Abstract
α-Crystallin-type small heat shock proteins (sHsps) are expressed in many bacteria, animals, plants, and archaea. Among mycoplasmas (Mollicutes), predicted sHsp homologues so far were found only in the Acholeplasmataceae family. In this report, we describe the cloning and functional characterization of a novel sHsp orthologue, IbpA protein, present in Acholeplasma laidlawii. Importantly, similar to the endogenously expressed sHsp proteins, the recombinant IbpA protein was able to spontaneously generate oligomers in vitro and to rescue chemically denatured bovine insulin from irreversible denaturation and aggregation. Collectively, these data suggest that IbpA is a bona fide member of the sHsps family. The immune-electron microscopy data using specific antibodies against IbpA have revealed different intracellular localization of this protein in A. laidlawii cells upon heat shock, which suggests that IbpA not only may participate in the stabilization of individual polypeptides, but may also play a protective role in the maintenance of various cellular structures upon temperature stress.
Keywords: Mycoplasmas, Acholeplasma laidlawii, Heat shock proteins, Oligomeric structures, Chaperone function, Immune-electron microscopy
Introduction
Heat shock proteins (Hsps) exert various functions in the cell, of which the most critical one is to protect and restore the structure of proteins upon heat shock. The vast majority of α-crystallin-type small Hsp family proteins (sHsps) have at least three properties in common. They (1) contain the α-crystallin domain in their amino acid sequence and (2) spontaneously form oligomers, and (3) oligomeric dynamics are important for their chaperone function. Under different kinds of stress, including heat shock, sHsps bind to polypeptide chains of target proteins, preventing their aggregation (Horwitz 1992). Unlike the GroEL and DnaK proteins, sHsps act in an ATP-independent manner (Jakob et al. 1993). sHsps appear to play a critical role in the regulation of cell membrane fluidity, as they are able to stabilize the bilayer liquid–crystal state and to sustain the membrane integrity during thermal fluctuations (Horváth et al. 1998; Tsvetkova et al. 2002). It was also shown that sHsps were able to interact with different enzymes (Ehrnsperger et al. 1997) and cytoskeleton proteins (actin, tubulin, intermediate filaments) (Nicholl and QuinIan 1994; Mounier and Arrigo 2002; Day et al. 2003), and protect them from denaturation under different types of stress. It is likely that sHsps are integrated into the multi-chaperone network that strictly controls the quality of cell proteins (Narberhaus 2002; Wong and Houry 2004).
Very little is known about Hsps and their regulation in mycoplasmas (class Mollicutes). Our earlier studies on Acholeplasma laidlawii cells have revealed an accumulation of several proteins upon heat shock (Borchsenius et al. 1990). Subsequently, these proteins were identified as various Hsps. The 72-kDa protein was identified as chaperone DnaK (bacterial homologue of a human Hsp70), and the 65- and 10-kDa proteins were established as orthologues of bacterial chaperones GroEL and GroES, respectively (Vonskii et al. 1993). We also noticed an increased level of the 17-kDa protein in the heat shock-treated acholeplasma cells (up to 7.2% of the total acholeplasma protein), whose identity was not immediately clear. The protein was tentatively named as p17. We hypothesized that p17 belongs to the family of α-crystallin-type Hsps.
In the present study, we have verified experimentally that the 17-kDa protein indeed belongs to sHsps. Actually, the presence of functionally active sHsp in Mollicutes is demonstrated at the first time. Furthermore, the localization of this protein in A. laidlawii cells before and after heat shock has been determined, and important ramifications of these results are discussed.
Materials and methods
Bacterial strains and plasmids
A. laidlawii PG8 (Cell Culture Collection of the Institute of Cytology, RAS) was cultivated in the modified PPLO broth with 10% horse serum (Freundt 1983). Cells of Escherichia coli BL21(DE3)pLysS strain (Novagen, USA) were grown in LB media supplemented with chloramphenicol, 25 μg/ml, and ampicillin, 100 μg/ml, when they were transformed with plasmid pibpA (pET-15b vector, Novagen, USA).
Identification and analysis of p17 full-length amino acid sequence
The search for an orf that corresponds to a gene encoding p17 protein (IbpA) was performed in the genome of A. laidlawii recently sequenced in full (GenBank: CP000896; Lazarev et al. 2011). A check for conservative domains and motifs within p17 amino acid sequence was done by using the Conserved Domain Database on NCBI server (www.ncbi.nlm.nih.gov). The search for homologous sequences was performed in EMBL, GenBank, and SWISS-PROT databases by using BLAST and PROSITE. Multiple alignments were done with BLAST.
Cloning of ibpA gene
To amplify the full-size ibpA gene for direct cloning into pET-15b vector, the following oligonucleotide primers (Lytech, Russia) were designed and synthesized: 5′ TTCTTATCATggattcTTAAGTTC 3′ (Tm = 44.3°C) and 5′ AGTGTAAAAcatatgTTGAGTTTATTG 3′ (Tm = 44.7°C) (the sites for BamHI and NdeI are indicated by lowercase letters). Amplification was carried out with Taq polymerase (Lytech, Russia). A. laidlawii genomic DNA was used as template, and PCR was performed for 30 cycles: 10 s, 93°C; 10 s, 44°C; and 10 s, 72°C. The amplified fragment of expected length, 411 bp, was cloned into pET-15b vector according to the pET System Manual. The enzymes were BamHI, NdeI, and DNA ligase of phage T4 (Fermentas, Lithuania). The insert was verified by sequencing, and the resulting plasmid was called pibpA.
