Abstract
The incidence of asthma and atopic dermatitis (AD) were evaluated in HIV-infected (n=451) compared to HIV-exposed (n=227) but uninfected (HEU) children and adolescents by abstraction from clinical charts. Asthma was more common in HIV-infected compared to HEU children by clinical diagnosis (25% vs. 20%, p = 0.101), by asthma medication use, (31% vs. 22%, p = 0.012), and by clinical diagnosis or both medication use, (34% vs. 25%, p = 0.012). HIV-infected children had a greater risk of asthma compared to HEU children (HR = 1.37, 95% CI: 1.01 to 1.86). AD was more common in HIV-infected than HEU children (20% vs. 12%, p = 0.009)) and children with AD were more likely to have asthma in both cohorts (41% vs. 29%, p = 0.010). HIV-infected children and adolescents in this study had a 30% increased incidence of asthma and AD, a finding critical for millions of HIV-infected children worldwide.
Keywords: Atopic dermatitis, asthma, pediatric HIV infection, inhaled corticosteroids, protease inhibitors
1. Introduction
Genetically susceptible patients with HIV infection exhibit an increased incidence and prevalence of asthma as measured by use of asthma medications[1]. Specifically, HIV-infected children who inherited HLA allele A68 were at increased risk of asthma. Contrarily, the HLA-Cw6 allele was predictive of lower risk of asthma. The increase in CD4+ T cell percent or counts associated with treating children with highly active antiretroviral therapy (HAART) appeared to be followed by the development of asthma in genetically predisposed children [2-4]. It is not clear, however, if immune-restoring or immune-preserving treatment of HIV infection results in the same risk of asthma in HIV-infected children as that expected for HIV-uninfected children or if infected children on HAART are, in fact, at increased risk of asthma [5].
It is not clear if what is identified as asthma in HIV-infected children has the same underlying pathophysiology as asthma in HIV-uninfected children. In comparing chimpanzee and human immune responses, Sato et al [6] comment on the association of T cell over-reactivity to immune stimuli that results in diseases, such as bronchial asthma, psoriasis, and rheumatoid arthritis. It is interesting that the same HLA antigens identified as important in the development of asthma in HIV-infected patients after HAART and immune reconstitution[1] are also associated with autoimmune diseases, such as psoriasis, psoriatic arthritis, and rheumatoid arthritis that develop in HIV-infected patients following immune reconstitution with HAART [7]. Thus a fundamental immune activation process appears to be operating in these patients that manifests itself as asthma in some patients and as autoimmune disease in others. Establishing that conditions associated with asthma, like atopic dermatitis, are more common in HIV-infected children with asthma would strengthen the evidence that asthma in HIV-infected children is related to the same mechanisms underlying asthma in HIV-uninfected children.
To further explore the relationships between HIV infection and the development of two of the components of the allergic triad - atopic asthma and dermatitis - in the HAART era, the current study compared the risk of asthma and atopic dermatitis in HIV-infected children and adolescents to the risk in HIV-uninfected children and adolescents.
2. Materials and methods
2.1. Patient population
The Pediatric AIDS/HIV Cohort Study (PHACS) Adolescent Master Protocol (AMP) is a prospective multicenter cohort study of the complications of both HIV infection and HAART in children and adolescents with perinatal HIV infection and a comparison group of perinatally HIV-exposed but uninfected (HEU) children. Fifteen clinical sites in the U.S. (including Puerto Rico) enrolled perinatally HIV-infected and HEU children aged 7 to16 years between March 2007 and December 2009. Children were eligible for enrollment in AMP if they had previously been in an approved natural history study [e.g., Pediatric AIDS Clinical Trial Group (PACTG) protocol 219/219C [8], Women and Infants Transmission Study (WITS) [9]] or had medical records pertinent to their HIV infection available from birth. This provided data from birth or early childhood on important exposures and outcomes.
2.2. Study data collection methods
2.2.1. Type and frequency of data collection
For this analysis, we included data collected before (WITS, PACTG 219/219C, and chart review) and during enrollment in PHACS. The data included sociodemographic information, ages at first occurrence of asthma diagnosis, asthma medication use and atopic dermatitis diagnosis, as well as available historical data for CD4 count (cells/mm3), HIV viral load (copies/ml), type of antiretroviral (ARV) therapy and lymphocytic interstitial pneumonitis (LIP) diagnosis.
The protocol-defined frequency of data collection in WITS and PHACS was the same for HIV-infected and HEU children. In 219C, visit frequency was the same for HIV-infected and HEU children until children reached one year of age, at which time HIV-infected children were seen every three months, whereas the HEU children were seen yearly.
