Abstract
Malaria is caused by Plasmodium species, whose transmission to vertebrate hosts is facilitated by mosquito vectors. The transition from the cold blooded mosquito vector to the host represents physiological stress to the parasite, and additionally malaria blood stage infection is characterised by intense fever periods. In recent years, it has become clear that heat shock proteins play an essential role during the parasite's life cycle. Plasmodium falciparum expresses two prominent heat shock proteins: heat shock protein 70 (PfHsp70) and heat shock protein 90 (PfHsp90). Both of these proteins have been implicated in the development and pathogenesis of malaria. In eukaryotes, Hsp70 and Hsp90 proteins are functionally linked by an essential adaptor protein known as the Hsp70–Hsp90 organising protein (Hop). In this study, recombinant P. falciparum Hop (PfHop) was heterologously produced in E. coli and purified by nickel affinity chromatography. Using specific anti-PfHop antisera, the expression and localisation of PfHop in P. falciparum was investigated. PfHop was shown to co-localise with PfHsp70 and PfHsp90 in parasites at the trophozoite stage. Gel filtration and co-immunoprecipitation experiments suggested that PfHop was present in a complex together with PfHsp70 and PfHsp90. The association of PfHop with both PfHsp70 and PfHsp90 suggests that this protein may mediate the functional interaction between the two chaperones.
Keywords: Plasmodium falciparum, Molecular chaperone, Hsp70–Hsp90 organising protein, Hsp70, Hsp90
Introduction
Heat shock proteins occur in all known life forms, and their main role is to facilitate the folding of other proteins both under normal and stressful conditions. Consequently, some heat shock proteins are induced by cellular stress, thus protecting cells against adverse effects. The role of heat shock proteins in the survival of intracellular parasites extends beyond maintenance of proteostasis, as they are also implicated in the development and pathogenesis of these organisms (reviewed by Shonhai et al. (2011). The aetiological agent of malaria tropica, Plasmodium falciparum, develops and thrives under physiologically divergent conditions throughout its life stages. Heat shock proteins are thought to play an important role in the development of P. falciparum as well as other human parasites (Sharma 1992; Shonhai et al. 2007; Shonhai et al. 2011).
Heat shock protein 70 (Hsp70) and Hsp90 are some of the most studied molecular chaperones, proteins which themselves are responsible for the folding of other proteins in the cell. Hsp70 binds non-native proteins whilst substrates of Hsp90 are usually in native-like forms (Wegele et al. 2006). Proteins that require both Hsp70 and Hsp90 to fold are thus transferred from Hsp70 to Hsp90 during the folding process. Eukaryotic Hsp90 participates in the conformational regulation of signal transduction molecules, such as tyrosine kinases and steroid hormone receptors. For example, steroid hormone receptors associate with Hsp90 in order for them to adopt conformational competence for hormone binding (Dittmar and Pratt 1997).
In eukaryotes, the essential interaction between Hsp70 and Hsp90 is mediated by the Hsp70–Hsp90 organising protein (Hop; Nicolet and Craig 1989). Both Hsp70 and Hsp90 possess C-terminally located EEVD motifs that interact with Hop via its tetratricopeptide repeat (TPR) domains, TPR1 and TPR2A motifs, respectively (Scheufler et al. 2000). It is most likely that the Hsp70–Hsp90 functional partnership in Plasmodium spp. facilitates the folding of key proteins in the parasite cell, possibly those involved in signal transduction. PfHsp90 is known to play an essential role in the survival of the parasite and the antibiotic geldanamycin is known to inhibit its function (Banumathy et al. 2003).
Of the six Hsp70-like proteins encoded by the P. falciparum genome, only the cytosol-nuclear localised chaperone, PfHsp70 possess the EEVD motif (Shonhai et al. 2007) that is crucial for interaction between Hsp70 and Hop. PfHsp90 occurs in the cytosol and migrates to the nucleus in response to heat stress (Acharya et al. 2007). Thus, PfHsp70 and possibly PfHsp90 possibly cooperate in the parasite cell. Although a Hop homologue (PF14_0324) has been identified in the P. falciparum genome (Acharya et al. 2007), its function has not been characterised. Banumathy et al. (2003) observed the occurrence of PfHsp90 and PfHsp70 in functional units that were complexed to two other species of proteins with a molecular mass of around 50 and 60 kDa, respectively. Although not directly verified by experimental data, the authors proposed that the two proteins associating with PfHsp70 and PfHsp90 were P. falciparum cyophilin and PfHop. In addition, at least one TPR-rich protein, PP5 phosphatase from P. Falciparum, has been shown to interact with PfHsp90 (Dobson et al. 2001; Kumar et al. 2003).
So far. there is no direct experimental evidence for the existence of a functional PfHsp70–PfHsp90 partnership. However, the presence of PfHsp90 and PfHsp70 in common cellular compartments, coupled to data based on predictive bioinformatics suggest that the two chaperones may functionally interact through a PfHop-mediated pathway (Pavithra et al. 2004). The possible existence of a Hop-mediated Hsp70–Hsp90 partnership in P. falciparum is important, given the essential roles of these proteins. Indeed, this pathway has been proposed as a potential anti-malarial drug target (Pesce et al. 2010; Shonhai 2010). The aim of the current study was to investigate the role of PfHop as a potential mediator of the PfHsp70–PfHsp90 pathway. We cloned, expressed and purified recombinant PfHop protein to facilitate its further characterisation. We further analysed the cellular localisation of PfHop and its association with PfHsp70 and PfHsp90.
Materials and methods
Bioinformatics
To map out the TPR domains in PfHop, Clustal W alignments of Hop homologues from Plasmodium species, human, mouse and yeast were performed utilizing the Bioedit program version 7.0.5.3 (Hall 1999). A three-dimensional model of PfHop was generated by SWISS-MODEL (Arnold et al. 2006; Guex and Peitsch 1997; Schwede et al. 2003). The images were subsequently visualised using the PyMol molecular graphics system, version 0.99rc6 (DeLano 2002).
Peptide-directed PfHop and PfHsp90 antibody design and synthesis
Anti-peptide antibodies specific to PfHop (PlasmoDB accession number: PF14_0324) and PfHsp90 (PlasmoDB accession number, PF07_0029) were generated commercially (Eurogentec) by immunisation of rabbits with the following synthetic peptides: TGEGNDAEERQRQQR, corresponding to amino acids 195–206 of the PfHop sequence and a mixture of two peptides (CIRYESITDTQKLSAE and CPKRAPFDM FENRKKR), corresponding to amino acids 45–59 and 364–377 of PfHsp90, respectively.
