Abstract
Considerable evidence supports the effectiveness of aspirin for chemoprevention of colorectal cancer (CRC) in addition to its well-established benefits in the prevention of vascular disease. Epidemiologic studies have consistently observed an inverse association between aspirin use and risk of CRC. A recent pooled analysis of a long-term post-trial follow-up of nearly 14,000 patients from 4 randomized, cardiovascular disease prevention trials showed that daily aspirin treatment for about 5 years was associated with a 34% reduction in 20-year CRC mortality. A separate meta-analysis of nearly 3,000 patients with a history of colorectal adenoma or cancer in 4 randomized adenoma prevention trials demonstrated that aspirin reduced the occurrence of advanced adenomas by 28% and any adenoma by 17%. Aspirin has also been shown to be beneficial in a clinical trial of patients with Lynch syndrome, a hereditary CRC syndrome; in those treated with aspirin for at least 2 years, there was a ≥ 50% reduction in the risk of CRC commencing 5 years after randomization and after aspirin had been discontinued. A few observational studies have shown an increase in survival among patients with CRC who use aspirin. Taken together, these findings strengthen the case for consideration of long-term aspirin use in CRC prevention. Despite these compelling data, there is a lack of consensus about the balance of risks and benefits associated with long-term aspirin use, particularly in low-risk populations. The optimal dose to use for cancer prevention and the precise mechanism underlying aspirin’s anticancer effect require further investigation.
Keywords: Aspirin, colorectal cancer, colorectal adenoma, Lynch syndrome, adjuvant chemotherapy
INTRODUCTION
Colorectal cancer (CRC) is the third most common cancer in the world, accounting for an estimated 9.8% of all new cancers (1.2 million cases annually) and 8.1% of all cancer mortality (1). Recognition of the efficacy of endoscopic polypectomy in preventing CRC by identifying and removing precancerous adenomas has led to a marked increase in the use of colonoscopy screening, particularly in the United States (2–4). However, the development of primary prevention strategies to reduce the risk of developing colorectal neoplasia remains an important goal, particularly in view of the inherent limitations of population-based secondary prevention programs that rely on detection and removal of adenomas.
Perhaps the most widely studied pharmacologic agent for the prevention of CRC in humans is aspirin. During October 2–3, 2010, an International Expert Roundtable, sponsored by Bayer Healthcare, convened in Berlin, Germany to discuss the evidence for aspirin’s role in CRC prevention. In this overview, members of this Roundtable summarize the meeting and discuss new data published through September, 2011. We address a number of key issues, including epidemiologic evidence of aspirin use in relation to CRC incidence; clinical trials examining the role of aspirin in prevention of CRC and adenoma; clinical trials of aspirin in patients with hereditary syndromes; observational studies of aspirin use in relation to survival among patients with established CRC; safety issues associated with long-term aspirin use; optimal dosing that balances chemopreventive efficacy with safety; mechanisms underlying the anticancer benefit of aspirin; and the potential role of aspirin in combination with other chemopreventive agents.
Epidemiologic studies of sporadic CRC
The vast majority of cohort and case-control studies have observed an inverse association between aspirin use and risk of CRC (5). An early analysis of 662,424 men and women enrolled in the Cancer Prevention Study II cohort showed that aspirin use at least 16 times per month was associated with a 40% reduced risk of colon cancer mortality over a 6-year period (6). An updated analysis of this cohort observed that daily use of at least 325 mg for at least 5 years was associated with a lower incidence of CRC compared with nonusers (rate ratio [RR], 0.68; 95% confidence interval [CI], 0.52–0.90), as well as reduced risks of other cancers (7). Another cohort study of 47,363 male US health professionals showed that regular aspirin users (≥ 2 times/week) had a 21% (RR, 0.79; 95% CI, 0.69–0.90) lower risk of CRC over 18 years of follow-up (8). Similar findings were observed in the US Nurses’ Health Study (NHS) cohort of 82,911 women; regular aspirin use (≥ two 325 mg tablets/week) was associated with a 23% reduced risk of CRC (RR, 0.77; 95% CI, 0.67–0.88) over 20 years of follow-up (9). In a study of 301,240 older United States men and women (mean age 62.8 years), a lower risk of CRC was seen among both weekly users (RR, 0.88; 95% CI, 0.80–0.97) and daily users (RR, 0.86; 95% CI, 0.79–0.94) of aspirin compared with nonusers (10). Smaller cohort studies and a large number of case-control studies have reported very similar associations (5). In a separate analysis of the NHS cohort, regular aspirin use was significantly associated with a 28% reduction in risk of death from CRC, a 12% reduction in risk of death from any cancer, and a 25% reduction in risk of death from all causes (11).
The trend for the benefits of aspirin to increase with a longer duration of exposure has been consistently observed in cohort studies (5). An analysis of 5,146 women from 7 cohort studies estimated that long-term aspirin use (about 20 years) reduced the risk of CRC by 15% (RR, 0.85; 95% CI, 0.78–0.92) (12). Several case-control studies have also reported a reduction in CRC risk associated with increasing duration of aspirin use (12–15). An analysis of 9,232 men from 11 case-control studies reported that aspirin use (about 20 years) reduced the risk of CRC by 41% (RR, 0.59; 95% CI, 0.54–0.64) (12).
Clinical trials of cardiovascular disease with cancer outcomes
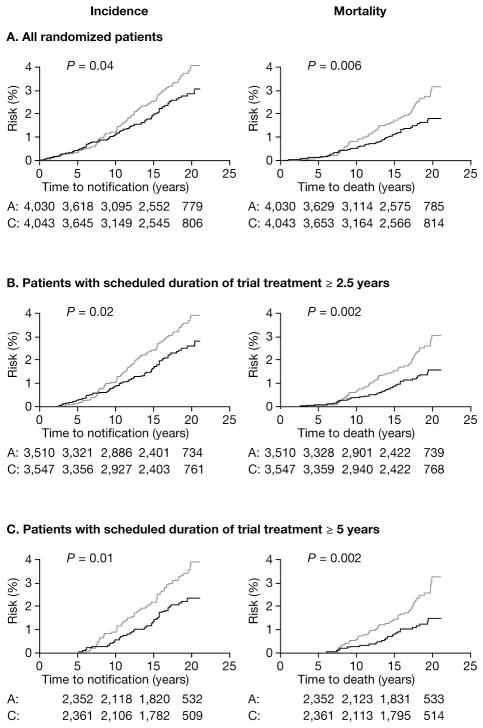
Rothwell et al. recently obtained long-term follow-up data on cancer outcomes for several randomized trials of aspirin that were originally designed to examine the effect of aspirin on cardiovascular (CV) disease prevention (16). An initial pooled analyses of individual patient data included four such trials, which each included at least 1,000 subjects assigned to daily aspirin treatment for at least 2.5 years: two primary prevention trials (British Doctor’s Trial [1988] and Thrombosis Prevention Trial [1998]) and two secondary prevention trials (Swedish Aspirin Low-dose Trial [SALT, 1991] and UK-Transient Ischaemic Attack [UK-TIA, 1991]) (17–20). These trials examined diverse populations, including men initially recruited with low CV risk (n = 10,224) (17, 18) and higher-risk men and women with a history of transient ischemic attack (TIA), minor stroke, or retinal artery occlusion (n = 3,809) (19, 20). The scheduled aspirin doses ranged from 75–1,200 mg/day (3 of the 4 trials were placebo controlled), and the median treatment duration was 2.6–6.9 years. Among the 4 trials, there were 391 documented CRC cases over a median follow-up of 18.3 years. Treatment with any aspirin dose between 75–500 mg/day reduced the 20-year risk of colon cancer by 24% and CRC-associated mortality by 35% overall, but with increasing benefit with longer durations of scheduled randomized treatment during the initial trial period (Fig. 1). There was a suggestion that the reduction in CRC incidence may be largely confined to an effect on the proximal colon (hazard ratio [HR], 0.45; 95% CI, 0.28–0.74) compared with the distal colon (HR, 1.10; 95% CI, 0.73–1.64) (P for difference = 0.04). Although there was no overall effect of aspirin on the risk of rectal cancer (HR, 0.90; 95% CI, 0.63–1.30), there appeared to be a reduced risk (HR, 0.47; 95% CI, 0.26–0.87, P = 0.01) among those with a scheduled treatment duration of at least 5 years.
Figure 1.
Pooled analysis of the effect of aspirin (thick line) versus control (thin line) on subsequent incidence and mortality due to colorectal cancer in all randomized patients (A) in three trials of low-dose aspirin versus placebo, in those with scheduled duration of trial treatment ≥ 2.5 years (B), and in those with scheduled duration of trial treatment ≥ 5 years (C). A, aspirin; C, control. Reprinted from The Lancet, 376, Rothwell P, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials, 1741-50, Copyright 2010, with permission from Elsevier.
A subsequent pooled analysis of individual patient data examined the effects of aspirin on mortality due to all cancers (21), including data from all 8 randomized trials of daily aspirin versus control (7 placebo controlled) with an initial scheduled trial treatment period of at least 4 years (17, 18, 20–26). Three of the studies enrolled 7,526 patients with type 1 or type 2 diabetes (22, 24, 25). These studies are summarized in Table 1. Randomized trials of aspirin administered on alternate days were not eligible for inclusion. Among the 8 included trials with a total of 25,570 patients and 674 cancer-related deaths during the trial periods, aspirin treatment at a dose ranging from 75 to 1200 mg/day was associated with a 21% lower risk of death from any cancer during the in-trial follow-up period (HR = 0.79; 95% CI, 0.68–0.92, P = 0.003). Benefit was only apparent after 5 years of follow-up (HR = 0.66; 95% CI, 0.50–0.87, P = 0.003), with the absolute reduction in 20-year risk of cancer death reaching 7.08% (95% CI, 2.42–11.74) at age 65 years. Among the 6 trials with data on sites of cancer occurrence, patients randomized to aspirin had a reduced risk of death due to CRC (HR, 0.41; 95% CI, 0.17–1.00, P = 0.05), beginning 5 years after the initiation of aspirin treatment.
