Abstract
While a good deal of information has been garnered in the last few decades regarding the neural and hormonal control of female sexual behavior, literature elucidating these mechanisms with respect to female sexual motivation has been scarce. We believe that one reason for this is the lack of a standardized paradigm that will quantify female sexual motivation while allowing for sexual interaction to occur. Here we describe a two-chambered apparatus that utilizes operant responding (nose poking) to quantify female sexual motivation. During the test, the female exhibits nose pokes to gain access to a sexually active male, with whom she is allowed to mate. Therefore, this apparatus allows for examination of sexual behavior as well as quantification of sexual motivation by assessing the number of nose pokes the female will exhibit within a fixed interval to gain access to the male.
We report that hormone priming significantly increases sexual motivation in the female as indicated by the number of nose pokes she will exhibit to gain access to the male. Additionally, hormone primed females enter the male compartment after a shorter period and spend more time in direct contact with the male compared to when they are not hormone primed. In contrast, when females are not hormone primed they spend more time in view, but out of reach, of the male.
This paradigm will help to advance the study of female sexual motivation, providing a method for quantifiable assessment of female sexual motivation while allowing for sexual activity to occur.
1. Introduction
Sexual motivation in the female rat: What is it, and how can it be measured?
Reproductive behavior can be broadly classified into two phases: the consummatory phase (the ability to engage in, or the act of copulation), and the appetitive phase (or motivation for reproduction). While female sexual ability is easy to observe and measure in the laboratory rat (e.g., lordosis), whether or not a female rat is sexually motivated, and the extent of motivation, is not as easy to quantify. A number of different paradigms have been used in the past to “measure” female sexual motivation. As we will discuss below, we believe there are significant drawbacks to each of these methods. In order to examine female sexual motivation, a method that quantitatively assesses motivation must be employed.
The field of sexual behavior research began with investigators focused on the male's behavior (Beach, 1942; Beach and Levinson, 1950; Beach, 1956) with little consideration for the female (Pfaff and Lewis, 1974). When attention turned to the female, Beach described female sexual behavior as being composed of attractivity, proceptivity and receptivity, with attractivity and proceptivity described as abstract concepts (Beach, 1976). Subsequently, proceptivity has been considered analogous to motivation, but Beach (1976) described proceptivity as “appetitive activities shown by females in response to stimuli received by males” (p. 114). So the behavior of the female was described relative to the male, rather than as an independent behavior intrinsic to the female. Advancements in the area of female sexual behavior research were lead by the groundbreaking experiments of Martha McClintock (McClintock and Adler, 1978; McClintock and Anisko, 1982; McClintock et al., 1982). McClintock showed that, when given the opportunity, a female rat controls the rate of the interaction with the male, pacing the sexual encounter. McClintock and colleagues identified specific behaviors exhibited by the female in a semi-natural paradigm that were not always evident during testing in a standard chamber: ear wiggling, hopping and darting, and approach toward, orientation to, and runaway from the male (McClintock and Adler, 1978). These female behaviors are efficient at soliciting an intromission from the male, indicating a role for the female in the sexual interaction that had not been identified before.
McClintock's work changed the way the field thought of the sexual encounter between a male and female rat, when a more naturalistic environment allowed for quantification of the full role of the female as it pertained to the interaction. The work of Mary Erskine went on to further refine measures of female sexual behavior in a testing situation that allowed the female to demonstrate an active role in the encounter (Erskine, 1989; Erskine et al., 1989; Coopersmith and Erskine, 1994).
The question that remains, however, is whether and/or which of the behaviors that were identified indicate sexual motivation vs. reflexive responses demonstrated by a sexually receptive female toward a sexually active male. A number of different experimental paradigms, and behaviors exhibited by the female, have been used in an attempt to identify and measure female sexual motivation. Some investigators have used whether a female will seek to be close to a sexually active male (McDonald and Meyerson, 1973; Meyerson et al., 1973; Clark et al., 1981; Edwards and Pfeifle, 1983; Williams et al., 1991). These proximity tests do not allow for copulation to occur so whether this is a measure of sexual motivation or driven by other social factors cannot be determined. Other experiments have examined how much electrical current a female rat is willing to withstand while crossing an electrified grid in order to gain access to a male (McDonald and Meyerson, 1973; Meyerson et al., 1973). As with the proximity measure above, the experimental female is exposed to olfactory, visual and auditory sensory stimulation from a sexually active male without copulating with him. Findings obtained with this type of a test, therefore, may indicate primarily social incentives rather than sexual ones.