Cloning of PfHop, over-expression and purification of PfHop
The coding sequence of PfHop (PlasmoDB accession number: PF14_0324) was PCR amplified from 3D7 gDNA using forward primer (5′-TGCATGCATGGTTAACAAAGAAGAAGCTC-3′) with a SphI site (in bold) and reverse primer (5′-TCTGCAGTTATCGTACCTTCAATATTCCAGC-3′) with a PstI site (in bold) and inserted into the SphI/PstI sites of pQE30 (Qiagen). Plasmid integrity was verified by restriction digest and automated DNA sequencing.
To facilitate purification of PfHop recombinant protein, the protein was first overexpressed in E. coli XL1 Blue cells. The E. coli XL1 Blue cells were first transformed with pQE30/PfHop plasmid. Subsequently, one isolated recombinant colony from the YT agar plate was inoculated to YT broth containing 100 μg/ml ampicillin (Roth) followed by incubation at 37°C shaking incubator. At OD600 of 0.6, the cells were induced with 1 mM isopropyl-β-d-1-thiogalactopyranoside [IPTG] (Roth). Five hours after induction, the cells were harvested by centrifugation at 5,000 g for 20 min at 4°C. They were then re-suspended in non-reducing lysis buffer (300 mM NaCl, 10 mM imidazole, 10 mM Tris, pH 8.0, 1 mM phenylmethylsulphonyl fluoride [PMSF], and 1 mM lysozyme). Cell lysis was allowed to proceed for 20 min at room temperature (22°C), after which the lysate was frozen at −80°C, overnight. The cells were then thawed by mild sonication at amplitude setting of 50 for 7 cycles with 15-second pulse and five-second rest after each cycle. Soluble cell extract was derived after clarification by centrifugation at 5,000 g for 30 min at 4°C. The His-tagged PfHop protein that was in the soluble cell extract was allowed to bind to nickel-charged sepharose beads at 4°C for 4 h. The beads were washed using wash buffer (10 mM Tris, pH 7.5, 300 mM NaCl, 50 mM imidazole), and PfHop recombinant protein was then eluted using elution buffer (10 mM Tris, pH 7.5, 300 mM NaCl, 1 M imidazole). The eluted protein was extensively dialysed at 4°C against a storage buffer (10 mM Tris, pH 7.5, 300 mM NaCl, 50 mM imidazole, 0.8 mM DTT and 10% glycerol). The protein was subsequently concentrated with polyethylene glycol.
Construction of plasmid expressing PfHop-GFP
The PfHop coding sequence was amplified by PCR from gDNA (clone 3D7) using forward primer (5′-GGCTCGAGATGGTTAACAAAGAAGAAGCTCAGAG-3′) with a XhoI site (in bold) and reverse primer (5′-GGCCTAGGTCGTACCTTCAATATTCCAGC-3′) with a AvrII site (in bold). The successful isolation and amplification of the PCR product were confirmed by agarose gel electrophoresis. The PCR product was recovered by gel purified and restricted with XhoI and AvrII. The PfHop encoding segment was inserted into the pARL-2-GFP vector (Przyborski et al. 2005) between XhoI and AvrII restriction sites located in the multiple cloning site. The product was inserted in frame with a downstream GFP coding sequence to generate pARL-2-GFP/PfHop plasmid construct. Diagnostic restriction digestions using XhoI and AvrII were conducted to confirm the integrity of the pARL-2-GFP/PfHop plasmid construct. The plasmid was also sequenced to confirm that the PfHop encoding segment was inserted in frame with the GFP encoding sequence.
Parasite culture and preparation of parasite fractions
P. falciparum 3D7 cells were cultured under standard conditions (Przyborski et al. 2005; Trager and Jensen, 1976). Sychronised parasites were harvested using Gelafundin flotation at the trophoite stage. Trophozoite-infected erythrocytes were subsequently washed in PBS (pH 7.4) and lysed using saponin (0.1%). Lysis was allowed to proceed on ice for 6 min with constant gentle mixing to facilitate the lysis. Parasites were collected by centrifugation at 36,000 g for 30 min at 4°C followed by an extensive wash step using PBS (pH 7.4). The parasite lysates were subjected to Western analysis to confirm the expression of PfHop. A host cell protein, glycophorin was also probed as a loading control. Images were acquired using X-ray film (Fuji super RX).
Immunofluorescence assays
Immunofluorescence assays to investigate the localisation of PfHop and PfHop-GFP in parasite-infected erythrocytes were carried out as previously described (Spork et al. 2009). Following fixation of parasite-infected red blood cells, localisation was examined using anti-PfHop primary antibody (purified IgG fraction, 1:100; this study), polyclonal anti-PfHsp70 primary antibodies (1:1,000; Pesce et al. 2008) or polyclonal rabbit anti-PfHsp90 antibody (purified IgG fraction 1:100; this study). After extensive washing steps, the erythrocytes were thereafter incubated with cy3-conjugated goat anti-rabbit IgG secondary antibodies (1:2,000; DAKO Hamburg, Germany) for 2 h at room temperature. The erythrocytes were observed with fluorescence microscope (cell observer) using a 63× oil immersion objective. The images were acquired using Axiovision software package version 4.8.2. Image J software was used for pseudo-colouring and for preparation of merged co-localisation images. Negative control samples of erythrocytes incubated with respective rabbit pre-immune serum and secondary antibody were used to validate the specificity of the localisation signal.
Size exclusion chromatography
Size exclusion chromatography was conducted as previously described (Pesce et al. 2008). Infected erythrocytes were suspended in PBS (pH 7.4) containing 0.15% (w/v) saponin for 10 min on ice followed by centrifugation at 5,000 g for 5 min. The pellet fraction of the parasite was washed three times with PBS (pH 7.4) followed by resuspension in 50 mM Tris, 150 mM NaCl (pH 7.4) including 1 mM PMSF and protease inhibitor cocktail (Pierce) and three freeze–thaw cycles. The parasite lysate was centrifuged at 20,000 g for 20 min at 4°C to separate soluble parasite lysate and cell debris. An aliquot containing 400 μg of total parasite lysate was injected into a 24-ml Superdex 200 column (GE Healthcare). Fractions of parasite cell lysate of 500 μl were recovered automatically. The proteins contained in the fractions were precipitated with 10% (w/v) trichloroacetic acid (TCA) on ice for 30 min and centrifuged. The protein pellet was subjected to Western analysis using anti-PfHsp90, anti-PfHsp70, and anti-PfHop antibodies. The secondary antibody used was HRP-conjugated goat anti-rabbit IgG (Dako, Germany, 1:1:2,000).
