Abstract
Objective
Low socioeconomic status (SES) early in life is one of the most well-established social predictors of poor health. However, little is understood about why some adults who grew up in low SES environments do not have poor health outcomes. This study examined whether the psychological characteristic of “shift-and-persist” protects adults from the physiological risks of growing up in low SES households. Shift-and-persist consists of reframing appraisals of current stressors more positively (shifting), while simultaneously persisting with a focus on the future. We hypothesized that this characteristic would be associated with reduced physiological risk in low childhood SES individuals.
Methods
A national sample of 1,207 adults (ages 25-74) from the Survey of Midlife Development in the U.S. (MIDUS) completed psychological questionnaires, and were queried about parent education. Biological assessments consisted of 24 different measures across seven physiological systems, from which a composite measure representing cumulative physiological risk (allostatic load) was derived.
Results
Among adults who grew up in low SES households, those who engaged in high shift and high persist strategies had the lowest allostatic load (b=-.15, p=.04). No benefit of shift-and-persist was found for those from higher childhood SES backgrounds (p=.36).
Conclusions
Identifying the health-related protective qualities that naturally occur in some low SES individuals represents one important approach for developing future health improvement interventions for those who start out life low in SES. Moreover, the psychological qualities that are protective from future disease risk for those from low SES backgrounds are different from those beneficial to high SES individuals.
Keywords: childhood socioeconomic status, allostatic load, protective factors, psychological, physiological
INTRODUCTION
Socioeconomic status (SES) is one of the most powerful social determinants of physical health in developed countries. For example, individuals living in low SES circumstances are consistently at greater risk for a variety of chronic medical illnesses, including cardiovascular disease (CVD), arthritis, and some cancers (1-3). The effects of low SES are evident across numerous countries throughout the world, including both those with and without universal health care (4).
Furthermore, research has identified the early years of life as a sensitive period, during which low SES seems to have especially potent and lasting effects on health. Indeed, low childhood SES increases people’s risks of developing infectious, respiratory, and cardiovascular diseases much later in life (5-8). Over the years, researchers have focused their efforts on investigating the pathways that can explain these effects of childhood SES (9-13).
However, one important question that has gone largely unexplored is why some adults are able to maintain physically healthy profiles despite having grown up under difficult life circumstances. This is the notion of resilience - that is, thriving under adversity (14, 15). Resilience has been extensively discussed within the mental health literature (16-18); however, the notion of resilience has not often been tested with respect to physical health-related outcomes.
Our group has been working to develop a theoretical account of the specific psychological characteristics that may protect low SES individuals from detrimental physical health consequences (19). Under traditional models, low SES typically evokes stress responses that over time can promote long-term pathogenic processes that result in chronic illnesses such as cardiovascular disease years later (1, 20-24).
In contrast, our model states that there is an approach to life that is adaptive specifically in a low SES context. That is, a “shift-and-persist” approach to dealing with life demands will be beneficial physiologically to those who come from low SES backgrounds. This approach balances adapting the self to life stressors together with maintaining a focus on the future. It entails both shifting (adjusting oneself to stressors through cognitive reappraisals and emotion regulation) and persisting (enduring life with strength by holding onto hopes for the future). This combination of approaches to dealing with life stressors is hypothesized to reduce physiological responses to stress acutely, and by doing so, mitigates the long-term progression of pathogenic processes that lead to chronic disease (19).
The shift-and-persist theory is based in part on lifespan theories of control which postulate that human beings strive for primary control (being able to change the environment so that it fits one’s needs and desires), but that when this type of control is not possible, they engage in secondary control efforts (attempts to bring oneself in line with one’s environment) (25, 26). Given the multitude of constraints that low SES individuals face in life, they are hypothesized to value efforts to control the self and to adjust oneself to the world and others around them (conjoint agency) more so than efforts to control the world (27, 28). Consistent with this notion, in the health psychology literature, active efforts to cope with stressors and being given control over the parameters of a stressor are not beneficial to cardiovascular risk profiles amongst low SES individuals (29-33).
In addition, efforts to maintain a focus on the future are also thought to be beneficial for those low in SES. This idea is derived from theories of resilience that postulate that finding meaning in life helps individuals to cope with traumatic or life-threatening events (34-36). Low SES may represent another circumstance in which broader perspectives on life, such as meaning making and a future orientation is beneficial, not only to well-being, but also to physical health. Consistent with this notion, low SES adults who reported greater purpose in life showed lower levels of systemic inflammatory markers (37).
Finally, we argue that it is not just the presence of one of the above approaches, but the combination, that is critical – that is, possessing both an approach that values shifting the self in response to stress together with persisting with hopes for one’s future will be more beneficial than either trait on its own for physiological responses to stress specifically among those who come from low SES backgrounds. This is because shift-and-persist is postulated to represent a good fit with the general environmental constraints for those low in SES. In contrast, among high SES individuals, proactive efforts at coping that are aimed at eliminating stressors may be more effective, given the greater resources, on average, that high SES individuals possess for engaging in preventive behaviors, resolving situations, and influencing outcomes (38-41). Furthermore, shifting without persisting has the potential to lead to learned helplessness (passive acceptance of all stressors), which has been associated with increased risk of depression and functional disability in patient populations (42, 43). Hence the label that we use, “shift-and-persist,” is intended to connote the fact that it is the combination of these characteristics, rather than either one on its own, that is most beneficial to health among those low in SES.