Examination of female sexual behavior using a female-paced paradigm (i.e., “pacing”), which allows the female to regulate the timing of sexual interactions by restricting the male to one side of an apparatus and allowing the female to freely traverse the chamber, has yielded a great deal of information. Specifically, these studies have helped to elucidate the female-preferred temporal pattern of copulatory behavior, identified brain regions and neurotransmitters important for the display of female sexual behavior, and highlighted the importance of female-paced copulation for reward and positive affect (Yang and Clemens, 1997 and 2000; Jenkins and Becker, 2001; Coria-Avila et al., 2005; Paredes and Vazquez, 1999; Pfaus and Coria-Avila, 2007; Georgescu and Pfaus, 2006). The use of the pacing paradigm to measure female sexual motivation, however, is limited. For example, a reduction in the number of times the female leaves the male after an intromission disrupts the pattern of copulation, but may not indicate increased motivation for sex and should not be interpreted as such. Further, experiments utilizing pacing in conjunction with in vivo microdialysis have demonstrated an increase in the release of dopamine in the reward pathway in anticipation of sexual contact. While this increase may be important for female sexual motivation, it does not assess sexual motivation in a quantitative way (Jenkins and Becker, 2001; Jenkins and Becker, 2003a; Jenkins and Becker, 2003b; Xiao and Becker, 1997).
More recently, the number of level changes in a bilevel apparatus (Pfaus et al., 1999; Afonso et al., 2007) has also been used as an index of sexual motivation. Using a bilevel chamber allows for the expression of sexual activity, but the “appetitive” measure used – number of level changes before introduction of the male – is open to interpretation. For example, changing levels may be due to an increase in general activity or anticipation of social interaction, and not to increased sexual motivation.
Another confound of examining female sexual motivation is that some of the behaviors that are used to “measure” sexual motivation may be more reflexive, rather than motivationally driven. For example, the mating posture lordosis has been used as an index of sexual motivation. Upon examining the neural circuitry controlling lordosis (Pfaff, 1980), it is clear that the exhibition of this posture is reflexive in nature. Alternatively, the number of precopulatory behaviors (hopping/darting and ear wiggling) exhibited by the female has been used as a measure of sexual motivation, as the display of these behaviors appears to be correlated to sexual receptivity. A female will exhibit these behaviors in a non-reproductive context, however, such as when triggered by manually stroking the hind flanks (personal observations). Therefore, it appears as though these behaviors are more reflexive than motivationally driven.
An ideal paradigm would allow for sexual activity to occur, while employing an objective, quantifiable measurement of sexual motivation. Because there have not been standardized paradigms to quantitatively assess motivation in a reproductive context in rodents, the neural circuitry and neurotransmitters responsible for mediating reproductive motivation in the female have not been clearly defined. In order to address this concern and advance the field of female reproduction, a technique that allows for direct quantifiable assessment of sexual motivation is needed.
Measuring motivation: Utilizing operant response
The motivation to demonstrate a specific behavior in order to receive reinforcement can be quantified by the use of an operant response paradigm. In an operant task an animal is trained to exhibit a behavioral response (e.g., a nose poke or a lever press) in order to receive a reward. Everitt and colleagues used a second order schedule of reinforcement to differentiate male sexual ability versus motivation. In these experiments, male rats were trained to lever press for a secondary reinforcer (a light cue) that had been previously paired with the primary reinforcer, a sexually receptive female (Everitt and Stacey, 1987; Everitt, 1990). With this paradigm, they were able to demonstrate the neuroanatomical dissociation of sexual motivation (bar pressing for access to the female) versus sexual ability. Prior to this, French et al. used operant responding to examine partner preference in females, training females to bar press for their preferred partner (French et al., 1972). Additionally, Bermant used lever-pressing to examine latencies to bar press after females received a mount from a male, compared with intromission or ejaculation (Bermant, 1961). Surprisingly, an operant paradigm has never been used to examine sexual motivation in females.
Quantitative assessment of female sexual motivation: Using an operant paradigm
We have addressed this need by developing an apparatus that utilizes operant responding (nose poking) to quantify female sexual motivation (Figure 1). Our paradigm allows for examination of sexual behavior (including traditional pacing and behavioral measures) as well as quantification of sexual motivation by assessing the number of nose pokes the female will exhibit to gain access to the male. During our first application of this paradigm, we found that hormone primed female rats are significantly more motivated to obtain access to a sexually active male, approach the male after a shorter duration, and spend more time in direct contact with the male rat than when they are not hormone primed.
Figure 1.