Immunoprecipitation analysis
Parasites released by saponin lysis were resuspended in IP-lysis buffer (150 mM NaCl, 50 mM Tris–HCl, 0.1% NP-40, 1 mM PMSF, and protease inhibitor cocktail (Pierce)), and subjected to three freeze/thaw cycles. A soluble supernatant was collected by centrifugation (4°C, 36,000 × g). Anti-PfHsp70 antisera were added and the sample split into two aliquots. One aliquot was adjusted to 5 mM ATP and the other received no extra ATP. Antibody binding was allowed to occur overnight at 4°C on a rocking platform. Following this, protein A/G beads (Pierce) were added, and binding was allowed to proceed for 1 h. The immunoprecipitate was then collected by centrifugation at 1,000 × g for 5 min at 4°C and the pellet washed extensively with PBS (pH 7.4) before preparation of the samples for SDS-PAGE and Western analysis. Specificity of binding was controlled by monitoring binding to control agarose beads.
Results
Mapping of the TPR domains in PfHop
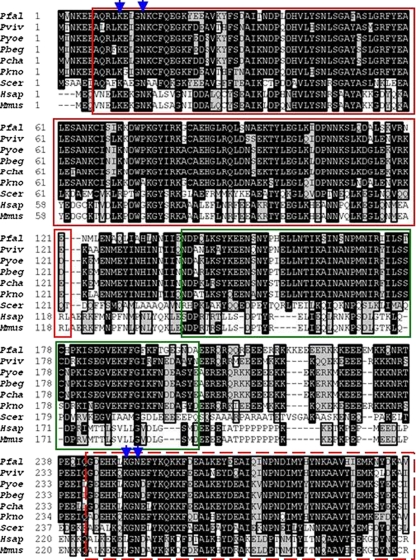
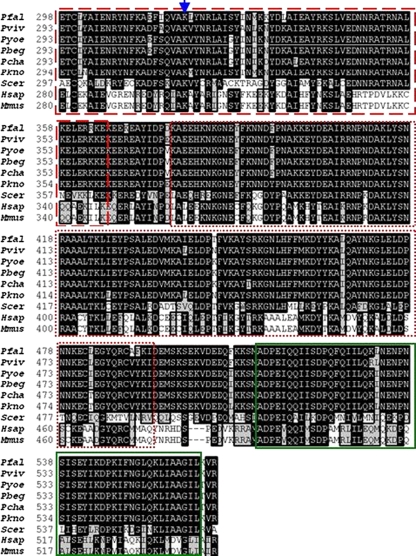
Based on the Clustal W alignments conducted, PfHop possesses three TPR domains: an N-terminal TPR1 domain composed of residues 7–121, a centrally located TPR2A, at position 243–365 and a C-terminal TPR2B, at position 378–494 (Fig. 1). Residues that are known to be important in the interaction of the TPR domains of mouse Hop with either Hsp70 or Hsp90 (Odunuga et al. 2003) are also conserved in PfHop (Fig. 1). Thus, based on sequence alignment data, PfHop presents itself as a typical member of these TPR-rich proteins. Although, Hop proteins whose sequences were analysed appear generally conserved across species, it is interesting to note that there is relative variation between plasmodial Hop proteins in comparison to the human, yeast and mouse homologues even within important functional domains such as the TPR segments and DP motifs (Fig. 1; Carrigan et al. 2005). DP motifs are known to be important in the global fold of Hop and their disruption has been found to impair Hop function (Nelson et al. 2003).
Fig. 1.
Multiple sequence alignment of plasmodial, yeast and mammalian Hop homlogues. Multiple sequence alignment of TPR domains of Hop homologues from Pfal (Plasmodium falciparum, PlasmoDB accession number, PF14_0324), Pviv (Plasmodium vivax, NCBI accession number, XP_001616631.1), Pkno (Plasmodium knowlesi, NCBI accession number, XP_002260669.1), Pcha (Plasmodium chabaudi, NCBI accession number, XP_745506.1), Pber (Plasmodium berghei, NCBI accession number, XP_677465.1), Pyoe (Plasmodium yoelii, NCBI accession number, XP_731105.1), Hsap (Homo sapiens, NCBI accession number, NP_006810.1), Mmus (Mus musculus, NCBI accession number, BC003794.1), and Scer (Saccharomyces cerevisiae, NCBI accession number, CBZ50259.1). TPR domains are highlighted as follows: TPR1 (red solid box), TPR2A (red dashed box) and TPR2B [red dotted box] (Scheufler et al. 2000). Amino acids that are implicated in TPR1–PfHsp70 and TPR2A–PfHsp90-1 interaction are indicated by blue arrows (Scheufler et al. 2000; Odunuga et al. 2003; 2004). Residues constituting DP1 and DP2 repeat (Carrigan et al. 2005) motifs are highlighted by green rectangles
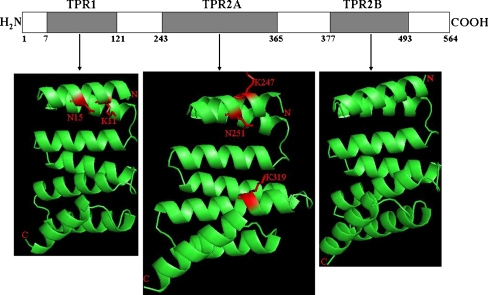
The concave surfaces that are formed by the TPR domains of Hop provide interactive sites that can accommodate specific peptide binding (Scheufler et al. 2000). Based on the three-dimensional model of TPR1 and TPR2A domains, PfHop possesses conserved residues in TPR1 (K11, N15) and TPR2A (K247, N251, K319) that are implicated in the interaction of Hop with Hsp70 and Hsp90 chaperones, respectively (Fig. 2; Scheufler et al. 2000; Odunuga et al. 2004). These residues appear to be surface-exposed on the TPR helical domains, suggesting that they may play an important role in binding PfHsp70 and PfHsp90 chaperones.
Fig. 2.
