetin et al. 2009).
Etiology of AA appears to be multifactorial (Dudda-Subramanya et al. 2007). There is strong evidence of a genetic basis for AA; the predisposition has been shown to be polygenic (Dudda-Subramanya et al. 2007). Associations have been found with multiple genes, including HLA genes, which encode the MHC molecules (Gilhar et al. 2007), and the IL-1 receptor antagonist gene (McDonagh and Tazi-Ahnini 2002). Some environmental factors, such as physiological and psychological stress, are suspected triggers of AA (Wasserman et al. 2007).
HSP70
While HSP70, which possesses both protein chaperoning and immunomodulatory domains, can act as an intercellular signaling molecule to promote the inflammatory response, it has also been implicated in an anti-inflammatory and cytoprotective role within the cell (de Jong et al. 2009; Roberts et al. 2010). This dual role in inflammation depends on the context in which HSP70 is found. On the one hand, when HSP70 is present extracellularly, it has been shown to promote the expression of inflammatory cytokines such as TNF-α and IL-1, to present peptides to APCs, and to activate macrophages, dendritic cells, and NK cells (de Jong et al. 2009; Molvarec et al. 2010). It has been suggested that extracellular HSP70 promotes the expression of proinflammatory cytokines by activating NF-κB (Asea et al. 2000). On the other hand, intracellular HSP70 can act in an anti-inflammatory and cytoprotective fashion as a molecular chaperone, an anti-apoptotic effector, an inhibitor upstream of NF-κB transcription, and an aid in proteasome-mediated protein degradation in times of cellular stress (Garrido et al. 2006; de Jong et al. 2009).
Release of endogenous HSPs into the extracellular fluid may be triggered by physical trauma or stress, and release can occur by active physiological secretion or by passive cell death during necrosis (de Jong et al. 2009). HSP70 is not secreted via the classical endoplasmic reticulum-Golgi pathway but is associated with export vesicles (De Maio 2011). After release into the extracellular fluid, HSPs may bind the surfaces of nearby cells and initiate signal transduction or transport of antigenic peptides. HSP70 has been shown to bind CD40 on T cells (de Jong et al. 2009). HSPs may also enter the bloodstream and act at distant sites in the body. During exposure to bacterial pathogens, prokaryotic HSPs released are the dominant antigens in the immune response; it has been speculated that bacterial HSPs may trigger an autoimmune response to mammalian HSPs. Interestingly, HSP70 presented by macrophages and neutrophils can act as an antigen for γδT cells (Hirsh and Junger 2008). Moreover, in addition to intracellular and extracellular localization, HSP70 has also been associated with the plasma membrane (De Maio 2011; Multhoff and Hightower 2011).
HSP70’s dual role in inflammation does not seem to be explained simply by its location (intracellular versus cell surface or secreted). Whether HSP70 acts to stimulate inflammatory cells or suppress them also depends on the microenvironment of concomitant inflammatory signals and the cells with which it interacts (Pockley et al. 2008).
Decreased HSP70 in the plasma during pregnancy is thought to promote immunological tolerance of mother to fetus (Molvarec et al. 2010). If HSP70 has a similar relationship to IP in the HF, we speculate that high levels of extracellular HSP70 could promote antigen recognition, loss of IP, and T cell infiltration, especially in the presence of costimulatory signals. Future studies are needed to elucidate the role of HSP70 in loss of IP in AA.