Expression and purification of recombinant IbpA
The E. coli BL21(DE3)pLysS cells were transformed with 6His-IbpA expressing plasmid. The protein expression was induced by addition of 0.1 mM of isopropyl-β-D-1-thiogalactopyranozide (IPTG) to the exponentially growing culture (OD600, ∼0.8) followed by incubation of the culture for 2 h. Cells were harvested by centrifugation, resuspended in 1/10 culture volume of buffer A (Tris–HCl, pH 7.4; 300 mM NaCl; 20 mM imidazole), and lysed by ultrasonic treatment at 22 kHz. The resulting cellular extract containing 6His-IbpA was clarified at 40,000 rpm for 45 min. The supernatant was loaded onto the Ni-sepharose column. The recombinant IbpA was eluted with the same buffer, supplemented with 300 mM imidazole. The resulting prep was further purified by gel filtration on the AKTA FPLC chromatography system using Superdex-200 PrepGrade Tricorn 10/300 column (GE Healthcare, USA) equilibrated with phosphate-buffered saline (PBS). All stages of the protein synthesis and purification were controlled by PA gel electrophoresis (PAGE).
Raising of polyclonal antibodies
The recombinant IbpA was used to raise antiserum in rabbits. Immunization of rabbits was carried out according to the method described in Ivanov and Fel (1984). The resulting immune serum was loaded onto the native protein A-sepharose column (GE Healthcare, USA) and washed by 20 mM NaH2PO4 (pH 7.0). The immunoglobulins were eluted with 0.1 M glycine (pH 3.0). To prevent the loss of immunoglobulins in the sediment, 1 M Tris–OH was added in the collected fraction to shift the pH to neutral values. Then polyclonal antibodies were transferred to PBS by gel filtration on Superdex-200 PrepGrade Tricorn 10/300 column.
Immunoblotting
Proteins were separated by 15% SDS-PAGE (Laemmli 1970) and transferred to nitrocellulose Hybond C (GE Healthcare, USA). The membrane was blocked with 3% skim milk in PBS for 30 min. The antibodies against IbpA were diluted 1:1,000. The secondary antibodies (goat anti-rabbit antibodies conjugated with horseradish peroxidase, Sigma, USA) were diluted 1:10,000. The membrane was incubated with primary and secondary antibodies for 1 h, and after each procedure, it was carefully washed with PBS. Blots were stained with DAB (Sigma, USA).
Heat shock
Induction of IbpA synthesis in A. laidlawii cells was mediated by switching temperature at exponential growth phase from 30°C up to 42°C for 90 min followed by incubation of culture at 37°C, 90 min. These growth conditions correspond to maximal accumulation of IbpA.
Size-exclusion characterization of IbpA-specific oligomeric forms
The size of IbpA oligomers was determined at room temperature and 43°C by gel filtration on Superdex-200 PrepGrade Tricorn 10/300 column using the AKTA FPLC system. The void volume of the column was estimated at 6 ml. The following protein molecular mass markers (GE Healthcare, USA) were used to calibrate the column: thyroglobulin (669 kDa), ferritin (401 kDa), phosphorylase b from rabbit muscle (97 kDa), egg albumin (45 kDa), and α-lactalbumin (14 kDa). The flow rate was 1 ml/min, and the protein elution was monitored by absorbance at 280 nm.
Evaluation of IbpA chaperone activity in vitro
Bovine insulin (kindly provided by Prof. K.K. Turoverov, Institute of Cytology, RAS) as a model substrate and egg albumin (both Sigma, USA) as a control were used in final concentrations 0.45 mg/ml (80 μM) and 0.5 mg/ml (11 μM), correspondingly. Dithiothreitol was added (final concentration, 1 mM) for destruction of disulfide bonds between the chains of insulin. Experiments were carried out at 25°C and 43°C for 30 min in a total volume of 100 μl (PBS 1×, pH 7.0). Samples (1 μl) were taken every 3 min, and light scattering measurements were done with NanoDrop 2000 (Thermo Scientific, USA) at 360 nm. Each experiment was repeated three times, and the statistical analysis was performed in Excel.
Immune-electron microscopy
A. laidlawii cells were fixed by addition of formaldehyde and glutaraldehyde to final concentration 2% and 0.1–0.2%, correspondingly. Cells were fixed at room temperature for 12 h and were collected by centrifugation (10,000 rpm, 10 min). The pellet was dehydrated with ethanol in increasing concentrations and embedded into LR-White resin (Polyscience, Inc., USA). LR-White was polymerized at 50°C. Immunocytochemistry was carried out on ultrathin sections according to the standard technique (Mironov et al. 1994). IbpA-specific antibodies were used in 1:50 dilutions in PBS supplemented with 0.1% BSA. To visualize the distribution of the IbpA protein, goat anti-rabbit antibodies conjugated with 15-nm colloid gold particles (EY Laboratories, Inc., USA) were used. Sections were stained with uranyl acetate and lead citrate and examined using JEM-100U electron microscope. Preparations lacking polyclonal antibodies or incubated with rabbit pre-immune serum served as negative controls.
