Abstract
Exposure to social stressors can cause profound changes in an individual’s physiology and behavior. In Syrian hamsters, even a single social defeat results in conditioned defeat, which includes an abolishment of territorial aggression and the emergence of high levels of submissive behavior. The purpose of the current study was to determine whether the lateral septum (LS) is a component of the putative neural circuit underlying conditioned defeat. Experiment 1 explored the possibility that plasticity in the LS is necessary for the induction of conditioned defeat. Infusions of the protein synthesis inhibitor, anisomycin, prior to defeat training, however, failed to alter conditioned defeat during testing on the following day, suggesting that synaptic plasticity in the LS is not critical for defeat-induced suppression of aggression. Experiment 2 tested whether the LS is necessary for the expression of conditioned defeat. Infusions of the GABAA agonist muscimol into the LS prior to testing significantly increased aggression and decreased submission in previously defeated animals suggesting that the LS is an important component of the neural circuit mediating the expression of both aggression and submission in conditioned defeat. Experiment 3 examined whether the effects of muscimol on aggression were dependent on prior social defeat. Non-defeated animals receiving muscmol infusions prior to testing with a non-aggressive intruder displayed significantly more aggression than did hamsters receiving control injections. Thus, these data suggest that the activation of GABAA receptors in the LS increases aggression regardless of whether or not a hamster has previously experienced social defeat.
1. Introduction
The social environment can be a potent source of stress in many animals including humans. Social stressors, such as defeat, often lead to robust changes in physiology and behavior. For example, Syrian hamsters display profound behavioral changes, which last at least a month in the majority of defeated animals, following a single social defeat by a larger, dominant male (Potegal et al., 1993). These behavioral changes, collectively known as conditioned defeat, include an abolishment of territorial aggression and the emergence of high levels of submissive behavior, even when the defeated animal is subsequently tested in its own home cage against a smaller, non-aggressive intruder (Huhman et al., 2003). We believe that conditioned defeat is a valuable, ethologically relevant model with which to examine the neurobiology of social stress-induced behavioral plasticity.
Much of the work with the conditioned defeat model has been devoted to elucidating the neural circuit underlying the acquisition and expression of conditioned defeat. Past research in our lab has suggested that a number of neuroanatomical regions are components of the conditioned defeat neural circuit including portions of the amygdala (central (CeA), basolateral complex (BLA), and medial (Me)), bed nucleus of the stria terminalis (BNST), nucleus accumbens (NAcc), medial prefrontal cortex, dorsal raphe nucleus, and ventral hippocampus (Jasnow and Huhman, 2001; Luckett et al., in press; Markham and Huhman, 2008; Markham et al., 2009; Markham et al., 2010; Markham et al., in press). While pharmacological manipulations of each of these regions have significantly increased, decreased or even blocked submissive behavior in defeated animals, manipulations of the majority of these brain regions has not produced a reinstatement of territorial aggression in defeated hamsters. In fact, the only brain region in which temporary inactivation has restored territorial aggression has been the NAcc (Luckett, et al., in press). The NAcc was not found to be necessary for the acquisition of conditioned defeat, however, indicating that another brain area must mediate the synaptic plasticity underlying the formation of the “defeat memory” that inhibits aggressive behavior.
The purpose of the present study was to explore the possibility that the lateral septum (LS) is a component of the conditioned defeat neural circuit. The LS sends GABAergic projections to a variety of limbic, diencephalic, and midbrain structures, and the LS has been implicated in the regulation of affect, various aspects of social behavior, and activity of the HPA axis (Herman and Cullinan, 1997; Numan, 2000; Sheehan et al., 2004; Sodetz and Bunnell, 1970). The LS also appears to contain a population of GABAergic interneurons that have the potential to induce generalized inhibition of septal neurons including GABAergic projection neurons (Lee and Gammie, 2009; Raggenbass, 2008). This region expresses a high density of receptors for chemical mediators of the stress response (e.g., corticotropin releasing factor, vasopressin, serotonin, catecholamines) and is highly responsive to a variety of stressors including social defeat (Bonaz and Tache, 1994; Campeau and Watson, 1997; Chen and Herbert, 1995; Duncan et al., 1993; Kollack-Walker et al., 1997; Veenema and Neumann, 2007). Numerous studies have demonstrated a role of the LS in the inhibition of behavioral responses to stress and fear-related stimuli, including the inhibition of defensive reactions such as startle, freezing, and defensive aggression (Pradhan, 1975; Thomas, 1988). Lesions of the septum increase intermale aggression in hamsters (Sodetz and Bunnell, 1970), and c-fos expression is significantly higher following social defeat in subordinate as compared to dominant hamsters (Kollack-Walker et al., 1997). Thus, the LS would appear to be a brain area within which defeat-induced changes in aggressive behavior might be mediated.