Diagnoses and medication use were recorded in different formats across studies. For children previously enrolled in WITS, diagnosis and medication information was collected from birth. The available WITS medication data did not include codes for unique medications but did indicate whether the medication was intended to treat asthma. Though the WITS diagnosis data were not coded using the Medical Dictionary for Regulatory Activities (MedDRA), specific respiratory diagnosis codes were recorded that served as indicators of asthma.
For patients previously enrolled in PACTG 219/219C, each medication had a unique drug code and the diagnosis data were MedDRA coded. At entry, medication information was collected since the last approved study visit for patients on previous protocols, or within the last year for new patients. At entry, all diagnoses since birth were recorded for patients not on a previous protocol, and any diagnoses since the last study visit were recorded for rollover patients. At follow up visits, any new information regarding medication or diagnoses that had occurred since the last visit was recorded.
In AMP, the medication data had unique drug codes and the diagnosis data were MedDRA coded. Prescription medication information was obtained through chart abstraction. At study entry, medications were recorded since the time of the patient’s last approved protocol (219/219C, WITS, etc.) or in the last 30 days if not previously on a PHACS approved protocol. At follow-up visits, medications taken since the last AMP visit were recorded. All diagnoses that were new, ongoing, or resolved since the last appropriate study evaluation (includes 219/219C, WITS study visits) or since birth if not on a previous protocol were recorded. At follow up visits, any new information regarding medication or diagnoses that had occurred since the last visit was recorded.
2.3. Study outcomes
2.3.1. Definition of asthma diagnosis and asthma medication use
Recorded diagnoses meeting the definition of asthma diagnosis included asthma, bronchial hyperreactivity, wheezing, exercise induced asthma, and status asthmaticus. Bronchodilators, inhaled corticosteroids, and the leukotriene modifier montelukast were the asthma medications included in this review. The asthma diagnosis and medication use definitions for all children are described below.
If the children had an asthma diagnosis first recorded at or after age three, these were considered valid asthma diagnoses and the onset date was the first recorded date on file. Asthma medication use beginning at or after age three was defined as use of at least one of the following drug regimens: 1) any use of inhaled corticosteroids, 2) repeated use (i.e., reported at multiple visits or multiple medication start dates) of albuterol or other bronchodilator, 3) use of montelukast, if accompanied by at least one use of albuterol and/or by asthma diagnosis, or 4) single albuterol use lasting for at least three months and/or accompanied by an asthma diagnosis.
Children whose first recorded asthma diagnosis or medication use occurred before age three were reviewed individually in order to confirm asthma diagnosis/medication status and age at onset. The purpose of this approach was to reduce misclassification of asthma, because in children younger than three years, transient wheezing in association with respiratory viral infections is common, often treated with asthma medications and frequently misdiagnosed as asthma. Asthma can have its onset prior to three years old, but asthma diagnosis can be established only if symptoms persist beyond three or even six years of age [10,11]. Thus, children whose first recorded asthma diagnosis occurred before age three were only classified as having an asthma diagnosis if they also had diagnosis of asthma recorded at or after age three; the onset date remained the first recorded data in these cases. Children whose only recorded asthma diagnosis was before age three were not classified as having asthma. If children had asthma medication use recorded before age three, they were classified starting asthma medication use before age three if: 1) they also used asthma medication after age three and no more than one year after the medication use that occurred before age three and/or, 2) they also had an asthma diagnosis after age three and the date of first asthma diagnosis was no more than one year after the medication use before age three. For all children with confirmed asthma medication use, the onset date was the first medication date on file
2.3.2. Definition of atopic dermatitis
The definition of atopic dermatitis was based on the following MedDRA preferred terms: dermatitis-allergic, dermatitis-atopic, eczema, eczema-infantile. WITS data had one diagnosis code: Eczema/Atopic Dermatitis.
2.4. Statistical methods
The sociodemographic status of the HIV-infected and HEU children were compared using the Wilcoxon rank sum test for continuous variables and the Chi-square Test for categorical variables. Markers of HIV disease severity and type of ARV in the HIV-infected children were described.
Three different definitions of asthma were used as outcomes: 1) clinical diagnosis of asthma, 2) asthma medication use, and 3) asthma diagnosis, asthma medication use, or both. First, the proportion who ever had asthma by each of the above outcomes and the age of onset of each outcome by HIV status was determined. The difference in proportion of each asthma outcome by HIV status was tested by the chi-square test and difference in age by Wilcoxon Rank Sum Test. The proportions of children with an asthma diagnosis who also took asthma medications and of children with asthma medication use who also had an asthma diagnosis were computed. Cox proportional hazards models were fit to estimate the association of HIV status with time from birth to asthma onset or until the time of last patient contact for each outcome. We included sex and race/ethnicity in each model to determine if there was confounding of the association between HIV status and the outcome. If the estimate for HIV changed more than 10% with inclusion of either or both of these variables than they were included in the final model. Analyses were repeated after removal of all cases of LIP from the data set to test the potential effect of misclassification of children with LIP as having a diagnosis of asthma [12]. An LIP case was defined by an LIP diagnosis recorded in the clinical record. From the Cox models, the cumulative incidence of asthma by diagnosis, or medication use, or both in HIV-infected and HEU children by age was estimated.
