Abstract
The intracellular domain of ErbB4 receptor tyrosine kinase is known to translocate to the nucleus of cells where it can regulate p53 transcriptional activity. The purpose of this study was to examine whether ErbB4 can localize to the nucleus of adult rat ventricular myocytes (ARVM), and regulate p53 in these cells. We demonstrate that ErbB4 does locate to the nucleus of cardiac myocytes as a full-length protein, although nuclear location occurs as a full-length protein that does not require Protein Kinase C or γ-secretase activity. Consistent with this we found that only the non-cleavable JM-b isoform of ErbB4 is expressed in ARVM. Doxorubicin was used to examine ErbB4 role in regulation of a DNA damage response in ARVM. Doxorubicin induced p53 and p21 was suppressed by treatment with AG1478, an EGFR and ErbB4 kinase inhibitor, or suppression of ErbB4 expression with small interfering RNA. Thus ErbB4 localizes to the nucleus as a full-length protein, and plays a role in the DNA damage response induced by doxorubicin in cardiac myocytes.
Keywords: ErbB4, doxorubicin, DNA damage, p53, p21WAF1/CIP1
INTRODUCTION
ErbB4 is a member of the epidermal growth factor receptor (EGFR) family including ErbB1/EGFR, ErbB2/HER2, ErbB3/HER3, and ErbB4. ErbB4 is expressed in many tissues including heart, skeletal muscle, and epithelial cells [1] and has diverse function in part due to alternative splicing (for review see [2]). Alternative splicing of ErbB4 at a cytoplasmic site results in the presence (CYT-1) or absence (CYT-2) of a PI-3-kinase interacting domain that couples ErbB4 to prosurvival and metabolic pathways. This functions of ErbB4 in the heart, including regulation cardiac development at the stage of trabeculation [3] and maintenance of cardiac function in the adult mouse [4], presumably involves CYT-1 ErbB4 given the critical role for signaling through PI-3-kinase in response to the ligand Neuregulin-1β (Nrg-1β) [5; 6].
A juxtamembrane (JM) splice site leads to JM-a and JM-b ErbB4 variants which can couple this receptor to other pathways. TACE (tumor necrosis factor- alpha converting enzyme) and γ-secretase both cleave ErbB4 JM-a in response to protein kinase C (PKC) activation through phorbol esters, generating a 120kDa receptor extracellular domain and a soluble 80kDa (s80) fragment [7; 8; 9]. The s80 fragment can translocate to the nucleus where it has pro-apoptotic activity involving the Mdm2-p53 dependent pathway [8; 10; 11; 12]. Nrg-1β is also known to stimulate the cleavage and nuclear translocation of s80 ErbB4 in some cell types [13]. Previous expression profiling work suggested the absence of JM-a isoform expression in the heart [7]. However immunolocalization studies in our laboratory suggested that ErbB4 can be found in the nucleus of cardiac myocytes. The purpose of this study was to examine in what form ErbB4 can locate to cardiac myocyte nuclei, and examine whether ErbB4 regulates DNA damage responses in these cells.
METHODS
Primary Culture of Ventricular Myocytes
ARVMs were isolated as previously reported [14; 15]. ARVMs were plated at densities of 80–150 myocytes/mm2 and maintained with Dulbecco’s modified Eagle Medium supplemented with 7% fetal calf serum (Gibco) for 7 to 10 days before serum starvation.
Immunohistochemistry
ARVMs were fixed with 4% paraformaldehyde for 15 min and permeabilized in 0.2% Triton X-100 for 5 min. Adult C57 BL6 mice hearts were embedded in O.C.T. medium (Tissue-Tek), and 5 µM sections were prepared with a cryotome. Nonspecific binding was blocked with 5% Bovine serum albumin in PBS for 1 h, and coverslips were incubated overnight with anti-ErbB4 (Upstate Cell Signaling). A secondary antibody conjugated with FITC and TXRD-conjugated phalloidin (Molecular Probe) was added for 1 hr. Coverslips were mounted using Vectashield (Vector laboratories). TO-PRO-3 staining (Molecular Probes) or DAPI (Vector laboratories) were used according to manufacturers instructions for nuclear staining. Images were generated with FV100 or LSM510 confocal microscope (CIRC, Vanderbilt University).
ErbB4 isoform identification by RT-PCR/plasmid cloning strategy
A cDNA pool was generated by reverse transcriptase (SuperScript First Strand Synthesis System, Gibco BRL) with oligo(dT) priming of total RNA isolated from ARVM primary cultures grown to confluence over 7–10 days. The cDNA underwent PCR amplification with primer sets designed from known rat exon sequences to a common upstream sequence targeting the juxtamembrane domain and a downstream sequence (see Table 1 for primer sequences). The PCR products were TA-cloned into the pCR 2.1-TOPO vector. Colonies were screened by PCR using primer pairs for each isoform, and sequences were confirmed at the Boston University Medical Center Gencore Sequencing Facility (ABI 377–96).
Table 1.
| Forward Primers: 5’-3’ | Reverse Primers: 5’-3’ | |
|---|---|---|
| ErbB41 | TGTCCTACAGGGAGCAAACA | TGGGCATTCCTTGTTGTGTA |
| JM-a2 | GCCTACAGGGAGCAAACAGT | GCATGTTGTGGTAAAGTGGAA |
| JM-b2 | ACCGGGACCTGACAACTGTA | GGCCGATGCAGTCTTCAATA |
| CYT-12 | GGACGCTGAGGAATATTTGG | CCTCTGGTATGGTGCTGGTT |
| CYT-22 | GGACGCTGAGGAATATTTGG | CCTCTGGTATGGTGCTGGTT |
Primers used for the cloning of 1454bp ErbB4.
Primers used for the screening of 167bp JM-a, 157bp JM-b, 221bp CYT-1 and 173bp CYT-2 isoforms.
Western Blot Analysis and Cellular Fractionation
For total protein isolation, cells were lysed in a modified RIPA buffer containing 1%NP-40 (Calbiochem), 0.25% deoxycholic acid, 50mM Tris-HCl (pH 7.4), 1mM EDTA, 150 mM NaCl, protease inhibitor cocktail (Roche). Protein concentrations were quantified with Bradford Reagent (Bio-Rad). 20–50ug of sample was run on a Tris-HCl ready gel (Bio-Rad), and transferred to a PVDF membrane (BioRad). Antibodies for actin (Sigma), ErbB4 (sc283, Santa Cruz), p53 Ab-1 (Calbiochem, #OP03T), bax Ab-5 (Neomarkers #MS1335), mdm2 (Santa Cruz, #sc965), p21WAF1/CIP1 (Santa Cruz), tubulin (Santa Cruz), topoisomerase (BD Bioscience), p53 phospho-serine 15 (Cell Signaling), Mdm2 serine 166 (Cell Signaling) were used for immunoblots using dilutions and blocking conditions as recommended by the supplier.
To obtain nuclear and cytoplasmic fractions, cells were lysed in homogenization buffer (10 mM HEPES pH 7.2, 10mM MgCl2, 24 mM KCl, protease inhibitor cocktail (Roche), 0.2% NP40 (Calbiochem)) and centrifuged (2600 rpm for 10 min). The supernatant was saved as the cytoplasmic fraction; the nuclear pellet was washed and resuspended in homogenization buffer, loaded on 1M sucrose solution and centrifuged (2600 rpm for 15 min). The pellet was resuspended in nuclear extraction buffer (50mM Hepes, 50mM KCl, 300mM NaCl, 10% glycerol plus inhibitors as above). Both the cytoplasmic and the nuclear fractions were then centrifuged (13,000 rpm for 10 min), and the pellet was discarded.
ErbB4 siRNA treatment
ErbB4 siRNA (Xeragon Oligoribonucleotides) was designed to target a common sequence present in all ErbB4 isoforms. Cell were serum starved for 24 hrs followed by RNA transfection (Qiagen TransMessenger Transfection Reagent) with either double-stranded randomly generated control siRNA (uucuccgaacgugucacgu) or ErbB4 siRNA (cgggaaucucaucuuucuu). Cells were lysed 90–96 hrs post-transfection and analyzed for ErbB4 expression by Western blot.