Table 1.
Patient characteristics and the effect of aspirin on all-cause mortality and cancer mortality in randomized trials of aspirin (with an initial treatment period ≥ 4 years) (Peto et al, 1988; Farrell et al, 1991; ETDRS Investigators, 1992; Juul-Möller et al, 1992; Thrombosis Prevention Trial, 1998; Ogawa et al, 2008; Belch et al, 2008; Fowkes et al, 2010; Rothwell et al, 2011)
| TPT | UK-TIA | BDT | ETDRS | JPAD | SAPAT | POPADAD | AAAT | |
|---|---|---|---|---|---|---|---|---|
| Placebo-controlled, double-blind | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Patients (n) | 5,085 | 2,435 | 5,139 | 3,711 | 2,539 | 2,035 | 1,276 | 3,350 |
| Median duration of scheduled treatment (years) | 6.9 | 4.4 | 6.0 | 5.0 | 4.4 | 4.2 | 6.7 | 8.2 |
| Aspirin dose (mg/day) | 75 | 300/1,200 | 500 | 650 | 81/100 | 75 | 100 | 100 |
| Odds ratio (95% CI) for any cancer death | 0.83 (0.62–1.11) | 0.45 (0.25–0.82) | 0.79 (0.55–1.14) | 1.14 (0.56–2.35) | 0.80 (0.47–1.37) | 0.53 (0.25–1.15) | 0.80 (0.47–1.37) | 0.86 (0.63–1.17) |
| Odds ratio (95% CI) for any death | 1.06 (0.87–1.29) | 0.90 (0.70–1.14) | 0.88 (0.72–1.09) | 0.91 (0.77–1.08) | 0.88 (0.55–1.40) | 0.77 (0.57–1.04) | 0.92 (0.68–1.25) | 0.94 (0.76–1.17) |
AAAT, Aspirin for Asymptomatic Atherosclerosis Trial; BDT, British Doctor’s Trial; ETDRS, Early Treatment Diabetic Retinopathy Study; JPAD, Japanese Primary Prevention of Atherosclerosis with Aspirin for Diabetes; POPADAD, Prevention of Progression of Arterial Disease and Diabetes; SAPAT, Swedish Angina Pectoris Aspirin Trial; TPT, Thrombosis Prevention Trial; UK-TIA, UK-Transient Ischaemic Attack; CI, confidence interval.
Although these data on the effects of daily aspirin are compelling, it should be acknowledged that these were secondary analyses of cardiovascular prevention trials not originally designed to examine cancer incidence or mortality. Hence, ascertainment of cancer-related endpoints may be less complete or accurate than would be expected in a clinical trial with defined cancer outcomes. Some results were also based upon post-trial follow-up of patients through linkage with death certificates or cancer registries and there was no information regarding post-trial usage of aspirin or nonaspirin NSAIDs or cancer screening. In addition, two large randomized trials of alternate-day aspirin, the Physicians’ Health Study (PHS) and Women’s Health Study (WHS) did not demonstrate a reduction in CRC incidence (27, 28). The PHS was a randomized, placebo-controlled trial designed to determine the effect of aspirin 325 mg/every other day on CV disease in 22,071 healthy male physicians. The WHS examined the effect of aspirin 100 mg/every other day on CV events and overall cancer incidence in 39,876 initially healthy women. There was no effect of aspirin on the incidence of CRC over a 10-year follow-up in either trial; the relative risk of CRC was 1.03 (95% CI, 0.83–1.28) in the PHS and 0.97 (95% CI, 0.77–1.24) in the WHS (29, 30).
The explanation for the contrast in results between the PHS and WHS incidence data with the findings from these 2 meta-analyses is unclear. First, both the PHS and WHS studies used alternate-day dosing regimens, in contrast to the daily dosing used in the studies included by Rothwell et al. It is possible that alternate-day dosing may be less effective than daily dosing in inhibiting carcinogenesis. Secondly, the duration of follow-up in both the PHS and WHS studies may have been insufficient to detect a difference in CRC incidence. In their meta-analysis determining CRC incidence (16), Rothwell et al. noted at least a 7-year delay after initiation of aspirin treatment before a reduction in CRC incidence even began to appear (Fig. 1), with a clear reduction not evident until after 10 years. Thus, it is possible that longer follow-up of the WHS or PHS may demonstrate a potential protective benefit. Thirdly, in the WHS, the equivalent daily dose of aspirin was 50 mg/day, lower than the 75 mg/day shown to be effective in both meta-analyses.
Clinical trials of sporadic colorectal adenoma
Adenomas are the precursors of the vast majority of CRC (31–33). To date, 4 placebo-controlled, randomized trials have examined the effects of aspirin in nearly 3,000 patients with either a history of colorectal adenoma or previous CRC (Table 2) (34–36). A meta-analysis of these trials showed that aspirin at any dose (81–325 mg/day) reduced the risk of any colorectal adenoma (defined as occurrence after randomization) by 17% (RR, 0.83; 95% CI, 0.72–0.96) over a median postrandomization follow-up of 33 months (37). The risk of advanced colorectal adenomas (defined as 1 cm or larger in size or with tubulovillous or villous histology, high-grade dysplasia, or invasive cancer) was reduced by 28% (RR, 0.72; 95% CI, 0.57–0.90). Additional studies are ongoing, including the Japan Colorectal Aspirin Polyps Prevention (J-CAPP) study, a multicenter, randomized, placebo-controlled study, which is examining the effect of aspirin 100 mg/day on occurrence of recurrent tumors 2 years after endoscopic removal of colorectal adenomas and cancers (38).
Table 2.
Patient characteristics and effect of aspirin on the reduction of colorectal adenoma risk (Baron et al, 2003; Benamouzig et al, 2003; Cole et al, 2009; Logan et al, 2008; Sandler et al, 2003)
| APACC | AFPPS | CALGB | ukCAP | |
|---|---|---|---|---|
| Design |
|
|
|
|
| Patients (n) | 272 | 1,121 | 635 | 945 |
| Adenoma inclusion criteria | Recent history of colorectal adenomas | Recent history of colorectal adenomas | Previous history of CRCs | Recent history of colorectal adenomas |
| Family history of adenomas (%) | 34.6 | 30.4 | Not reported | 14.1 |
| Risk ratio (95% CI) for any adenoma* | 0.95 (0.75–1.21) | 0.88 (0.77–1.02) | 0.61 (0.44–0.86) | 0.79 (0.63–0.99) |
| Risk ratio (95% CI) for advanced adenoma* | 0.91 (0.51–1.60) | 0.74 (0.52–1.06) | 0.77 (0.29–2.05) | 0.63 (0.43–0.91) |
Versus placebo or folate (based on colonoscopic follow-up). AFPPS, Aspirin/Folate Polyp Prevention Study; APACC, Association pour la Préventionparl’Aspirine du Cancer Colorectal; CALGB, Colorectal Adenoma prevention study originated in the cooperative trials group cancer and Leukemia Group B; ukCAP, United Kingdom Colorectal Adenoma Prevention; CRC, colorectal cancer; CI, confidence interval.
Clinical trials of hereditary colorectal neoplasia
Aspirin has also been investigated in clinical trials among patients with hereditary conditions with a known genetic basis, such as familial adenomatous polyposis (FAP) or Lynch syndrome (hereditary nonpolyposis colorectal carcinoma). In classic FAP, patients typically develop hundreds to thousands of adenomatous polyps throughout the colon, often beginning as early as the second decade of life. Colorectal adenocarcinomas inevitably develop in FAP patients, typically by age 40 years, or approximately 10–15 years after the initial appearance of polyposis (39). A germline mutation in the adenomatous polyposis coli (APC) gene underlies FAP and is a primary molecular event in up to 85% of sporadic cancers; thus, chemoprevention studies in these patients have relevance for colorectal carcinogenesis in the general population.
Agents that can delay adenoma development or growth and progression to cancer could play a vital role in delaying prophylactic colectomy and preventing polyposis in a retained rectum or ileoanal pouch after colectomy (40). Early randomized control trials have demonstrated the efficacy of the nonsteroidal anti-inflammatory drug (NSAID), sulindac, as well as the cyclooxygenase-2 (COX-2) selective inhibitors, celecoxib and rofecoxib, in reducing the mean size of colorectal polyps and the mean number of colorectal polyps after 6 to 9 months of treatment in FAP patients (41–43). Aspirin has also been investigated in the Colorectal Adenoma/carcinoma Prevention Programme 1 (CAPP1) study, a randomized, placebo-controlled trial of aspirin 600 mg/day and/or resistant starch 30 g/day in a 2-by-2 factorial design. Among 133 evaluable patients, aspirin treatment resulted in a nonsignificant reduction in polyp number (RR = 0.77; 95% CI, 0.54–1.10) compared with non-aspirin, and a significant reduction in polyp size among patients treated with aspirin for more than 1 year (44). The efficacy of lower doses of aspirin (100 mg/day) in FAP patients is currently being explored in the Japan Familial Adenomatous Polyposis Prevention II (J-FAPP II) trial.