Current treatment of AA
Intralesional corticosteroids, which act as immunosuppressants, are first-line therapy for AA when <50% of scalp is involved (Wasserman et al. 2007). Topical application, which is usually used in pediatric patients, has been reported to have varying levels of efficacy and very high relapse rates (Wasserman et al. 2007). Systemic administration, on the other hand, has been linked to significant adverse effects (Wasserman et al. 2007). Younger patients appeared to respond better to Minoxidil than older patients (Wasserman et al. 2007). Contact immunotherapy seems best for severe acute AA; however, only a small percentage of patients maintain long-term results (Garg and Messenger 2009). Dithranol has been shown to have a <25% response rate (Garg and Messenger 2009). Conflicting results have been reported for response to psoralen and ultraviolet A (Wasserman et al. 2007). Immunomodulatory drugs such as cyclosporine, methotrexate, and sulfasalazine can be used in combination with corticosteroids; however, risk of adverse effects is very high (Alkhalifah et al. 2010b). Multiple biologics, including adalimumab, etanercept, efalizumab, and infliximab, have also been tested for the treatment of AA, but none have shown satisfactory results (Alkhalifah et al. 2010b). The 308-nm excimer laser, which is thought to be capable of inducing T cell apoptosis in vitro (Ohtsuki et al. 2010), has also been used to treat scalp AA. In a study of 18 patients, regrowth was found in 41.5% (17 of 41) of scalp patches (Al-Mutairi 2007). In a pediatric study of nine patients with 30 recalcitrant patches, 60% (18 of 30) of patches showed regrowth (Al-Mutairi 2009). Although laser therapy is promising, there is no conclusive evidence that it can prevent AA, and like intralesional steroids and other therapies, laser therapy requires repeated office visits. Complementary and alternative therapies, such as psychotherapy, segmental massage, energy healing including acupuncture, topical onion juice, topical garlic gel, vitamin A acid, and polyphenolic compounds in green tea, have shown some promise; however, to date many of the published studies did not satisfy objective quality requirements (van den Biggelaar et al. 2010). However, it is important to note that onions, garlic, and tea are all natural sources of quercetin (Slimestad et al. 2007; Marzouki et al. 2010; Egert et al. 2011; Sun et al. 2011), and hair regrowth was observed in some AA patients treated topically with onion juice (Sharquie and Al-Obaidi 2002) or a combination of topical garlic gel and betamethasone valerate cream (Hajheydari et al. 2007).
Potential use of quercetin as therapy for AA
In this study, we showed that subcutaneous injections of quercetin induced hair regrowth in preexisting alopecic lesions of C3H/HeJ mice with spontaneous AA. We also showed that quercetin prevented the onset of heat-induced AA in C3H/HeJ mice. In addition, we have observed significantly reduced alopecic area in mice receiving systemic quercetin treatment as compared with sham-treated mice that developed spontaneous AA.
Furthermore, we found that quercetin reduced HSP70 levels in lesional skin of heat-induced alopecic mice. The anti-inflammatory properties of quercetin suggest that in AA, one mechanism by which quercetin may counter inflammation is by inhibiting HSP70 induction. Quercetin is also known to downregulate NF-κB, which normally enhances the expression of pro-inflammatory cytokines such as TNF-α and IL-1 (Nam 2006). Quercetin has been observed to counter NF-κB directly and also through its antioxidant role in neutralizing reactive oxygen species (Boots et al. 2008). Additionally, quercetin has been shown to modulate the immune response, such as by suppressing dendritic cells (Huang et al. 2010; Nickel et al. 2011). Quercetin treatment, therefore, demonstrates the potential to counter the breakdown of the IP in AA.
Quercetin is found in many foods. Rich sources of quercetin include broccoli, parsley, onions, apples, grapes, dark berries, cherries, raisins, cabbage, beans, tomatoes, tea, and red wine (Erdman et al. 2007; Egert et al. 2011; Lee et al. 2011). In the USA, average daily intake of quercetin is estimated at 10–100 mg and can be as high as 200–500 mg (Erdman et al. 2007; Harwood et al. 2007). In addition to natural food sources, quercetin is also available as a dietary supplement, with recommended daily doses of 200–1,200 mg (Harwood et al. 2007; Egert et al. 2011). It comes in pill, soft chew, or water-soluble form (Bigelman et al. 2011; Lee et al. 2011). Following oral administration, quercetin derivatives including glycosides are absorbed from the small intestine, and as much as 60% of total quercetin can be absorbed (Harwood et al. 2007). No significant adverse health effects have been reported following oral administration of quercetin to humans, at doses up to 1,000 mg/day for up to 12 weeks (Harwood et al. 2007). Unlike current treatments such as corticosteroid injections that require multiple office visits, should oral quercetin prove to be effective in the treatment of preexisting AA in clinic, its ready availability may result in increased patient compliance and consequently positive outcome for AA. Prevention of heat-induced AA in our mouse model also suggests a potential use of quercetin in the prevention of recurrent AA.
Acknowledgments
We gratefully acknowledge the Locks of Love Foundation (J.J.J.) for their support in this investigation. T.C.W. is supported by a Career Development Award from NIH/NIAMS (AR-050487).
Conflict of interest The authors state no conflict of interest.