Though perhaps better known for its role in the regulation of affect and agonistic behavior, the LS is also involved in learning and memory. Specifically, the LS has been implicated in such processes as social recognition, pair bond formation, and context-dependent fear conditioning (Bielsky and Young, 2004; Everts and Koolhaas, 1999; Fischer et al., 2002; Lee et al., 1992; Liu et al., 2001). In rats, injections of vasopressin (AVP) in the LS prolong the time over which the memory of a male conspecific is retained, while V1a receptor antagonists significantly impair such recognition (Everts and Koolhaas, 1999). In the prairie vole model of pair bonding, administration of AVP in the LS can induce partner preference in the absence of mating, while the administration of a V1a antagonist in this region blocks mating-induced partner preference (Liu et al., 2001). Interestingly, the application of AVP in the LS stimulates long-term potentiation both in vitro and in vivo (Joels and Urban, 1984; Urban, 1998; Van den Hooff and Urban, 1990). In addition to its involvement in social memory, manipulations of serotonergic function in the LS prior to conditioning can enhance or impair retention of the association between contextual cues and electric shock in rats (Lee et al., 1992). Furthermore, blocking cyclin-dependent kinase 5, a protein that is markedly upregulated in response to stressors such as restraint or shock, in the LS prevents the acquisition of conditioned context-dependent fear in mice (Fischer et al., 2002). Thus, protein synthesis in the LS may be a critical component of the mechanisms responsible for conditioned defeat.
The purpose of the present study was to examine the role of protein synthesis within the LS in the acquisition in conditioned defeat and to determine whether activation of GABAA receptors in the LS modulate the expression of conditioned defeat. Experiment 1 tested the hypothesis that protein synthesis in the LS is necessary for the acquisition of conditioned defeat. Experiment 2 investigated whether bilateral infusions of the GABAA agonist muscimol into the LS prior to social interaction would reduce the expression of conditioned defeat and Experiment 3 examined whether the ability of muscimol to increase aggression are dependent on prior social defeat.
2. Results
2.1 Experiment 1: Anisomycin in the LS does not affect plasticity related to conditioned defeat
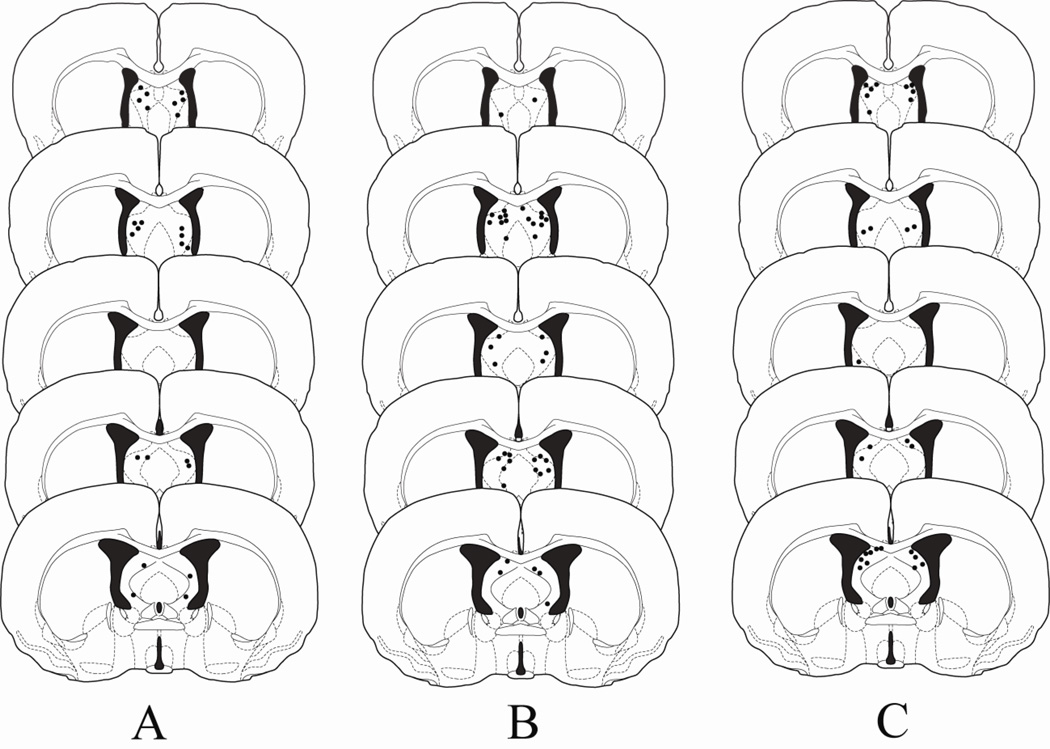
Figure 1A shows the injection sites for animals in Experiment 1. The injection needles were localized within 0.3 mm of the lateral septum in 13 of 15 animals. Injection sites for two animals were unable to be verified as a result of an obstruction in the cannula preventing dye infusion. One animal with injection sites located bilaterally in the LS was attacked by the non-aggressive intruder (NAI) and was subsequently removed from the study. Thus, a total of 12 animals were used for data analysis.
Figure 1.
Histological reconstructions of injection sites for animals receiving infusions of muscimol into the LS in Experiments 1 (A), 2 (B) and 3 (C). Black dots represent injection sites in the LS. Drawings are adapted from Morin and Wood (2001).