A role for aspirin has also been studied in patients with Lynch syndrome, a distinct autosomal dominantly inherited condition in which germline mutations in mismatch repair genes confer a high lifetime risk of cancers of the colorectum as well as other organs, including the uterus, small intestine, and ovaries. It is estimated that 1–5% of CRC cases arise as a result of Lynch syndrome (45). Information on chemoprevention in this high-risk group with germline susceptibility may be relevant to the 1 in 6 CRCs in which acquired silencing of a mismatch repair gene is the primary molecular driver of carcinogenesis. The CAPP2 randomized controlled trial was the first to have cancer prevention as a primary end point. A factorial design was used to evaluate aspirin 600 mg/day and/or 30 g of the resistant starch Novelose® In over 80% of patients, recruitment was based on molecular genetic identification of the underlying mismatch repair gene defect. Of the 1,007 eligible carriers randomized, 937 patients with Lynch syndrome commenced treatment and were the basis for analysis. Analysis at the end of the intervention phase revealed that aspirin did not reduce the risk of colorectal adenoma or carcinoma over a mean treatment duration of 29 months (46). The study design involved a prolonged double-blind, postintervention follow-up. When the first recruits reached the target of 10 years, a review of cancer incidence was performed (47). Over a mean follow-up of 55.7 months, despite routine clinical surveillance, 48 participants developed 53 primary CRCs (18/427 randomized to aspirin, 30/434 to aspirin placebo). Intention to treat analysis of time to first CRC showed an HR of 0.63 (95% CI, 0.35–1.13, P = 0.12). The primary end point of the trial was the number and stage of CRCs after 2 years of aspirin treatment. In a per-protocol analysis of individuals who consumed a minimum of 1,400 tablets as a marker of adherence to at least 2 years of treatment, the HR of CRC was 0.41 (95% CI, 0.19–0.86, P = 0.02) (Fig. 2). Secondary analysis revealed fewer Lynch syndrome-related cancers in those on aspirin for at least 2 years (incident RR = 0.42; 95% CI, 0.25–0.72, P = 0.001).
Figure 2.
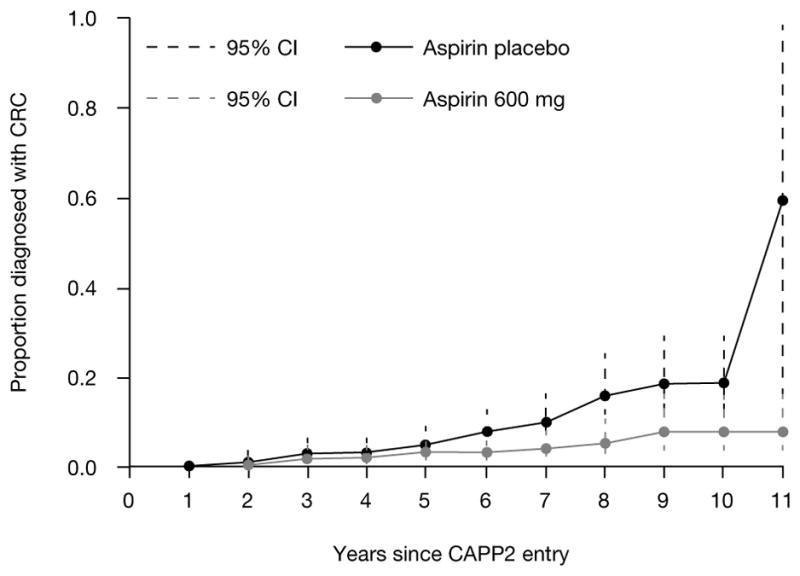
CAPP2: Time to first colorectal cancer in those randomized to aspirin compared with those randomized to the aspirin placebo. In each case, Kaplan-Meier analysis was restricted to participants who had taken ≥ 2 years’ intervention and the analysis was adjusted for gender (hazard ratio 0.41 (95% confidence interval, 0.19–0·86), P = 0·02). Each point on the plot shows the estimated cumulative incidence by years of follow-up, together with the corresponding 95% confidence interval.
Studies of patients with previous CRC
In a placebo-controlled, randomized trial of patients with a history of nonmetastatic colon or rectal cancer after resection of their primary tumor, daily treatment with standard-dose (325 mg) aspirin was associated with a 35% reduction in risk of recurrent adenoma or carcinoma at 3 years (38). These results suggested a possible role for aspirin the prevention of disease recurrence or death in CRC patients, a concept that was further supported by a study of 1,279 male and female health professionals diagnosed with stage I–III CRC. Among this cohort, regular aspirin use after diagnosis was associated with improved CRC-specific survival (48). During a median follow-up of 11.8 years, the HR (adjusted for cancer stage and location, sex, age, and body mass index) for CRC mortality was lower in aspirin users compared with nonusers (RR, 0.71; 95% CI, 0.53–0.95); the effect was particularly pronounced in primary cancers that over-expressed COX-2 (RR, 0.39 [COX-2 positive] vs. RR, 1.22 [COX-2 negative]). The effect of aspirin on disease-free survival and disease recurrence has also been examined in the Cancer and Leukemia Group B (CALGB) 89803, a randomized, multicenter study originally designed to compare regimens of adjuvant 5-fluorouracil/leucovorin (5-FU/LV) with or without irinotecan in relation to the overall survival and disease-free survival in 830 patients with stage III colon cancer. Within this trial, disease recurrence was lower among patients who reported consistent aspirin use compared with nonuse (HR, 0.45; 95% CI, 0.21–0.97 (49). Two additional cohort studies that only assessed aspirin use patterns before diagnosis observed that prediagnosis aspirin use was associated with lower CRC-specific mortality (50, 51). Randomized trial evidence of a benefit for aspirin in the adjuvant treatment of CRC will be forthcoming. The ASpirin for Dukes’ C and high-risk Dukes’ B cOLorecTal cancers—an international, multicentre, double-blind, randomized placebo-controlled phase 3 trial (ASCOLT) is an ongoing, phase III, placebo-controlled trial being conducted in several Asian centers that will examine the effect of aspirin 200 mg/day on disease-free and overall survival among patients with Dukes’ C and high-risk Dukes’ B CRC.
Safety profile for chemoprevention
The safety profile of aspirin for CV disease prevention has been well established and extensively reviewed (52, 53). The most frequently reported serious adverse events associated with regular aspirin use are related to gastrointestinal (GI) bleeding; the vast majority of which are not life threatening. A recent meta-analysis of 35 randomized controlled trials of aspirin using doses of 75 to 325 mg per day estimated an HR for a major GI bleed of 1.55 (95% CI, 1.27–1.90) compared to inert control reagants. For average-risk individuals, this translates into 1–2 GI bleeds per 1,000 person-years (54), although the absolute risk may be higher among individuals with additional baseline risk factors. Some (55–57), but not all (58–60), studies find that such toxicities are largely dose related, with the HR for bleeding complications being generally higher at 300–325 mg doses of aspirin rather than 75–162.5 mg doses (20, 61–65). Nonetheless, the risk of GI toxicity with low-dose aspirin remains significant (59). The relative (RR, 1.43; 95% CI, 0.85–2.42) and absolute (1–2 intracranial bleeds per 10,000 patient-years) risk of intracranial bleeding with low-dose aspirin use is lower than the corresponding risk of GI bleeding (59, 66). However, the generally more severe consequences of intracranial bleeding weigh heavily in overall considerations of risk and benefit. Thus, based on available data and largely due to concerns about the adverse consequences of long-term aspirin use, the United States Preventative Services task force recommended against the routine use of aspirin for CRC prevention in 2007 (67).
Such risk–benefit calculations will require reconsideration based on the recent evidence supporting a benefit of daily aspirin use in the prevention of death from several cancers, including those of the colorectum (21). In the pooled analysis of individual patient data from 8 randomized controlled trials of aspirin versus control, daily aspirin for 5–10 years, reduced in-trial cancer deaths after 5 years by 34% (P = 0.003), with a 10% reduction in all-cause mortality during the trials, and reduced the 20-year risk of cancer death by 20% (P < 0.0001). Based on these data, it would appear that, for most individuals, the benefits of long-term use of daily aspirin for prevention of chronic disease would likely outweigh the consequences associated with the increased risk of bleeding. Thus, a formal risk-benefit analysis that fully incorporates the cumulative hazards of prolonged use of aspirin is warranted. Moreover, clinical trial data are still lacking and are unlikely to become available regarding the risks and benefits associated with the daily use of aspirin in low-risk populations over a duration beyond 10 years, as might be expected in routine clinical practice. A clinical trial to examine the full range of risks and benefits directly over such a prolonged duration of use would almost certainly be impractical. As a result, the present data may be considered sufficiently compelling by some to warrant a broader recommendation for routine aspirin use for the prevention of chronic disease. For individuals at high risk of CRC, such as those with Lynch syndrome, the results of the CAPP2 trial suggest a clear case for aspirin use.
Dosing for chemoprevention
Because the adverse effects of aspirin appear to be largely dose related as noted above, the minimally effective dose required for CRC prevention remains a critically important question. The Rothwell meta-analysis found that typical regimens of daily aspirin used for vascular disease prevention (75–325 mg daily) were as effective as high-dose (1,200 mg/day) aspirin (16). However, the short-term follow-up data from the two trials of alternate-day aspirin that were not eligible for inclusion in the meta-analysis (i.e., aspirin 325 mg in the PHS study and 100 mg in the WHS study) did not show a reduction in risk of CRC (27, 28). Although the negative findings of these studies could be attributed to their relatively short follow-up and/or alternate-day dosing, they do leave some uncertainty regarding the effects of low-dose regimens.
The adenoma trials indicate that aspirin doses in the range of 81–325 mg daily reduce risk. However, the dose–response patterns in the studies are difficult to integrate. Two trials compared higher (300–325 mg/day) and lower (81–160 mg/day) doses of aspirin; a reduction in the risk of all recurrent adenomas was found only with the lower (81–160 mg/day) doses (37). Nonetheless, two other trials that only studied the higher (300–325 mg/day) doses of aspirin both reported reductions in risk of all adenomas from the active treatment. Over all trials, the summary estimates for the risk reduction associated with lower (81–160 mg) and higher (300–325 mg) dose aspirin were similar both for all adenomas and for advanced adenomas.
Although the posttrial follow-up of the randomized trials of daily aspirin in prevention of CV disease and the results of the adenoma recurrence trials demonstrate with relative consistency that 75–81 mg daily doses of aspirin may be sufficient for optimal CRC prevention, observational data are not clear on this point. Some studies suggest that 300–325 mg/day may be required (5, 7–9, 68). Unfortunately, in most of these observational studies, information regarding the doses and duration of use is incomplete, and many of the analyses of dose–response patterns have not taken into account the duration of use and the follow-up apparently required for prevention of CRC. In 2 prospective cohort studies that could examine use of aspirin over a long duration, greater efficacy was observed with intake as high as 14 (325 mg) tablets per week (8, 9).