RESULTS
ErbB4 is present in the nucleus of cardiac myocytes as a full-length protein
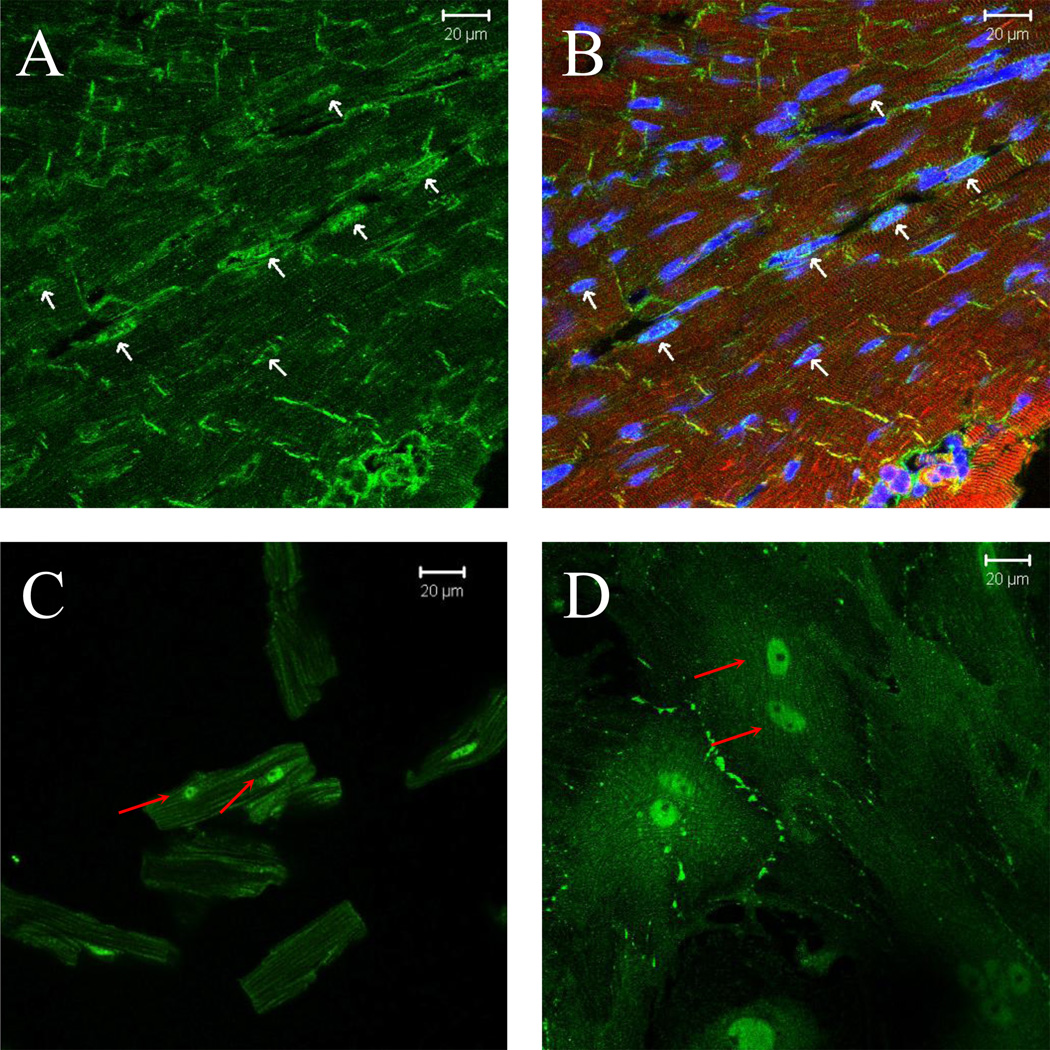
We examined the cellular localization of ErbB4 in heart tissue and in cultured cardiac myocytes by immunostaining and cell fractionation. In the intact heart, ErbB4 was localized primarily in cellular membrane of myocytes with pronounced staining at the intercalated disk (Fig 1A, B). Low levels of nuclear ErbB4 staining was also apparent in some myocyte nuclei. Nuclear ErbB4 staining was present in all cardiac myocytes immediately after isolation (Fig 1C), and increased further when myocytes were kept in culture (Fig 1D). ErbB4 nuclear staining was confirmed in these cells using a second polyclonal C-terminal anti-erbB4 antibody that gave identical pattern of localization (data not shown).
Figure 1. ErbB4 cellular localization and processing.
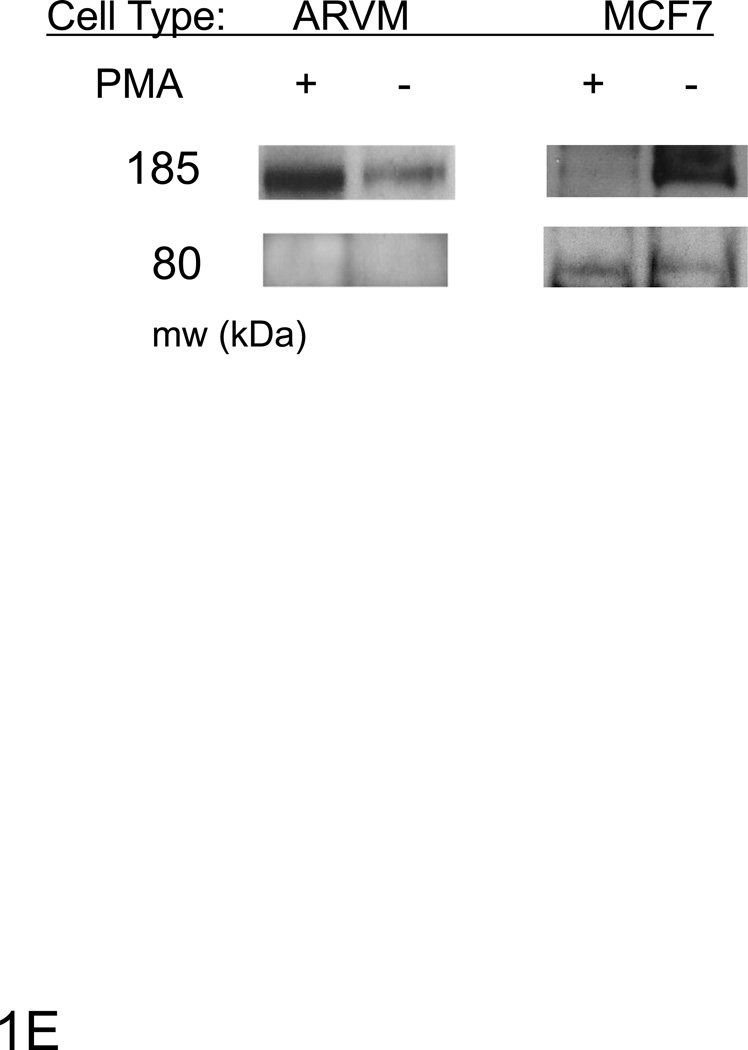
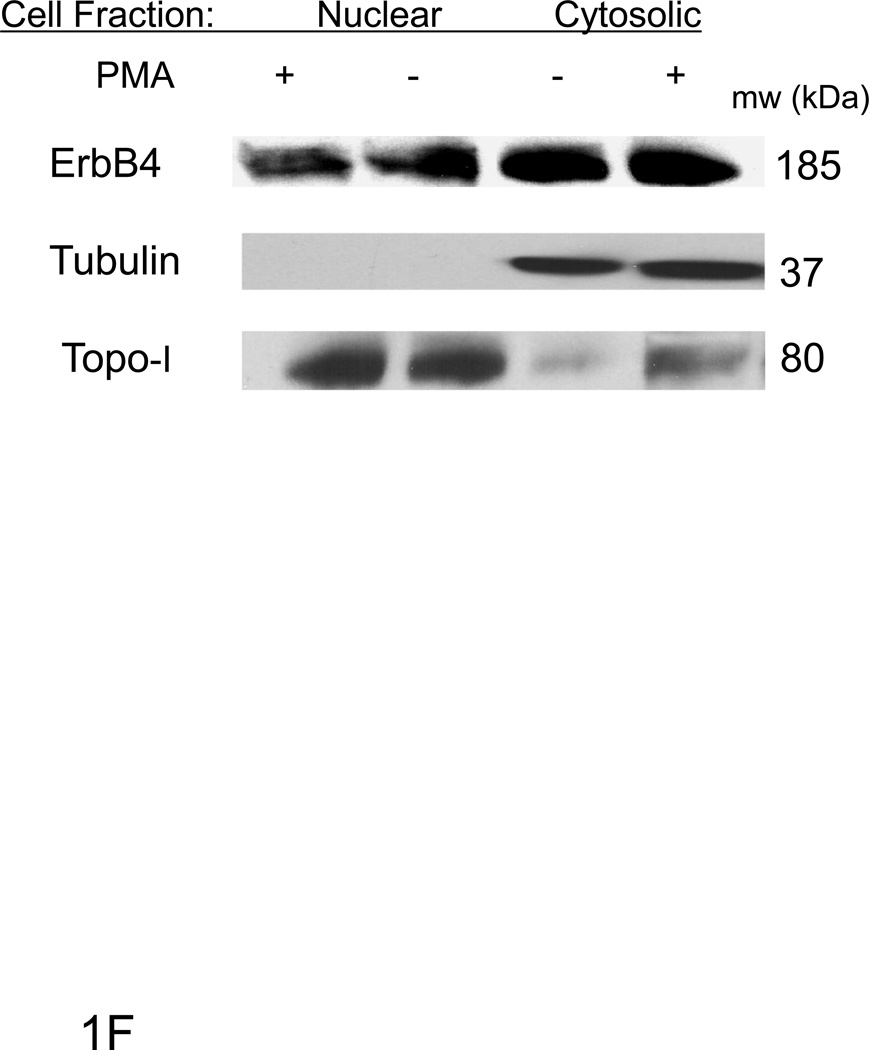
Cross-section of mouse heart (A&B) or short term (C) or long term cultured ARVMs (D) were immunostained for ErbB4 (green), actin was stained with phalloidin (red) and visualized by confocal microscopy. TO-PRO-3 or DAPI was used for nuclear staining (blue). Controls lacking primary anti-ErbB4 antibody showed minimal background staining (data not shown). The bar represents 20 µM. E) ARVM or MCF7 cells were serum starved overnight following PMA (10µM) treatment for 3 hours. Cells were lysed with TGH buffer and lysates were analyzed by immunoblot. For cellular fractionation (F) cells were lysed and separated into nuclear and cytoplasmic fractions. Tubulin (cytoplasmic) and topoisomerase-1 (nuclear) antibodies were used to assess the purity of fractions. Photomicrographs and blots are representative of at least three independent experiments.