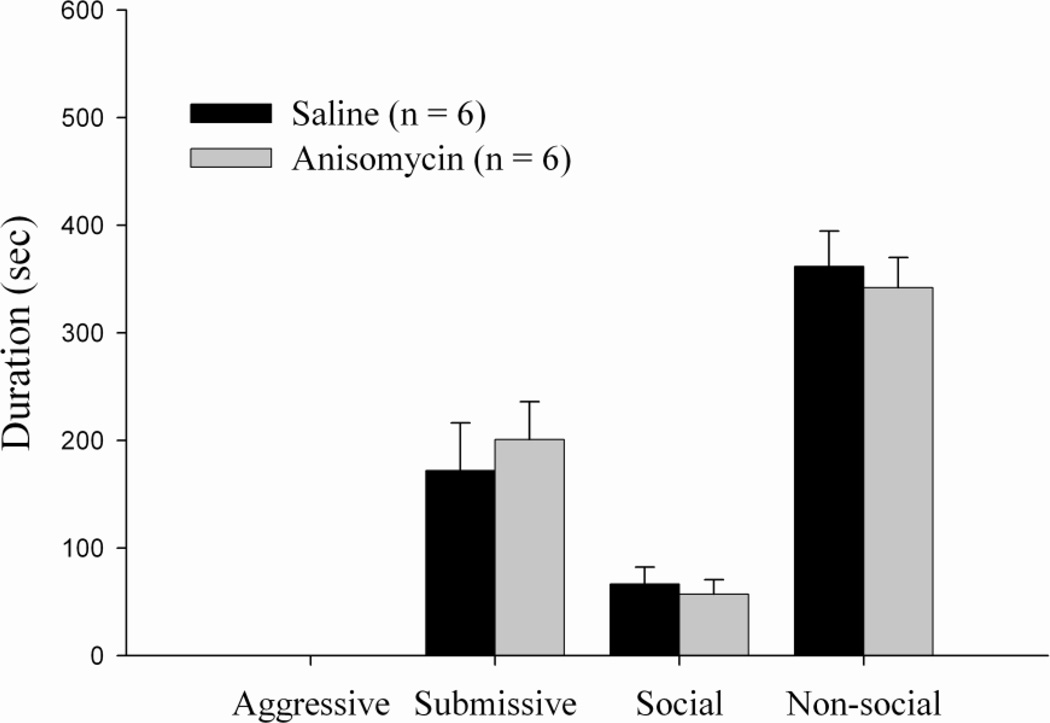
Animals receiving bilateral injections of anisomycin in the LS immediately before training exhibited no significant differences in the duration of aggression, submission, social behavior, or nonsocial behavior as compared to the vehicle group (Figure 2).
Figure 2.
Total duration (mean ± SEM) of behaviors exhibited during the 10-min test with a non-aggressive intruder (NAI). Animals received bilateral infusions of anisomycin or saline into the LS immediately prior to conditioned defeat training. There were no significant differences between drug groups.
As indicated in Table 1, there was also no significant difference in the aggressive behavior of the resident aggressor (RA) toward the animals given anisomycin versus saline controls. Thus, anisomycin infusions did not appear to alter the overall defeat experience of the experimental animals.
Table 1.
Total attack duration and latency to first attack of resident aggressors during training in Experiment 1.
| Experimental Condition | Latency to First attack (sec) |
Total duration of aggression (sec) |
|---|---|---|
| Saline control | 35.1 ± 18.2 | 390.5 ± 59.1 |
| Anisomycin | 21.9 ± 2.1 | 375.8 ± 60.4 |
2.2 Experiment 2: Muscimol in the LS Reduces the Expression of Conditioned Defeat
Figure 1B shows the injection sites for animals in Experiment 2. The injection needles were bilaterally localized within 0.3 mm of the lateral septum in 18 of the 27 cannulated animals. Injection sites that were within the lateral ventricles were considered “misses”, and these animals were excluded from data analysis. Of the 9 animals excluded from data analysis, 7 animals had unilateral placements in the LS while 2 animals had cannula placements that missed the LS bilaterally.