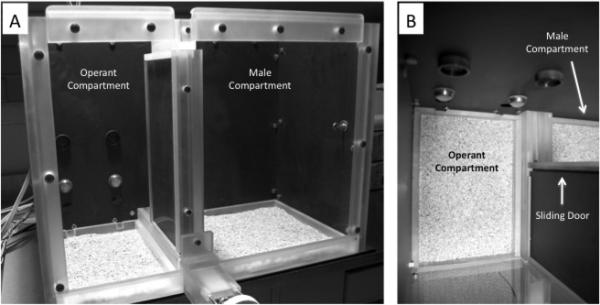
Photographs of the apparatus. Panel A shows a front angled view of the apparatus. On the left is the Operant Compartment, containing the two nose pokes holes (located 7.5 cm from the ground) with associated lights and optional tone (located above the holes). Operant responses (i.e., nose pokes) in the appropriate hole open the door, which separates the Operant Compartment from the Male Compartment (door opens 12 cm, open in these photos). Panel B is a view looking down into the Operant Compartment. The Male Compartment is clearly visible through the open door. (Photographs courtesy of Christel Westenbroek.)
2. Materials and Methods
2.1 Animals and Preparation
Experimental animals were eight Long-Evans female rats, 55-60 days of age upon arrival, obtained from Charles River (Portage, MI). Eight adult, proven breeder males were used as the stimulus males (Charles River, Portage, MI). Animals were housed in same-sex pairs in standard laboratory cages and maintained on a 14:10, light:dark cycle (lights off at 10:00 am) with free access to rat chow and water. Rooms were maintained at a constant temperature of 20–22°C. All procedures were conducted in accordance with the National Institutes of Health (NIH) guidelines on laboratory animal use and care, using a protocol approved by the University of Michigan Committee on Use and Care of Animals.
Females were bilaterally ovariectomized under isoflurane anesthesia (5%, inhalation) via a single dorsal incision. Beginning 5 days after surgery, vaginal epithelium was sampled daily via saline lavage and examined under a microscope to ensure complete ovariectomy. Males were left intact. Hormone priming consisted of subcutaneous injections of 10 μg of 17 β-estradiol benzoate (EB; in 0.1 ml peanut oil) 48 hr prior to testing, and 500 μg of progesterone (P; in 0.1 ml peanut oil) 4-6 hr before testing.
2.2 Apparatus
The apparatus was designed by our laboratory and built by ArborWind Farm (ArborWind Farm, L.L.C., Grass Lake, MI; Figure 1). The apparatus is a dual compartment chamber constructed of Plexiglas. Three of the sides of the chamber are made of black quarter-inch thick Plexiglas with one long side (the front of the chamber) made of clear Plexiglas to allow subjects to be viewed. The smaller of the two compartments (the Operant Compartment) measures 22 cm wide × 30 cm deep × 45 cm high, and the larger compartment (the Male Compartment) measures 36 cm wide × 30 cm deep × 45 cm high. A door that slides horizontally opens from one side separating the two compartments (opened doorway is 12 cm wide). When open the female can travel from one compartment to the other. The wall of the operant compartment opposite the clear front wall contains two nose poke holes (MedAssociates, Inc) positioned 7.5 cm above the ground, 15 cm apart. Three cm above each hole is a light associated with the hole, which becomes illuminated when the animal nose pokes in the hole below. The door that separates the compartments is to the right of the nose pokes holes, and the hole closest to the door is always the active hole. The hole furthest from the door is the inactive hole. The male is tethered to the corner of the chamber furthest from the door by a strong wire that attaches to a removable suede vest. The tethering allows for the male to exhibit the full complement of sexual behavior (mounting, intromissions, ejaculations, chasing) while limiting only his ability to cross into to the operant compartment.
Animals are tracked using a Sony Mini DV HandyCam that interfaces with the computer and ANY-maze tracking software, utilizing a custom-made interface box (ANY-maze; Stoelting Co., Inc., Wood Dale, IL) to control the operation of the chamber.
2.3 Training
Testing occurred once per week, with at least 72 hr between a testing session and the next exposure to EB. For sessions one to six and Test 1, females received the hormone priming as described above. For Test 2, females did not receive any exposure to hormones. Males were always allowed a 5 min acclimation period before the female was introduced into her chamber. For the first three consecutive training sessions, the male was tethered on his side of the apparatus and the door between the chambers remained opened, allowing the female to become familiar with the apparatus and to “pace” the sexual encounter, as she moved between the two compartments of the chamber and engaged in sexual activity with the male.
Sessions 1 – 6 lasted for 30 min, while Tests 1 and 2 lasted 15 min. The 15 min test this allowed for one and in many cases two ejaculations for each female in the ‘hormone primed’ group, while reducing the amount of time the females are in the chamber during the ‘no hormone’ tests. (In preliminary tests, females remaining in the apparatus longer than 15 min when not hormone primed began jumping out of the chamber.)