Chi-square test was used to test 1) the proportion of children with atopic dermatitis by cohort; and 2) whether children with a history of atopic dermatitis were more likely to have a history of asthma compared to those without a history of atopic dermatitis, overall and stratified by cohort.
Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC) based on data collected as of July 1, 2010.
3. Results
3.1. General characteristics of PHACS AMP cohort: HIV-infected vs. HEU children
Most of the 451 HIV-infected and 227 HEU AMP participants were non-Hispanic black and the proportion was greater in the HIV-infected cohort (Table 1). At the entry visit in PHACS, HIV-infected participants were approximately 2.2 years older than HEU participants. Most HIV-infected children had a peripheral blood percent of CD4 T-cells ≥ 25% and a plasma RNA copy number (viral load) of ≤ 400 copies per mL. Most were currently receiving HAART (86%) and had a median of 8.6 years of lifetime exposure to HAART. In the HIV-infected cohort, the older children were less likely than younger children to have CD4 counts and HIV viral load measurements available before they began HAART.
Table 1.
Characteristics of HIV-infected and HIV-exposed, uninfected (HEU) children at entry into the Adolescent Master Protocol (AMP)
| Median, (25th, 75th) or N (%) | HIV-infected N=451 |
HEU N=227 |
P-value1 | |
|---|---|---|---|---|
| Demographic Characteristics | ||||
|
| ||||
| Age, years | 12.2 (9.9, 14.2) | 10.0 (8.2, 12.0) | <0.001 | |
| Male | 210 (47%) | 118 (52%) | 0.183 | |
| Race/Ethnicity | White Non-Hispanic | 28 (6%) | 16 (7%) | 0.029 |
| Black Non-Hispanic | 298 (66%) | 123 (54%) | ||
| Hispanic (Regardless of Race) | 109 (24%) | 80 (35%) | ||
| Other2 | 16 (4%) | 8 (4%) | ||
|
| ||||
| HIV-Related Characteristics | ||||
|
| ||||
| CDC Class3 | N3 | 72 (16%) | ||
| A | 145 (32%) | |||
| B | 122 (27%) | |||
| C | 108 (24%) | |||
| CD4 count,cells/mm3 | 731 (530, 966) | |||
| CD4 % | 33 (27, 38) | |||
| Nadir CD4 Count, cells/mm3 | 411 (239, 603) | |||
| Nadir CD4% | 19 (13, 26) | |||
| HIV Viral Load , copies/mL | 0-400 | 305 (68%) | ||
| 401-5000 | 76 (17%) | |||
| 5001-50000 | 50 (11%) | |||
| 50001+ | 17 (4%) | |||
| ARV4 regimen | HAART5 with PI6 | 309 (69%) | ||
| HAART without PI | 75 (17%) | |||
| Non-HAART ARV | 35 (8%) | |||
| Not on ARV | 32 (7%) | |||
| Cumulative HAART5 duration, years |
8.6 (6.0, 10.1) | |||
P-value for categorical and continuous variables and based on the Chi-square test and Wilcoxon rank sum test, respectively;
Includes both other and not known or not-reported;
Center for Disease Control HIV Disease Progression Classification;
Antiretroviral;
Highly active antiretroviral therapy;
Protease Inhibitor. Note missing data on: nadir CD4, 5 children; CDC class at entry, 4 children; CD4 and viral load at entry, 3 children.
3.2. Asthma classification by asthma diagnosis and by medication use
By clinical diagnosis (Table 2), 25% of the HIV-infected children were classified as ever having asthma versus 20% of the HEU children (p=0.101); the median ages at asthma diagnosis for HIV-infected and HEU children were similar (4.5 vs. 4.3 years, p=0.730), respectively. By medication use, 31% of the HIV-infected children ever had asthma compared to 22% of the HEU children (p=0.012), and the median age of asthma onset was similar for the HIV-infected (6.7 years) and HEU children (5.8 years) (p=0.294). When asthma was defined by clinical diagnosis, medication use, or both, HIV-infected children had a significantly higher asthma prevalence compared to HEU children (34% vs. 25%, p=0.012). There was a substantial overlap between asthma diagnosis and medication use, such that 141 out of 160 (88%) who ever had an asthma diagnosis also had used asthma medications. Of the 191 children who ever used asthma medications, 74% also had an asthma diagnosis. These percentages were similar for both HIV-infected and HEU children.