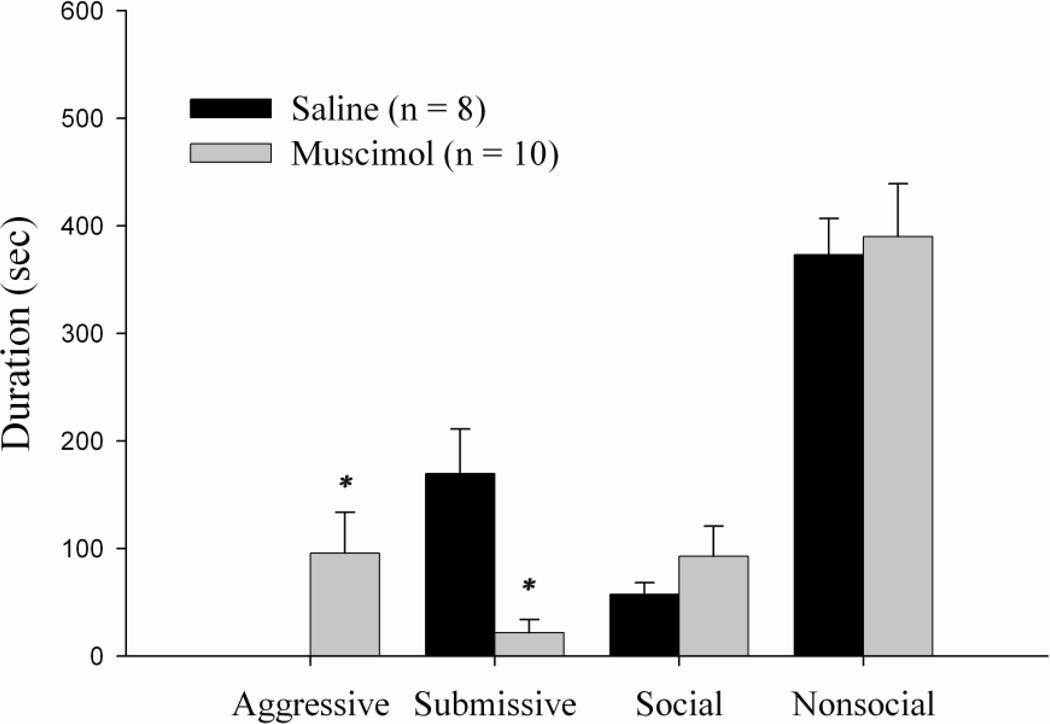
Previously defeated animals that received muscimol into the LS 5 min before behavioral testing displayed a significant decrease in the total duration of submissive behavior and a significant increase in levels of aggression compared to the vehicle group (t(16) = 0.001, p < 0.005 and t(16) = 0.003, p < 0.005, respectively (see Fig. 3)). No significant differences in either the duration of social behavior or nonsocial behavior was observed.
Figure 3.
Total duration (mean ± SEM) of behaviors exhibited during the 10-min test with a non-aggressive intruder (NAI). Animals received bilateral infusions of muscimol or saline into the LS 5 min prior to behavioral testing. * indicates significant differences compared to vehicle controls (p < 0.005).
2.3 Experiment 3: Muscimol in the LS increases aggression in undefeated animals
Figure 1C shows the injection sites for animals in Experiment 3. The injection needles were bilaterally localized within 0.3 mm of the lateral septum in 17 of the 26 cannulated animals. A total of 9 animals were excluded from data analysis. Seven of these animals had unilateral placements in the LS, and one animal had cannula placements that missed the LS bilaterally. The brain of one animal was compromised during slicing and injections sites could not be verified.
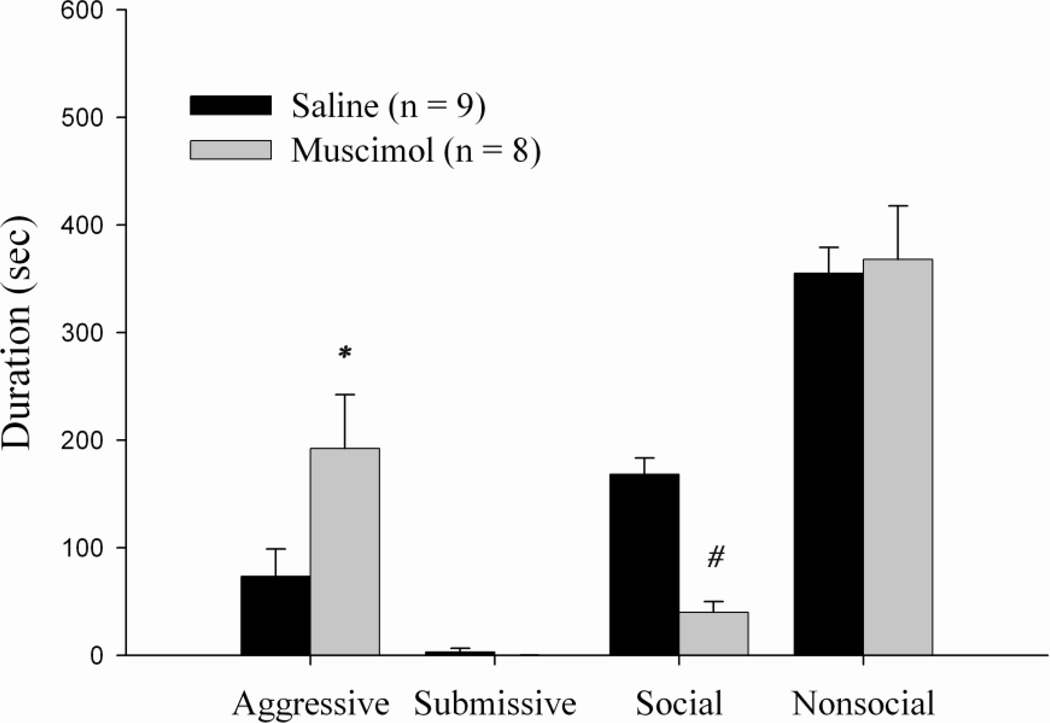
Animals receiving bilateral injections of muscimol in the LS 5 min before behavioral testing displayed a significant increase in the duration of aggression, t(15) = 0.045, p < 0.05. In addition, animals receiving muscimol exhibited a significant decrease in social behavior as compared to the vehicle group, t(15) = 0.000, p < 0.001 (Figure 4). No significant differences in either the duration of submissive behavior or nonsocial behavior were observed.