We examined whether ErbB4 localizes to cardiac myocyte nuclei via a PKC/γ-secretase pathway as occurs in other cell types [11]. ARVMs were treated for 30 with PMA, and western blots were performed in total cell lysates (Fig 1E) as well as nuclear and cytoplasmic fractions (Fig 1F). In MCF7 cells PMA treatment results in a decrease in full-length ErbB4 with increased expression of an ~80 kDa protein, consistent with γ-secretase dependent ErbB4 cleavage. In contrast, we did not observe the 80 kDa cleavage product in ARVMs, and treatment with phorbol-12-myristate-13-acetate (PMA) did not decrease expression of full length ErbB4. In fact we found that PMA induced an increase in the expression of ErbB4 (Fig 1E). ErbB4 was present in nuclear fractions as a ~185 kDa protein consistent with full-length ErbB4, and this did not change after PMA treatment (Fig 1F). Treatment of ARVM with the γ-secretase inhibitor Compound E also did not change nuclear localization of ErbB4 (data not shown). Thus it appears that nuclear ErbB4 is not a result of γ-secretase-dependent cleavage of ErbB4 in ARVM.
To determine whether the γ-secretase cleavable JM-a isoform of ErbB4 is expressed in ARVM, we assessed the relative expression of ErbB4 isoforms in ARVMs by cloning ErbB4 from ARVMs. We amplified all ErbB4 variants expressed in ARVM as described in methods, and screened clones for specific CYT-1/2, JM-a/b isoform expression. We detected both known cytoplasmic variants CYT-1 and CYT-2 in ARVMs, with CYT-1 isoform appearing more often than CYT-2 (ratio of 4:1). In contrast, 25 of 25 clones screened were JM-b isoform. Thus we conclude that in ARVMs full-length JM-b ErbB4 is able to localize to the nucleus.
Doxorubicin induces a DNA damage response involving p53 and p21/WAF1/CIP1