The present paper sought to empirically test the shift-and-persist theory in a national sample of midlife adults in the U.S. To examine the notion that shift-and-persist strategies evolve out of adverse childhood circumstances and can have long-term health benefits, we focused on participants who grew up in low SES households during childhood, and examined whether shift-and-persist strategies were associated their physiological profiles as adults. Because SES disparities are observed for a wide range of health outcomes, we focus in this paper on multiple system indices of physiological risk, captured by allostatic load. The concept of allostatic load refers to the long-term physiological wear and tear that results from the body’s efforts to maintain homeostasis in response to stressors (44). Over time, this wear-and-tear manifests in the dysregulation of multiple physiological systems, including the cardiovascular, autonomic, metabolic, and inflammatory systems (45). Allostatic load composites reflecting these systems predict the onset of diseases such as CVD, as well as all-cause mortality (46-48). Capturing the biological effects of low SES across multiple regulatory systems is important, given that low SES has been linked to a wide array of health conditions that are likely affected by multiple physiological systems. In the present study, we hypothesized that the combination of shift-and-persist strategies would be associated with lower allostatic load among adults who grew up in low SES households, but not among adults who grew up in higher SES households.
METHODS
Participants
Participants were from the Midlife in the United States (MIDUS) survey, a national sample from the 48 contiguous states of individuals ages 25-74, selected via random-digit telephone dialing (Brim, Ryff, & Kessler, 2004). MIDUS I was conducted in 1995–1996 and MIDUS II in 2004–2006. In MIDUS I, 7,108 noninstitutionalized adults were selected, with 1,914 being twins. 75% of these individuals participated in MIDUS II. Biological data were collected on a subset of MIDUS II participants (N=1,255) who completed the telephone and mail surveys and were able and willing to travel to one of three General Clinical Research Centers (GCRC) for an overnight visit. 43% of those invited agreed to participate. Those who participated in the biological protocol were not different from the overall sample in terms of age, sex, race, marital status, or income (though they were more likely to have a college degree) (49). This study was approved by the Institutional Review Boards at UCLA, University of Wisconsin Madison, Georgetown University, and Brandeis University.
The current analyses focused on 1,207 MIDUS II participants who both provided biological data and information about their childhood SES. 300 of these participants were low SES, and 907 were higher SES (see below for SES definitions). See Table 1.
Table 1.
Sample characteristics of MIDUS II participants (N=1,207)
| Low Childhood SES (n=300) | High Childhood SES (n=907) | |||||
|---|---|---|---|---|---|---|
| % | M | SD | % | M | SD | |
| Age | 58.50 | 12.12 | 53.19 | 11.32*** | ||
| Gender (% male) | 40.8 | 44.6 | ||||
| Ethnicity (% white) | 68.6 | 84.2*** | ||||
| Current education | ||||||
| < high school | 9.7 | 3.5*** | ||||
| High school | 33.6 | 17.8 | ||||
| Some college | 28.9 | 30.4 | ||||
| ≥ university degree | 27.9 | 48.3 | ||||
| Current smoker | 15.7 | 12.5 | ||||
| Diabetes (ever diagnosed) | 14.1 | 8.7** | ||||
| Cardiovascular disease (ever diagnosed) | 22.1 | 12.7*** | ||||
| Shift – positive reappraisals | 3.16 | 0.65 | 3.10 | 0.62 | ||
| Shift – emotion regulation | 8.38 | 5.86 | 8.85 | 2.29 | ||
| Persist – future orientation | 2.60 | 0.78 | 2.85 | 0.71*** | ||
| Sympathetic Nervous System | ||||||
| Epinephrine (ug/g creatine) | 2.04 | 1.40 | 1.95 | 1.24 | ||
| Norepinephrine (ug/g creatine) | 29.36 | 13.89 | 26.79 | 13.83** | ||
| Parasympathetic Nervous System | ||||||
| SDRR (msec) | 34.39 | 17.03 | 36.34 | 17.86 | ||
| RMSSD | 23.64 | 18.14 | 22.72 | 18.10 | ||
| LFHRV | 357.86 | 501.69 | 453.52 | 655.59* | ||
| HFHRV | 304.63 | 635.65 | 323.49 | 777.59 | ||
| Hypothalamic Pituitary Adrenal Axis | ||||||
| Cortisol (ug/g creatine) | 15.32 | 16.13 | 15.61 | 15.19 | ||
| DHEA-S (ug/dL) | 96.03 | 72.04 | 108.92 | 78.75* | ||
| Inflammation | ||||||
| CRP (ug/mL) | 3.57 | 5.59 | 2.84 | 4.52* | ||
| IL-6 (pg/mL) | 3.60 | 3.47 | 2.82 | 2.90** | ||
| Fibrinogen (mg/dL) | 359.86 | 91.05 | 344.46 | 86.86** | ||
| sICAM-1 (ng/Ml) | 302.63 | 124.24 | 284.86 | 110.92* | ||
| sE-selectin (ng/Ml) | 45.05 | 24.16 | 42.62 | 21.80 | ||
| Cardiovascular | ||||||