Figure 4.
Total duration (mean ± SEM) of behaviors exhibited by non-defeated animals during the 10-min test with a non-aggressive intruder (NAI). Animals received bilateral infusions of muscimol or saline into the LS 5 min prior to behavioral testing. * indicates significantly different than saline controls (p < 0.05) and # indicates significantly different than saline controls (p < 0.001).
3. Discussion
The present results do not support the hypothesis that plasticity within the LS is necessary for the acquisition of conditioned defeat. In contrast, injection of muscimol into the LS in previously defeated animals significantly reduced the expression of conditioned defeat and increased aggression. Consistent with previously published work (Pradhan, 1975; Sodetz and Bunnell, 1970), a qualitative analysis of the aggressive behavior induced by muscimol in both the defeated and undefeated hamsters suggests that it has features that are similar to the aggression typically associated with the ‘septal rage’ response, rather than aggression characterized by territoriality. Animals infused with muscimol appeared to be hyper-vigilant, and highly reactive to the movement of the NAI. In contrast, control animals in the no-defeat group exhibited normal territorial aggression which terminated when the NAI displayed a submissive posture. Thus, although it appears that the LS may be a component of the complex neural circuit mediating the expression of agonistic behavior following social defeat, it does not appear to be a critical site within which memory of the social defeat experience is formed, and it also appears to be important site for the modulation of aggressive behavior irrespective of previous social experience.
While the present study provides the first evidence that activation of GABAA receptors in the LS increases inter-male aggression, a recent study found that the GABAA antagonist bicuculline injected into the LS reduces maternal aggression in mice (Lee and Gammie, 2009). Although lesions of the LS have long been reported to produce extraordinarly high levels of aggression in a variety of species (Albert and Richmond, 1976; Albert and Chew, 1980; Pradhan, 1975; Sodetz and Bunnell, 1970), a closer examination of many of these lesion studies is beginning to suggest that this may be an overgeneralization (Sheehan and Numan, 2000). Rather, the effects of LS lesions on aggression are complex and can depend upon the prior social experience of the lesioned animal, the time interval between lesioning and testing, and the context of social interaction (Albert and Chew, 1980; Fried, 1973; Gotsick and Marshall, 1972; Potegal et al., 1981; Sodetz and Bunnell, 1970). The present data are important because they suggest that activation of GABAA receptors in the LS can induce aggression in males irrespective of prior social experiences. As such, GABAergic mechanisms in the LS may be a critical component in the neural circuitry regulating aggression as well as conditioned defeat
With microinjection studies, there is always a possibility that the drug may have diffused to an adjacent brain region limiting the anatomical specificity of the findings, particularly when the injected brain area borders the ventricular system. In order to assess this possibility, we examined data from five animals in the muscimol group that were also anatomical “misses” (cannula placements located 300µm or more from the LS). Of these animals, 3 had one cannula in the LS and the other in the lateral ventricle, one had a cannula in the precommissural fornix and the other in the LS, and the remaining animal was a bilateral miss with both cannulae in the ventricles. The animals with unilateral placements in the LS (n=4) exhibited intermediate levels of aggression, though this was not statistically different from controls. Previous research in our lab has also shown, however, that the same dose and volume of muscimol (2.2 nmol, 200nl) infused directly into the lateral ventricles has no effect on aggression or submission in defeated animals (Markham et al., 2009). No aggression was observed in the one animal with bilateral placements in the ventricles. Thus, we maintain that the action of muscimol within the LS specifically mediates the current behavioral effects.
In Experiment 1, animals receiving bilateral infusions of anisomycin into the LS prior to defeat showed no changes in conditioned defeat as compared to controls, indicating that the LS is not a site of synaptic plasticity underlying the acquisition of conditioned defeat. One possible explanation for the lack of an effect is that the dose or volume of anisomycin administered was insufficient to compromise protein synthesis enough to effectively alter the acquisition of defeat-induced changes in aggression. However, this possibility seems unlikely as this dose and volume of anisomycin has been shown to block conditioned defeat when infused into the BLA (Markham and Huhman, 2008). Although some studies have found that protein synthesis in the LS is crucial for the formation of context-dependent fear memory (Fischer et al., 2002), the current data do not support a role for protein synthesis in the LS in the learned suppression of aggression following social defeat.
In summary, the current data show that while the LS can affect conditioned defeat, it is unclear whether the LS is part of the neural circuit that specifically inhibits aggression following social defeat or whether the effect on aggression is more global and experience-independent. Importantly, however, we believe that the present data show that manipulations of the LS can enhance aggression in defeated, as well as non-defeated, animals. Future studies will continue to explore the anatomical location wherein the memory for social defeat is formed which then inhibits territorial aggression in previously defeated animals.