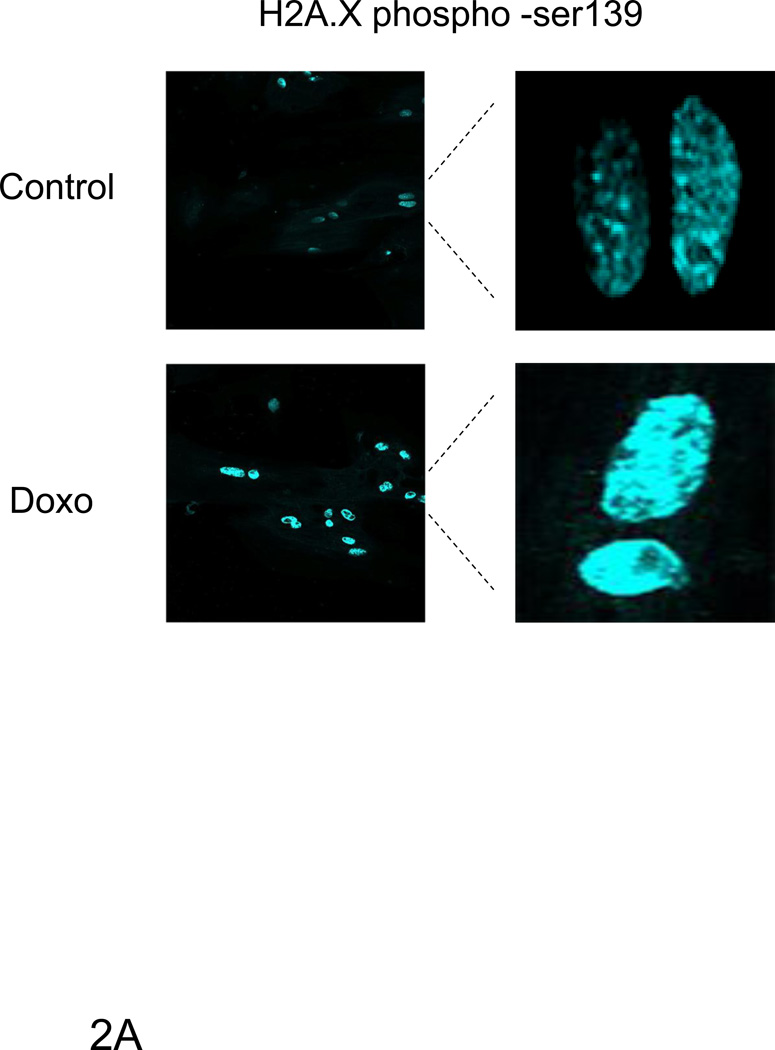
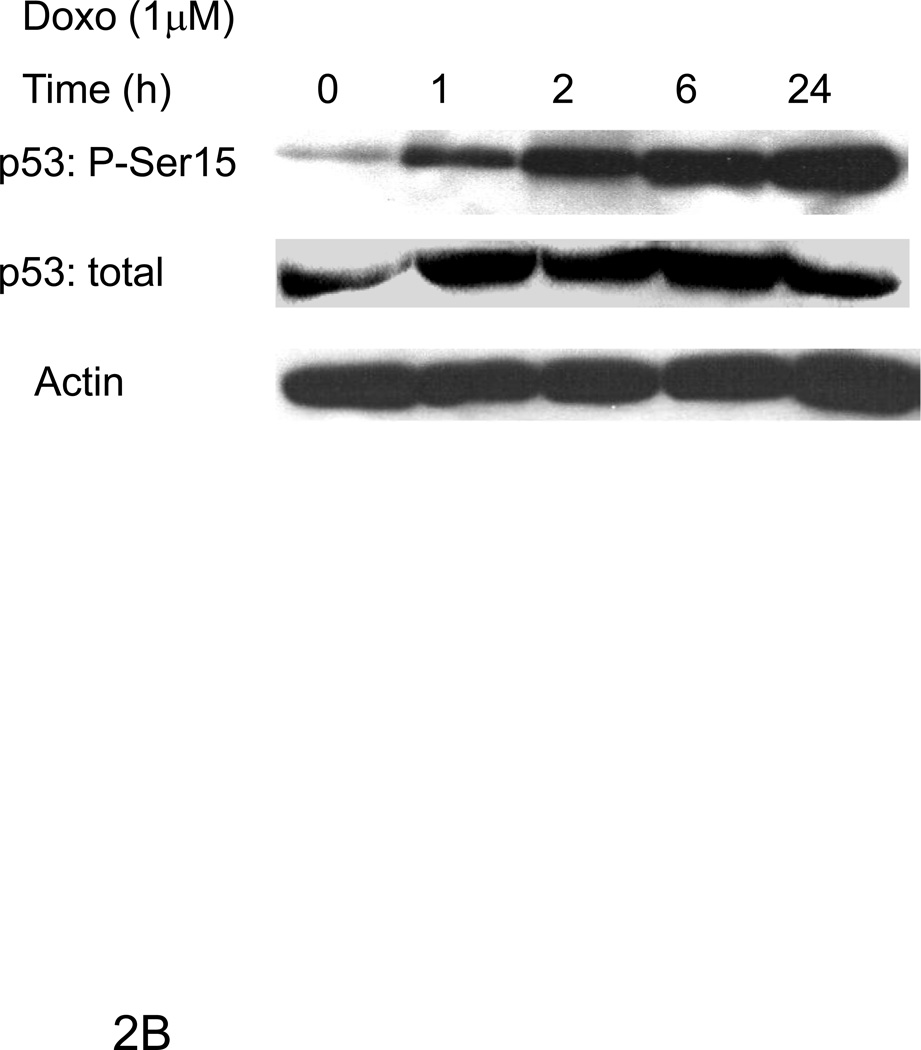
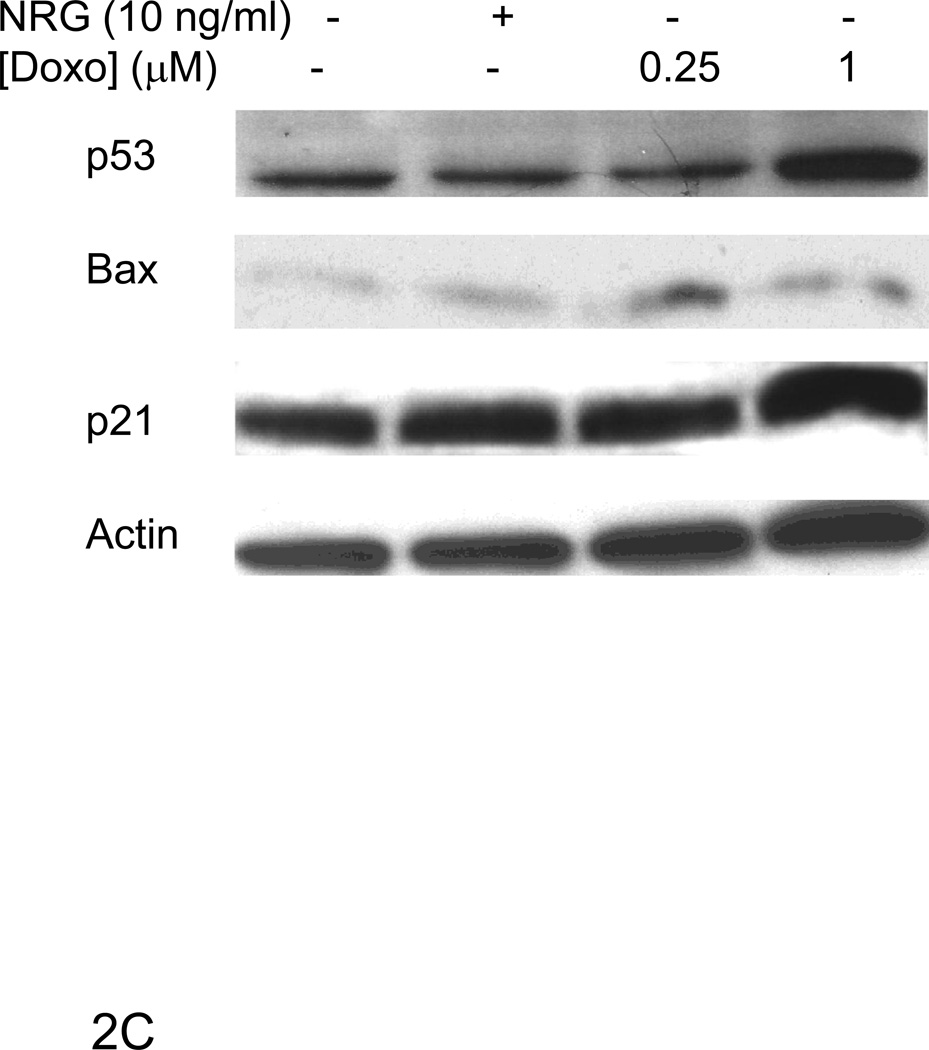
ErbB4 can regulate transcriptional activity of p53 [10; 16]. To examine whether ErbB4 can play a similar role in myocytes we characterized myocyte response to doxorubicin, an inducer of DNA damage and p53 activation. Doxorubicin treatment increased H2A.X phosphorylation at serine 139 (Fig 2A), a marker of DNA damage [17; 18]. Doxorubicin treatment also induced p53 phosphorylation at serine 15 (Fig 2B), a site known to cause p53 stabilization and increased activity [19; 20; 21; 22; 23; 24]. While doxorubicin treatment increased expression of p53, there was no change in the expression of bax, and a variable change in expression of p21WAF1/CIP1 at 24 h (compare Fig 2C and 4A). Treatment with Nrg-1β had no effect on expression of any of these proteins under these conditions.
Figure 2. Doxorubicin induces a DNA damage in ARVMs.
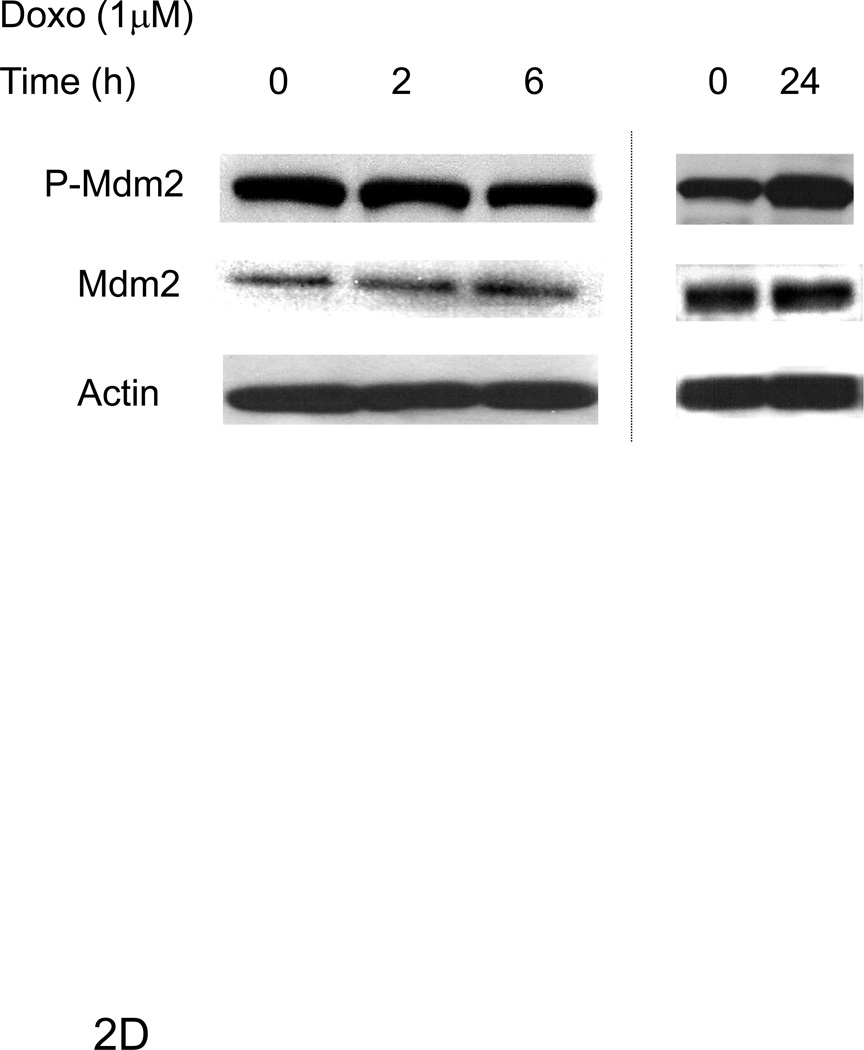
Confocal microscopy images were taken of ARVMs maintained in the absence or presence of doxorubicin (Doxo) for 24 hours. Cells were fixed and stained with anti-phospho Ser139 H2A.X antibody (blue) (A). No staining was seen in the absence of primary antibody (data not shown). ARVMs were serum starved for 24 hrs followed by treatment with doxorubicin (1µM) for indicated durations (B, D) or 30 min with doxorubicin or Neuregulin-1β (Nrg-1β) at the indicated concentrations (C). Cells were lysed in modified RIPA buffer and subjected to Western blot analysis to assess p53 phospho-Ser 15, p53, Bax, p21WAF1/CIP1, Mdm2 phospho-Ser166, Mdm2 and actin contents. Photomicrographs and blots are representative of at least three independent experiments.