For session four, the door between the chambers was closed prior to the introduction of the animals. As the female approached the active nose poke hole for the first time, the experimenter illuminated the associated light and the door was opened. The light remained on for 5 s, and the door remained opened until the female re-entered her side of the chamber. As the session progressed, the female was required to come closer and closer to the nose poke hole in order for the experimenter to illuminate the appropriate light and open the door. In all cases, the female successfully poked her nose in the active hole, and thus opened the door, within the 45 min test. Session five was similar to session four, although all chamber outputs (i.e., illumination of lights, opening and closing of the door) were controlled by the computer. This session continued to run on a fixed ratio 1 (FR1) schedule, in which every nose poke resulted in immediate illumination of the associated light and opening of the door.
Session six was similar to five, in that the computer controlled all outputs. However, the paradigm was adjusted in order to obtain a quantitative measure of motivation for the female to gain access to the male: the first nose poke in the active hole started a 15 s timer (fixed interval 15 s, FI15s). During this interval, nose pokes were recorded but had no consequence. The first active nose poke following the completion of the 15 s interval opened the door to the male chamber. The addition of the FI 15 s schedule allowed for a variable number of nose pokes to occur before the door was opened.
2.4 Testing
All data presented in this paper were obtained from the seventh and eight sessions, utilizing a FI 15 s schedule (as in session 6). For session seven (Test 1), females were hormone primed with EB and P as they were for all previous sessions. For session eight (Test 2), females did not receive any hormone priming prior to the test (therefore, the last exposure to EB was 9 days before the Test, and the last exposure to P was 7 days prior to the test). The following measures were recorded: the latency to the first nose poke, latency to first door opening, latency to enter the male side, number of times the door was opened, number of pokes per door opening, percentage of openings that resulted in the female crossing to the male side, duration of time spent in the operant side (out of view of the male), duration of time spent on the male side (in direct contact with the male), and duration of time spent in the doorway between the chambers (in view, but out of reach, of the male). All measures were analyzed using within subjects paired comparisons (t-tests).
2.5 Data Analysis
Data for the number of nose pokes, door openings, and initial latencies were obtained from the ANY-maze software program. All durations and sexual behavior data were acquired by watching recorded videos in real-time and scoring the behavior using the JWatcher Program (UCLA Office of Instructional Development). The videos were analyzed by the same observer and were checked by a second observer to ensure accuracy (inter-observer reliability >90%). Data were analyzed using paired t-tests (with hormone condition as the comparison) using GraphPad Prism 5.
3. Results
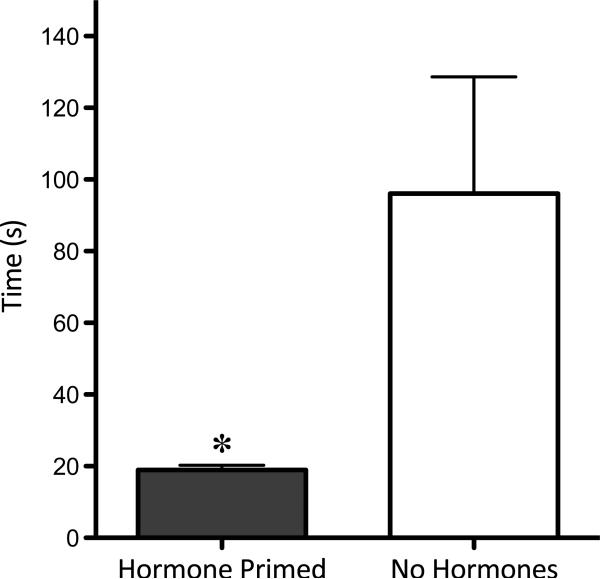
There was no effect of hormone priming on the latency to first nose poke or door opening (data not shown). Hormone priming, however, did affect the latency to enter the male side of the apparatus (p=0.05), with females crossing to the male side significantly faster after hormone priming than when not treated with hormones (Figure 2).
Figure 2.
Latency to enter male compartment. Females that received prior exposure to estradiol benzoate (10 μg, 24 hr prior to test) and progesterone (500μg, 4-6 hr prior to test) entered the male side of the apparatus significantly faster than they did when they had not been hormone primed (p=0.05).