Table 2.
Proportion of participants with a history of asthma and age at first occurrence by 3 different criteria, stratified by HIV status
| Method of Asthma Classification | HIV-infected N=451 |
HEU1 N=227 |
P-value2 |
|---|---|---|---|
| Diagnosis | |||
| N (%) | 115 (25%) | 45 (20%) | 0.101 |
| Age, Median, years (25th, 75th) | 4.5 (2.0, 8.5) | 4.3 (2.4, 7.0) | 0.730 |
| Medication use | |||
| N (%) | 141 (31%) | 50 (22%) | 0.012 |
| Age, Median, years (25th, 75th) | 6.7 (3.4, 9.4) | 5.8 (2.4, 9.1) | 0.294 |
| Diagnosis, medication use, or both | |||
| N (%) | 154 (34%) | 56 (25%) | 0.012 |
| Age, Median, years (25th, 75th) | 4.6 (2.6, 7.9) | 4.4 (2.0, 7.3) | 0.650 |
HEU: HIV exposed, uninfected
Chi-square test for categorical variable and Wilcoxon test
Bronchodilators were used by nearly all HIV-infected and HEU children who used asthma medications, while controller medications were used in only about half of both groups (Table 3). Inhaled corticosteroids were the most commonly used controller medications for HEU children, of which fluticasone was most common; conversely, montelukast was the most commonly used controller medication among HIV-infected participants, who used fluticasone and other inhaled steroids less commonly. Two (5%) HIV-infected patients on ritonavir at study entry were also receiving fluticasone.
Table 3.
Asthma Medication Use at entry into the Adolescent Master Protocol (AMP), by HIV status. Within the HIV-infected group, asthma medication use is shown overall and by type of antiviral regimen.
| HIV-Infected | HEU1 | ||||
|---|---|---|---|---|---|
| Asthma Medication | RTV2 (N=41) |
Other PI (N=16) |
Not on PI (N=32) |
Overall (N=89) |
(N=39) |
| Bronchodilators | 36 (88%) | 16 (100%) | 32 (100%) | 84 (94%) | 38 (97%) |
| Controller Medications | 17 (41%) | 12 (75%) | 19 (59%) | 48 (54%) | 20 (51%) |
| Inhaled Fluticasone | 2 (5%) | 3 (19%) | 3 (9%) | 8 (9%) | 7 (18%) |
| Other Inhaled Corticosteroid3 | 3 (7%) | 2 (13%) | 2 (6%) | 7 (8%) | 1 (3%) |
| Montelukast | 7 (17%) | 5 (31%) | 10 (31%) | 22 (25%) | 5 (13%) |
| Fluticasone + Montelukast | 0 (0%) | 1 (6%) | 3 (9%) | 4 (4%) | 4 (10%) |
| Other inhaled steroid + Montelukast | 5 (12%) | 1 (6%) | 1 (3%) | 7 (8%) | 3 (8%) |
HEU: HIV-exposed, uninfected
RTV: Ritonavir
Includes beclomethasone, budesonide, mometasone, triamcinalone
3.3. Incidence of asthma by age and HIV status
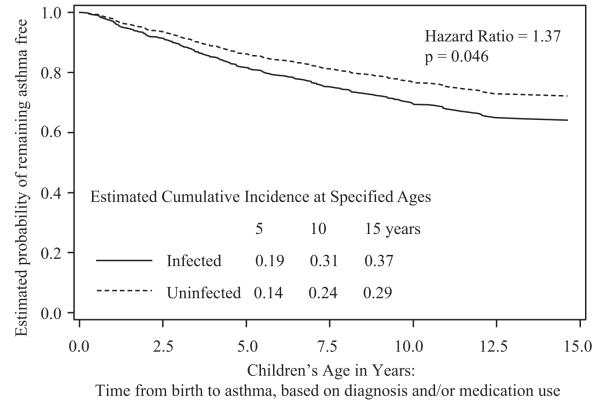
The unadjusted cumulative incidence of asthma by diagnosis and/or medication use, from the Cox model, is shown in Figure 1 for the HIV-infected and HEU children. This demonstrates the significantly increased risk in the HIV-infected children. For example, by age 15 years, the cumulative incidence was 37% in the HIV-infected and 29% in the HEU children. HIV-infected children had a 1.37 (95% CI 1.01, 1.86, p= 0.046) times greater risk of asthma by diagnosis, medication use, or both than HEU children (Table 4). While race/ethnicity was significantly associated with risk of asthma (defined by medication use) in univariate analysis (HR = 1.30, 95% CI: 0.96 to 1.76, p = 0.09), it was not a confounder of the association between HIV-status and any of the asthma outcomes in the Cox models (as defined in the methods). Sex was not associated with any outcome (p > 0.21) and was not a confounder. Thus, neither variable was included in the final models.