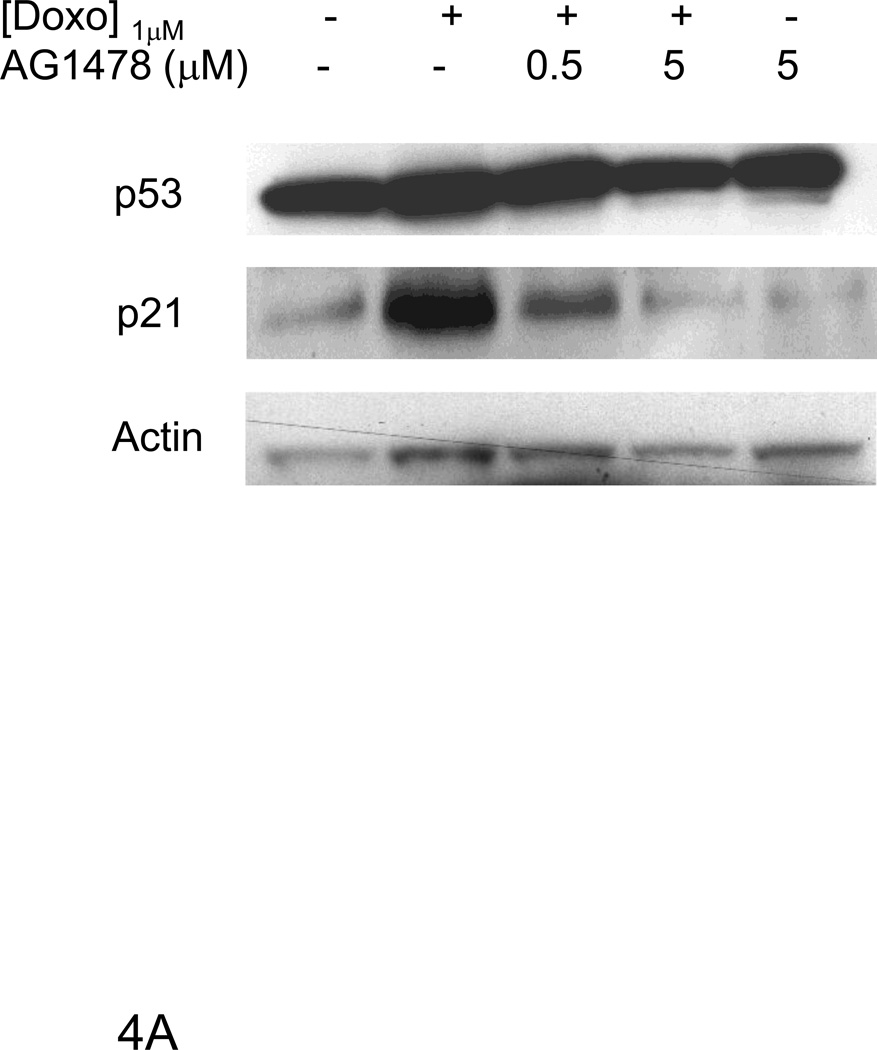
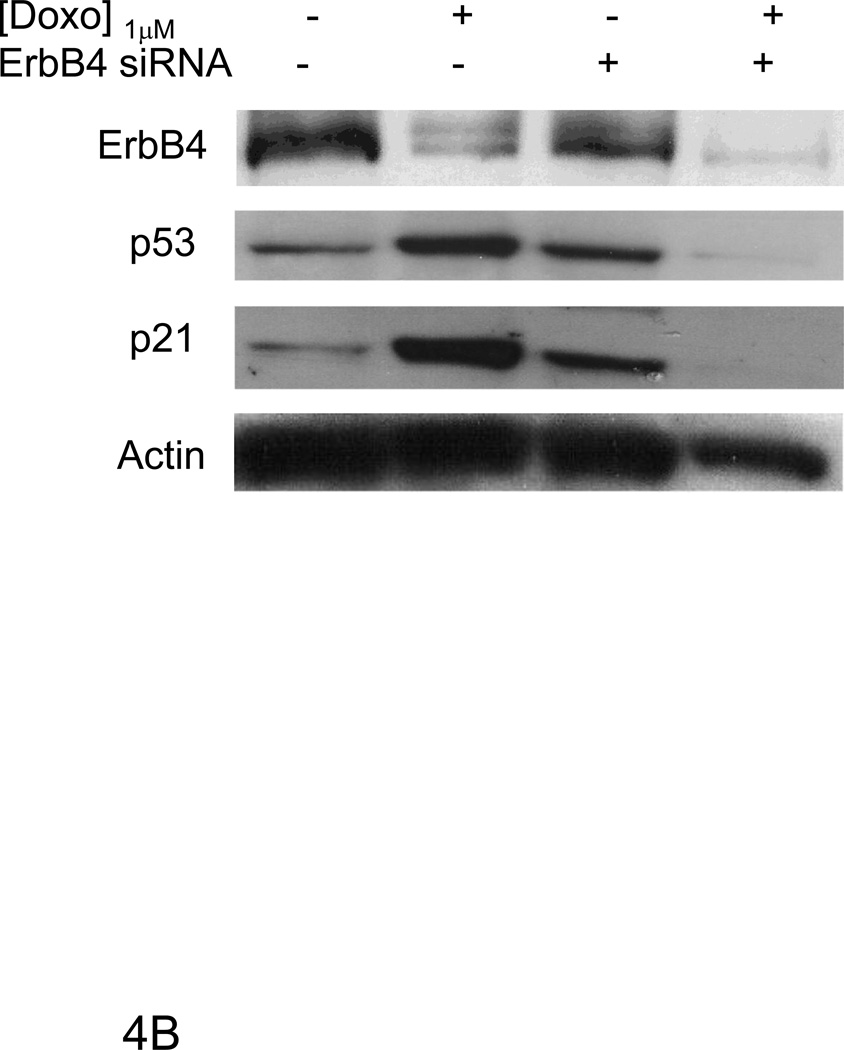
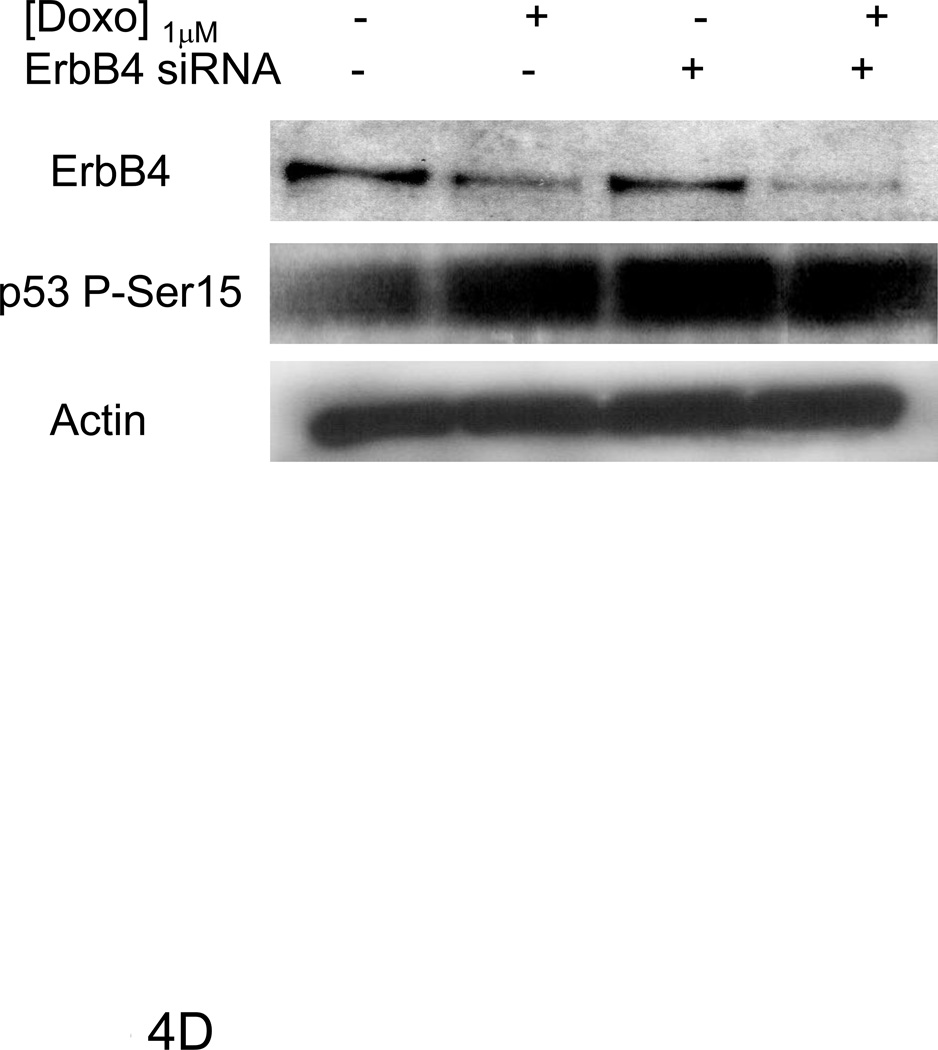
Figure 4. AG1478 and ErbB4 siRNA inhibited doxorubicin induced p53 and p21WAF1/CIP1 protein levels.
Primary cultures of ARVMs serum starved for 24 hours were pretreated with AG1478 for 1hr followed by doxorubicin for 24 hrs at indicated concentrations (A, C). ARVMs were transfected with ErbB4 (100 nM) siRNA for 96 hrs. During the last 24hrs of the siRNA treatment, cells were treated with Doxorubicin (B, D). Cells were lysed in TGH buffer and subjected to Western blot analysis. Immunoblot analyses were performed for ErbB4, p53, p21WAF1/CIP1, p53 phospho-Ser 15 and actin expression as indicated. Blots are representative of at least three independent experiments.
Mdm2 is an E3 ubiquitin ligase that induces the degradation of p53 (and itself) [25] thereby negatively regulating p53 [26]. Phosphorylation of Mdm2 at serine 166 can enhance its ubiquitin ligase activity [13; 27]. There was no effect of doxorubicin on Mdm2 phosphorylation until late (24h) after treatment (Fig 2D). Thus doxorubicin increases p53 expression early, as well as activates counter-regulatory pathways that lead to p53 degradation at later time points.
Role of ErbB4 and its kinase activity in the doxorubicin DNA damage response