Glossary
- AA
Alopecia areata
- APC
Antigen-presenting cell
- AT
Alopecia totalis
- AU
Alopecia universalis
- HF
Hair follicle
- HSP
Heat shock protein
- HSP70
Heat shock protein 70
- IL-1, IL-2, IL-6, IL-10
Interleukins 1, 2, 6, 10
- IFN-γ
Interferon-gamma
- IGF-1
Insulin-like growth factor 1
- IP
Immune privilege
- α-MSH
Melanocyte-stimulating hormone
- MHC
Major histocompatibility complex
- NK
Natural killer
- NF-κB
Nuclear factor kappa B
- PUVA
Psoralen and ultraviolet A
- TGF-β1
Transforming growth factor beta 1
- TNF-α
Tumor necrosis factor alpha
- Th1
T helper 1
- Th2
T helper 2
References
- Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part I. Clinical picture, histopathology, and pathogenesis. J Am Acad Dermatol. 2010;62:177–188. doi: 10.1016/j.jaad.2009.10.032. [DOI] [PubMed] [Google Scholar]
- Alkhalifah A, Alsantali A, Wang E, McElwee KJ, Shapiro J. Alopecia areata update: part II. Treatment. J Am Acad Dermatol. 2010;62:191–202. doi: 10.1016/j.jaad.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Al-Mutairi N. 308-nm excimer laser for the treatment of alopecia areata. Dermatol Surg. 2007;33:1483–1487. doi: 10.1111/j.1524-4725.2007.33320.x. [DOI] [PubMed] [Google Scholar]
- Al-Mutairi N. 308-nm excimer laser for the treatment of alopecia areata in children. Pediatr Dermatol. 2009;26:547–550. doi: 10.1111/j.1525-1470.2009.00980.x. [DOI] [PubMed] [Google Scholar]
- Arck PC, Gilhar A, Bienenstock J, Paus R. The alchemy of immune privilege explored from a neuroimmunological perspective. Curr Opin Pharmacol. 2008;8:480–489. doi: 10.1016/j.coph.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Asea A, Kraeft SK, Kurt-Jones EA, Stevenson MA, Chen LB, Finberg RW, Koo DC, Calderwood SK. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- Bhaskar S, Shalini V, Helen A. Quercetin regulates oxidized LDL induced inflammatory changes in human PBMCs by modulating the TLR-NF-κB signaling pathway. Immunobiology. 2011;216:367–373. doi: 10.1016/j.imbio.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Bigelman AK, Chapman DP, Freese EC, Trilk JL, Curetin KJ. Effects of 6 weeks of quercetin supplementation on energy, fatigue, and sleep in ROTC cadets. Mil Med. 2011;176:565–572. doi: 10.7205/milmed-d-09-00230. [DOI] [PubMed] [Google Scholar]
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Cetin ED, Savk E, Uslu M, Eskin M, Karul A. Investigation of the inflammatory mechanisms in alopecia areata. Am J Dermatopathol. 2009;31:53–60. doi: 10.1097/DAD.0b013e318185a66e. [DOI] [PubMed] [Google Scholar]
- Jong PR, Schadenberg AW, Jansen NJ, Prakken BJ. Hsp70 and cardiac surgery: molecular chaperone and inflammatory regulator with compartmentalized effects. Cell Stress Chaperones. 2009;14:117–131. doi: 10.1007/s12192-008-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio A. Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: a form of communication during injury, infection, and cell damage. Cell Stress Chaperones. 2011;16:235–249. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudda-Subramanya R, Alexis AF, Siu K, Sinha AA. Alopecia areata: genetic complexity underlies clinical heterogeneity. Eur J Dermatol. 2007;17:367–374. doi: 10.1684/ejd.2007.0231. [DOI] [PubMed] [Google Scholar]
- Egert S, Wolffram S, Schulze B, Langguth P, Hubbermann EM, Schwarz K, Adolphi B, Bosy-Westphal A, Rimbach G, Muller MJ (2011) Enriched cereal bars are more effective in increasing plasma quercetin compared with quercetin from powder-filled hard capsules. Br J Nutr. doi:10.1017/s0007114511003242 [DOI] [PubMed]