4. Materials and Methods
4.1 Animals and Housing Conditions
Subjects were adult male Syrian hamsters (Mesocricetus auratus; Charles River Laboratories, Wilmington, MA) that weighed 120–140 g and were approximately 9–10 weeks old at the beginning of the experiment. All subjects were housed in groups of 5–6 per cage for one week (as a quarantine measure) prior to surgery in a temperature (20±2° C) and humidity-controlled room with free access to food and water. Following surgery, all subjects were singly housed. Animals were kept on a 14:10 hr light:dark cycle. All training and testing sessions were performed under dim red illumination during the first 3 hrs of the dark phase of the light dark cycle to minimize circadian effects. RA’s used for defeat training were older (>6 months), singly-housed males weighing between 160 and 180 g. NAI’s used for conditioned defeat testing were younger (2 months), group-housed (5–6 per cage) males weighing between 100–110 g. The cages of the experimental animals and the resident aggressors were not changed for one week prior to testing to allow them to establish residence by scent marking their cages. All procedures and protocols were approved by the Georgia State University Institutional Animal Care and Use Committee and are in accordance with the standards outlined in the National Institutes of Health Guide for Care and Use of Laboratory Animals.
4.2 Surgical Procedures
Subjects were anesthetized with sodium pentobarbital (Lundbeck, Inc., Deerfield, IL, 90 mg/kg, i.p.) and placed into a stereotaxic frame. Stainless steel guide cannula (26-gauge) were bilaterally implanted into the brain and aimed at the lateral septum. The angle of approach was 10° toward the midline to prevent cannulas from touching one another. Stereotaxic coordinates were 1.7 mm posterior to bregma, 1.5 mm lateral to bregma, and 3.0 mm below dura. To avoid damaging the site of injection, the injection needle (33-gauge) projected an additional 1.2 mm below the cannula to a total depth of 4.2mm below dura. Following surgery, post-operative care consisting of subcutaneous injections of 0.1 ml of 10 mg/ml Ketofen and 0.9 ml of physiological saline was administered. After surgery, dummy stylets were placed in the guide cannula to help prevent clogging. Hamsters were allowed 7–10 d to recover from surgery prior to the start of behavioral testing. During this period, animals were handled each day by gently restraining them and removing and replacing the dummy stylet in order to maintain patency and habituate the subjects to the injection procedure.
4.3 Social Defeat and Behavioral Testing
Our conditioned defeat protocol has been extensively described elsewhere (Jasnow and Huhman, 2001; Markham and Huhman, 2008) and is briefly described here. On the day of conditioned defeat training, animals were transported to a testing suite where they were allowed to acclimate for 1 hr. The defeat procedure consisted of a single resident/intruder pairing in which subjects were placed into the cage of the RA for 15 min. The RA reliably attacked and defeated the experimental animals which displayed submissive and defensive behaviors toward the RA. No-defeat controls were given a 15-min exposure to the RA’s empty cage. Behavioral testing occurred 24 h after training and consisted of one, 10-min encounter with a novel NAI in the experimental animal’s home cage. An animal was considered to show conditioned defeat if it exhibited a significant increase in submissive/defensive behavior compared to no-defeat controls and if no aggressive behavior was exhibited when the NAI was introduced into its home cage. In contrast, non-defeated animals typically exhibit little to no submissive behavior and instead produce high levels of territorial aggression directed toward the NAI. All testing sessions were videotaped via a camera mounted overhead and later scored by two observers blind to condition using the behavioral scoring program Noldus Observer (inter-rater reliability > 90%). The total duration of four classes of behaviors were scored during the test session: (1) social behavior (stretch, approach, sniff, nose touching, and flank marking); (2) non-social behavior (locomotion, exploration, grooming, nesting, feeding, and sleeping); (3) submissive/defensive behaviors (flight, avoidance, tail up, upright, side defense, full submissive posture, stretch attend, head flag, attempted escape from cage); and (4) aggressive behaviors (upright and side offense, chase and attack, including bite).
4.4 Experiment 1: Plasticity Related to Conditioned Defeat
The purpose of Experiment 1 was to determine whether the infusion of anisomycin into the lateral septum would attenuate or block the acquisition of conditioned defeat. Animals (n=15) were matched by weight and randomly assigned to drug or vehicle group. Animals received infusions (as described in the Methods section) of either anisomycin (Sigma-Aldrich, 50µg/1µl in 200 nl saline) or vehicle (200 nl saline) immediately before conditioned defeat training. This dose of anisomycin effectively blocks the acquisition of conditioned defeat when administered into the BLA (31). All animals were tested with a NAI for 10 min in their own homecage 24 h later.