PfHop model displays surface exposed residues implicated in interaction with PfHsp70 and PfHsp90. Schematic representation of PfHop showing the TPR domain as indicated by rectangles highlighted in grey. The numbers represent the starting and the ending amino acid residues of the three TPR domains. Ribbon representation of the three dimensional models of TPR1, TPR2A and TPR2B domains. Residues known to be crucial in the interaction of TPR1 and TPR2A with Hsp70 and Hsp90, respectively are represented in sticks. The models were generated by SWISS-MODEL (Arnold et al. 2006; Guex and Peitsch 1997; Schwede et al. 2003) and rendered using PyMol (DeLano 2002)
Overexpression and purification of recombinant PfHop
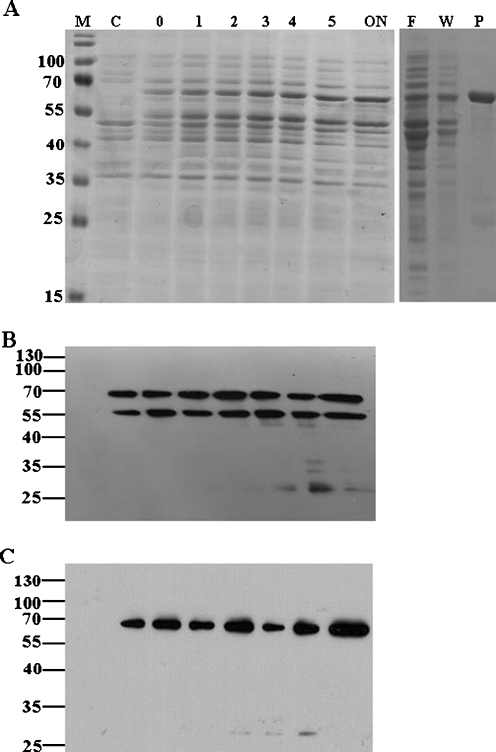
Recombinant PfHop protein was expressed in E. coli XL1 Blue cells as a species of approximately 66 kDa (Fig. 3). However, we also noticed a protein species of approximately 55 kDa, produced in E. coli together with the full length PfHop recombinant protein. The expressed lower molecular weight species, like the full length PfHop protein was produced upon induction of the cells with IPTG and did not appear in the control cells. Anti-PfHop antibodies were able to recognize both the full length Hop protein and the lower molecular weight species as confirmed by Western blotting (Fig. 3b). We concluded that the lower molecular species was either a truncated version of the full length PfHop protein or a product of an incomplete synthesis. We proceeded to purify PfHop by nickel affinity chromatography and were able to recover the full length PfHop protein (Fig. 3a). Fortunately, the species of lower molecular weight did not co-purify with PfHop protein, suggesting that this species had no polyhistidine tag attached (Fig. 3a, c). Therefore, it is likely that the species of lower molecular weight was an N-terminally truncated version of PfHop.
Fig. 3.
Expression and purification of recombinant PfHop protein. a SDS-PAGE analysis for the expression and purification of recombinant PfHop protein. PfHop was expressed in E. coli XL1 Blue cells transformed with pQE30/PfHop plasmid. Lane M represents molecular weight markers (in kDa) as indicated on the left hand side; lane C, represents the total extract for cells transformed with pQE30 plasmid after IPTG induction; lane 0, represents total extract for cells transformed with pQE30/PfHop plasmid prior to IPTG induction; lanes 1–ON, represent total extract for cells transformed with pQE30/PfHop plasmid collected hourly and overnight, respectively; lane F, represents the flow through; lane W, represents wash sample; and lane P, represents eluted PfHop protein. b Western analysis of PfHop using anti-PfHop and c Western analysis of PfHop using anti-His antibodies. The results are representative of at least three independent experiments
Localisation of PfHop
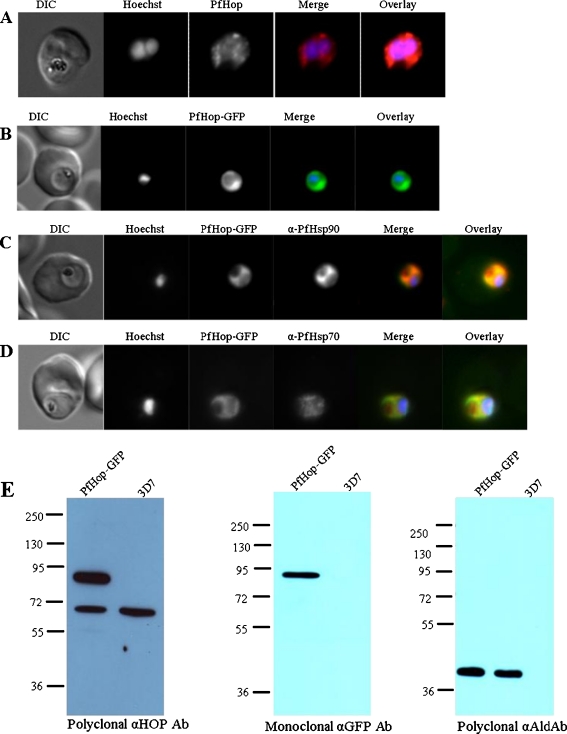
The localisation of PfHop in P. falciparum cells at the trophozoite stage was investigated by immunofluorescence analysis (IFA) using anti-PfHop antibodies that we developed (Fig. 4a). The fluorescence signal (red signal) was observed in the cytosol of parasites that had been cultured at 37°C. To further investigate the cellular localisation of PfHop, we created a transgenic parasite line expressing PfHop attached to a C-terminal GFP tag. PfHop-GFP localised largely to the body of the parasite (Fig. 4b). The GFP signal was not detected in the nucleus, suggesting that PfHop may not localise to the nucleus under normal growth conditions. By indirect immunofluorescence, we investigated whether PfHop-GFP co-localises with PfHsp90. It appears that PfHop-GFP and PfHsp90 co-localise in the cytosol and their distribution assumed a uniform pattern in the cell (Fig. 4c). PfHop-GFP and PfHsp70 co-localised in the cytosol; however, they associated less uniformly than PfHop-GFP and PfHsp90 (Fig. 4c, d). The expression of PfHop-GFP by the transfected parasites was confirmed by Western blot analysis (Fig. 4e). As loading control, a Western blot was conducted using antibodies recognising P. falciparum aldolase (Fig. 4e, third panel).
Fig. 4.