- Erdman JW, Jr, Balentine D, Arab L, Beecher G, Dwyer JT, Folts J, Harnly J, Hollman P, Keen CL, Mazza G, Messina M, Scalbert A, Vita J, Williamson G, Burrowes J. Flavonoids and heart health: proceedings of the ILSI North America Flavonoids Workshop. J Nutr. 2007;137:718S–737S. doi: 10.1093/jn/137.3.718S. [DOI] [PubMed] [Google Scholar]
- Garg S, Messenger AG. Alopecia areata: evidence-based treatments. Semin Cutan Med Surg. 2009;28:15–18. doi: 10.1016/j.sder.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Gilhar A. Collapse of the immune privilege in alopecia areata: coincidental or substantial? J Investig Dermatol. 2010;130:2535–2537. doi: 10.1038/jid.2010.260. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Kalish RS. Alopecia areata: a tissue specific autoimmune disease of the hair follicle. Autoimmun Rev. 2006;5:64–69. doi: 10.1016/j.autrev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Gilhar A, Paus R, Kalish RS. Lymphocytes, neuropeptides, and genes involved in alopecia areata. J Clin Invest. 2007;117:2019–2027. doi: 10.1172/JCI31942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou S, Papafragkaki D, Kontochristopoulos G, Rallis E, Kalogeromitros D, Rigopoulos D (2010) Cytokines and other mediators in alopecia areata. Mediators Inflamm 2010:928030 [DOI] [PMC free article] [PubMed]
- Hajheydari Z, Jamshidi M, Akbari J, Mohammadpour R. Combination of topical garlic gel and betamethasone valerate cream in the treatment of localized alopecia areata: a double-blind randomized controlled study. Indian J Dermatol Venereol Leprol. 2007;73:29–32. doi: 10.4103/0378-6323.30648. [DOI] [PubMed] [Google Scholar]
- Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Hirsh MI, Junger WG. Roles of heat shock proteins and gamma delta T cells in inflammation. Am J Respir Cell Mol Biol. 2008;39:509–513. doi: 10.1165/rcmb.2008-0090TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang RY, Yu YL, Cheng WC, OuYang CN, Fu E, Chu CL. Immunosuppressive effect of quercetin on dendritic cell activation and function. J Immunol. 2010;184:6815–6821. doi: 10.4049/jimmunol.0903991. [DOI] [PubMed] [Google Scholar]
- Jimenez JJ, Roberts SM, Mejia J, Mauro LM, Munson JW, Elgart GW, Connelly EA, Chen Q, Zou J, Goldenberg C, Voellmy R. Prevention of chemotherapy-induced alopecia in rodent models. Cell Stress Chaperones. 2008;13:31–38. doi: 10.1007/s12192-007-0005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kos L, Conlon J. An update on alopecia areata. Curr Opin Pediatr. 2009;21(4):475–480. doi: 10.1097/MOP.0b013e32832db986. [DOI] [PubMed] [Google Scholar]
- Lee KH, Park E, Lee HJ, Kim MO, Cha YJ, Kim JM, Lee H, Shin MJ. Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers. Nutr Res Pract. 2011;5:28–33. doi: 10.4162/nrp.2011.5.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzouki SM, Almagro L, Sabater-Jara AB, Ros Barcelo A, Pedreno MA. Kinetic characterization of a basic peroxidase from garlic (Allium sativum L.) cloves. J Food Sci. 2010;75:C740–C746. doi: 10.1111/j.1750-3841.2010.01848.x. [DOI] [PubMed] [Google Scholar]
- McDonagh AJ, Tazi-Ahnini R. Epidemiology and genetics of alopecia areata. Clin Exp Dermatol. 2002;27:405–409. doi: 10.1046/j.1365-2230.2002.01077.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Hoffmann R, Freyschmidt-Paul R, Wenzel E, Kissling S, Sundberg JP, Zoller M. Resistance to alopecia areata in C3H/HeJ mice is associated with increased expression of regulatory cytokines and a failure to recruit CD4+ and CD8+ cells. J Invest Dermatol. 2002;119:1426–1433. doi: 10.1046/j.1523-1747.2002.19620.x. [DOI] [PubMed] [Google Scholar]
- McElwee KJ, Freyschmidt-Paul P, Sundberg JP, Hoffman R. The pathogenesis of alopecia areata in rodent models. J Investig Dermatol Symp Proc. 2003;8:6–11. doi: 10.1046/j.1523-1747.2003.12164.x. [DOI] [PubMed] [Google Scholar]