Figure 1.
Time to first occurrence of asthma, defined as diagnosis, medication use, or both from Cox regression analysis. Curves represent the estimated probability of remaining asthma free by HIV status. The estimated cumulative incidence of asthma at 5, 10, and 15 years of age are presented in the body of the graph as is the p-value for the hazard ratio
Table 4.
Risk of asthma from birth in HIV-infected vs. HIV-exposed, uninfected children, from Cox regression analysis
| Method of Asthma Classification | Hazard Ratio (95% CI) | P-value |
|---|---|---|
| Diagnosis | 1.22 (0.86, 1.72) | 0.264 |
| Medication use | 1.32 (0.96, 1.83) | 0.089 |
| Diagnosis, medication use, or both | 1.37 (1.01, 1.86) | 0.046 |
3.4. Impact of lymphoid interstitial pneumonitis diagnosis
Only 10% (16 cases) of HIV-infected participants classified as having asthma also had a recorded diagnosis of LIP. Among HIV-infected children not classified as an asthma case, 9% had an LIP diagnosis. As expected, no HEU child had an LIP diagnosis. When we excluded cases of LIP from our Cox models, the results did not change.
3.5. Atopic dermatitis incidence and association with asthma
The proportion of HIV-infected participants with a history of atopic dermatitis was higher than that in the HEU children(20% vs. 12%, p=0.009). The median age of atopic dermatitis onset was 2.85 years for HIV-infected and 5.03 years for HEU children (p=0.248). Among the 117 HIV-infected and HEU children with a history of atopic dermatitis, 48 (41%) had a history of asthma by diagnosis and/or medication use. In comparison, 162 of the 561 (29%) children without atopic dermatitis had a history of asthma by this definition (p = 0.010). (Table 5). The trends were the same when stratified by HIV status (not shown).
Table 5.
Prevalence of asthma history by three definitions among those with and without a history of atopic dermatitis 1
| Method of Asthma Classification |
Atopic Dermatitis N=117 |
No Atopic Dermatitis N=561 |
P-value2 |
|---|---|---|---|
| Diagnosis | 36 (31%) | 124 (22%) | 0.045 |
| Medication use | 45 (38%) | 146 (26%) | 0.007 |
| Diagnosis and/or medication use |
48 (41%) | 162 (29%) | 0.010 |
The diagnosis of atopic dermatitis may have occurred before, at the same time, or after the first occurrence of asthma
Chi-square test
4. Discussion
This study demonstrates that the risks of atopic dermatitis and asthma are about 30% higher in HIV-infected children treated with antiretroviral drugs than in HIV-uninfected children. A recent study of HIV-infected adults reveals at least a 20% prevalence of asthma compared to that of 8.8% in the general population [13]. Given the WHO estimates of 2.5 million HIV-infected children and 30.8 million HIV-infected adults (http://www.who.int/hiv/data/en), the number of additional HIV-infected patients seeking treatment for asthma and atopic dermatitis in children is potentially staggering.
The increased co-incidence of atopic dermatitis and asthma lends further evidence supporting a hyperallergic state brought about by treated HIV infection. The finding that atopic dermatitis is associated with asthma in patients with or without HIV infection suggests that what is being classified as asthma in HIV infection is the same condition classified as asthma outside the HIV context, and not a different, asthma-like condition. Our finding of the close association of atopic dermatitis and asthma is in harmony with the observation of a multicenter study of 1314 children (HIV-uninfected) followed for 7 years in which early appearance of atopic dermatitis was associated with asthma at school age [14].
The observations of asthma made in the PHACS AMP Study support the hypothesis of inflammatory mechanisms causing bronchospasm in HIV-infected patients whose cellular immunity is preserved or restored by HAART. In a study of AIDS and asthma, patients lose their potential to develop asthma with an unopposed loss of CD4+ T cells (e.g. <100 cells/μL in adults), but regain that potential with a return of CD4+ T cells upon treatment with HAART. This inverse correlation between the development of asthma and the degree of HIV-induced immunosuppression pattern underlines the important role of cellular immunity in airway hyperreactivity [15-17]. Evidence points to a generalized state of inflammation with HAART-induced immunoreconstitution or immunopreservation (i.e., HIV-infected infant treated with HAART before the loss of CD4 T cells) likely to involve the release of inflammatory cytokines, production of activated CD4+ and CD8+ T cells, and loss of T regulatory (TReg) cells and tolerance as the cause of pulmonary hyper-responsiveness. This process may take place over many months as seen in children with the immune reconstitution inflammatory syndrome [18]. At this critical time of immune imbalance, it is likely that patients will become highly sensitized to powerful antigens, an appropriate description for certain microorganisms that are ubiquitous in nature and are well established allergens in human [19] and animal asthma models. Such immune dysregulation may result in creation of a hyperallergic condition as observed clinically in drug allergy, including sensitivity to antimicrobial drugs and antiretroviral agents, [20] atopic dermatitis,[21] and allergic rhinitis/sinusitis [22].