Negative staining
The IbpA protein was negatively stained on grids coated with collodion films that were discharged in UV prior to application of 5 μl of IbpA solution (10 μg/ml protein in PBS, pH 7.4). The protein solution was blotted off after 2 min, immediately followed by adding and blotting off after 1 min of 5 μl 2% aqueous uranyl acetate. Negatively stained grids were visualized in a Libra 120 electron microscope (Zeiss, Germany) at 50,000 magnification.
Results and discussion
Identification of p17 and analysis of its amino acid sequence
The first 15 amino acids of p17 (MLSLLNKNRSFFDXF; X—unknown residue) were identified previously by extraction of p17 from polyacrilamide gel and subsequent amino sequencing of its N-terminus (Vonskii 2001). We found the almost identical sequence MLSLLNKNRSFFDDF in the predicted product of the only one orf from A. laidlawii genome (recently sequenced in full), ACL_0421. The calculated molecular mass of the full-length product of ACL_0421 is 15.2 kDa. It roughly corresponds to the electrophoretically determined molecular mass of p17. Thus, the predicted product of ACL_0421 with high probability is p17.
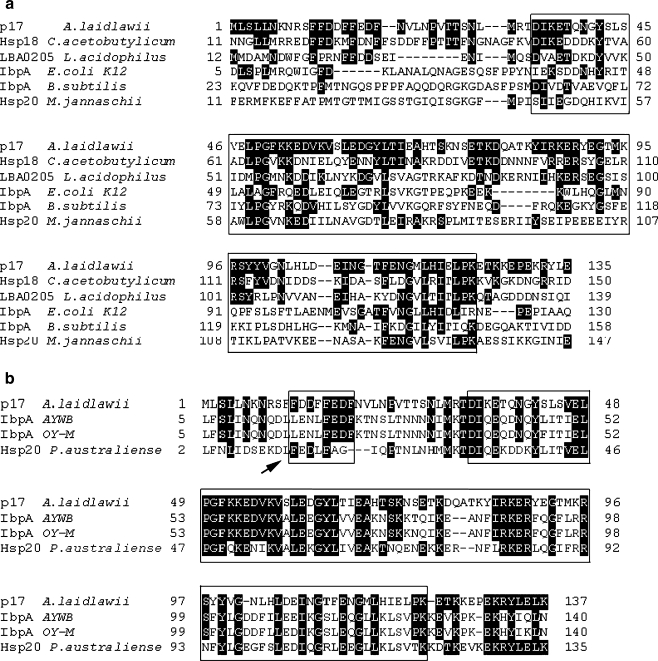
The amino acid sequence of p17 was checked for presence of conservative regions. At the middle of the p17, the so-called α-crystallin domain was determined. This domain consists of 90 amino acid residues and was flanked by 33 N-terminal and 14 C-terminal residues (Fig. 1a). Furthermore, a putative (WDPF) motif was found in the N-terminus of p17 (Fig. 1b). WDPF motifs are assumed to be involved in oligomerization of sHsps (Lambert et al. 1999).
Fig. 1.
Comparison of p17 A. laidlawii with eubacterial sHsps, Hsp20 Methanococcus jannaschii (a), and three predicted α-crystallin-type proteins from Mollicutes (b). AYWB Aster yellows witches’-broom phytoplasma, OY-M Onion yellows phytoplasma (Candidatus Phytoplasma asteris str. AYWB and OY-M). The identical amino acid residues are marked with black. α-Crystallin domain and a putative WDPF motif are framed (a, b). In addition, the WDPF motif is indicated with an arrow (b)
The search for genes and proteins, homologous to p17 of A. laidlawii, was performed in all mycoplasma genomes sequenced to date. We found three predicted proteins with 44% (IbpA AYWB—Aster yellows witches’-broom phytoplasma), 45% (IbpA OY-M—Onion yellows phytoplasma), and 41% (Hsp20 Candidatus Phytoplasma australiense) similarity to p17 A. laidlawii (Fig. 1b). All three proteins belong to phytoplasmas (Acholeplasmataceae), which are in close relationship with mycoplasma A. laidlawii. According to the authors' opinion, the predicted proteins can serve as molecular chaperones (Oshima et al. 2004; Bai et al. 2006; Tran-Nguyen et al. 2008). Additionally, we found only a few of predicted open reading frames encoding proteins with very weak similarity to p17 of A. laidlawii in other mycoplasma genomes. Among them, a putative ABC transporter (Mycoplasma synoviae), putative σ-factor RpoD (Mycoplasma alligatoris and Mycoplasma pulmonis), factor RbfA (M. pulmonis), and hypothetical proteins with unknown functions: MAGa7990 (Mycoplasma agalactiae) and HF1_03290 (Mycoplasma haemofelis). Since p17 has a high level of similarity with predicted IbpA proteins of phytoplasmas AYWB and OY-M, we renamed it as IbpA.