- Molvarec A, Tamasi L, Gyorgy L, Madach K, Prohaszka Z, Rigo J., Jr Circulating heat shock protein 70 (HSPA1A) in normal and pathological pregnancies. Cell Stress and Chaperones. 2010;15:237–247. doi: 10.1007/s12192-009-0146-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Multhoff G, Hightower LE. Distinguishing integral and receptor-bound heat shock protein 70 (Hsp70) on the cell surface by Hsp70-specific antibodies. Cell Stress Chaperones. 2011;16:251–255. doi: 10.1007/s12192-010-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam NH. Naturally occurring NF-kB inhibitors. Mini-Reviews in Medicinal Chemistry. 2006;6:945–951. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- Nickel T, Hanssen H, Sisic Z, Pfeiler S, Summo C, Schmauss D, Hoster E, Weis M. Immunoregulatory effects of the flavonol quercetin in vitro and in vivo. Eur J Nutr. 2011;50:163–172. doi: 10.1007/s00394-010-0125-8. [DOI] [PubMed] [Google Scholar]
- Ohtsuki A, Hasegawa T, Ikeda S. Treatment of alopecia areata with 308-nm excimer lamp. J Dermatol. 2010;37:1032–1035. doi: 10.1111/j.1346-8138.2010.00942.x. [DOI] [PubMed] [Google Scholar]
- Paus R, Ito N, Takigawa M, Ito T. The hair follicle and immune privilege. J Investig Dermatol Symp Proc. 2003;8:188–194. doi: 10.1046/j.1087-0024.2003.00807.x. [DOI] [PubMed] [Google Scholar]
- Pockley AG, Muthana M, Calderwood SK. The dual immunoregulatory roles of stress proteins. Trends Biochem Sci. 2008;33:71–79. doi: 10.1016/j.tibs.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Price VH. Alopecia areata: clinical aspects. J Invest Dermatol. 1991;96:68S. doi: 10.1111/1523-1747.ep12471869. [DOI] [PubMed] [Google Scholar]
- Roberts RJ, Agius C, Saliba C, Bossier P, Sung YY. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: a review. J Fish Dis. 2010;33:789–801. doi: 10.1111/j.1365-2761.2010.01183.x. [DOI] [PubMed] [Google Scholar]
- Safavi KH, Muller SA, Suman VJ, Moshell AN, Melton LJ., 3rd Incidence of alopecia areata in Olmsted County, Minnesota, 1975 through 1989. Mayo Clin Proc. 1995;70:628–633. doi: 10.4065/70.7.628. [DOI] [PubMed] [Google Scholar]
- Sharquie KE, Al-Obaidi HK. Onion juice (Allium cepa L.), a new topical treatment for alopecia areata. J Dermatol. 2002;29:343–346. doi: 10.1111/j.1346-8138.2002.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Slimestad R, Fossen T, Vagen IM. Onions: a source of unique dietary flavonoids. J Agric Food Chem. 2007;55:10067–10080. doi: 10.1021/jf0712503. [DOI] [PubMed] [Google Scholar]
- Sun J, Chen P, Lin LZ, Harnly JM. A non-targeted approach to chemical discrimination between green tea dietary supplements and green tea leaves by HPLC/MS. J AOAC Int. 2011;94:487–497. [PMC free article] [PubMed] [Google Scholar]
- Biggelaar FJ, Smolders J, Jansen JF. Complementary and alternative medicine in alopecia areata. Am J Clin Dermatol. 2010;11:11–20. doi: 10.2165/11530040-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Wang RE, Kao JLF, Hilliard CA, Pandita RK, Roti Roti JL, Hunt CR, Taylor JS. Inhibition of heat shock induction of heat shock protein 70 and enhancement of heat shock protein 27 phosphorylation by quercetin derivatives. J Med Chem. 2009;52:1912–1921. doi: 10.1021/jm801445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman D, Guzman-Sanchez DA, Scott K, McMichael A. Alopecia areata. Int J Dermatol. 2007;46:121–131. doi: 10.1111/j.1365-4632.2007.03193.x. [DOI] [PubMed] [Google Scholar]
- Wikramanayake TC, Alvarez-Connelly E, Simon J, Mauro LM, Guzman J, Elgart G, Schachner LA, Chen J, Plano LR, Jimenez JJ. Heat treatment increases the incidence of alopecia areata in the C3H/HeJ mouse model. Cell Stress and Chaperones. 2010;15:985–991. doi: 10.1007/s12192-010-0209-7. [DOI] [PMC free article] [PubMed] [Google Scholar]