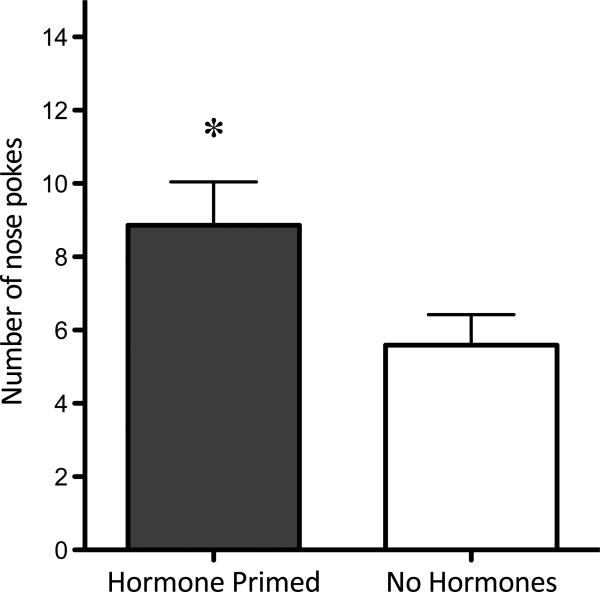
There was no effect of hormone priming on the number of times the females open the door (data not shown). When females received hormone priming they made more nose pokes per door opening compared to when they were not hormone primed (p=0.02; Figure 3).
Figure 3.
Sexual motivation. When hormone primed prior to the test, females worked significantly harder to obtain access to the male (i.e., demonstrated significantly more nose pokes per door opening) than when not primed (p=0.0247).
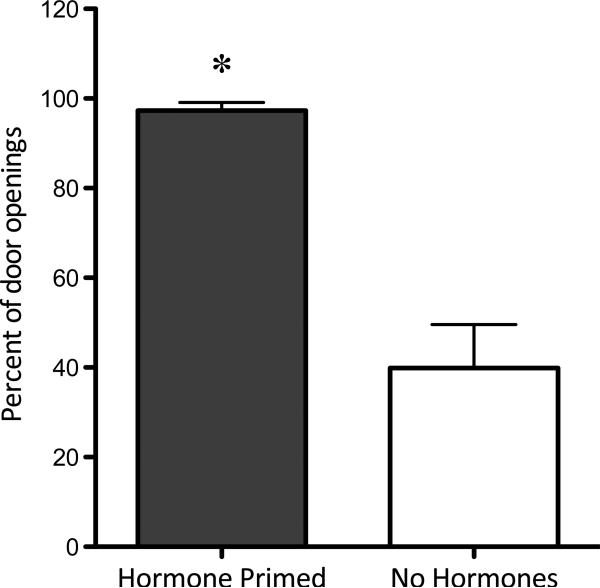
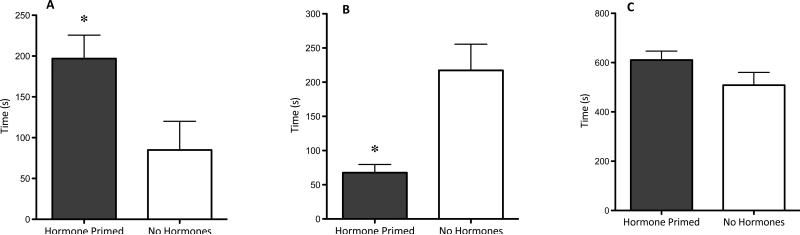
Hormone primed females also crossed to the male side a greater percentage of the time upon door opening than when they were not hormone primed (p=0.001; Figure 4). Once on the male side of the apparatus, hormone primed females remained on the male side of the apparatus longer than when not hormone primed (p=0.016; Figure 5) and reduced the amount of time spent in the doorway of the apparatus (that is, in view, but out of reach, of the male; p=0.008). Hormone priming did not alter the total amount of time the female spent on the initial side of the apparatus (out of view of the male). Hormone priming also affected sexual behavior, with 100% of the hormone primed females exhibiting lordosis and hopping/darting, and 0% of the non-primed females exhibiting the behaviors (data not shown).
Figure 4.
Percent entries to male compartment. The percentage of door openings that result in the females’ crossing to the male compartment is significantly higher when the females are hormone primed prior to the test compared to when they are not (p=0.001).
Figure 5.
Time spent in apparatus compartment. Hormone primed females spend significantly more time in contact with the male (p=0.016; panel A) and significantly less time in the doorway of the apparatus (in view, but out of reach, of the male) (p=0.008; panel B) than when they are not primed with hormones. Hormone exposure does not change the total amount of time the female spends on the operant side of the apparatus (p=0.136; panel C).
Discussion
We have described a novel testing apparatus and paradigm for use in quantification of female rat sexual motivation and sexual behavior. Utilizing an operant response paradigm to gain access to a sexually active male, the number of nose pokes the female makes is a quantitative measure of the female's sexual motivation. This measure can be combined with the data obtained when the female chooses to engage (or not engage) in sexual activity with the male to determine both sexual motivation and sexual ability.