We were unable to model the relationship of asthma to immune status in HIV-infected children as had been done in previous studies [2] because up to one third of children did not have CD4 counts available before asthma diagnosis or asthma medication use, especially among the older cohort of children. Additionally, almost all HIV-infected children had been taking HAART from a very young age, and so we were unable to study the effect of initiation of HAART on asthma incidence. While HIV-infected and HEU children had different visit schedules in their prior approved studies, all children we seen at least yearly. Thus, we think that there would not be differential capture of asthma cases across cohorts because of its clinical importance.
In this observational cohort we carefully reviewed the data when classifying cases of asthma by medication use and/or diagnosis to capture the maximum number of cases and to decrease misclassification error. In addition, we found consistent results across the three outcomes and were strongest when we used the most inclusive definition. Observations of the development of asthma in an immune dysregulated murine model have implicated a common household mold proteinase molecule as a cause for pulmonary hyperreactivity [23-25]. The HAART-induced oligoclonal return of CD4+T cells may confront such fungal proteinase antigens with CD8+ T cells and inflammatory Th2 cytokine release thus leading to bronchospastic pulmonary reactions. Animal model studies of asthma indicate that the most important of these microorganisms are fungi (collected from the house dust of asthmatic children), which are typically viewed as harmless contaminants of respiratory specimens. In fact, they represent potential pathogens capable of producing active lower respiratory tract infections leading to asthma. Our future studies will test the hypothesis that such microorganisms infect the immunocompromised airways of AIDS patients, leading to asthma after immunoreconstitution of the Th2 effector function. Studies of specific allergic symptoms, skin test to common airborne allergens, and pulmonary function tests would provide information on functional consequences of atopic diseases in patients with HIV infection.
Finally, we note that the use of asthma controller medications in HIV-infected children is complicated by an interaction of inhaled fluticasone – the most commonly used asthma controller medication in children – and ritonavir-containing HAART, which can result in adrenal suppression and other features of Cushing syndrome [26,27]. The data from this study suggest that clinicians are appropriately more likely to choose a different inhaled corticosteroid, montelukast, or both as the anti-inflammatory controller medication for their HIV-infected children with asthma compared to their practice for HIV-uninfected asthmatic children. Furthermore, the overall use of controller medications was similar for the two groups (54% in HIV-infected and 51% in HIV-uninfected), suggesting that controller medication use may still be suboptimal but it is no worse because of HIV infection status. It is notable, however, that, despite warnings against using fluticasone in combination with ritonavir [28-30], two HIV-infected patients with asthma on ritonavir at study entry were also receiving fluticasone.
In conclusion, this study has uncovered an important link between asthma and atopic dermatitis in a perinatally HIV-infected patient population whose lymphocyte development has been significantly interrupted by HIV infection, but then rescued by potent antiretroviral medications. It is reasonable to propose that a sudden return of immunity to an infant or child may produce a dysregulated immune state with lymphocyte sensitization to HIV itself and environmental antigens causing pulmonary hypersensitivity and atopic dermatitis. This hyperallergenic state deserves not only optimal and safe therapy of HIV infection, but also of atopic disease. The public health consequences of these findings on a world scare will demand close scrutiny.
Highlights.
HIV infection increases the risk for asthma and atopic dermatitis by 30%.
This represents an enormous clinical challenge for 33 million HIV-infected patients.
Appropriate management will help reduce the burden of skin and respiratory tract morbidity.