4.5 Experiment 2: Expression of Conditioned Defeat
Animals (n = 27) were matched by weight and randomly assigned to drug or vehicle condition. All hamsters were placed into the cage of the RA for 15 min for conditioned defeat training. On the following day, animals received bilateral infusions of either muscimol (Sigma-Aldrich, 2.2 nmol in 200 nl saline) or vehicle (200 nl saline) into the LS 5 min before being tested in their own home cage for 10 min with a NAI. Infusions were made over a 1 min period with a Hamilton syringe mounted on a syringe pump (Harvard apparatus PHD 2000, Holliston, MA, USA) connected to a 33-gauge needle via polyethylene tubing. The needle was kept in place for an additional 1-min before being removed to ensure complete diffusion of the drug. This dose of muscimol has been regularly used in our laboratory to temporarily inactivate specific brain regions (Markham et al., 2009; Markham et al., 2010).
4.6 Experiment 3: No-Defeat Control
Because the effect of muscimol on the expression of conditioned defeat could be independent of the defeat experience and a result of a non-specific stimulation of aggression, we determined whether the infusion of muscimol into the lateral septum increased aggression in non-defeated animals. Animals (n = 26) were matched by weight and randomly assigned to drug or control groups. All hamsters were given a 15-min exposure to the RA’s empty cage. On the following day, animals received infusions of muscimol (Sigma-Aldrich, 2.2 nmol in 200 nL) or vehicle (200 nL) before testing with the NAI as described in Experiment 2.
4.7 Site verification
At the end of each experiment, hamsters were given a lethal dose of sodium pentobarbital euthanasia solution (Sleepaway, Fort Dodge Animal Health, Fort Dodge, IA) and were infused with 200 nL of India ink in order to verify the placement of the injections. The brains were then rapidly removed and fixed in 10% buffered formalin for 3 d before being sectioned on a cryostat. Sections (30 µm) were stained with cresyl violet and coverslipped with DPX and then examined under a light-microscope for placement verification. Only animals with injection sites within 300 µm of the LS were included in the statistical analysis.
4.8 Statistical Analysis
Total durations (in seconds) of submissive, aggressive, social, and nonsocial behaviors were analyzed separately using independent sample t tests. When variance between groups were not homogenous, data was analyzed using Mann-Whitney U test. All comparisons were two-tailed, and alpha was set at p < 0.05.
Highlights.
Inhibition of protein synthesis in the LS did not affect acquisition of CD
Infusion of muscimol in the LS impaired expression of CD and increased aggression
Infusion of muscimol in the LS increased aggression in non-defeated animals
The LS is involved in the expression of aggression regardless of social defeat experience
It appears that plasticity to the defeat experience does not involve the LS
Acknowledgements
This work was supported by NIH RO1 MH62044 to KLH. This paper is dedicated to the memory of Dr. Bradford N. Bunnell (1929–2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert DJ, Richmond SE. Hyperreactivity and aggressiveness following infusion of local anesthetic into the lateral septum or surrounding structures. Behav. Biol. 1976;18:211–226. doi: 10.1016/s0091-6773(76)92118-0. [DOI] [PubMed] [Google Scholar]
- Albert DJ, Chew GL. The septal forebrain and the inhibitory modulation of attack and defense in the rat. A review. Behav. Neural Biol. 1980;30:357–388. doi: 10.1016/s0163-1047(80)91247-9. [DOI] [PubMed] [Google Scholar]
- Bielsky IF, Young LJ. Oxytocin, vasopressin, and social recognition in mammals. Peptides. 2004;25:1565–1574. doi: 10.1016/j.peptides.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bonaz B, Tache Y. Water-avoidance stress-induced c-fos expression in the rat brain and stimulation of fecal output: role of corticotrophin-releasing factor. Brain Res. 1994;641:21–28. doi: 10.1016/0006-8993(94)91810-4. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: Correlations with cardiovascular, hypothermic, and endocrine responses. Neurosci. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Johnson KB, Breese GR. Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake and expression of fos-like immunoreactivity. J. Neurosci. 1993;13:3932–3943. doi: 10.1523/JNEUROSCI.13-09-03932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts HG, Koolhaas JM. Differential modulation of lateral septal vasopressin receptor blockade in spatial learning, social recognition, and anxiety-related behaviors in rats. Behav. Brain Res. 1999;99:7–16. doi: 10.1016/s0166-4328(98)00004-7. [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J. Cyclin-dependent kinase 5 is required for associative learning. J. Neurosci. 2002;22:3700–3707. doi: 10.1523/JNEUROSCI.22-09-03700.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried PA. The septum and hyper-reactivity: a review. Br. J. Psychol. 1973;64:267–275. doi: 10.1111/j.2044-8295.1973.tb01351.x. [DOI] [PubMed] [Google Scholar]