Localisation and expression of PfHop-GFP by parasites in human red blood cells. a Localisation of PfHop in P. falciparum cells; panels show a DIC image, nuclear stain (Hoechst), distribution of PfHop, merge and overlay. b Panels show a DIC image, nuclear stain (Hoechst), distribution of PfHop-GFP, merge and overlay. c Panels show a DIC image, nucleus (Hoechst), distribution of PfHop-GFP, distribution of PfHsp90, merge and overlay for PfHsp90–PfHop-GFP co-localisation. d Panels show DIC image, stain (Hoechst), distribution of PfHop-GFP, distribution of PfHsp70, merge and overlay. e Western blot analyses to confirm the expression of PfHop-GFP. First panel, Western blot conducted using polyclonal anti-PfHop antibodies; second panel, Western blot conducted using monoclonal anti-GFP antibodies; and third panel, Western blot conducted as loading control conducted using polyclonal anti-PfAldolase antibodies. The results are representative of at least three independent experiments
Size exclusion chromatography and co-immunoprecipitation analysis
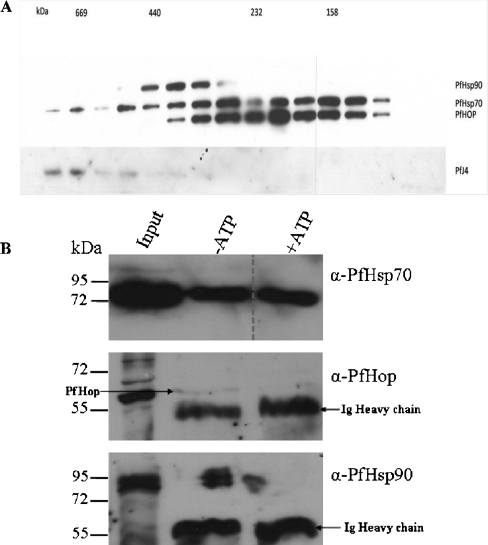
Size exclusion chromatography was performed to investigate whether PfHop, PfHsp70, PfHsp90 and PfJ4, a potential Hsp40 co-chaperone of PfHsp70 (Pesce et al. 2008) existed as a complex in parasite cells. The eluted fractions obtained were subjected to Western analysis using anti-PfHop, anti-PfHsp70, anti-PfHsp90 and anti-PfJ4 antibodies (Pesce et al. 2008; Fig. 5a). PfHsp90, PfHsp70 and PfHop all appeared in an apparent fraction of approximately 400 kDa in size. Several possibilities may underpin the observed findings. One interpretation is that all the three proteins are in a common complex. It is also possible that PfHsp90, PfHsp70 and PfHop may have been eluted in independent complexes that were apparently of similar sizes, thus eluting in the same fraction. Alternatively, this could represent independent homo-oligomers that approximated each other in size, and thus were eluted in the same fractions, but were not necessarily associated with one another. A previous study suggested that a type II (Cheetham and Caplan 1998) Hsp40 protein, PfJ4 associated with PfHsp70 (Pesce et al. 2008). However, it seemed that PfJ4 was not part of the putative complex made up of PfHop, PfHsp70 and PfHsp90 (Fig. 5a).
Fig. 5.
PfHop, PfHsp70, PfHsp90 and PfJ4 may associate in a heterologous complex. a The presence of PfHop, PfHsp70, PfHsp90 and PfJ4 in the elution fractions obtained by size exclusion chromatography was determined by Western analysis using corresponding antibodies. Approximate molecular weights (in kDa) associated with select fractions are given (top panel). b Co-immunoprecipitation of PfHop, PfHsp70 and PfHsp90 using anti-PfHop antibodies, in the absence and presence of ATP. PfHop and immunoglobulin heavy chain are shown by the arrows. The results are representative of at least three independent experiments
Formation of the Hsp70–Hop–Hsp90 complex is known to be nucleotide sensitive. As a result, we sought to establish whether PfHop, PfHsp70 and PfHsp90 association was nucleotide-sensitive. A co-immunoprecipitation study was conducted using anti-PfHsp70 antibodies and passing parasite lysate through the column. In one instance, 5 mM ATP was added to the parasite lysate before conducting the immunoprecipitation (lane ‘+ATP’, Fig. 5b) and the experiment was repeated without adding ATP to the lysate (lane ‘−ATP’, Fig. 5b). Hop associates preferably with Hsp70 when the latter is bound to ADP. In the absence of added ATP, it likely that most of the PfHsp70 protein in the lysate was either bound to ADP or was in a nucleotide-free state. In the absence of added ATP, PfHsp70 immunoprecipitated along with PfHop (lane ‘−ATP’, Fig. 5b). When ATP was added to the lysate, we could not detect PfHop in the PfHsp70 complex (lane ‘+ATP’, Fig. 5b). PfHsp90 was detected when its association with PfHsp70 was investigated both in the presence and absence of added ATP. PfHsp90 appeared on the Western blot as a species of approximately 90 kDa (Fig. 5b). However, in the absence of ATP, more PfHsp90 protein associated with PfHsp70 than when ATP was added to the parasite lysate (Fig. 5b).
Discussion
PfHsp70 and PfHsp90 chaperones of Plasmodium spp. are known to play essential roles in the development of the parasites. PfHsp70 and PfHsp90 have been characterised extensively and their roles in the development of P. falciparum are well documented (Banumathy et al. 2003; Sharma 1992; Shonhai et al. 2005; 2007). It is likely that these chaperones are functionally linked. In mammals, the TPR-rich Hop protein is known to modulate interaction of Hsp70 and Hsp90 chaperones. To the best of our knowledge, this is the first study to provide experimental evidence suggesting a possible role for the P. falciparum Hop homologue in mediating the functional partnership between PfHsp70 and PfHsp90.
Based on bioinformatics analysis, PfHop possesses conserved TPR1 and TPR2A domains that are implicated in facilitating the interaction of Hop with Hsp70 and Hsp90 in mammalian cells (Fig. 1; Odunuga et al. 2003; 2004). Residues that have direct contact with Hsp70 and Hsp90 are conserved in PfHop and they protrude on the helical surfaces of the respective TPR domains (Fig. 2), thus positioning them for the possible interaction of PfHop with its chaperone clients. Overall, PfHop exhibits conserved structural features of a typical Hop protein. However, although most structural motifs are conserved in Hop proteins, it is interesting to note that plasmodial Hop proteins exhibit select distinct functional domains, compared to their counterparts of yeast and mammalian origin. The relative variations in sequences exhibited between plasmodial Hop proteins and their counterparts from mouse, human as well as yeast even in important domains such as TPR and DP motifs (Fig. 1), could represent potential functional variation in the Hop-mediated pathways in these species. For example, DP repeats have been found to modulate Hop function by influencing the global folding status of Hop (Nelson et al. 2003) and thus variation within these domains could mirror functional specialisation of Hop proteins. It is thought that the less conserved segments of Hop outside its TPR domains have influence on the overall conformations of the helical turns of the TPR domains, thus are capable of imparting unique structural features to Hop molecules from different species (D'Andrea and Regan 2003). Therefore, the sequence variations observed between PfHop and human Hop may make PfHop amenable for selective inhibition using chemicals that may not interfere with human counterpart.
In mouse cells, Hop predominantly occurs in the cytosol under normal growth conditions and shuttles between the nucleus and the cytosol (Lässle et al. 1997; Longshaw et al. 2000; 2004). An investigation into the cellular distribution of PfHop in parasites at the trophozoite stage showed that the protein is localised to the cytosol (Fig. 4). As PfHop is implicated in mediating the interaction between PfHsp70 and PfHsp90, we investigated the distribution and co-localisation of these three proteins. Indeed, PfHop displayed a similar cytosolic localisation profile to PfHsp90 (Fig. 5b), suggesting that the two proteins may associate. Although, PfHop-GFP and PfHsp70 exhibited overlapping cytosolic co-localisation signals, the PfHsp90–PfHop-GFP co-localisation signal was more uniform than that for PfHsp70–PfHop-GFP (Fig. 4b, c). It is possible that PfHop associates more closely with PfHsp90 than with PfHsp70. Cytosolic co-localisation of Hop and Hsp70 homologues from Trypanosoma cruzi, another member of the apicocomplexan family, has been reported (Schmidt et al. 2011). Previously, it was proposed that PfHsp70 and PfHsp90 were found to co-elute complexed to a 60 kDa species, which was predicted to be PfHop (Banumathy et al. 2003). Findings from this study point to the possible existence of a PfHop-mediated partnership between PfHsp70 and PfHsp90 as has been previously proposed (Pesce et al. 2010; Shonhai 2010).