Evaluation of polyclonal antibodies against the recombinant IbpA protein
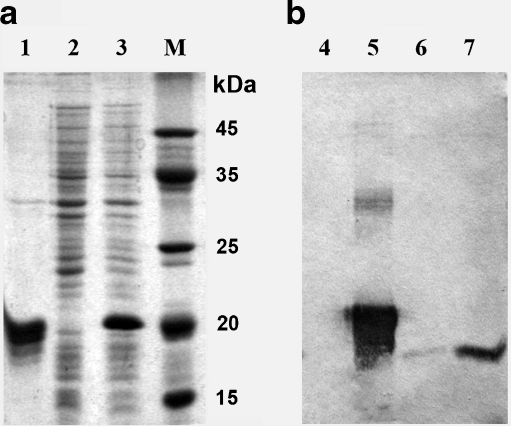
To investigate the functional properties of the IbpA protein in A. laidlawii cells, we decided to generate polyclonal antibodies against this protein in rabbits. To this end, we have generated a vector expressing 6His-tagged version of IbpA protein in E. coli. As shown in Fig. 2a (lane 3) a polypeptide with the molecular mass of about 20 kDa appears in the cell extract of E. coli transformed with IbpA expressing plasmid. The recombinant IbpA was isolated from the cell extract by metal chelate chromatography followed by gel filtration (Fig. 2a, lane 1). The molecular mass of this recombinant protein was defined experimentally (20 kDa) and closely matched the predicted one (19.65 kDa), composed of the full-length IbpA sequence (15.2 kDa) fused in frame with the translated region (4 kDa) of pET-15b, which contains the “histidine tail.” The specificity of the purified polyclonal antibodies was verified by immunoblotting. As seen from Fig. 2b, the antibodies recognize both native (lanes 6, 7) and recombinant (lane 5) IbpA proteins. Importantly, the level of IbpA protein in acholeplasma cells strongly increased after heat shock.
Fig. 2.
Validation of the polyclonal antibodies against the recombinant protein IbpA of A. laidlawii. a Coomassie staining of various samples of E. coli expressing IbpA: 1 the purified recombinant protein IbpA (19.65 kDa), 2 cell extract of E. coli BL21 (DE3)pLysS, 3 cell extract of E. coli, transformed by pibpA, + IPTG. b Specificity of the polyclonal rabbit antisera against IbpA: 4 cell extract of E. coli; 5 purified recombinant protein IbpA; 6 cell extract of A. laidlawii, normal conditions of growth; 7 the same, after heat shock. Lines 6 and 7 show single clear bands that correspond to native IbpA (15.2 kDa)
Protein oligomers, spontaneously formed by IbpA subunits
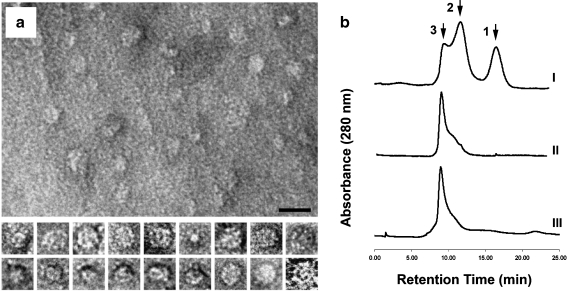
It is known that sHsps of many organisms, including plants, perform their chaperon function in the state of different oligomeric forms (Nakamoto and Vígh 2007). Probably, these forms exist in a dynamic equilibrium with each other. We have determined the molecular mass of the recombinant IbpA monomer to be around 20 kDa. It was interesting to test whether the recombinant IbpA protein is able to oligomerize. We have found that the monomers of IbpA spontaneously formed oligomers in solution at the concentration of 1 mg/ml in PBS at 4°C. High-resolution electron microscopy revealed that the sizes of the oligomers (globule projection) vary between 3 and 30 nm, most of them being about 15–20 nm in diameter (Fig. 3a). We have also evaluated the molecular mass of IbpA oligomers by gel filtration immediately after the protein purification. Three chromatographic peaks were detected (Fig. 3b, I). Peak 1 corresponds to a fraction of monomers or dimers (20–40 kDa), peak 2 represents some intermediate complexes that are formed during the oligomerization process (approx. 250 kDa), and peak 3 probably corresponds to a mature oligomeric form of IbpA (more than 400 kDa). When the gel filtration was repeated after 24 h after IbpA purification, the chromatographic pattern of the oligomers changed (Fig. 3b, II). There were no monomers, the intensity of peak 2 was noticeably decreased, and almost all the protein shifted to peak 3. Many of bacterial sHsps consist of 24 monomers, and all known sHsps use dimers as building blocks in their oligomer assemblies (Nakamoto and Vígh 2007). Taking in mind that our evaluation of the oligomer molecular masses is only approximate, we propose that the mature oligomeric form of IbpA may consist of 24 subunits. Similar oligomers (in size and image) were found in solution of the recombinant Hsp26 from yeasts (Haslbeck et al. 1999; White et al. 2006). The presence in solution of the purified recombinant IbpA oligomers of different size during the multimerization process (Fig. 3b, I and II) may be explained by the assumption that these sHsp forms are in a dynamic equilibrium with each other. It has been shown earlier that murine sHsp25 exists in a concentration-dependent equilibrium with tetramers and dimers, indicating that both types of particles are present during the assembly pathway, maintaining the substrate proteins in a folding-competent state (Ehrnsperger et al. 1997, 1999). Besides, it was established for Hsp26 from Saccharomyces cerevisiae that the sHsp dissociates to dimers when heated to 43°C (Haslbeck et al. 1999). We also tested the effect of heating (at 43°C) on the IbpA (Fig. 3b, III). However, the chromatographic pattern in this case was almost the same as on Fig. 3b, II. So, the heat treatment does not cause the dissociation of the IbpA oligomers.