Nose poking is a valid measure of motivation used throughout the field of drug abuse in order to test the motivation of animals to obtain a specific reward (e.g., an intravenous infusion of cocaine; (Becker and Hu, 2008)). The fixed interval schedule allows for a variable number of nose pokes to occur and, thus, a quantitative measure of motivation; females that poke more during that interval are more motivated to gain access to the male. We tested females with (10 μg estradiol benzoate, EB; and 500 μg progesterone, P) and without hormone priming to examine the effect of EB and P on sexual motivation. While hormone priming did not alter measures of general activity such as latency to first door opening and number of door openings per test, we found effects of hormones on measures specific to sexual motivation. Hormone primed females worked harder to obtain access to the sexually active male, demonstrated reduced latencies to contact the male, crossed over to the male side a greater percentage of the time, and spent more time in direct contact with the male compared to when the females do not receive hormone priming.
It has been well established that hormone priming increases a female's receptivity, allowing for sexual activity to occur (Hardy and DeBold, 1971; Parsons et al., 1980; Erskine, 1985). This is the first time, however, that an operant paradigm has been used to quantify the female's motivation for a sexually active male, and to show that motivation increases with hormone priming. As discussed in the Introduction, a number of experiments have examined sexual behavior in the female rat using a variety of techniques so we know a great deal about the neural systems involved in the consummatory components of copulation in the female. Examination of sexual motivation, however, has been limited in that previous methods do not quantify motivation. We describe a new apparatus that utilizes female-paced copulation and includes a quantifiable measurement of motivation: operant response. This addition allows for the more naturalistic female-paced copulation with traditional pacing measures, while including a technique to measure motivation. As demonstrated with the experiment presented here, the increased effort on the part of the female to gain access to the male coupled with the decreased latency to contact the male and sexual behavior exhibited when with the male clearly indicate increased sexual motivation of the female.
Importantly, our paradigm shows that time spent near a male is not necessarily the same as increased sexual motivation, as others have indicated (Edwards and Pfeifle, 1983; de Jonge and van de Poll, 1986; Williams et al., 1991). Females that are not sexually motivated spend less time in direct contact with the male but spend more time in close proximity to (but out of reach of) the male. In this test, the females spend more time in the doorway of the apparatus when they are not hormone primed, and less time with the male. Because our test allows for sexual interaction to occur, the female would have engaged in sexual activity after the door opened if she was motivated to do so. By the female choosing to not engage in copulation but to remain close to the male may indicate that she still enjoys some aspects of the social interaction that the chamber allows. Similarly, the non-hormone primed females will continue to poke to open the door to the male side, although to a significantly smaller extent than when hormone primed. Some aspect of the social interaction may be driving this continuation to respond to see the male. On the other hand, both rats and mice have been shown to exhibit operant responses for visual stimuli (Winterbauer and Balleine, 2007; Olsen and Winder, 2009). Therefore, the females may simply be poking to see and hear the visual stimuli that accompany the active pokes (illumination of the active light, and opening of the door).
The discussion of McClintock's work in the Introduction highlights the benefits of implementing a new, forward-thinking paradigm, which allowed for significant advancements in the field of female sexual behavior. In order to continue advancing the field, there is a need for a paradigm that builds on previous techniques while measuring female sexual motivation. The apparatus we present in this paper addresses that need. While this chamber does not represent a naturalistic mating situation, especially for the tethered male, it employs characteristics of female-preferred paced copulation and allows the female to escape from the male, as is typically seen in the wild. In addition, it makes use of operant responding to measure the female's motivation for sex and allows for examination of traditional sexual behavior. It is possible that restricting the male's movement to one chamber of the apparatus may alter the male/female interactions. This restriction is necessary, however, in order to achieve the primary goal of this paradigm, which is to evaluate sexual motivation of the female. Additionally, we have not found any significant differences in male sexual activity when he is tethered in one chamber compared to when he can move about one chamber in a pacing apparatus, after he has gained sexual experience in the apparatus with a stimulus female. Tethering is necessary in this paradigm for the automation of the apparatus.
Further Applications
This first set of experiments has shown that hormone priming affects sexual motivation in the female. Additionally, we have demonstrated that time spent near a male does not necessarily equate to increased sexual motivation. This paradigm can be used to answer a number of other long-standing questions that remain with respect to female sexual behavior and motivation, adding to current explanations and knowledge by expanding upon findings. For example, this paradigm can delineate the difference between social and sexual motivation, and can quantitatively assess an animal's preference for a specific partner. When used in conjunction with electrophysiological techniques, acute events that occur during the test can elucidate the involvement of brain regions, neurotransmitters and monoamine in appetitive vs. consummatory behaviors.