- Gotsick JE, Marshall RC. Time course of the septal rage syndrome. Physiol. Behav. 1972;9:685–687. doi: 10.1016/0031-9384(72)90033-9. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE. Neurocircuitry of stress: central control of the hypothalamo-pituitary-adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Solomon MB, Janicki M, Harmon AC, Lin SM, Israel JE, Jasnow AM. Conditioned defeat in male and female Syrian hamsters. Horm. Behav. 2003;44:293–299. doi: 10.1016/j.yhbeh.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Huhman KL. Activation of GABA(A) receptors in the amygdala blocks the acquisition and expression of conditioned defeat in Syrian hamsters. Brain Res. 2001;920:142–150. doi: 10.1016/s0006-8993(01)03054-2. [DOI] [PubMed] [Google Scholar]
- Joels M, Urban IJA. Arginine-vasopressin enhances the responses of lateral septal neurons in the rat to excitatory amino acids and fimbria-fornix stimuli. Brain Res. 1984;311:201–209. doi: 10.1016/0006-8993(84)90084-2. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J. Neurosci. 1997;17:8842–8855. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EH, Lin WR, Chen HY, Shiu WH, Liang KC. Fluoxetine and 8-OH-DPAT in the lateral septum enhances and impairs retention of an inhibitory avoidance response in rats. Physiol. Behav. 1992;51:681–688. doi: 10.1016/0031-9384(92)90103-9. [DOI] [PubMed] [Google Scholar]
- Lee G, Gammie SC. GABA(A) receptor signaling in the lateral septum regulates maternal aggression in mice. Behav. Neurosci. 2009;123:1169–1177. doi: 10.1037/a0017535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Curtis JT, Wang Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster) Behav. Neurosci. 2001;115:910–919. doi: 10.1037//0735-7044.115.4.910. [DOI] [PubMed] [Google Scholar]
- Luckett CA, Norvelle A, Huhman KL. The role of the nucleus accumbens in the acquisition and expression of conditioned defeat in Syrian hamsters. doi: 10.1016/j.bbr.2011.10.012. In press, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Huhman KL. Is the medial amygdala part of the neural circuit modulating conditioned defeat in Syrian hamsters? Learn. Mem. 2008;15:6–12. doi: 10.1101/lm.768208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Norvelle A, Huhman KL. Role of the bed nucleus of the stria terminalis in the acquisition and expression of conditioned defeat in Syrian hamsters. Behav. Brain Res. 2009;198:69–73. doi: 10.1016/j.bbr.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Taylor SL, Huhman KL. Role of amygdala and hippocampus in the neural circuit subserving conditioned defeat in Syrian hamsters. Learn. Mem. 2010;17:109–116. doi: 10.1101/lm.1633710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham CM, Luckett C, Huhman KL. The medial prefrontal cortex is both necessary and sufficient for the acquisition of conditioned defeat in Syrian hamsters. doi: 10.1016/j.neuropharm.2011.09.026. In press, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Wood RI. A stereotaxic atlas of the golden hamster brain. Academic Press; San Diego: 2001. [Google Scholar]
- Numan R. The behavioral neuroscience of the septal region. Springer-Verlag; New York: 2000. [Google Scholar]
- Potegal M, Blau A, Glusman M. Inhibition of intraspecific aggression in male hamsters by septal stimulation. Physiol. Psychol. 1981;9:213–218. doi: 10.1016/0031-9384(81)90167-0. [DOI] [PubMed] [Google Scholar]
- Potegal M, Huhman K, Moore T, Meyerhoff J. Conditioned defeat in the Syrian golden hamster (Mesocricetus auratus) Behav. Neural Biol. 1993;60:93–102. doi: 10.1016/0163-1047(93)90159-f. [DOI] [PubMed] [Google Scholar]
- Pradhan SN. Aggression and central neurotransmitters. Int. Rev. Neurobiol. 1975;18:213–262. doi: 10.1016/s0074-7742(08)60036-7. [DOI] [PubMed] [Google Scholar]
- Raggenbass M. Overview of cellular electrophysiological actions of vasopressin. Eur. J. Pharmacol. 2008;583:243–254. doi: 10.1016/j.ejphar.2007.11.074. [DOI] [PubMed] [Google Scholar]
- Sheehan T, Numan M. Numan R. The Behavioral Neuroscience of the Septal Region. Springer; New York: 2000. The septal region and social behavior; pp. 175–209. [Google Scholar]
- Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: implications for neuropsychiatry. Brain Res. Rev. 2004;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Sodetz FJ, Bunnell BN. Septal ablation and the social behavior of the golden hamster. Physiol. Behav. 1970;5:79–86. IN21–IN22, 81–88. doi: 10.1016/0031-9384(70)90017-x. [DOI] [PubMed] [Google Scholar]
- Thomas E. Forebrain mechanisms in the relief of fear: the role of the lateral septum. Psychobiol. 1988;16:36–44. [Google Scholar]
- Urban IJ. Effects of vasopressin and related peptides on neurons of the rat lateral septum and ventral hippocampus. Prog. Brain Res. 1998;119:285–310. doi: 10.1016/s0079-6123(08)61576-9. [DOI] [PubMed] [Google Scholar]
- Van den Hooff P, Urban IJ. Vaospressin facilitates excitatory transmission in slices of the rat dorso-lateral septum. Synapse. 1990;51:201–206. doi: 10.1002/syn.890050305. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Neumann ID. Neurobiological mechanisms of aggression and stress coping: a comparative study in mouse and rat selection lines. Brain Behav. Evol. 2007;70:274–285. doi: 10.1159/000105491. [DOI] [PubMed] [Google Scholar]