We performed size exclusion chromatography to investigate if PfHop may occur in complexes with PfHsp90 and PfHsp70 (Fig. 5a). We obtained fractions in which PfHop was present along with PfHsp70 and PfHsp90. This suggests the possible existence of a PfHsp70–PfHop–PfHsp90 complex in P. falciparum. However, we cannot formally rule out the possibility that the three proteins may have occurred in independent complexes that may have given rise to oligomeric units of similar apparent sizes.
Unusually, we also obtained a fraction containing a complex of about 440 kDa in which PfHsp90 and PfHsp70 occurred in the absence of PfHop (Fig. 5a). Whilst this may represent possible elution of PfHsp90 and PfHsp70 in independent complexes, the interaction of Hsp70 and Hsp90 through a Hop independent partnership has been reported in other living organisms such as Neurospora (Freitag et al. 1997). Interestingly, we also yielded several fractions of varying sizes in which PfHsp70 and PfHop occurred in the absence of PfHsp90. This was unexpected, as it is known that Hsp90 improves the affinity of Hsp70 for Hop binding (Hernández et al. 2002a, b). However, it is also known that binding of Hsp90 to Hsp70–Hop complex, reduces the number of Hsp70 binding sites on Hop from two to one binding site, although overall the binding of Hsp90 improves affinity of Hsp70 for Hop (Hernández et al. 2002b). Thus, Hsp90 binding may also be perceived as putting a strain on the Hsp70–Hop complex. In addition, in the absence of Hsp90, the Hsp70–Hop complex is stabilised by other protein interactors, particularly Hsp40. This may further mirror the complexity of the mechanism by which the Hsp70–Hop–Hsp90 pathway is governed. For example, a non-canonical Hop protein with a missing TPR1 domain from Caenorhabditis elegans (CeHop) has been described as capable of binding both Hsp70 and Hsp90 through the TPR2A domain (Gaiser et al. 2009). However, this essential protein could not bind both Hsp70 and Hsp90 at once. This could suggest that Hop may independently associate with Hsp90 and Hsp70. Interestingly, none of the fractions we obtained had PfHsp90 and PfHop in the absence of PfHsp70, in spite of the fact that Hsp90 is known to interact with Hop in the absence of Hsp70 (Hernández et al. 2002b). This may be due to the transient and dynamic nature of the association between PfHsp90 and PfHop since the immunofluorescence data suggested a strong association between PfHsp90 and PfHop (Fig. 4b).
Previous studies proposed that Hsp40 co-chaperones (belonging to the type I and type II subfamilies; Cheetham and Caplan 1998) are involved in the assembly of the Hsp70–Hop–Hsp90 complex (Cintron and Toft 2006). In this study, we sought to investigate if PfJ4, a type II Hsp40 known to associate with PfHsp70 (Pesce et al. 2008), would occur in the PfHsp70–PfHop–PfHsp90 complex. PfJ4 was not eluted in the fraction in which both PfHsp70 and PfHsp90 occurred (Fig. 5a). This suggests that PfJ4 may not be involved in the formation of this complex. We recently described a type I Hsp40 from P. falciparum (PfHsp40) that functionally co-operates with PfHsp70 in vitro (Botha et al. 2011). PfHsp40 may possibly be one of the P. falciparum Hsp40 proteins that are involved in the formation of the PfHsp70–PfHop complex, but this possibility still needs to be experimentally verified.
Based on co-immunoprecipitation analysis, PfHsp70 associated with both PfHsp90 and PfHop (Fig. 5b). As expected, in the absence of added ATP, PfHsp70 interacted with more PfHop protein than in the presence of added ATP (Fig. 5b). This is in agreement with a previous study that proposed that Hop binds to Hsp70 when the latter is complexed to ADP (Johnson et al. 1998). It seems in the presence of added ATP, less PfHsp90 was part of the PfHsp70 complex than was involved in the formation of this complex in the absence of added ATP (Fig 5b). Thus, ATP may have inhibited the association between PfHsp70 and PfHop, resulting in less PfHsp90 protein associating with PfHsp70. It seems that the possible association between PfHop and PfHsp70 (Fig. 5b) was sensitive to nucleotide, suggesting that PfHop and PfHsp70's association represents a functional interaction.
A study by Famin and Ginsburg (2003), observed that PfHsp70 and PfHsp90 both associate with ferriprotoporphyrin IX, a receptor of chloroquine that accumulates in chloroquine treated parasites. Ferriprotoporphyrin IX is thought to modulate the susceptibility of the parasite to chloroquine (Fitch 1989). This suggests that PfHsp70 and PfHsp90 may jointly facilitate the folding of proteins of parasitic origin that are implicated in regulating antimalarial drug efficacy. Interestingly, PfHsp90, PfHsp70 and PfHop were previously found to be distinctly upregulated in a group of clinical malaria patients (Pallavi et al. 2010). This suggests that the expression of these proteins may share a common regulatory trigger, and that their expression correlates with clinical malaria progression. As further evidence of their important role, plasmodial heat shock proteins have been described as potential antimalarial drug targets (reviewed in Pesce et al. (2010) and Shonhai (2010). Plasmodial heat shock proteins may be targeted in combination therapies by developing drugs that target these molecules and other malarial protein drug targets that fold through pathways that are facilitated by the parasite's chaperone machinery (reviewed in Pesce et al. 2010; Shonhai 2010).
The possible role of PfHop in coordinating the PfHsp70–PfHsp90 functional pathway might lead to interesting prospects in the search for alternative antimalarial therapies. For example, some pyrimidinones with antimalarial activity and are known to bind to the EEVD motif of Hsp70. These compounds may be able to inhibit interaction of Hsp70 with its co-chaperones such as Hop, Hsp40 and client substrates (Shonhai 2010). Thus, an understanding of the PfHop mediation of PfHsp70–PfHsp90 pathway could present a potential antimalarial drug target. This is particularly an important pathway to target, as inhibition of the Hsp70–Hsp90 pathway is known to lead to degradation of client proteins that depend on this pathway for folding (Whitesell and Lindquist 2005). Since both PfHsp70 and Hsp90 play essential roles in the folding of malarial proteins (Pavithra et al. 2007; Shonhai et al. 2007), targeting this pathway has conceivable deleterious effects to the parasite.