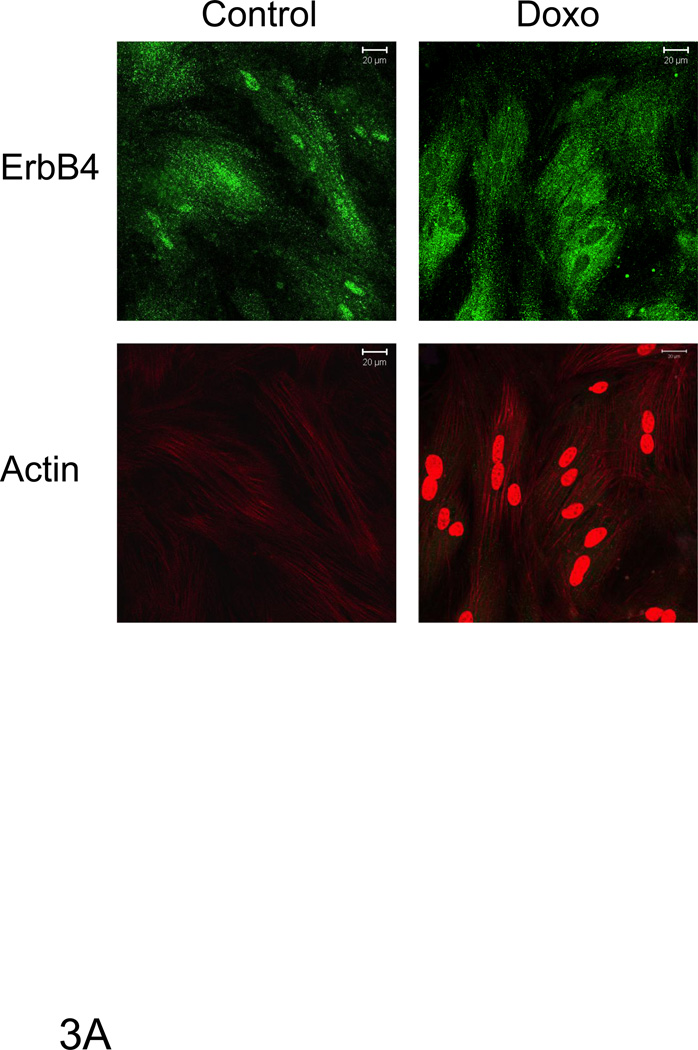
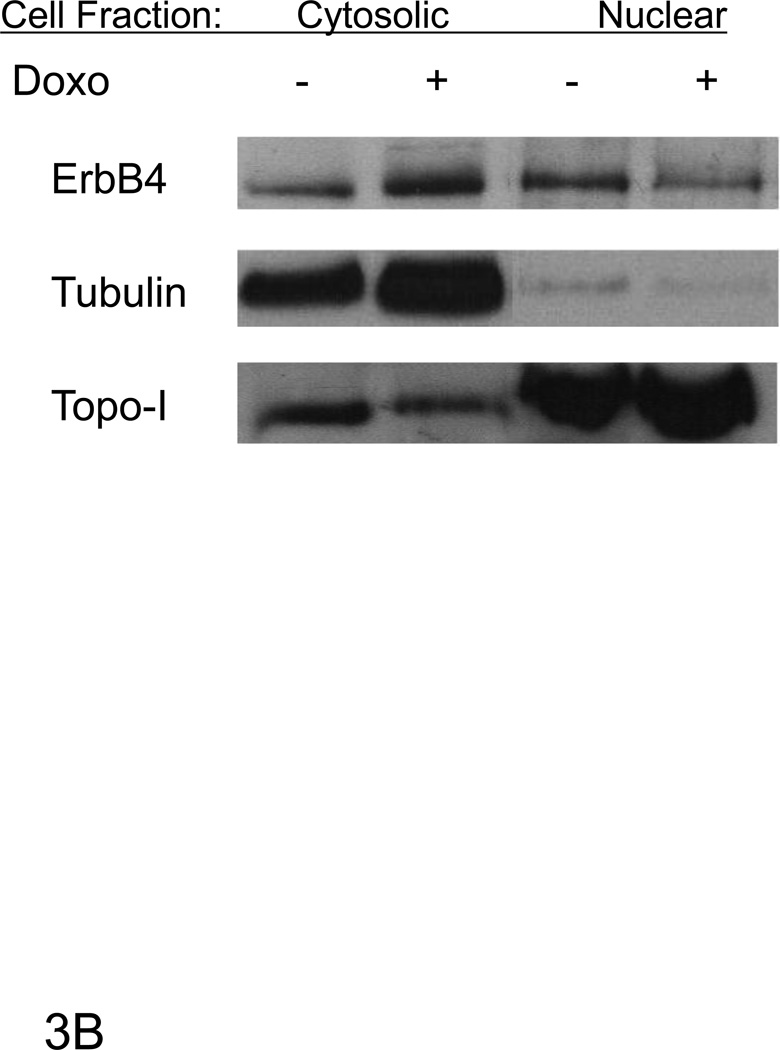
Following doxorubicin (1µM) treatment for 24 hrs, we observed a decrease in total as well as nuclear ErbB4 levels compared to the untreated control that was evident by immunofluorescent staining as well as western blot (Fig 3). To examine the role of ErbB4 in the regulation of p53 and p21WAF1/CIP1, we used tyrphostin AG1478, which we have shown inhibits ErbB4 signaling in myocytes [5; 6]. AG1478 inhibited doxorubicin-induced p53 and p21WAF1/CIP1 protein levels (Fig 4A). To confirm the role of ErbB4 in this pathway, we used ErbB4 siRNA to suppress ErbB4 expression. SiRNA treatment led to ~50% suppression of ErbB4 expression (e.g. Fig 4B and 4D). In the presence of doxorubicin and erbB4 siRNA, ErbB4 expression decreased ~90%, Treatment with ErbB4 siRNA decreased doxorubicin-induced p53 and p21WAF1/CIP1 expression. Although we observed lower actin levels when ARVMs were treated with ErbB4 siRNA and doxorubicin, quantification of the signal intensity normalized to actin still showed ~80% inhibition of p21WAF1/CIP. We observed no effect of inhibition of ErbB4 kinase activity or ErbB4 siRNA on phospho-Ser15 p53 (Fig 4C and 4D). Thus ErbB4 in ARVMs appears to play a role in regulating p53 accumulation and activity in response to doxorubicin.