Acknowledgments
We thank the children and families for their participation in PHACS, and the individuals and institutions involved in the conduct of PHACS. The study was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute of Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, and the National Institute on Alcohol Abuse and Alcoholism through cooperative agreements with the Harvard University School of Public Health (HD052102, 3 U01 HD052102-05S1, 3 U01 HD052102-06S3) (Principal Investigator: George Seage; Project Director: Julie Alperen) and the Tulane University School of Medicine (HD052104, 3U01HD052104-06S1) (Principal Investigator: Russell Van Dyke; Co-Principal Investigator: Kenneth Rich; Project Director: Patrick Davis). Additional support was received from the National Institutes of Health grants P30AI36211 and RR0188. Data management services were provided by Frontier Science and Technology Research Foundation (PI: Suzanne Siminski), and regulatory services and logistical support were provided by Westat, Inc (PI: Julie Davidson). The following institutions, clinical site investigators and staff participated in conducting PHACS AMP in 2010, in alphabetical order: Baylor College of Medicine: William Shearer, Mary Paul, Norma Cooper, Lynette Harris; Bronx Lebanon Hospital Center: Murli Purswani, Mahboobullah Baig, Anna Cintron; Children’s Diagnostic & Treatment Center: Ana Puga, Sandra Navarro, Doyle Patton, Deyana Leon; Children’s Hospital, Boston: Sandra Burchett, Nancy Karthas, Betsy Kammerer; Children’s Memorial Hospital: Ram Yogev, Margaret Ann Sanders, Kathleen Malee, Scott Hunter; Jacobi Medical Center: Andrew Wiznia, Marlene Burey, Molly Nozyce; St. Christopher’s Hospital for Children: Janet Chen, Latreca Ivey, Maria Garcia Bulkley, Mitzie Grant; St. Jude Children’s Research Hospital: Katherine Knapp, Kim Allison, Megan Wilkins; San Juan Hospital/Department of Pediatrics: Midnela Acevedo-Flores, Heida Rios, Vivian Olivera; Tulane University Health Sciences Center: Margarita Silio, Medea Jones, Patricia Sirois; University of California, San Diego: Stephen Spector, Kim Norris, Sharon Nichols; University of Colorado Denver Health Sciences Center: Elizabeth McFarland, Emily Barr, Robin McEvoy; University of Maryland, Baltimore: Douglas Watson, Nicole Messenger, Rose Belanger; University of Medicine and Dentistry of New Jersey: Arry Dieudonne, Linda Bettica, Susan Adubato; University of Miami: Gwendolyn Scott, Patricia Bryan, Elizabeth Willen.
Abbreviations
- AMP
Adolescent Master Protocol
- HAART
Highly active antiretroviral therapy
- HEU
HIV-exposed but uninfected (children)
- LIP
lymphoid interstitial pneumonitis
- PACTG 219/219C
Pediatric AIDS Clinical Trials Group 219/219C Late Outcomes Study
- PHACS
Pediatric HIV/AIDS Cohort Study
- WITS
Women and Infants Transmission Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest None of the authors have conflicts with the content of this report.
Note: The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or U.S. Department of Health and Human Services.
References
- [1].Foster SB, Lu M, Thompson B, et al. Association between HLA inheritance and asthma medication use in HIV-positive children. AIDS. 2010;24:2133–2135. doi: 10.1097/QAD.0b013e32833cba08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Foster SB, McIntosh K, Thompson B, et al. Increased incidence of asthma in HIV-infected children treated with highly active antiretroviral therapy in the National Institutes of Health Women and Infants Transmission Study. J Allergy Clin Immunol. 2008;122:159–165. doi: 10.1016/j.jaci.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Foster SB, Paul ME, Kozinetz CA, et al. Prevalence of asthma in children and young adults with HIV infection. J Allergy Clin Immunol. 2007;119:750–752. doi: 10.1016/j.jaci.2007.01.002. [DOI] [PubMed] [Google Scholar]
- [4].Gutin F, Butt A, Alame W, et al. Asthma in immune-competent children with human immunodeficiency virus. Ann Allergy Asthma Immunol. 2009;102:438. doi: 10.1016/S1081-1206(10)60518-2. [DOI] [PubMed] [Google Scholar]
- [5].Rudikoff D. The relationship between HIV-infection and atopic dermatitis. Curr Allergy Asthma Rep. 2002;2:275–281. doi: 10.1007/s11882-002-0050-x. [DOI] [PubMed] [Google Scholar]
- [6].Soto PC, Stein LL, Hurtado-Ziola N, et al. Relative over-reactivity of human versus chimpanzee lymphocytes: Implications for the human diseases associated with immune activation. J Immunol. 2010;184:4185–4195. doi: 10.4049/jimmunol.0903420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Reveille JD, Williams FM. Infection and musculoskeletal conditions: Rheumatologic complications of HIV infection. Best Pract Res Clin Rheumatol. 2006;20:1159–1179. doi: 10.1016/j.berh.2006.08.015. [DOI] [PubMed] [Google Scholar]