Thus, taking the clinical trial and observational data together, there is very strong evidence that aspirin in doses as low as 325 mg per day reduces CRC risk. There is clear clinical trial evidence for daily doses as low as 75 mg, but the observational data—admittedly, incomplete—are inconsistent. Additional data regarding the effectiveness of 100 mg aspirin every other day may soon be available through longer-term follow-up of the WHS and PHS, which is currently underway (29). In addition, 2 ongoing placebo-controlled trials of aspirin (100 mg/day), the Aspirin in Reducing Events in the Elderly (ASPREE) study and the Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE), might yield important insights, although each of these studies may not offer follow-up beyond 5 years. A dose-finding study regarding the risk–benefit balance of different aspirin doses among individuals with Lynch syndrome is currently being planned.
It is important to consider that, for CV disease prevention, 75–81 mg aspirin appears equivalent to 300–325 mg aspirin (69). Thus, even if 300–325 mg aspirin is more effective than 75–81 mg aspirin for CRC prevention, any lower efficacy for 75–81 mg aspirin may be offset by its more favorable safety profile. In any case, the large magnitude of the benefits for vascular disease prevention may dominate those for CRC at either dose. Moreover, incorporation of potential benefits associated with the development of an efficacious and cost-effective means of reducing the GI risks of long-term aspirin use (e.g., concurrent administration of gastroprotective agents) will also likely require future consideration in defining optimal dosing.
The mechanism of action of aspirin for chemoprevention
Aspirin’s most well-characterized pharmacologic activity is the permanent modification of the prostaglandin-endoperoxide synthetase (PTGS) or COX enzymes, which are rate limiting for the metabolic conversion of arachidonic acid to prostaglandins and related eicosanoids. The COX-1 isoenzyme is constitutively expressed in many tissues, whereas growth factors, oncogenes, tumor promoters, and inflammatory cytokines induce the COX-2 isoenzyme. Aspirin’s vascular benefits appear largely due to acetylation of platelet-activated PTGS-1 or COX-1, and inhibition of COX-1 generated thromboxane A2 (TXA2) that occurs with low doses (< 75 mg/day) (70). In contrast, the mechanism of aspirin’s antineoplastic effect is less clear, with substantial evidence supporting both COX-dependent and COX-independent mechanisms. Moreover, data supporting the importance of COX-dependent mechanisms are not entirely consistent concerning the relative importance of the COX-1 and COX-2 isoforms in carcinogenesis.
Some of the primary effectors of COX-dependent mechanisms in carcinogenesis are likely to be prostaglandins, particularly prostaglandin E2 (PGE2), which increases cellular proliferation, migration, and invasiveness, promotes angiogenesis, induces resistance to apoptosis, and modulates cellular and humoral immunity. In animal models, PGE2 administration reverses aspirin-induced adenoma regression and enhances carcinogen-induced tumor incidence, whereas genetic deletion of PGE2 receptors EP1 and EP4 confers resistance to formation of aberrant crypt foci, polyps, and cancers (71). Examination of the polyps in ApcMin/+ mice reveals that both COX-1 and COX-2 contribute to PGE2 formation in polyps, but only COX-1 contributes to PGE2 production in normal tissue (72). In humans, aspirin doses sufficient to inhibit COX-1 but not COX-2 (73) appear to effectively inhibit prostaglandin synthesis in the colon (74). This suggests that, if the anticancer benefit of aspirin is primarily mediated through the suppression of prostaglandins, inhibition of COX-1 is likely to play a role. In support of this hypothesis, disruption of both COX-1 and COX-2 genes is associated with reduced colorectal polyp formation by ~80% in the ApcMin/+ murine model of FAP (72).
Nonetheless, a number of studies have shown that COX-2 is directly implicated in colorectal tumorigenesis (75, 76). In Apc Δ716 knockout mice, which mimic human FAP, deletion of the COX-2 gene reduced the number of intestinal polyps compared with controls (77). Human data also support a central role for COX-2 in colorectal carcinogenesis. First, COX-2 selective inhibitors have been shown to be effective in preventing colorectal adenoma (78–80). Furthermore, aspirin inhibits COX-2 in epithelial cells at higher doses compared with those required to acetylate platelets (70). This is consistent with the observational finding that higher doses of aspirin are more strongly inversely associated with CRC than lower doses, particularly for tumors that overexpress the COX-2 protein (81). COX-2’s influence in neoplasia may also be mediated through mechanisms other than inhibition of prostaglandin synthesis. Possible effects of aspirin include acetylated-COX-2-dependent generation of aspirin-triggered lipoxins, which inhibit cell proliferation (82), transcription-mediated expression of COX-2, and attenuation of COX-2/peroxidase-mediated activation of carcinogens, such as polycyclic aromatic hydrocarbons (83).
Aspirin may also indirectly influence COX-2 through its effect on platelets. It has been hypothesized that aspirin-mediated inactivation of platelets may restore antitumor reactivity by blocking the release of paracrine lipid and protein mediators that induce COX-2 expression in adjacent nucleated cells at sites of mucosal injury (84). Ex vivo studies in human volunteers have shown that aspirin inhibits thromboxane-mediated release of the biologically active lipid, sphingosine-1-phosphate (S-1P) from platelets (85). S-1P promotes tumor growth, neovascularization, and inflammation (86). An antineoplastic effect of aspirin mediated through its effect of platelets would also provide a mechanistic basis for findings of clinical trials showing a lower risk of colorectal neoplasia associated with lower, anti-platelet doses of aspirin that would not be expected to substantially inhibit COX-2.
There are also a number of non-COX-related pathways that may underlie aspirin’s antineoplastic effects. These may include direct modulation of oncogene-induced expression of transcription factors, such as nuclear factor kappa B (NFkB) and induction of spermidine/spermine N1-acetyltransferase resulting in modulation of polyamine synthesis (87, 88). In in vitro models, aspirin has been shown to reduce microsatellite instability in CRC cells deficient for a subset of the human mismatch repair genes (89) and to uncouple oxidative phosphorylation, which is necessary for cell proliferation (90, 91). Aspirin may also increase apoptosis of tumor cells, possibly via complex interactions with tumor promoters and suppressors and deoxyribonucleic acid (DNA) repair genes and through modulation of the Wnt/β-catenin (ceramide) pathway (90). An inhibition of angiogenesis through a Cox-independent mechanism has been observed in vitro (92).
Despite the large body of data regarding the potential mode of action for aspirin in chemoprevention, understanding of the mechanisms remains incomplete. A significant limitation of much of the data derived from in vitro studies is the high doses examined, which may not be physiologically relevant in vivo. In humans, 300 mg/day of aspirin corresponds to peak plasma levels of unchanged aspirin of less than 50 μM and levels of the primary metabolite, salicylate, of less than 0.5 mM, with the great majority (> 90%) of both compounds bound to other proteins. In in vitro studies, the typical aspirin concentrations used in protein-free media are generally much higher, primarily in the low millimolar range. Nonetheless, further investigation into aspirin’s anticancer mechanism continues to be a high research priority. An improved understanding of mechanisms will inform decisions about optimal dose, frequency of administration, and combination therapy with other agents. An overview of the possible modes of action of aspirin in cancer chemoprevention is illustrated in Figure 3.
Figure 3.
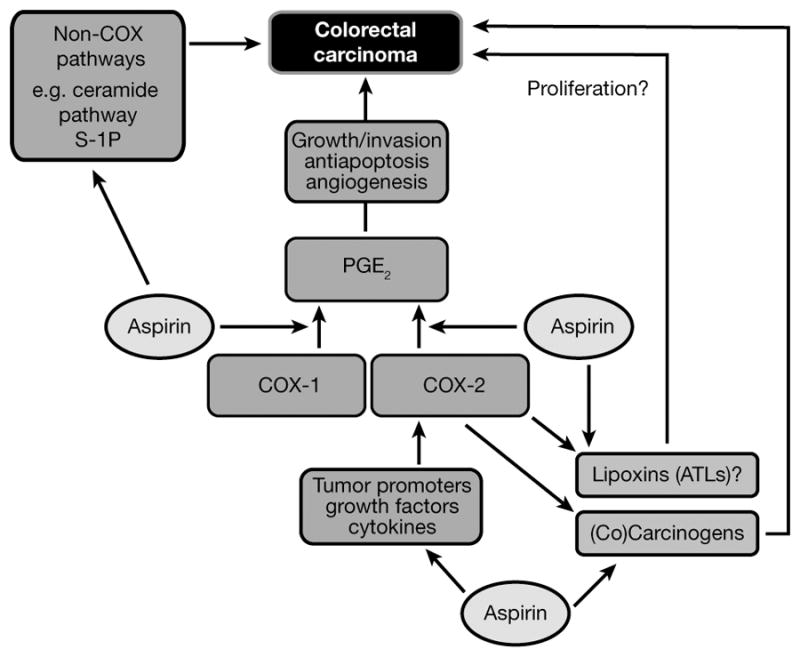
Possible sites of action of aspirin in the prevention of colorectal cancer. COX-dependent and independent targets of aspirin are likely to be present in both epithelial and stromal cell compartments in colorectal adenomas and cancers. ATLs, aspirin-triggered lipoxins; COX, cyclooxygenase; PGE2, prostaglandin E2; S-1P, sphingosine-1-phosphate.
The fact that aspirin reduces the risk of sporadic colorectal adenomas within a few years, but requires 7–10 years for an effect on invasive cancer suggests that at least some of aspirin’s chemopreventive effect occurs relatively early in the carcinogenesis process. However, it is noteworthy that the CAPP2 trial did not demonstrate a reduction in the number of Lynch syndrome gene carriers reporting adenoma development, but that reports of CRC were reduced after a delay, as in the sporadic setting (47). This is compatible with the possibility that, at least in individuals with Lynch syndrome, aspirin may have a long-term impact on the risk of cancer development which is not primarily mediated through adenoma prevention.