Fig. 3.
Characterization of the protein oligomers spontaneously formed by IbpA subunits. a Electron micrographs of the oligomers, negative staining (1% aqueous uranyl acetate). At the upper part, a panoramic picture with the oligomers is displayed. The bar corresponds to 20 nm. At the lower part, representative examples of selected individual oligomers (approximately 15 nm in diameter each) exposed at different angles to the plane of a grid. b Evaluation of the oligomers molecular mass by gel filtration (1 × PBS, 1 ml/min). I Chromatographic pattern 10 min after purification of the recombinant IbpA from E. coli cell extract, II 24 h after purification (storage at +4°C), III the same prep under heat treatment (43°C). Peak 1 corresponds to a fraction of monomers or dimers (20–40 kDa), peak 2 represents some intermediate complexes that are formed during the oligomerization process (approx. 250 kDa), and peak 3, obviously, corresponds to a mature oligomeric form of IbpA (more than 400 kDa)
Chaperone activity of the IbpA in vitro
The results of a large number of experiments based on light scattering data have demonstrated the properties of sHsps as molecular chaperones; many of the sHsps efficiently protect proteins against heat denaturation and also against chemical denaturation at low temperatures in vitro. An example of such activity is the protective effect of sHsp on the insulin stability upon treatment with dithiothreitol (DTT) (Haslbeck et al. 1999).
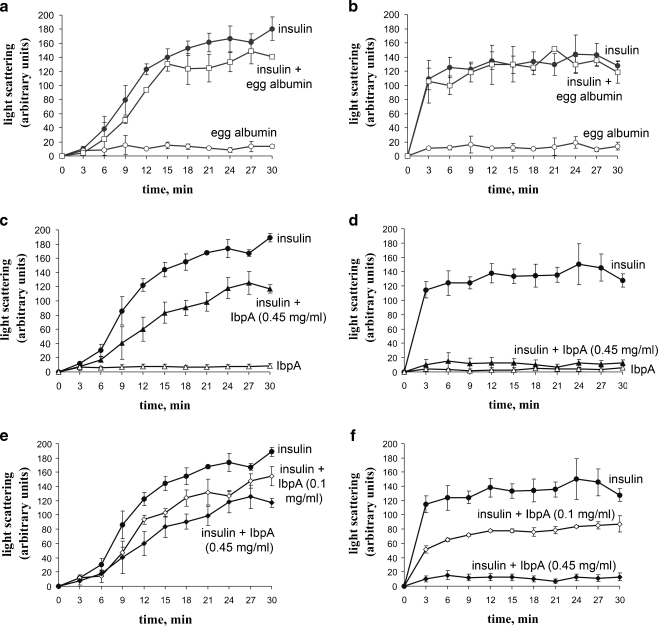
We studied the effect of the recombinant IbpA from A. laidlawii on the denaturation and aggregation of insulin at different temperatures, provoked by the addition of DTT to the reaction mixture. Pure insulin, egg albumin, and IbpA preps were used as control and insulin mixed with egg albumin or IbpA as experimental variable. Both IbpA and egg albumin did not aggregate at 25°C (Fig. 4a, c) and 43°C (Fig. 4b, d). At the same temperatures, egg albumin did not interfere with insulin denaturation and aggregation when they were mixed (Fig. 4a, b). On the contrary, at 43°C and a molar ratio close to 1:4, the recombinant IbpA protein completely prevented the aggregation of insulin (Fig. 4d), whereas at 25°C, IbpA had little effect on the process (Fig. 4c). Such a difference in the effect of IbpA and egg albumin on the aggregation of the model substrate indicates the presence of a specific temperature-dependent chaperone activity in the studied protein. In addition, the effect of IbpA on the aggregation of insulin depended on the concentration of the chaperone (Fig. 4e, f). At 43°C, this dependence appears to be much more pronounced (Fig. 4f). Chaperone activity of the IbpA before and after freeze-thawing was also checked, and the same level of the activities was detected in both cases (data not shown). Overall effect of the recombinant IbpA from A. laidlawii on the aggregation of insulin reminds that of Hsp26 from S. cerevisiae (Haslbeck et al. 1999). Hsp26 is a temperature-dependent chaperone, effective only at elevated temperatures. Similar to Hsp26 from yeast, IbpA from A. laidlawii showed a much higher level of activity in suppressing the aggregation of insulin at 43°C compared to 25°C. However, in our case, it was not related to the dissociation of oligomers to dimers (see previous section). More recently, on the same Hsp26, it was demonstrated that the rearrangements necessary for shifting this chaperone from a low to a high affinity state for binding non-native proteins occur also without dissolving the oligomer (Franzmann et al. 2005). So, we are not surprised with the results we obtained. It is believed that the increase in temperature causes structural changes in the complexes of sHsps accompanied by exposure to solvent of hydrophobic residues that influence the rate of exchange of subunits and are involved in the binding of partially denatured proteins (Raman et al. 1995).