In conclusion, we have provided evidence that PfHop potentially exists in association with PfHsp70 and PfHsp90. Further studies need to be undertaken in order to understand the mechanism by which PfHop modulates this pathway in the malaria parasite and to understand if there may be variations between the two pathways in the host and malaria parasite towards the development of possible malaria intervention strategies.
Acknowledgements
AS, GLB and JMP were in part supported by the DFG “German–African Cooperation Projects in Infectology” programme (Li402/12-1), which also supported GWG and PM as postgraduate students. Further funding to support this study was provided by the University of Zululand Research Committee. This work was supported in part by an equipment grant provided to AS by the Department of Science and Technology/National Research Foundation (NRF) of South Africa. We wish to further acknowledge Mr. James Njunge and Dr. Adrienne Edkins, based at the BioBRU Research Unit, Rhodes University for expert advice on cell culture- and microscopy-based studies. We also wish to thank Mr. Benjamin El Harim who provided technical input during the development of the co-immunoprecipitation protocols that we used.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- Hsp70
Heat shock protein 70
- Hsp90
Heat shock protein 90
- Hop
Hsp70–Hsp90 organising protein
- Hsp40
Heat shock protein 40
- YT medium
Yeast–tryptophan medium
- IPTG
Isopropyl-1-thio-β-d-galactopyranoside
- PBS
Phosphate-buffered saline
- PMSF
Phenyl methyl sulfonyl fluoride
- SDS-PAGE
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- GFP
Green fluorescent protein
- IgG
Immunoglobulin G
- his-tag
Polyhistidine tag
- ATP
Adenosine triphosphate
- ADP
Adenosine diphosphate
- TCA
Trichloroacetic acid
- HRP
Horseradish peroxidase
- IP
Immunoprecipitation
- OD
Optical density
References
- Acharya P, Kumar R, Tatu U. Chaperoning a cellular upheaval in malaria: heat shock proteins in Plasmodium falciparum. Mol Biochem Parasitol. 2007;153:85–94. doi: 10.1016/j.molbiopara.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL Workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Banumathy G, Singh V, Pavithra S, Tatu U. Heat shock protein 90 is essential for Plasmodium falciparum growth in human erythrocytes. J Biol Chem. 2003;278:18336–18345. doi: 10.1074/jbc.M211309200. [DOI] [PubMed] [Google Scholar]
- Botha M, Chiang AN, Needham PG, Stephens LL, Hoppe HC, Külzer S, Przyborski JM, Lingelbach K, Wipf P, Brodsky JL, Shonhai A, Blatch GL. Plasmodium falciparum encodes a single cytosolic type I Hsp40 that functionally interacts with Hsp70 and is upregulated by heat shock. Cell Stress Chaperones. 2011;16:389–401. doi: 10.1007/s12192-010-0250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan PE, Riggs DL, Chinkers M, Smith DF. Functional comparison of human and Drosophila Hop reveals novel role in steroid receptor maturation. J Biol Chem. 2005;280:8906–8911. doi: 10.1074/jbc.M414245200. [DOI] [PubMed] [Google Scholar]
- Cheetham ME, Caplan AJ. Structure, function and evolution of DnaJ: conservation and adaptation of chaperone function. Cell Stress Chaperones. 1998;3:28–36. doi: 10.1379/1466-1268(1998)003<0028:SFAEOD>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cintron NS, Toft D. Defining the requirements for Hsp40 and Hsp70 in the Hsp90 chaperone pathway. J Biol Chem. 2006;281:26235–26244. doi: 10.1074/jbc.M605417200. [DOI] [PubMed] [Google Scholar]
- D'Andrea L, Regan L. TPR proteins: the versatile helix. Trends Biochem Sci. 2003;28:655. doi: 10.1016/j.tibs.2003.10.007. [DOI] [PubMed] [Google Scholar]
- DeLano WL (2002) The PyMOL molecular graphics system. DeLanoScientific, San Carlos, CA, USA:http://www.pymol.org
- Dittmar KD, Pratt WB. Folding of the glucocorticoid receptor by the reconstituted Hsp90-based chaperone machinery. The initial hsp90.p60.hsp70-dependent step is sufficient for creating the steroid binding conformation. J Biol Chem. 1997;272:13047–13054. doi: 10.1074/jbc.272.20.13047. [DOI] [PubMed] [Google Scholar]
- Dobson S, Kar B, Kumar R, Adams B, Barik S. A novel tetratricopeptide repeat (TPR) containing PP5 serine/threonine protein phosphatase in the malaria parasite. Plasmodium falciparum. BMC Microbiol. 2001;1:3. doi: 10.1186/1471-2180-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famin O, Ginsburg H. The treatment of Plasmodium falciparum-infected erythrocytes with chloroquine leads to accumulation of ferriprotoporphyrin IX bound to particular parasite proteins and to the inhibition of the parasite's 6-phosphogluconate dehydrogenase. Parasite. 2003;10:39–50. doi: 10.1051/parasite/2003101p39. [DOI] [PubMed] [Google Scholar]
- Freitag DG, Ouimet PM, Girvitz TL, Kapoor M. Heat shock protein 80 of Neurospora crassa, a cytosolic molecular chaperone of the eukaryotic stress 90 family, interacts directly with heat shock protein 70. Biochemistry. 1997;36:10221–10229. doi: 10.1021/bi963030g. [DOI] [PubMed] [Google Scholar]
- Fitch CD. Ferriprotoporphyrin IX: role in chloroquine susceptibility and resistance in malaria. Prog Clin Biol Res. 1989;313:45–52. [PubMed] [Google Scholar]
- Gaiser AM, Brandt F, Richter K. The non-canonical Hop protein from Caenorhabditis elegans exerts essential functions and forms binary complexes with either Hsc70 or Hsp90. J Mol Biol. 2009;391:621–634. doi: 10.1016/j.jmb.2009.06.051. [DOI] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modelling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Hall TA. Bioedit: a user friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Hernández MP, Chadli A, David O, Toft DO. HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J Biol Chem. 2002;277:11873–11881. doi: 10.1074/jbc.M111445200. [DOI] [PubMed] [Google Scholar]
- Hernández MP, Sullivan WP, Toft DO. The assembly and intermolecular properties of the hsp70–Hop–hsp90 molecular chaperone complex. J Biol Chem. 2002;277:38294–38304. doi: 10.1074/jbc.M206566200. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Schumacher RJ, Ross ED, Toft DO. Hop modulates hsp70/hsp90 interactions in protein folding. J Biol Chem. 1998;273:3679–3686. doi: 10.1074/jbc.273.6.3679. [DOI] [PubMed] [Google Scholar]