Potential combinations with aspirin for chemoprevention
There are a number of additional candidates for CRC prevention, such as calcium, other NSAIDs (e.g., celecoxib), statins, difluoromethylornithine (DFMO), and fish oil for which there are some data suggesting the potential for enhanced prevention when used in combination with aspirin. In the Calcium Polyp Prevention Study (CPPS) of 930 patients with a recent history of colorectal adenoma, randomization to calcium 1,200 mg/day reduced the risk of a recurrent adenoma at a 4-year colonoscopy by 19% (RR, 0.81; 95% CI, 0.67–0.99) (93). A potential synergistic effect of calcium and aspirin on adenoma risk was observed in a combined analysis of data from the CPPS and the Aspirin/Folate Polyp Prevention Study (AFPPS) (94).
Considerable epidemiological data support an inverse association between use of non-aspirin NSAIDs such as ibuprofen and colorectal cancer (95). Selective COX-2 inhibitors, including celecoxib, have been shown to reduce the risk of recurrent colorectal adenomas in 3 randomized, controlled trials (78–80). Despite this demonstrated efficacy, a dose-dependent association with CV events that was uncovered in these studies hampers the routine use of these agents for widespread chemoprevention (96–99). In a preclinical study, administration of combinations of low doses of celecoxib and aspirin reduced colorectal adenomas, apoptosis, and proliferation more effectively than high doses of these agents given individually in the azoxymethane-treated rat model of CRC (100). However, human data supporting synergistic chemopreventive efficacy with a combination of aspirin and celecoxib are lacking. In the placebo-controlled Adenoma Prevention with Celecoxib (APC) trial, 2,035 patients after resection of a colorectal adenoma were stratified according to their use of cardioprotective doses of aspirin (≤ 162.5 mg/day) and randomized to 2 doses of celecoxib. There was no significant difference in efficacy or toxicity according to concurrent use of low-dose aspirin, either at the end of treatment or after 5 years of follow-up (98). In the parallel-designed Prevention of Colorectal Sporadic Adenomatous Polyps (PreSAP) trial, which randomized 1,561 patients following adenoma removal to celecoxib 400 mg/day, there was no difference in the efficacy or toxicity of celecoxib among aspirin users (≤ 162.5 mg/day) compared with non-aspirin users at either 3 years or 5 years (80, 99). Similar results were observed in a placebo-controlled randomized study of the COX-2 selective agent, rofecoxib, in the Adenomatous Polyp Prevention on Vioxx (APPROVe) trial (78).
It is important to note that in the APC, PreSAP, and APPROVe trials, aspirin users were defined according to their self-selected use of aspirin at the time of their baseline examination. Thus, it is reasonable to assume that most subjects developed their initial qualifying adenoma in the setting of low-dose aspirin use, suggesting that they may already have been relatively “resistant” to the antineoplastic benefit of aspirin. Therefore, the possibility remains for enhanced benefit with the combination of aspirin and celecoxib among patients who have never been exposed to these agents. Moreover, further studies are needed to determine the need for prolonged use of combination treatment. In the APC, PreSAP, and APPROVe trials, patients who were withdrawn from COX-2 inhibitor treatment had a higher rate of recurrent adenoma compared with patients withdrawn from placebo, findings suggesting a “rebound” of neoplasia at an accelerated rate after release of COX-2 inhibition (78, 98, 99). This rate of “rebound” was particularly pronounced among patients who initially developed their adenoma despite using aspirin and had a UGT1A6 variant genotype associated with impaired aspirin (101).
Statins have also been considered in combination with aspirin for disease prevention, as is currently recommended for many patients with CV disease. In many case-control studies, statins have been associated with a reduction in the risk of CRC, but most cohort studies and secondary analyses of randomized clinical trials have not shown a benefit (102, 103). Preclinical studies have shown that statins combined with aspirin are more effective in inhibiting experimental colorectal carcinogenesis than either agent alone (100). In a German case-control study of 540 patients with CRC and 614 controls, there was a marked reduction in CRC when both aspirin and statins were used in combination for at least 5 years (odds ratio, 0.38; 95% CI, 0.15–0.97) (104). However, there was no apparent interaction between aspirin and statins in 2 other case-control studies (105, 106) and in a prospective cohort study (107). The recently launched National Surgical Adjuvant Bowel Project P-5 phase III study in patients with resected stage I or II colon cancer will specifically examine rosuvastatin, with and without concurrent aspirin use, in relation to risk of colorectal adenomas and cancer.
Another promising agent for use in combination with aspirin for chemoprevention is DFMO. Aspirin and DFMO are hypothesized to synergistically reduce levels of colonic mucosal polyamines (e.g., putrescine, spermidine, and spermine), which may be procarcinogenic (108). DFMO inhibits polyamine synthesis (109), whereas aspirin increases polyamine acetylation and export (88). In a previous small clinical trial of patients with a history of colorectal adenoma, treatment with DFMO (500 mg/day) and the NSAID, sulindac (150 mg/day), for 36 months was associated with an impressive 70% reduction in the risk of adenoma recurrence (110). A total of 38.9% of patients were also using concurrent aspirin (≤ 81 mg/day or ≤ 325 mg twice weekly) at the time of enrollment. Although this concurrent aspirin use did not affect the total number of adenomas in either group, it remains possible that this study population was preferentially resistant to aspirin, since patients likely developed their baseline adenoma in the setting of low-dose aspirin use. Further analysis of aspirin and DFMO in a well-designed, randomized trial is needed, particularly since this combination may have a lower risk of CV toxicity than the DFMO and sulindac regimen (51).
Because omega-3 polyunsaturated fatty acids (PUFAs) and aspirin may both interrupt prostaglandin synthesis through complementary pathways (111), combined use of aspirin and PUFAs including eicosapentaenoic acid (EPA) and docosahexaenoic acid has been proposed. Evidence demonstrating a lower risk of colorectal neoplasia with PUFAs has been recently reviewed (112). In a clinical trial of 55 FAP patients, EPA, in the free fatty acid form, has been demonstrated to reduce polyp number and size in the retained rectum after 6 months of treatment (113). The magnitude of benefit was similar to that observed previously with celecoxib (42). The recently launched Systematic Evaluation of Aspirin and Fish Oil polyp prevention trial (SeAFOod) trial is designed specifically to examine daily use of aspirin (300 mg/day) and EPA (2 g/day) in a placebo-controlled 2-by-2 factorial design among sporadic colorectal adenoma patients following clearance colonoscopy of ≥ 5 small polyps or ≥ 3 polyps with at least one at ≥ 1 cm.
Summary and future directions
CRC accounts for a significant proportion of cancer deaths worldwide, which highlights an urgent need for preventive strategies. Despite uncertainty about the precise mechanisms that underlie aspirin’s anticancer benefit, the evidence supporting the effectiveness of aspirin for the prevention of CRC is substantial. Long-term follow-up of patients enrolled in CV disease prevention trials has recently shown that allocation to daily aspirin for at least 5 years reduced the 20-year risk of CRC by 32% and 20-year mortality by 43% (16). A separate pooled analysis of individual patient data from approximately 20,000 patients from 8 trials demonstrated that scheduled daily aspirin treatment (75–1,200 mg) for a duration of 5 or more years reduced the risk of CRC mortality by 21% after a 10-year follow-up and by 40% after a 20-year follow-up (21). The CAPP2 randomized trial found almost a 60% reduction in new cancers among 1,000 Lynch syndrome gene carriers, an effect that became apparent around 5 years from randomization in those who took 600 mg aspirin daily for a minimum of 2 years (44). Data from 4 randomized trials that enrolled nearly 3,000 patients clearly indicates that aspirin (75–325 mg/day for 3 years) reduced the risk of any recurrent colorectal adenoma by 17% and advanced adenoma by 28% (37).
Some authorities encourage routine use of aspirin among individuals for whom the risk of myocardial infarction or ischemic stoke outweigh the risk of gastrointestinal hemorrhage (114). However, these same bodies do not advise the routine use of aspirin for prevention of CRC primarily due to concerns about toxicity and continued uncertainty about the optimal dose, duration, and frequency of use, and age of initiation that may maximize the benefits of aspirin while minimizing the risk (12, 67). These recommendations were developed, however, prior to the recent data from long-term follow-up of randomized trials of daily aspirin (16, 21). The potential benefit of aspirin in both prevention of cancer at multiple sites and vascular disease may tip the scale in favor of aspirin for broader chronic disease prevention. It is likely that the benefits of such disease prevention in terms of morbidity and mortality will outweigh concerns about GI bleeding, which is rarely life threatening, and cerebral bleeding, which is extremely rare. Thus, consideration should be made to discontinue the practice of issuing separate recommendations for the routine use of aspirin for the prevention of vascular disease or cancer in favor of broader recommendations encouraging use of aspirin for prevention of multiple chronic diseases, in certain populations for whom the benefits outweigh the risks. Additional data regarding the balance of risk and benefits will likely be available from ongoing studies of aspirin and colorectal neoplasia as summarized in Table 3. Continued research, however, does not preclude immediate consideration of aspirin use for the prevention of CRC in the context of an individualized assessment of risks and benefits.
Table 3.