Conclusion
Here we report on a novel paradigm that utilizes operant response to quantify female sexual motivation. We find that hormone priming significantly increases female sexual motivation, as determined by the female's willingness to work harder to obtain access to a sexually active male and the behavior she exhibits after gaining access to the male chamber. These findings are the first of their kind to use operant response to measure sexual motivation in the female rat. We believe that the versatility of this chamber and paradigm will allow for significant advances in the study of female sexual behavior and motivation.
Highlights.
We describe a novel operant testing apparatus to measure female rat sexual motivation.
Sexual motivation and sexual activity are quantitatively and qualitatively assessed.
Here, sexual motivation is examined in females with and without hormone priming.
Females work harder to get to a male and approach him faster when hormone primed.
ACKNOWLEDGEMENTS
Financial support for this research was contributed by NIH grant R01-DA012677 to JBB and NIH grant 5R21-DA27924-2 to JBB and JAC. We thank Pfizer, Inc. for a grant that contributed to the construction and initial testing of the chamber. The authors would also like to thank Brandon Luma for his technical expertise in the set-up and continuous maintenance of the chamber.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121:515–526. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- Beach FA. Analysis of factors involved in the arousal, maintenance and manifestation of sexual excitement in male animals. Psychosom Med. 1942;4:173–198. [Google Scholar]
- Beach FA. Characteristics of masculine “sex drive.”. In: Jones MR, editor. Nebraska Symposium on Motivation. Univ. Nebraska Press; Lincoln, NB: 1956. pp. 1–31. [Google Scholar]
- Beach FA. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm Behav. 1976;7:105–138. doi: 10.1016/0018-506x(76)90008-8. [DOI] [PubMed] [Google Scholar]
- Beach FA, Levinson G. Effects of androgen on the glans penis and mating behavior of castrated male rats. J Experiment Zoology. 1950;114:159–168. [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermant G. Response latencies of female rats during sexual intercourse. Science. 1961;133:1771–1773. doi: 10.1126/science.133.3466.1771. [DOI] [PubMed] [Google Scholar]
- Clark AS, Pfeifle JK, Edwards DA. Ventromedial hypothalamic damage and sexual proceptivity in female rats. Physiol Behav. 1981;27:597–602. doi: 10.1016/0031-9384(81)90228-6. [DOI] [PubMed] [Google Scholar]
- Coopersmith C, Erskine MS. Influence of paced mating and number of intromissions on fertility in the laboratory rat. J Reprod Fertil. 1994;102:451–458. doi: 10.1530/jrf.0.1020451. [DOI] [PubMed] [Google Scholar]
- Coria-Avila GA, Ouimet AJ, Pacheco P, Manzo J, Pfaus JG. Olfactory conditioned partner preference in the female rat. Behav Neurosci. 2005;119:716–725. doi: 10.1037/0735-7044.119.3.716. [DOI] [PubMed] [Google Scholar]
- de Jonge FH, van de Poll NE. On the involvement of progesterone in sexually rewarded choice behavior of the female rat. Physiol Behav. 1986;37:93–98. doi: 10.1016/0031-9384(86)90389-6. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Pfeifle JK. Hormonal control of receptivity, proceptivity and sexual motivation. Physiol Behav. 1983;30:437–443. doi: 10.1016/0031-9384(83)90150-6. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Effects of paced coital stimulation on estrus duration in intact cycling rats and ovariectomized and ovariectomized-adrenalectomized hormone-primed rats. Behav Neurosci. 1985;99:151–161. doi: 10.1037//0735-7044.99.1.151. [DOI] [PubMed] [Google Scholar]
- Erskine MS. Solicitation behavior in the estrous female rat: a review. Horm Behav. 1989;23:473–502. doi: 10.1016/0018-506x(89)90037-8. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Kornberg E, Cherry JA. Paced copulation in rats: effects of intromission frequency and duration on luteal activation and estrus length. Physiol Behav. 1989;45:33–39. doi: 10.1016/0031-9384(89)90163-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–232. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): II. Effects of preoptic area lesions, castration, and testosterone. J Comp Psychol. 1987;101:407–419. [PubMed] [Google Scholar]
- French D, Fitzpatrick D, Law OT. Operant Investigation of Mating Preference in Female Rats. J Comp Physiol Psychol. 1972;81:226–232. doi: 10.1037/h0033535. [DOI] [PubMed] [Google Scholar]