Fig. 4.
Effect of recombinant IbpA on the aggregation of insulin in vitro. Proteins' preps were used in the following concentrations: insulin and egg albumin (both Sigma, USA)—0.5 mg/ml, IbpA—0.1 and 0.45 mg/ml. Experiments were performed at 25°C (a, c, e) or 43°C (b, d, f)
Localization of IbpA in A. laidlawii cells
Chaperones of the sHsp family are able to interact with many targets in the cell. To uncover the potential targets of Acholeplasma sHsp, IbpA, and its intracellular localization, we applied the immune-electron microscopy method using specific polyclonal antibodies against the IbpA protein. We know only two examples of such attempts to localize sHsps in prokaryotic cells (Lünsdorf et al. 1995; Cunningham and Spreadbury 1998). As to Mollicutes, there is the only one example of determined location of Hsp in the mycoplasma cell: HspA1 (Hsp70 analogue) of Mycoplasma suis (Hoelzle et al. 2007).
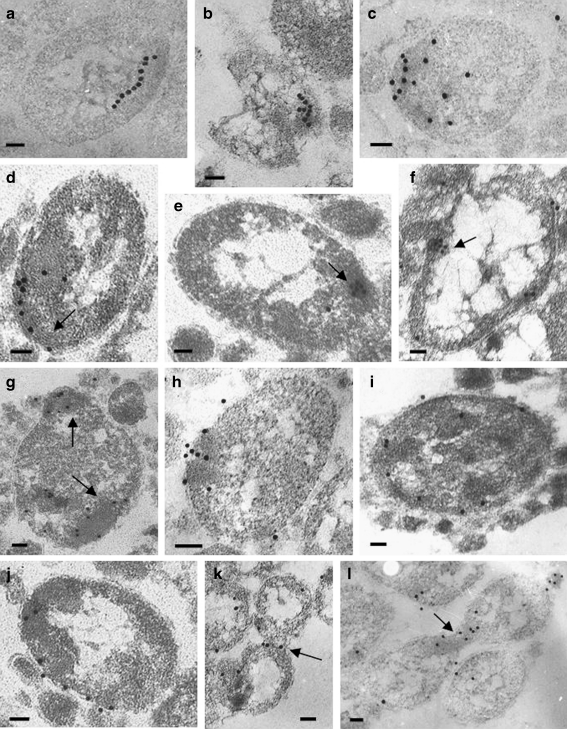
Examination of the cells grown under normal conditions revealed only a small amount of IbpA protein (not shown). On the contrary, in the cells subjected to heat shock, an intensive labeling was observed (Fig. 5). Forty percent of the label was dispersed in cytoplasm irregularly. However, it is known that sHsps can chaperone a variety of partially denatured proteins, including a number of enzymes. We reasoned that in the acholeplasma cell, such chaperoning activity may also occur, resulting in such irregular staining pattern. The other 60% of the label was associated with defined cell structures, and we conventionally distinguished four patterns of the label distribution.
Ten percent of these colloidal gold particles (and correspondingly IbpA molecules) were located along inner regular structures yet unidentified (Fig. 5a–f). Extended “chains” of label at the periphery of the cell (Fig. 5a), branched (Fig. 5b) and curved (Fig. 5c) “threads” of gold particles, located sometimes close to the cell poles, were also observed. Sometimes the structures were clearly visible. The label was associated with them in cases where the cuts were performed along (Fig. 5d) or across (Fig. 5e, f) the structures. A similar pattern of localization was described for the 16-kDa α-crystallin homolog from Mycobacterium bovis BCG (Cunningham and Spreadbury 1998). These authors proposed that sHsp interacted with the peptidoglycan layer of the mycobacterial cell. However, there is no peptidoglycan layer in mycoplasma cells because these organisms lack a cell wall. Earlier in the cells of strain A. laidlawii NCTC 10116, the elements resembling microtubules were found (Meloni et al. 1980). We have shown the presence of similar elements in the cells of strain A. laidlawii PG8, and in some cases, we did observe the label associated with these elements (Fig. 5d–f). Thus, it is tempting to speculate that the label indicates the association of IbpA with cytoskeleton-like intracellular structures. It also possible that IbpA protein might take part in the maintenance of these structures under stress conditions.
Sixty-five percent of the IbpA label was found in the electron-dense regions (Fig. 5g, h), so-called granular bodies; in some cases, the label seems to be organized in intracellular clusters. The clustering of sHsps in the cytoplasm was also reported in M. bovis (Cunningham and Spreadbury 1998) and in Stigmatella aurantiaca (Lünsdorf et al. 1995). The nature of the “granular bodies or granular regions” described in acholeplasma cells many years ago is yet unclear. One may hypothesize that electron-dense regions, termed “granular bodies,” represent aggregated proteins, as they are formed during heat stress. But it is not the case because similar structures are clearly seen in A. laidlawii cells under normal conditions. It was proposed that these regions could possibly be either a form of septum (because they are occasional found in the constriction area) or some kind of intracellular storage granule (Maniloff 1970). In any case, IbpA could participate in protection of contents of the “granular bodies” under stress.