An overview of major ongoing trials of aspirin (status: April 2011)
| Title | Identifier | Patients | Interventions | Aspirin dose | Design/phase | Start date | End date | Primary outcome | Follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Phase IIA Clinical Biomarker Trial of Aspirin and Arginine Restriction in Colorectal Cancer Patients | NCT00578721 | Resected CRC | Aspirin/arginine restriction | 325 mg/day | Phase II | September 2008 | December 2012 | Change in markers (putrescine) of polyamine reduction | 3 years |
| Spectral Markers in Aspirin Chemoprevention of Colonic Neoplasia | NCT00468910 | History of CRC/adenomas | Aspirin | 325 mg/day | Phase II, RCT | March 2007 | April 2014 | Change in spectral slope and fractal dimension in rectal biopsies | 3 months |
| Randomized Phase II Trial of Aspirin and Difluoromethylornithine (DFMO) in Patients at High Risk of Colorectal Cancer | NCT00983580 | History of CRC/adenomas | Aspirin and difluoromethyl oorithine | 325 mg/day | Phase II, RCT | August 2009 | June 2015 | Adenoma recurrence rate | 1 year |
| The Systematic Evaluation of Aspirin and Fish Oil polyp prevention (SeAFOod) | SRCTN05926847 | High-risk colorectal adenoma patients | Aspirin and eicosapen-taenoic acid-free fatty acid | 300 mg/day | Phase III, RCT | September 2011 | October 2014 | Number of colorectal adenomas | 1–3 years |
| Japan Colorectal Aspirin Polyps Prevention (J-CAPP) | UMIN000000697 | Resected CRC | Aspirin | 100 mg/day | Phase III, RCT | January 2007 | December 2012 | Newly developed tumors | 2–3 years |
| Aspirin for Dukes C and High Risk Dukes B Colon Cancer–An International, Multi-Centre, Double-blind, Randomised, Placebo-controlled, Phase III trial (ASCOLT) | NCT00565708 | Dukes B, C colon or rectal cancer | Aspirin | 200 mg/day | Phase III, RCT | December 2008 | December 2019 | Disease-free survival | 5 years |
| Aspirin in Reducing Events in the Elderly (ASPREE) | NCT01038583 | Aged ≥70 years | Aspirin 100 mg daily | 100 mg/day | Phase III, RCT | January 2010 | August 2016 | Any cause mortality , dementia, or persistent physical disability | Every 3–6 months |
| Aspirin to Reduce Risk of Initial Vascular Events (ARRIVE) | NCT00501059 | Males aged ≥ 55 years with 2 cardiac risk factors and females ≥ 60 years with 3 cardiac risk factors | Aspirin 100 mg daily | 100 mg/day | Phase III, RCT | July 2007 | January 2014 | Any composite outcome of MI, stroke or CV death | ~ 5 years |
| Cancer Prevention Programme project 3 (CAPP3) | In development | Carriers of a germline mismatch repair gene defect, age 18–60 years | Aspirin | 100 mg, 300 mg or 600 mg daily for 5 years | Phase II/III RCT | 2012 | 2020 | Lynch syndrome cancer | At least 10 years |
CRC, colorectal cancer; RCT, randomized controlled trial; CV, cardiovascular.
Acknowledgments
Financial support
Nadir Arber, John Baron, John Burn, Andrew Chan, John Whay-Kuang Chia, Peter Elwood, Mark Hull, Richard Logan, Peter Rothwell and Karsten Schrör have all acted as external consultants to Bayer, Berlin, Germany. Mark Hull has received commercial grants from SLA Pharma.
The authors would like to acknowledge the contributions from: Shinkan Tokudome, National Institute of Health and Nutrition, Shinjuku-ku, Tokyo, Japan; Hideki Ishikawa, Department of Molecular-Targeting Cancer Prevention, Graduate School of Medical Science, Kyoto, and Prefectural University of Medicine, Osaka, Japan; Raghib Ali, INDOX Cancer Research Network, University of Oxford, Oxford, UK; Gabriela Möslein, St Josef’s Hospital Bochum Linden, Nordrhein-Westfalen, Germany; Carlo Patrono, Department of Pharmacology, Catholic University School of Medicine, Rome, Italy
Footnotes
Statement of authorship and conflict of interest statements
Nadir Arber, John Baron, John Burn, Andrew Chan, John Whay-Kuang Chia, Peter Elwood, Mark Hull, Richard Logan, Peter Rothwell and Karsten Schrör have all contributed to the content development and review of this article. Editorial assistance and preparation of figures was supported by Bayer, Berlin, Germany.
Contributor Information
Andrew T. Chan, Email: achan@partners.org.
Nadir Arber, Email: narber@post.tau.ac.il.
John Burn, Email: john.burn@ncl.ac.uk.
John Whay-Kuang Chia, Email: nmocwk@nccs.com.sg.
Peter Elwood, Email: ElwoodPC@cf.ac.uk.
Mark A. Hull, Email: m.a.hull@leeds.ac.uk.
Richard F. Logan, Email: richard.logan@nottingham.ac.uk.
Peter M. Rothwell, Email: peter.rothwell@clneuro.ox.ac.uk.
Karsten Schrör, Email: karsten.schroer@uni-duesseldorf.de.
John A. Baron, Email: John.A.Baron@Dartmouth.edu.
References
- 1.GLOBOCAN 2008. Feb 2, 2011. p. 2011. [Google Scholar]
- 2.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 3.Screening for colorectal cancer: U S Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Colorectal cancer screening. V1, 2010. Vol. 2011. Feb 2, 2011. [Google Scholar]
- 5.Flossmann E, Rothwell PM. Effect of aspirin on long-term risk of colorectal cancer: consistent evidence from randomised and observational studies. Lancet. 2007;369:1603–1613. doi: 10.1016/S0140-6736(07)60747-8. [DOI] [PubMed] [Google Scholar]
- 6.Thun MJ, Namboodiri MM, Heath CW., Jr Aspirin use and reduced risk of fatal colon cancer. N Engl J Med. 1991;325:1593–1596. doi: 10.1056/NEJM199112053252301. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE. A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst. 2007;99:608–615. doi: 10.1093/jnci/djk132. [DOI] [PubMed] [Google Scholar]
- 8.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Wu K, Fuchs CS. Aspirin dose and duration of use and risk of colorectal cancer in men. Gastroenterology. 2008;134:21–28. doi: 10.1053/j.gastro.2007.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan AT, Giovannucci EL, Meyerhardt JA, Schernhammer ES, Curhan GC, Fuchs CS. Long-term use of aspirin and nonsteroidal anti-inflammatory drugs and risk of colorectal cancer. JAMA. 2005;294:914–923. doi: 10.1001/jama.294.8.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruder EH, Laiyemo AO, Graubard BI, Hollenbeck AR, Schatzkin A, Cross AJ. Non-steroidal anti-inflammatory drugs and colorectal cancer risk in a large, prospective cohort. Am J Gastroenterol. 2011;106:1340–1350. doi: 10.1038/ajg.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan AT, Manson JE, Feskanich D, Stampfer MJ, Colditz GA, Fuchs CS. Long-term aspirin use and mortality in women. Arch Intern Med. 2007;167:562–572. doi: 10.1001/archinte.167.6.562. [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol. 2009;10:501–507. doi: 10.1016/S1470-2045(09)70035-X. [DOI] [PubMed] [Google Scholar]
- 13.Johnson CC, Hayes RB, Schoen RE, Gunter MJ, Huang WY. Non-steroidal anti-inflammatory drug use and colorectal polyps in the Prostate, Lung, Colorectal, And Ovarian Cancer Screening Trial. Am J Gastroenterol. 2010;105:2646–2655. doi: 10.1038/ajg.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Din FV, Theodoratou E, Farrington SM, Tenesa A, Barnetson RA, Cetnarskyj R, et al. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59:1670–1679. doi: 10.1136/gut.2009.203000. [DOI] [PubMed] [Google Scholar]
- 15.Kune GA. The Melbourne Colorectal Cancer Study: reflections on a 30-year experience. Med J Aust. 2010;193:648–652. doi: 10.5694/j.1326-5377.2010.tb04093.x. [DOI] [PubMed] [Google Scholar]
- 16.Rothwell PM, Wilson M, Elwin CE, Norrving B, Algra A, Warlow CP, et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet. 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 17.Peto R, Gray R, Collins R, Wheatley K, Hennekens C, Jamrozik K, et al. Randomised trial of prophylactic daily aspirin in British male doctors. Br Med J (Clin Res Ed) 1988;296:313–316. doi: 10.1136/bmj.296.6618.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thrombosis prevention trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. The Medical Research Council’s General Practice Research Framework. Lancet. 1998;351:233–241. [PubMed] [Google Scholar]
- 19.Swedish Aspirin Low-Dose Trial (SALT) of 75 mg aspirin as secondary prophylaxis after cerebrovascular ischaemic events. The SALT Collaborative Group. Lancet. 1991;338:1345–1349. [PubMed] [Google Scholar]
- 20.Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–1054. doi: 10.1136/jnnp.54.12.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 22.Aspirin effects on mortality and morbidity in patients with diabetes mellitus. Early Treatment Diabetic Retinopathy Study report 14. ETDRS Investigators. JAMA. 1992;268:1292–1300. doi: 10.1001/jama.1992.03490100090033. [DOI] [PubMed] [Google Scholar]
- 23.Juul-Moller S, Edvardsson N, Jahnmatz B, Rosen A, Sorensen S, Omblus R. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet. 1992;340:1421–1425. doi: 10.1016/0140-6736(92)92619-q. [DOI] [PubMed] [Google Scholar]
- 24.Ogawa H, Nakayama M, Morimoto T, Uemura S, Kanauchi M, Doi N, et al. Low-dose aspirin for primary prevention of atherosclerotic events in patients with type 2 diabetes: a randomized controlled trial. JAMA. 2008;300:2134–2141. doi: 10.1001/jama.2008.623. [DOI] [PubMed] [Google Scholar]