- [8].van Dyke RB, Wang L, Williams PL. Pediatric ACTG 219c Team. Toxicities associated dual nucleoside reverse-transcriptase inhibitor regimens in HIV-infected children. J Infect Dis. 2008;198(11):1599–1608. doi: 10.1086/593022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Aldrovandi GM, Chu C, Shearer WT, et al. Antiretroviral exposure and lymphocyte mtDNA content among uninfected infants of HIV-1-infected women. Pediatrics. 2009;124(6):e1189–1197. doi: 10.1542/peds.2008-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Martinez FD, Wright AL, Taussig LM, et al. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- [11].Castro-Rodriguez JA, Holberg CJ, Wright AL, et al. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162:1403–1406. doi: 10.1164/ajrccm.162.4.9912111. [DOI] [PubMed] [Google Scholar]
- [12].Brand PL, Baraldi E, Bisgaard H, et al. Definition, assessment and treatment of wheezing disorders in preschool children: an evidence-based approach. Eur Respir J. 2008;32:1096–1110. doi: 10.1183/09031936.00002108. [DOI] [PubMed] [Google Scholar]
- [13].MR Gingo, SE Wenzel, C Steele, et al. Asthma diagnosis and airway bronchodilator response in HIV-infected individuals. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.11.015. (accepted for publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Illi S, von Mutius E, Lau S, et al. Multicenter Allergy Study Group. The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. J Allergy Clin Immunol. 2004;113(5):925–31. doi: 10.1016/j.jaci.2004.01.778. [DOI] [PubMed] [Google Scholar]
- [15].Paul ME, Shearer WT. Human immunodeficiency virus and allergic disease. In: Adkinson NF Jr., Yunginger JW, Busse WW, Bochner Bs, Holgate ST, Simons FER, editors. Middelton’s Allergy: Principles and Practice. 7th Edition Volume II. Mosby; St. Louis: 2009. pp. 831–843. [Google Scholar]
- [16].Lin RY, Lazarus TS. Asthma and related atopic disorders in outpatients attending an urban HIV clinic. Ann Allergy Asthma Immunol. 1995;74:510–515. [PubMed] [Google Scholar]
- [17].Galli L, Sabatino G, Zappa M, et al. Reduced frequency of wheezing respiratory illness in infants with perinatal human immunodeficiency virus-type 1 infection: a model for immunologic and inflammatory mechanisms of airway obstruction? Pediatr Allergy Immunol. 2003;14:42–49. doi: 10.1046/j.1439-0388.2003.02104.x. [DOI] [PubMed] [Google Scholar]
- [18].Zar HJ. Chronic lung disease in human immunodeficiency virus (HIV) infected children. Pediatric Pulmonol. 2008;43(1):1–10. doi: 10.1002/ppul.20676. [DOI] [PubMed] [Google Scholar]
- [19].Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med. 2007;357:1487–1495. doi: 10.1056/NEJMoa052632. [DOI] [PubMed] [Google Scholar]
- [20].Davis CM, Shearer WT. Diagnosis and management of HIV drug hypersensitivity. J Allergy Clin Immunol. 2008;121:826–832. doi: 10.1016/j.jaci.2007.10.021. [DOI] [PubMed] [Google Scholar]
- [21].Daniel TD, Levy ML. Cutaneous manifestations of pediatric HIV infection. In: Shearer WT, Hanson IC, editors. Medical Management of AIDS in Children. Saunders; Philadelphia: 2003. pp. 271–289. [Google Scholar]
- [22].Porter JP, Patel AA, Dewey CM, et al. Prevalence of sinonasal symptoms in patients with HIV infection. Am J Rhinol. 1999;13:203–208. doi: 10.2500/105065899781389696. [DOI] [PubMed] [Google Scholar]
- [23].Lamhamedi-Cherradi S-E, Martin RE, Ito T, et al. Frugal proteases induce Th2 polarization through limited dendritic cells maturations and inhibition of IL-12 production. J Immunol. 2008;180:6000–6009. doi: 10.4049/jimmunol.180.9.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kheradmand F, Corry DB. Discovery of novel markers in allergic lung inflammation through proteomic-based technologies. Expert Rev Proteomics. 2008;5:9–12. doi: 10.1586/14789450.5.1.9. [DOI] [PubMed] [Google Scholar]
- [25].Porter P, Susarla SC, Polikepahad S, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immun. 2009;2:504–517. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Arrington-Sanders R, Hutton N, Siberry GK. Ritonavir-fluticasone interaction causing Cushing syndrome in HIV-infected children and adolescents. Pediatr Infect Dis J. 2006;25:1044–1048. doi: 10.1097/01.inf.0000242929.95258.69. [DOI] [PubMed] [Google Scholar]
- [27].Johnson SR, Marion AA, Vrchoticky T, et al. Cushing syndrome with secondary adrenal insufficiency from concomitant therapy with ritonavir and fluticasone. J Pediatr. 2006;148:386–388. doi: 10.1016/j.jpeds.2005.11.034. [DOI] [PubMed] [Google Scholar]
- [28].Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children [August 16, 2010];Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. :1–219. Available at http://aidsinfo.nih.gov/ContentFiles/PediatricGuidelines.pdf.
- [29].Flovent [package insert] GlaxoSmithKline; Research Triangle Park, NC: 2011. [Google Scholar]
- [30].Kaletra [package insert] Abbott Laboratories; North Chicago, IL: 2011. [Google Scholar]