- Georgescu M, Pfaus JG. Role of glutamate receptors in the ventromedial hypothalamus in the regulation of female rat sexual behaviors - I. Behavioral effects of glutamate and its selective receptor agonists AMPA, NMDA and kainate. Pharmacol Biochem Behav. 2006;83:322–332. doi: 10.1016/j.pbb.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Hardy DF, DeBold JF. The relationship between levels of exogenous hormones and the display of lordosis by the female rat. Horm Behav. 1971;2:287–297. [Google Scholar]
- Jenkins WJ, Becker JB. Role of the striatum and nucleus accumbens in paced copulatory behavior in the female rat. Behav Brain Res. 2001;121:119–128. doi: 10.1016/s0166-4328(00)00394-6. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. European J Neurosci. 2003a;18:1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003b;43:503–507. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- McClintock MK, Adler NT. The role of the female during copulation in wild and domestic Norway rsts (Rattus norvegicus). Behavior. 1978;67:67–96. [Google Scholar]
- McClintock MK, Anisko JJ. Group mating among Norway rats. I. Sex differences in the pattern and neuroendocrine consequences of copulation. Anim Behav. 1982;30:398–409. [Google Scholar]
- McClintock MK, Anisko JJ, Adler NT. Group mating among Norway rats. II. The social dynamics of copulation: Competition, cooperation, and mate choice. Anim Behav. 1982;30:410–425. [Google Scholar]
- McDonald PG, Meyerson BJ. Effect of Estradiol, Testosterone and Dihydrotestosterone on Sexual Motivation in Ovariectomized Female Rat. Physiol Behav. 1973;11:515–520. doi: 10.1016/0031-9384(73)90038-3. [DOI] [PubMed] [Google Scholar]
- Meyerson BJ, Lindstrom L, Nordstrom EB, Agmo A. Sexual Motivation in Female Rat after Testosterone Treatment. Physiol Behav. 1973;11:421–428. doi: 10.1016/0031-9384(73)90026-7. [DOI] [PubMed] [Google Scholar]
- Olsen CM, Winder DG. Operant sensation seeking engages similar neural substrates to operant drug seeking in C57 mice. Neuropsychopharmacol. 2009;34:1685–1694. doi: 10.1038/npp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes RG, Vazquez B. What do female rats like about sex? Paced mating. Behav Brain Res. 1999;105:117–127. doi: 10.1016/s0166-4328(99)00087-x. [DOI] [PubMed] [Google Scholar]
- Parsons B, Maclusky NJ, Krey L, Pfaff DW, McEwen BS. The temporal relationship between estrogen-inducible progestin receptors in the female rat-brain and the time course of estrogen activation of mating behavior. Endocrinology. 1980;107:774–779. doi: 10.1210/endo-107-3-774. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Estrogens and brain function: Neural analysis of hormone-controlled mammalian reproductive behavior. Springer-Verlag Press; New York, NY: 1980. [Google Scholar]
- Pfaff DW, Lewis C. Film analyses of lordosis in female rats. Horm Behav. 1974;5:317–335. doi: 10.1016/0018-506x(74)90018-x. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Coria-Avila GA. Neuronal activation by stimuli that predict sexual reward in female rats. Neurosci. 2007;148:623–632. doi: 10.1016/j.neuroscience.2007.05.052. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Smith WJ, Coopersmith CB. Appetitive and consummatory sexual behaviors of female rats in bilevel chambers - I. A correlational and factor analysis and the effects of ovarian hormones. Horm Behav. 1999;35:224–240. doi: 10.1006/hbeh.1999.1516. [DOI] [PubMed] [Google Scholar]
- Williams GW, Goldman J, McGinnis MY, Possidente B, Lumia AR. Effects of ovarian hormones on sexual receptivity, proceptivity, and motivation in olfactory bulbectomized female rats. Physiol Behav. 1991;50:751–755. doi: 10.1016/0031-9384(91)90013-e. [DOI] [PubMed] [Google Scholar]
- Winterbauer NE, Balleine BW. The influence of amphetamine on sensory and conditioned reinforcement: evidence for the re-selection hypothesis of dopamine function. Front Integr Neurosci. 2007;1:9. doi: 10.3389/neuro.07.009.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Becker JB. Hormonal activation of the striatum and the nucleus accumbens modulates paced mating behavior in the female rat. Horm Behav. 1997;32:114–124. doi: 10.1006/hbeh.1997.1412. [DOI] [PubMed] [Google Scholar]
- Yang LY, Clemens LG. Function of intromissions on intromission-return latency of female rats during paced sexual behavior. Physiol Behav. 1997;61:889–894. doi: 10.1016/s0031-9384(96)00614-2. [DOI] [PubMed] [Google Scholar]
- Yang LY, Clemens LG. MPOA lesions affect female pacing of copulation in rats. Behav Neurosci. 2000;114:1191–1202. [PubMed] [Google Scholar]