Twenty percent of the label was visualized on membranes (Fig. 5i, j). This distribution of IbpA does not contradict its proposed role in sustaining the membrane integrity: sHsps are deemed to play the critical role in regulation of the cell membrane fluidity and are able to stabilize the bilayer liquid–crystal state during thermal fluctuations (Horváth et al. 1998; Tsvetkova et al. 2002). This is especially important in the case of the mycoplasma cell because of the lack of a cell wall. If the role of IbpA in sustaining the cell membrane integrity will be confirmed, then potentially it may be used as a target for suppression of plant diseases caused by Acholeplasmataceae.
We found some examples (5% of the label) of localization close to the constriction area of dividing cells (Fig. 5k, l). We hypothesize that IbpA may protect and stabilize some proteins involved in the septation process.
Fig. 5.
Localization of the IbpA protein in A. laidlawii cells. Pattern I: The label was visualized on: some regular structures at the periphery of a cell (a, b), on a cell pole (c), along the structures (probably, cytoskeleton-like) (d), across the structures (e, f). Pattern II: The label was observed at the electron-dense regions of the cell (g, h). Pattern III: The label was present mainly on the membranes (i, j). Pattern IV: The label is predominantly on constrictions of the dividing cells (k, l). Intracellular structures (d–f), electron-dense regions (g), and constrictions (k, l) are pointed by arrows. Bars correspond to 100 nm
In all the cases studied, little or no labeling was associated with the nucleoid area or chromosomal DNA. These data are in agreement with localization of other sHsps (Lünsdorf et al. 1995; Cunningham and Spreadbury 1998).
Our data strongly suggest that IbpA (p17) is a novel valid sHsp. A. laidlawii is the only known mycoplasma found in nature outside the host organism. This “ubiquitous” mycoplasma was isolated from soil, compost, and sewage, as well as from the tissues of humans, animals, and plants (Razin et al. 1998). Because of its independent existence, cells of A. laidlawii must adequately answer to rapid temperature changes. Probably, this feature of A. laidlawii is associated with the presence of the most complete HSP set compared to the other mycoplasmas, including the functionally active sHsp (IbpA). The patterns of the A. laidlawii IbpA protein localization suggest that it might have multiple targets in the cells and is probably involved in stabilization of different cell structures during the heat shock. Future experiments should reveal the identity of these structures and the function of IbpA in A. laidlawii cells.
Acknowledgments
We are grateful to Dr. D. Bobkov, Dr. M. Vonskii, and A. L. Runov for the creative assistance. The authors also thank Dr. I. Freedlanskaya and Dr. N. Barlev for their helpful comments on the manuscript.
References
- Bai X, Zhang J, Ewing A, Miller SA, Radek AJ, Shevchenko DV, Tsukerman K, Walunas T, Lapidus A, Campbell JW, Hogenhout SA. Living with genome instability: the adaptation of phytoplasmas to diverse environments of their insect and plant hosts. J Bacteriol. 2006;188:3682–3696. doi: 10.1128/JB.188.10.3682-3696.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchsenius SN, Budantseva EV, Vonsky MS. The heat-shock proteins of Acholeplasma laidlawii. In: Stanek G, Cassell G, Tully JG, Whitcomb RF, editors. Resent advances in mycoplasmology. Stuttgart: Gustav Fisher; 1990. pp. 657–658. [Google Scholar]
- Cunningham AF, Spreadbury CL. Mycobacterial stationary phase induced by low oxygen tension: cell wall thickening and localization of the 16-kilodalton α-crystallin homolog. J Bacteriol. 1998;180:801–808. doi: 10.1128/jb.180.4.801-808.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day RM, Gupta JS, MacRae TH. A small heat shock/α-crystallin protein from encysted Artemia embryos suppresses tubulin denaturation. Cell Stress Chaperones. 2003;8:183–193. doi: 10.1379/1466-1268(2003)008<0183:ASHCPF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Gräber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrnsperger M, Lilie H, Gaestel M, Buchner J. The dynamics of Hsp25 quaternary structure—structure and function of different oligomeric species. J Biol Chem. 1999;274:14867–14874. doi: 10.1074/jbc.274.21.14867. [DOI] [PubMed] [Google Scholar]
- Franzmann TM, Wuhr M, Richter K, Walter S, Buchner J. The activation mechanism of Hsp26 does not require dissociation of the oligomer. J Mol Biol. 2005;350:1083–1093. doi: 10.1016/j.jmb.2005.05.034. [DOI] [PubMed] [Google Scholar]
- Freundt E. Culture media for classical mycoplasmas. In: Rasin S, Tully JG, editors. Methods in mycoplasmology. New York: Academic; 1983. pp. 127–136. [Google Scholar]