- Kumar R, Musiyenko A, Barik S. The heat shock protein 90 of Plasmodium falciparum and antimalarial activity of its inhibitor, geldanamycin. Malaria J. 2003;2:30. doi: 10.1186/1475-2875-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lässle M, Blatch GL, Kundra V, Takatori T, Zetter BR. Stress-inducible, murine protein mSTI1: characterization of binding domains for heat shock proteins and in vitro phosphorylation by different kinases. J Biol Chem. 1997;272:1876–1884. doi: 10.1074/jbc.272.3.1876. [DOI] [PubMed] [Google Scholar]
- Longshaw VM, Chapple JP, Balda MS, Cheetham ME, Blatch GL. Nuclear translocation of the Hsp70/Hsp90 organizing protein mSTI1 is regulated by cell cycle kinases. J Cell Sci. 2004;117:701–710. doi: 10.1242/jcs.00905. [DOI] [PubMed] [Google Scholar]
- Longshaw VM, Dirr HW, Blatch GL, Lässle M. The in vitro phosphorylation of the co-chaperone mSTI1 by cell cycle kinases substantiates a predicted casein kinase II-p34cdc2-NLS (CcN) motif. Biol Chem. 2000;381:1133–1138. doi: 10.1515/BC.2000.139. [DOI] [PubMed] [Google Scholar]
- Nelson GM, Huffman H, Smith DF. Compariosn of the carboxy-terminal DP-repeat region in the co-chaperones Hop and Hip. Cell Stress chaperones. 2003;8:125–133. doi: 10.1379/1466-1268(2003)008<0125:COTCDR>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolet CM, Craig EA. Isolation and characterization of STI1, a stress-inducible gene from Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:3638–3646. doi: 10.1128/mcb.9.9.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odunuga OO, Hornby JA, Bies C, Zimmermann R, Pugh DJ, Blatch GL. Tetratricopeptide repeat motif-mediated Hsc70-mSTI1 interaction: molecular characterization of the critical contacts for successful binding and specificity. J Biol Chem. 2003;278:6896–6904. doi: 10.1074/jbc.M206867200. [DOI] [PubMed] [Google Scholar]
- Odunuga OO, Longshaw VM, Blatch GL. Hop: more than an Hsp70/Hsp90 adaptor protein. Bioessays. 2004;26:1058–1068. doi: 10.1002/bies.20107. [DOI] [PubMed] [Google Scholar]
- Pallavi R, Acharya P, Chandran S, Daily JP, Tatu U. Chaperone expression profiles correlate with distinct physiological states of Plasmodium falciparum in malaria patients. Malaria J. 2010;9:236. doi: 10.1186/1475-2875-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavithra SR, Banumathy G, Joy O, Singh V, Tatu U. Recurrent fever promotes Plasmodium falciparum development in human erythrocytes. J Biol Chem. 2004;279:46692–46699. doi: 10.1074/jbc.M409165200. [DOI] [PubMed] [Google Scholar]
- Pavithra SR, Kumar R, Tatu U. Systems analysis of chaperone networks in the malarial parasite Plasmodium falciparum. PLoS Comput Biol. 2007;3:1701–1715. doi: 10.1371/journal.pcbi.0030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce ER, Acharya P, Tatu U, Nicoll WS, Shonhai A, Hoppe HC, Blatch GL. The Plasmodium falciparum heat shock protein 40, Pfj4, associates with heat shock protein 70 and shows similar heat induction and localisation patterns. Int J Biochem Cell Biol. 2008;40:2914–2926. doi: 10.1016/j.biocel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Pesce ER, Cockburn IL, Goble JL, Stephens LL, Blatch GL. Malaria heat shock proteins: drug targets that chaperone other drug targets. Infect Disord Drug Targets. 2010;10:147–157. doi: 10.2174/187152610791163417. [DOI] [PubMed] [Google Scholar]
- Przyborski JM, Miller SK, Pfahler JM, Henrich PP, Rohrbach P, Crabb BS, Lanzer M. Trafficking of STEVOR to the Maurer's clefts in Plasmodium falciparum-infected erythrocytes. EMBO J. 2005;24:2306–2317. doi: 10.1038/sj.emboj.7600720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I. Structure of TPR domain–peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell. 2000;101:199–210. doi: 10.1016/S0092-8674(00)80830-2. [DOI] [PubMed] [Google Scholar]
- Schmidt JC, Soares MJ, Samuel Goldenberg S, Pavoni DP, Krieger MA. Characterization of TcSTI-1, a homologue of stress-induced protein-1, in Trypanosoma cruzi. Mem Inst Oswaldo Cruz. 2011;106:70–77. doi: 10.1590/S0074-02762011000100012. [DOI] [PubMed] [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:338–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma YD. Structure and possible function of heat-shock proteins in Plasmodium falciparum. Comp Biochem Physiol B. 1992;102B:437–444. doi: 10.1016/0305-0491(92)90033-N. [DOI] [PubMed] [Google Scholar]
- Shonhai A. Plasmodial heat shock proteins: targets for chemotherapy. FEMS Immunol Med Microbiol. 2010;58:61–74. doi: 10.1111/j.1574-695X.2009.00639.x. [DOI] [PubMed] [Google Scholar]
- Shonhai A, Boshoff A, Blatch GL (2005) Plasmodium falciparum heat shock protein 70 is able to suppress the thermosensitivity of an Escherichia coli DnaK mutant strain. Mol Genet Genomics 274:70–78 [DOI] [PubMed]
- Shonhai A, Boshoff A, Blatch GL. The structural and functional diversity of Hsp70 proteins from Plasmodium falciparum. Protein Sci. 2007;16:1803–1818. doi: 10.1110/ps.072918107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonhai A, Maier AG, Przyborski J, Blatch GL. Intracellular protozoan parasites of humans: the role of molecular chaperones in development and pathogenesis. Protein Pept Lett. 2011;15:1117–1125. doi: 10.2174/092986608786071067. [DOI] [PubMed] [Google Scholar]
- Spork S, Hiss JA, Mandel K, Sommer M, Kooij TW, Chu T, Schneider G, Maier UG, Przyborski JM. An unusual ERAD-like complex is targeted to the apicoplast of Plasmodium falciparum. Eukaryotic Cell. 2009;8:1134–1145. doi: 10.1128/EC.00083-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wegele H, Wandinger SK, Schmid AB, Reinstein J, Buchner J. Substrate transfer from the chaperone Hsp70 to Hsp90. J Mol Biol. 2006;356:802–811. doi: 10.1016/j.jmb.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Lindquist SL. Hsp90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]