- 25.Belch J, MacCuish A, Campbell I, Cobbe S, Taylor R, Prescott R, et al. The prevention of progression of arterial disease and diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fowkes FG, Price JF, Stewart MC, Butcher I, Leng GC, Pell AC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–848. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 27.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 29.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the Women’s Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 30.Gann PH, Manson JE, Glynn RJ, Buring JE, Hennekens CH. Low-dose aspirin and incidence of colorectal tumors in a randomized trial. J Natl Cancer Inst. 1993;85:1220–1224. doi: 10.1093/jnci/85.15.1220. [DOI] [PubMed] [Google Scholar]
- 31.Morson BC. The evolution of colorectal carcinoma. Clin Radiol. 1984;35:425–431. doi: 10.1016/s0009-9260(84)80033-1. [DOI] [PubMed] [Google Scholar]
- 32.Eide TJ. Natural history of adenomas. World J Surg. 1991;15:3–6. doi: 10.1007/BF01658952. [DOI] [PubMed] [Google Scholar]
- 33.Neugut AI, Johnsen CM, Forde KA, Treat MR. Recurrence rates for colorectal polyps. Cancer. 1985;55:1586–1589. doi: 10.1002/1097-0142(19850401)55:7<1586::aid-cncr2820550729>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 34.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–899. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 35.Benamouzig R, Deyra J, Martin A, Girard B, Jullian E, Piednoir B, et al. Daily soluble aspirin and prevention of colorectal adenoma recurrence: one-year results of the APACC trial. Gastroenterology. 2003;125:328–336. doi: 10.1016/s0016-5085(03)00887-4. [DOI] [PubMed] [Google Scholar]
- 36.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Cole BF, Logan RF, Halabi S, Benamouzig R, Sandler RS, Grainge MJ, et al. Aspirin for the chemoprevention of colorectal adenomas: meta-analysis of the randomized trials. J Natl Cancer Inst. 2009;101:256–266. doi: 10.1093/jnci/djn485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–890. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 39.Gibbons DC, Sinha A, Phillips RK, Clark SK. Colorectal cancer: no longer the issue in familial adenomatous polyposis? Fam Cancer. 2011;10:11–20. doi: 10.1007/s10689-010-9394-x. [DOI] [PubMed] [Google Scholar]
- 40.Ishikawa H, Nakamura T, Kawano A, Gondo N, Sakai T. Chemoprevention of colorectal cancer in Japan: a brief introduction to current clinical trials. J Gastroenterol. 2009;44 (Suppl 19):77–81. doi: 10.1007/s00535-008-2286-2. [DOI] [PubMed] [Google Scholar]
- 41.Giardiello FM, Hamilton SR, Krush AJ, Piantadosi S, Hylind LM, Celano P, et al. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993;328:1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- 42.Steinbach G, Lynch PM, Phillips RK, Wallace MH, Hawk E, Gordon GB, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–1952. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 43.Hallak A, Alon-Baron L, Shamir R, Moshkowitz M, Bulvik B, Brazowski E, et al. Rofecoxib reduces polyp recurrence in familial polyposis. Dig Dis Sci. 2003;48:1998–2002. doi: 10.1023/a:1026130623186. [DOI] [PubMed] [Google Scholar]
- 44.Burn J, Bishop DT, Chapman PD, Elliott F, Bertario L, Dunlop MG, et al. A randomized placebo-controlled prevention trial of aspirin and/or resistant starch in young people with familial adenomatous polyposis. Cancer Prev Res (Phila) 2011;4:655–665. doi: 10.1158/1940-6207.CAPR-11-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moslein G. Hereditary nonpolyposis colorectal carcinoma (HNPCC): surgical aspects. Praxis (Bern 1994 ) 2001;90:483–489. [PubMed] [Google Scholar]
- 46.Burn J, Bishop DT, Mecklin JP, Macrae F, Moslein G, Olschwang S, et al. Effect of aspirin or resistant starch on colorectal neoplasia in the Lynch syndrome. N Engl J Med. 2008;359:2567–2578. doi: 10.1056/NEJMoa0801297. [DOI] [PubMed] [Google Scholar]
- 47.Burn J, Gerdes A-M, Macrae F, Mecklin J-P, Moeslein G, Olschwang S. The long-term impact of aspirin on cancer risk in carriers of hereditary colorectal cancer: the CAPP2 randomized control trial. Lancet. 2011 doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fuchs C, Meyerhardt JA, Heseltine DL, Niedzwiecki D, Hollis D, Chan AT, et al. Influence of regular aspirin use on survival for patients with stage III colon cancer: Findings from Intergroup trial CALGB 89803. J Clin Oncol. 2005 Jan 6;23(16S):3530. [Google Scholar]
- 50.Coghill AE, Newcomb PA, Campbell PT, Burnett-Hartman AN, Adams SV, Poole EM, et al. Prediagnostic non-steroidal anti-inflammatory drug use and survival after diagnosis of colorectal cancer. Gut. 2011;60:491–498. doi: 10.1136/gut.2010.221143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zell JA, Ziogas A, Bernstein L, Clarke CA, Deapen D, Largent JA, et al. Nonsteroidal anti-inflammatory drugs: effects on mortality after colorectal cancer diagnosis. Cancer. 2009;115:5662–5671. doi: 10.1002/cncr.24705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sostres C, Lanas A. Gastrointestinal effects of aspirin. Nat Rev Gastroenterol Hepatol. 2011;8:385–394. doi: 10.1038/nrgastro.2011.97. [DOI] [PubMed] [Google Scholar]
- 53.Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:199S–233S. doi: 10.1378/chest.08-0672. [DOI] [PubMed] [Google Scholar]
- 54.Lanas A, Wu P, Medin J, Mills EJ. Low doses of acetylsalicylic Acid increase risk of gastrointestinal bleeding in a meta-analysis. Clin Gastroenterol Hepatol. 2011;9:762–768. doi: 10.1016/j.cgh.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 55.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Baggish JS, Bhatt DL, et al. Analysis of risk of bleeding complications after different doses of aspirin in 192,036 patients enrolled in 31 randomized controlled trials. Am J Cardiol. 2005;95:1218–1222. doi: 10.1016/j.amjcard.2005.01.049. [DOI] [PubMed] [Google Scholar]
- 56.Peters RJ, Mehta SR, Fox KA, Zhao F, Lewis BS, Kopecky SL, et al. Effects of aspirin dose when used alone or in combination with clopidogrel in patients with acute coronary syndromes: observations from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE) study. Circulation. 2003;108:1682–1687. doi: 10.1161/01.CIR.0000091201.39590.CB. [DOI] [PubMed] [Google Scholar]
- 57.Topol EJ, Easton D, Harrington RA, Amarenco P, Califf RM, Graffagnino C, et al. Randomized, double-blind, placebo-controlled, international trial of the oral IIb/IIIa antagonist lotrafiban in coronary and cerebrovascular disease. Circulation. 2003;108:399–406. doi: 10.1161/01.CIR.0000084501.48570.F6. [DOI] [PubMed] [Google Scholar]
- 58.Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of aspirin: meta-analysis. BMJ. 2000;321:1183–1187. doi: 10.1136/bmj.321.7270.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119:624–638. doi: 10.1016/j.amjmed.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 60.Steinhubl SR, Bhatt DL, Brennan DM, Montalescot G, Hankey GJ, Eikelboom JW, et al. Aspirin to prevent cardiovascular disease: the association of aspirin dose and clopidogrel with thrombosis and bleeding. Ann Intern Med. 2009;150:379–386. doi: 10.7326/0003-4819-150-6-200903170-00006. [DOI] [PubMed] [Google Scholar]
- 61.A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. The Dutch TIA Trial Study Group. N Engl J Med. 1991;325:1261–1266. doi: 10.1056/NEJM199110313251801. [DOI] [PubMed] [Google Scholar]
- 62.Roderick PJ, Wilkes HC, Meade TW. The gastrointestinal toxicity of aspirin: an overview of randomised controlled trials. Br J Clin Pharmacol. 1993;35:219–226. doi: 10.1111/j.1365-2125.1993.tb05689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang ES, Strate LL, Ho WW, Lee SS, Chan AT. A prospective study of aspirin use and the risk of gastrointestinal bleeding in men. PLoS One. 2010;5:e15721. doi: 10.1371/journal.pone.0015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang ES, Strate LL, Ho WW, Lee SS, Chan AT. Long-term use of aspirin and the risk of gastrointestinal bleeding. Am J Med. 2011;124:426–433. doi: 10.1016/j.amjmed.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weil J, Colin-Jones D, Langman M, Lawson D, Logan R, Murphy M, et al. Prophylactic aspirin and risk of peptic ulcer bleeding. BMJ. 1995;310:827–830. doi: 10.1136/bmj.310.6983.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gorelick PB, Weisman SM. Risk of hemorrhagic stroke with aspirin use: an update. Stroke. 2005;36:1801–1807. doi: 10.1161/01.STR.0000174189.81153.85. [DOI] [PubMed] [Google Scholar]
- 67.Routine aspirin or nonsteroidal anti-inflammatory drugs for the primary prevention of colorectal cancer: U S Preventive Services Task Force recommendation statement. Ann Intern Med. 2007;146:361–364. [PubMed] [Google Scholar]
- 68.Mahipal A, Anderson KE, Limburg PJ, Folsom AR. Nonsteroidal anti-inflammatory drugs and subsite-specific colorectal cancer incidence in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev. 2006;15:1785–1790. doi: 10.1158/1055-9965.EPI-05-0674. [DOI] [PubMed] [Google Scholar]
- 69.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med. 2005;353:2373–2383. doi: 10.1056/NEJMra052717. [DOI] [PubMed] [Google Scholar]
- 71.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55:115–122. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, et al. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res. 2000;60:4705–4708. [PubMed] [Google Scholar]
- 73.Patrono C. Measurement of cyclooxygenase isozyme inhibition in humans: exploring the clinical relevance of biochemical selectivity. Clin Exp Rheumatol. 2001;19:S45–S50. [PubMed] [Google Scholar]