Figure 3. Doxorubicin decreased full length nuclear ErbB4 levels.
A) Confocal microscopy images of ARVMs maintained in the absence or presence of doxorubicin as described previously. Cells were fixed and stained with anti-ErbB4 antibody (green) (A) or phalloidin (red). Doxorubicin fluorescence was detected in the nucleus of Doxorubicin treated myocytes (red). The bar represents 20 µm. B) Western blots of lysates of similarly treated ARVM showed a decrease in the relative amount of ErbB4 in the nuclear fraction. Tubulin (cytoplasmic) and Topoisomerase-1 (Topo-1, nuclear) antibodies were used to check the cross-contamination between cytoplasmic and nuclear fractions. Photomicrographs and blots are representative of at least three independent experiments.
DISCUSSION
ErbB4 is required for cardiac development and mediates a number of adaptive responses in cardiac myocytes when activated by ligands such as Nrg-1β [3; 5; 6; 28; 29]. In this study we demonstrate that ErbB4 localizes to the nucleus in cardiac myocytes, and plays a role in regulating p53 activity in response to DNA damage. While these findings are conceptually similar to what has been described for ErbB4 in cancer cells [10], the molecular details are quite distinct.
Previous studies have shown that the JM-a isoform of ErbB4 can be cleaved by the action of TACE, a disintegrin and metalloprotease (ADAM) family protease and γ-secretase [7; 30], and the soluble 80 kD cytoplasmic domain of ErbB4 translocates into the nucleus [8; 11; 31]. We did not find expression of JM-a ErbB4 mRNA in ARVMs, confirming prior expression profiling [7]. Moreover, ErbB4 protein nuclear localization was not sensitive to treatment with PKC inhibitors of γ-secretase. Rather nuclear ErbB4 in cardiac myocytes appears to be full-length ErbB4. This is similar to other receptor tyrosine kinases that localize to the nucleus as full-length proteins, i.e. EGF receptor and FGF (fibroblast growth factor) receptors [32].
Nuclear ErbB4 increased upon isolation of cardiac myocytes compared to the intact rodent heart. The marked increase in nuclear ErbB4 in ARVM with cell isolation suggests that this may be an indicator of cell stress as has been observed for other receptor tyrosine kinases. In liver tissue, for example, a very low fraction of cells demonstrate EGFR staining in the nucleus at baseline, with a marked increase after liver injury [33]. Similarly ionizing radiation, oxidative stress, and heat all stimulate nuclear localization of full-length EGFR in an epithelial cancer cell line where it regulates the DNA damage response [34; 35].
The cellular mechanisms for nuclear translocation of full-length type I transmembrane proteins are still unclear. Nuclear EGFR appears to be derived from perinuclear EGFR, and not from the plasma membrane. Alternatively, ErbB4 has been observed to internalize in neuronal cells via an endocytosis related pathway after Nrg stimulation [36]. Similarly ErbB2 is also known to be internalized into an endosomal compartment that results in transfer of the receptor from an early-endosomal compartment into the nucleus [37]. Chaperone proteins such as importin beta1, Sec61β a member of the Sec61 translocon, and Nup358 can bind to the receptor tyrosine kinases, assisting endocytosis and/or nuclear entry [37; 38]. Further work is necessary to fully understand the mechanisms for ErbB4 nuclear localization in cardiac myocytes.
Prior work has demonstrated the role of ErbB4 in transmitting signals that regulated myocyte survival, growth, glucose uptake and formation of focal adhesions (for review see [39]). The present results suggest a more complicated description of ErbB4, coupling extracellular signals for tissue growth and metabolism under favorable conditions, and DNA repair, cell-cycle arrest and/or suppression of growth under conditions of cellular or tissue stress. For example, recent studies suggest potential roles of p21WAF1/CIP1 that are independent of its function in cell cycle regulation [40], including actin cytoskeleton organization [41; 42; 43]. Thus ErbB4 dependent p53 activity may serve to regulate myocyte cytoarchitecture and/or growth via regulation of this and/or other pathways.
Highlights.