- Haslbeck M, Walke S, Stromer T, Ehrnsperger M, White HE, Chen SX, Saibil HR, Buchner J. Hsp26: a temperature-regulated chaperone. EMBO J. 1999;18:6744–6751. doi: 10.1093/emboj/18.23.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzle LE, Hoelzle K, Harder A, Ritzmann M, Aupperle H, Schoon HA, Heinritzi K, Wittenbrink MM. First identification and functional characterization of an immunogenic protein in unculturable haemotrophic Mycoplasmas (Mycoplasma suis HspA1) FEMS Immunol Med Microbiol. 2007;49:215–223. doi: 10.1111/j.1574-695X.2006.00184.x. [DOI] [PubMed] [Google Scholar]
- Horváth I, Glatz A, Varvasovszki V, Török Z, Páli T, Balogh G, Kovács E, Nádasdi L, Benkö S, Joó F, Vigh L. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci USA. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. α-Crystallin can function as a molecular chaperone. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VA, Fel VJ. On some similarity between membrane antigens of the cell of Zajdela hepatoma and liver of rats subjected to a single 4-dimethylaminoazobenzene injection. Neoplasma. 1984;27:745–750. [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J Biol Chem. 1999;274:9378–85. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- Lazarev VN, Levitskii SA, Basovskii YI, Chukin MM, Akopian TA, Vereshchagin VV, Kostrjukova ES, Kovaleva GY, Kazanov MD, Malko DB, Vitreschak AG, Sernova NV, Gelfand MS, Demina IA, Serebryakova MV, Galyamina MA, Vtyurin NN, Rogov SI, Alexeev DG, Ladygina VG, Govorun VM (2011) Complete genome and proteome of Acholeplasma laidlawii. J Bacteriol. 193:4943–4953 [DOI] [PMC free article] [PubMed]
- Lünsdorf H, Schairer HU, Heidelbach M. Localization of the stress protein SP21 in indole-induced spores, fruiting bodies, and heat shocked cells of Stigmatella aurantiaca. J Bacteriol. 1995;177:7092–7099. doi: 10.1128/jb.177.24.7092-7099.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniloff J. Ultrastructure of Mycoplasma laidlawii during culture development. J Bacteriol. 1970;102:561–572. doi: 10.1128/jb.102.2.561-572.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meloni GA, Bertoloni G, Busolo F, Conventi L. Colony morphology, ultrastructure and morphogenesis in Mycoplasma hominis, Acholeplasma laidlawii and Ureaplasma urealyticum. J Gen Microbiol. 1980;116:435–443. doi: 10.1099/00221287-116-2-435. [DOI] [PubMed] [Google Scholar]
- Mironov AA, YaYu K, Mironov VA. Methods of electron microscopy in biology and medicine. Saint-Petersburg: Nauka; 1994. [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7:167–176. doi: 10.1379/1466-1268(2002)007<0167:ACASHS>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto H, Vígh L. The small heat shock proteins and their clients. Cell Mol Life Sci. 2007;64:294–306. doi: 10.1007/s00018-006-6321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F. α-Crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholl ID, QuinIan RA. Chaperone activity of α-crystallins modulates intermediate filament assembly. EMBO J. 1994;13:945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Kakizawa S, Nishigawa H, Jung HY, Wei W, Suzuki S, Arashida R, Nakata D, Miyata S, Ugaki M, Namba S. Reductive evolution suggested from the complete genome sequence of a plant-pathogenic phytoplasma. Nat Genet. 2004;36:27–29. doi: 10.1038/ng1277. [DOI] [PubMed] [Google Scholar]
- Raman B, Ramakrishna T, Rao CM. Temperature dependent chaperone-like activity of alpha-crystallin. FEBS Lett. 1995;365:133–136. doi: 10.1016/0014-5793(95)00440-K. [DOI] [PubMed] [Google Scholar]
- Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Kube M, Schneider B, Reinhardt R, Gibb KS. Comparative genome analysis of ‘Candidatus Phytoplasma australiense’ (subgroup tuf-Australia I; rp-A) and ‘Ca. Phytoplasma asteris’ strains OY-M and AY-WB. J Bacteriol. 2008;190:3979–3991. doi: 10.1128/JB.01301-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsvetkova NM, Horváth I, Török Z, Wolkers WF, Balogi Z, Shigapova N, Crowe LM, Tablin F, Vierling E, Crowe JH, Vígh L. Small heat-shock proteins regulate membrane lipid polymorphism. Proc Natl Acad Sci USA. 2002;99:13504–13509. doi: 10.1073/pnas.192468399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonskii MS (2001) Heat shock proteins of mycoplasmas: cloning and expression of dnaK gene. PhD Thesis (in Russian) 121
- Vonskii MS, Astvatsaturiants GV, Borkhsenius SN. Expression of heat shock proteins in mycoplasma. Dokl Akad Nauk (in Russian) 1993;331:112–115. [PubMed] [Google Scholar]
- White HE, Orlova EV, Chen S, Wang L, Ignatiou A, Gowen B, Stromer T, Franzmann TM, Haslbeck M, Buchner J, Saibil HR. Multiple distinct assemblies reveal conformational flexibility in the small heat shock protein Hsp26. Structure. 2006;14:1197–1204. doi: 10.1016/j.str.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Wong P, Houry WA. Chaperone networks in bacteria: analysis of protein homeostasis in minimal cells. J Struct Biol. 2004;146:79–89. doi: 10.1016/j.jsb.2003.11.006. [DOI] [PubMed] [Google Scholar]