- 74.Ruffin MT, Krishnan K, Rock CL, Normolle D, Vaerten MA, Peters-Golden M, et al. Suppression of human colorectal mucosal prostaglandins: determining the lowest effective aspirin dose. J Natl Cancer Inst. 1997;89:1152–1160. doi: 10.1093/jnci/89.15.1152. [DOI] [PubMed] [Google Scholar]
- 75.Chulada PC, Loftin CD, Winn VD, Young DA, Tiano HF, Eling TE, et al. Relative activities of retrovirally expressed murine prostaglandin synthase-1 and -2 depend on source of arachidonic acid. Arch Biochem Biophys. 1996;330:301–313. doi: 10.1006/abbi.1996.0257. [DOI] [PubMed] [Google Scholar]
- 76.Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res. 1996;56:4566–4569. [PubMed] [Google Scholar]
- 77.Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–809. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 78.Baron JA, Sandler RS, Bresalier RS, Quan H, Riddell R, Lanas A, et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology. 2006;131:1674–1682. doi: 10.1053/j.gastro.2006.08.079. [DOI] [PubMed] [Google Scholar]
- 79.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Solomon SD, Kim K, et al. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006;355:873–884. doi: 10.1056/NEJMoa061355. [DOI] [PubMed] [Google Scholar]
- 80.Arber N, Eagle CJ, Spicak J, Racz I, Dite P, Hajer J, et al. Celecoxib for the prevention of colorectal adenomatous polyps. N Engl J Med. 2006;355:885–895. doi: 10.1056/NEJMoa061652. [DOI] [PubMed] [Google Scholar]
- 81.Chan AT, Ogino S, Fuchs CS. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356:2131–2142. doi: 10.1056/NEJMoa067208. [DOI] [PubMed] [Google Scholar]
- 82.Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 83.Craven PA, DeRubertis FR. Effects of aspirin on 1,2-dimethylhydrazine-induced colonic carcinogenesis. Carcinogenesis. 1992;13:541–546. doi: 10.1093/carcin/13.4.541. [DOI] [PubMed] [Google Scholar]
- 84.Patrono C, Patrignani P, Garcia Rodriguez LA. Cyclooxygenase-selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read-outs. J Clin Invest. 2001;108:7–13. doi: 10.1172/JCI13418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ulrych T, Bohm A, Polzin A, Daum G, Nusing RM, Geisslinger G, et al. Release of sphingosine-1-phosphate from human platelets is dependent on thromboxane formation. J Thromb Haemost. 2011;9:790–798. doi: 10.1111/j.1538-7836.2011.04194.x. [DOI] [PubMed] [Google Scholar]
- 86.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 87.Babbar N, Gerner EW, Casero RA., Jr Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem J. 2006;394:317–324. doi: 10.1042/BJ20051298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinez ME, O’Brien TG, Fultz KE, Babbar N, Yerushalmi H, Qu N, et al. Pronounced reduction in adenoma recurrence associated with aspirin use and a polymorphism in the ornithine decarboxylase gene. Proc Natl Acad Sci U S A. 2003;100:7859–7864. doi: 10.1073/pnas.1332465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ruschoff J, Dietmaier W, Bocker T, Wallinger S, Kullmann F, Beham A, et al. Molecular cancer disposition diagnosis exemplified by colorectal carcinoma. What is the contribution of pathology? Pathologe. 1998;19:269–278. doi: 10.1007/s002920050283. [DOI] [PubMed] [Google Scholar]
- 90.Schror K. Acetylsalicyclic acid. Weinheim, Germany: Wiley-Blackwell. WILEY-VCH Verlag GmbH & Co. KGaA; 2009. [Google Scholar]
- 91.Deng L, Hu S, Baydoun AR, Chen J, Chen X, Cong X. Aspirin induces apoptosis in mesenchymal stem cells requiring Wnt/beta-catenin pathway. Cell Prolif. 2009;42:721–730. doi: 10.1111/j.1365-2184.2009.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Borthwick GM, Johnson AS, Partington M, Burn J, Wilson R, Arthur HM. Therapeutic levels of aspirin and salicylate directly inhibit a model of angiogenesis through a Cox-independent mechanism. FASEB J. 2006;20:2009–2016. doi: 10.1096/fj.06-5987com. [DOI] [PubMed] [Google Scholar]
- 93.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 94.Grau MV, Baron JA, Barry EL, Sandler RS, Haile RW, Mandel JS, et al. Interaction of calcium supplementation and nonsteroidal anti-inflammatory drugs and the risk of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2005;14:2353–2358. doi: 10.1158/1055-9965.EPI-05-0003. [DOI] [PubMed] [Google Scholar]
- 95.Rostom A, Dube C, Lewin G, Tsertsvadze A, Barrowman N, Code C, et al. Nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: a systematic review prepared for the U.S. Preventive Services Task Force. Ann Intern Med. 2007;146:376–389. doi: 10.7326/0003-4819-146-5-200703060-00010. [DOI] [PubMed] [Google Scholar]
- 96.Solomon SD, Pfeffer MA, McMurray JJ, Fowler R, Finn P, Levin B, et al. Effect of celecoxib on cardiovascular events and blood pressure in two trials for the prevention of colorectal adenomas. Circulation. 2006;114:1028–1035. doi: 10.1161/CIRCULATIONAHA.106.636746. [DOI] [PubMed] [Google Scholar]
- 97.Bresalier RS, Sandler RS, Quan H, Bolognese JA, Oxenius B, Horgan K, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–1102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 98.Bertagnolli MM, Eagle CJ, Zauber AG, Redston M, Breazna A, Kim K, et al. Five-year efficacy and safety analysis of the Adenoma Prevention with Celecoxib Trial. Cancer Prev Res (Phila) 2009;2:310–321. doi: 10.1158/1940-6207.CAPR-08-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arber N, Spicak J, Racz I, Zavoral M, Breazna A, Gerletti P, et al. Five-year analysis of the prevention of colorectal sporadic adenomatous polyps trial. Am J Gastroenterol. 2011;106:1135–1146. doi: 10.1038/ajg.2011.116. [DOI] [PubMed] [Google Scholar]
- 100.Reddy BS, Wang CX, Kong AN, Khor TO, Zheng X, Steele VE, et al. Prevention of azoxymethane-induced colon cancer by combination of low doses of atorvastatin, aspirin, and celecoxib in F 344 rats. Cancer Res. 2006;66:4542–4546. doi: 10.1158/0008-5472.CAN-05-4428. [DOI] [PubMed] [Google Scholar]
- 101.Chan AT, Hsu M, Zauber AG, Hawk ET, Bertagnolli MM. The influence of UGT1A6 variants and aspirin use in a randomized trial of celecoxib for the prevention of colorectal adenoma. Cancer Prev Res. 2011 doi: 10.1158/1940-6207.CAPR-11-0337. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bonovas S, Filioussi K, Flordellis CS, Sitaras NM. Statins and the risk of colorectal cancer: a meta-analysis of 18 studies involving more than 1. 5 million patients. J Clin Oncol. 2007;25:3462–3468. doi: 10.1200/JCO.2007.10.8936. [DOI] [PubMed] [Google Scholar]
- 103.Bardou M, Barkun A, Martel M. Effect of statin therapy on colorectal cancer. Gut. 2010;59:1572–1585. doi: 10.1136/gut.2009.190900. [DOI] [PubMed] [Google Scholar]
- 104.Hoffmeister M, Chang-Claude J, Brenner H. Individual and joint use of statins and low-dose aspirin and risk of colorectal cancer: a population-based case-control study. Int J Cancer. 2007;121:1325–1330. doi: 10.1002/ijc.22796. [DOI] [PubMed] [Google Scholar]
- 105.Vinogradova Y, Hippisley-Cox J, Coupland C, Logan RF. Risk of colorectal cancer in patients prescribed statins, nonsteroidal anti-inflammatory drugs, and cyclooxygenase-2 inhibitors: nested case-control study. Gastroenterology. 2007;133:393–402. doi: 10.1053/j.gastro.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 106.Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–2192. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 107.Lee JE, Baba Y, Ng K, Giovannucci EL, Fuchs C, Ogino S, et al. Statin use and colorectal cancer risk according to molecular subtypes in two large prospective cohorts. Cancer Prev Res. 2011 doi: 10.1158/1940-6207.CAPR-11-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Thompson PA, Wertheim BC, Zell JA, Chen WP, McLaren CE, LaFleur BJ, et al. Levels of rectal mucosal polyamines and prostaglandin E2 predict ability of DFMO and sulindac to prevent colorectal adenoma. Gastroenterology. 2010;139:797–805. 805. doi: 10.1053/j.gastro.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Meyskens FL, Jr, Gerner EW, Emerson S, Pelot D, Durbin T, Doyle K, et al. Effect of alpha-difluoromethylornithine on rectal mucosal levels of polyamines in a randomized, double-blinded trial for colon cancer prevention. J Natl Cancer Inst. 1998;90:1212–1218. doi: 10.1093/jnci/90.16.1212. [DOI] [PubMed] [Google Scholar]
- 110.Meyskens FL, Jr, McLaren CE, Pelot D, Fujikawa-Brooks S, Carpenter PM, Hawk E, et al. Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila) 2008;1:32–38. doi: 10.1158/1940-6207.CAPR-08-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Serhan CN, Yacoubian S, Yang R. Anti-inflammatory and proresolving lipid mediators. Annu Rev Pathol. 2008;3:279–312. doi: 10.1146/annurev.pathmechdis.3.121806.151409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cockbain AJ, Toogood GJ, Hull MA. Omega-3 polyunsaturated fatty acids for the treatment and prevention of colorectal cancer. Gut. 2011 doi: 10.1136/gut.2010.233718. [DOI] [PubMed] [Google Scholar]
- 113.West NJ, Clark SK, Phillips RK, Hutchinson JM, Leicester RJ, Belluzzi A, et al. Eicosapentaenoic acid reduces rectal polyp number and size in familial adenomatous polyposis. Gut. 2010;59:918–925. doi: 10.1136/gut.2009.200642. [DOI] [PubMed] [Google Scholar]
- 114.Aspirin for the prevention of cardiovascular disease: U. S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;150:396–404. doi: 10.7326/0003-4819-150-6-200903170-00008. [DOI] [PubMed] [Google Scholar]