ErbB4 localizes to cardiac myocyte nuclei as a full-length receptor
Cardiac myocytes express predominantly JM-a/CYT-1 ErbB4
Myocyte p53 activation in response to doxorubicin requires ErbB4 activity
ACKNOWLEDGEMENTS
We thank Sandra Zinkel, David Seldin, Mary Jo Murnane and Konstantin Kandror for helpful comments. This work was supported by grants HL068144 from the National Institutes of Health, an Established Investigator Award from the American Heart Association to Douglas B. Sawyer, and the Department of Medicine, Boston University Medical Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Srinivasan R, Poulsom R, Hurst HC, Gullick WJ. Expression of the c-erbB-4/HER4 protein and mRNA in normal human fetal and adult tissues and in a survey of nine solid tumour types. J Pathol. 1998;185:236–245. doi: 10.1002/(SICI)1096-9896(199807)185:3<236::AID-PATH118>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter G. ErbB-4: mechanism of action and biology. Exp Cell Res. 2003;284:66–77. doi: 10.1016/s0014-4827(02)00100-3. [DOI] [PubMed] [Google Scholar]
- 3.Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, Lemke G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378:390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Rivello H, Taranda J, Said M, Cabeza-Meckert P, Vila-Petroff M, Scaglione J, Ghio S, Chen J, Lai C, Laguens RP, Lloyd KC, Hertig CM. Dilated cardiomyopathy in Erb-b4-deficient ventricular muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1153–H1160. doi: 10.1152/ajpheart.00048.2005. [DOI] [PubMed] [Google Scholar]
- 5.Fukazawa R, Miller TA, Kuramochi Y, Frantz S, Kim YD, Marchionni MA, Kelly RA, Sawyer DB. Neuregulin-1 protects ventricular myocytes from anthracycline-induced apoptosis via erbB4-dependent activation of PI3-kinase/Akt. J Mol Cell Cardiol. 2003;35:1473–1479. doi: 10.1016/j.yjmcc.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, Sawyer DB. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- 7.Elenius K, Corfas G, Paul S, Choi CJ, Rio C, Plowman GD, Klagsbrun M. A novel juxtamembrane domain isoform of HER4/ErbB4. Isoform-specific tissue distribution and differential processing in response to phorbol ester. J Biol Chem. 1997;272:26761–26768. doi: 10.1074/jbc.272.42.26761. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Jung KM, Huang YZ, Bennett LB, Lee JS, Mei L, Kim TW. Presenilin-dependent gamma-secretase-like intramembrane cleavage of ErbB4. J Biol Chem. 2002;277:6318–6323. doi: 10.1074/jbc.M110371200. [DOI] [PubMed] [Google Scholar]
- 9.Vecchi M, Baulida J, Carpenter G. Selective cleavage of the heregulin receptor ErbB-4 by protein kinase C activation. J Biol Chem. 1996;271:18989–18995. doi: 10.1074/jbc.271.31.18989. [DOI] [PubMed] [Google Scholar]
- 10.Arasada RR, Carpenter G. Secretase-dependent tyrosine phosphorylation of Mdm2 by the ErbB-4 intracellular domain fragment. J Biol Chem. 2005;280:30783–30787. doi: 10.1074/jbc.M506057200. [DOI] [PubMed] [Google Scholar]
- 11.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 12.Vidal GA, Naresh A, Marrero L, Jones FE. Presenilin-dependent gamma-secretase processing regulates multiple ERBB4/HER4 activities. J Biol Chem. 2005;280:19777–19783. doi: 10.1074/jbc.M412457200. [DOI] [PubMed] [Google Scholar]
- 13.Zhou BP, Liao Y, Xia W, Zou Y, Spohn B, Hung MC. HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–982. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 14.Communal C, Singh K, Pimentel DR, Colucci WS. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the beta-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- 15.Nishida M, Carley WW, Gerritsen ME, Ellingsen O, Kelly RA, Smith TW. Isolation and characterization of human and rat cardiac microvascular endothelial cells. Am J Physiol. 1993;264:H639–H652. doi: 10.1152/ajpheart.1993.264.2.H639. [DOI] [PubMed] [Google Scholar]
- 16.Naresh A, Long W, Vidal GA, Wimley WC, Marrero L, Sartor CI, Tovey S, Cooke TG, Bartlett JM, Jones FE. The ERBB4/HER4 intracellular domain 4ICD is a BH3-only protein promoting apoptosis of breast cancer cells. Cancer Res. 2006;66:6412–6420. doi: 10.1158/0008-5472.CAN-05-2368. [DOI] [PubMed] [Google Scholar]
- 17.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–967. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 18.Thiriet C, Hayes JJ. Chromatin in need of a fix: phosphorylation of H2AX connects chromatin to DNA repair. Mol Cell. 2005;18:617–622. doi: 10.1016/j.molcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 19.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 20.Siliciano JD, Canman CE, Taya Y, Sakaguchi K, Appella E, Kastan MB. DNA damage induces phosphorylation of the amino terminus of p53. Genes Dev. 1997;11:3471–3481. doi: 10.1101/gad.11.24.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shieh SY, Taya Y, Prives C. DNA damage-inducible phosphorylation of p53 at N-terminal sites including a novel site, requires tetramerization. Embo J. 1999;18:1815–1823. doi: 10.1093/emboj/18.7.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi K, Herrera JE, Saito S, Miki T, Bustin M, Vassilev A, Anderson CW, Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, Smorodinsky NI, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. doi: 10.1126/science.281.5383.1674. [DOI] [PubMed] [Google Scholar]
- 24.Dumaz N, Meek DW. Serine15 phosphorylation stimulates p53 transactivation but does not directly influence interaction with HDM2. Embo J. 1999;18:7002–7010. doi: 10.1093/emboj/18.24.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58. doi: 10.1016/s1044-579x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 26.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–299. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 27.Ogawara Y, Kishishita S, Obata T, Isazawa Y, Suzuki T, Tanaka K, Masuyama N, Gotoh Y. Akt enhances Mdm2-mediated ubiquitination and degradation of p53. J Biol Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 28.Meyer D, Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- 29.Zhao YY, Sawyer DR, Baliga RR, Opel DJ, Han X, Marchionni MA, Kelly RA. Neuregulins promote survival and growth of cardiac myocytes-Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273:10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 30.Rio C, Buxbaum JD, Peschon JJ, Corfas G. Tumor necrosis factor-alpha-converting enzyme is required for cleavage of erbB4/HER4. J Biol Chem. 2000;275:10379–10387. doi: 10.1074/jbc.275.14.10379. [DOI] [PubMed] [Google Scholar]
- 31.Komuro A, Nagai M, Navin NE, Sudol M. WW domain-containing protein YAP associates with ErbB-4 and acts as a co-transcriptional activator for the carboxyl-terminal fragment of ErbB-4 that translocates to the nucleus. J Biol Chem. 2003;278:33334–33341. doi: 10.1074/jbc.M305597200. [DOI] [PubMed] [Google Scholar]
- 32.Myers JM, Martins GG, Ostrowski J, Stachowiak MK. Nuclear trafficking of FGFR1: a role for the transmembrane domain. J Cell Biochem. 2003;88:1273–1291. doi: 10.1002/jcb.10476. [DOI] [PubMed] [Google Scholar]
- 33.Marti U, Hug M. Acinar and cellular distribution and mRNA expression of the epidermal growth factor receptor are changed during liver regeneration. J Hepatol. 1995;23:318–327. [PubMed] [Google Scholar]
- 34.Dittmann K, Mayer C, Rodemann HP. Inhibition of radiation-induced EGFR nuclear import by C225 (Cetuximab) suppresses DNA-PK activity. Radiother Oncol. 2005;76:157–161. doi: 10.1016/j.radonc.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 35.Dittmann K, Mayer C, Fehrenbacher B, Schaller M, Raju U, Milas L, Chen DJ, Kehlbach R, Rodemann HP. Radiation-induced epidermal growth factor receptor nuclear import is linked to activation of DNA-dependent protein kinase. J Biol Chem. 2005;280:31182–31189. doi: 10.1074/jbc.M506591200. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Tao YM, Woo RS, Xiong WC, Mei L. Stimulated ErbB4 internalization is necessary for neuregulin signaling in neurons. Biochem Biophys Res Commun. 2007;354:505–510. doi: 10.1016/j.bbrc.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giri DK, Ali-Seyed M, Li LY, Lee DF, Ling P, Bartholomeusz G, Wang SC, Hung MC. Endosomal transport of ErbB-2: mechanism for nuclear entry of the cell surface receptor. Mol Cell Biol. 2005;25:11005–11018. doi: 10.1128/MCB.25.24.11005-11018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao HJ, Carpenter G. Role of the Sec61 translocon in EGF receptor trafficking to the nucleus and gene expression. Mol Biol Cell. 2007;18:1064–1072. doi: 10.1091/mbc.E06-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pentassuglia L, Sawyer DB. The role of Neuregulin-1beta/ErbB signaling in the heart. Exp Cell Res. 2009;315:627–637. doi: 10.1016/j.yexcr.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denicourt C, Dowdy SF. Cip/Kip proteins: more than just CDKs inhibitors. Genes Dev. 2004;18:851–855. doi: 10.1101/gad.1205304. [DOI] [PubMed] [Google Scholar]
- 41.Chow KC, King CK, Ross WE. Abrogation of etoposide-mediated cytotoxicity by cycloheximide. Biochem Pharmacol. 1988;37:1117–1122. doi: 10.1016/0006-2952(88)90519-9. [DOI] [PubMed] [Google Scholar]
- 42.Thakkar NS, Potten CS. Abrogation of adriamycin toxicity in vivo by cycloheximide. Biochem Pharmacol. 1992;43:1683–1691. doi: 10.1016/0006-2952(92)90697-h. [DOI] [PubMed] [Google Scholar]
- 43.Thakkar NS, Potten CS. Inhibition of doxorubicin-induced apoptosis in vivo by 2-deoxy-D-glucose. Cancer Res. 1993;53:2057–2060. [PubMed] [Google Scholar]