| SBP (mmHg) | 133.98 | 17.64 | 130.30 | 18.10** | ||
| DBP (mmHg) | 75.50 | 10.98 | 75.54 | 10.58 | ||
| Heart rate (bpm) | 71.67 | 12.08 | 70.84 | 10.91 | ||
| Glucose metabolism | ||||||
| Glucose (mg/dL) | 105.08 | 29.45 | 101.01 | 28.01* | ||
| Insulin resistance (HOMA-IR) | 3.84 | 3.67 | 3.40 | 3.64 | ||
| HbA1c% | 6.35 | 1.34 | 6.00 | 1.08*** | ||
| Lipid metabolism | ||||||
| LDL (mg/dL) | 102.23 | 33.72 | 106.84 | 35.82 | ||
| HDL (mg/dL) | 54.13 | 16.98 | 55.46 | 18.25 | ||
| Triglycerides (mg/dL) | 130.30 | 79.76 | 133.96 | 147.11 | ||
| BMI | 30.99 | 7.85 | 29.28 | 6.11** | ||
| WHR | 0.90 | 0.10 | 0.89 | 0.10* | ||
| Allostatic load score | 2.02 | 1.00 | 1.66 | 1.02*** | ||
p<.05.
p<.01.
p<.001, for differences between low childhood and high childhood SES groups.
SDRR=standard deviation of the RR interval. RMSSD=root mean squared successive difference. LFHRV=low frequency heart rate variability. HFLRV=high frequency heart rate variability. DHEA-S= dehydroepiandrosterone sulfate. CRP=C reactive protein. IL-6=interleukin 6. sICAM-1=soluble intercellular adhesion molecule 1. SBP=systolic blood pressure. DBP=diastolic blood pressure. HbA1c%=hemoglobin A1c %. LDL=low density lipoprotein. HDL=high density lipoprotein. BMI=body mass index. WHR=waist hip ratio.
Protocol
Participants completed demographic and psychosocial questionnaires by mail and telephone interview. At the GCRC, individuals provided a complete medical history and medication information, underwent a physical exam with a physician, and provided blood, urine, and saliva samples, along with cardiovascular and heart rate variability measurements. Fasting morning blood was collected around 7:00am (prior to any caffeine or nicotine consumption). Urine was collected during a 12-hour overnight protocol beginning at 7:00pm and ending at 7:00am. Informed consent was obtained for all research participants.
Measures
SES
To index childhood SES, participants were queried about their parents’ highest level of educational attainment. We used parent educational attainment because this is recommended for use in retrospective SES studies of adult samples and is more likely to be accurately remembered from childhood than other SES measures (such as family income) (50). Second, we sought to identify a group of clearly low SES adults, so we classified those who reported both parents as having less than a high school diploma as low SES (n=300). The remainder of the MIDUS II sample was categorized as higher SES (n=907). Current SES was used as a covariate in analyses, and was coded based on the participant’s own level of educational attainment (less than high school diploma, high school grad, some college, or bachelor’s degree or higher). This allowed us to test whether effects were due to childhood circumstances, or whether childhood SES merely served as a proxy for current SES.
Measures of shift
Two measures were combined to capture the tendency to shift oneself in response to stressors. One was the tendency to reappraise stressful situations more positively. This was measured at time 2 using the Positive Reappraisals scale of the Primary and Secondary Control questionnaire (51). Four items (e.g., “I can find something positive, even in the worst situations”) were queried on a 4 point scale (ranging from “not at all” to “a lot”). Items were coded such that higher scores indicated higher tendency to positively reappraise stressful situations. Cronbach’s alpha for the scale in MIDUS I was .78. Ten year test-retest reliability (from MIDUS I to MIDUS II) was .57, a value that is comparable to the stability of personality traits over this type of interval (52). This measure has been shown to have validity in previous studies in terms of correlating significantly with other related constructs, such as mastery (51), and in terms of predicting positive adjustment and positive affect among those who have more constrained life opportunities (e.g., older adults; (51, 53, 54)).
Second, we used a questionnaire measure of emotional reactivity to stress to get at emotion regulation (55). The stress reactivity subscale of the Multidimensional Personality Questionnaire (MPQ) taps the extent to which participants control their emotions in response to the stressors of daily life (e.g., “My mood often goes up and down,” and “Minor setbacks sometimes irritate me too much”). Three items were rated on a 4 point scale (true, somewhat true, somewhat false, false) at time 2. Items were recoded such that higher scores indicated a greater ability to control one’s emotions in the face of hassles. These items represent a shortened version of the MPQ-BF (55), which was necessary, given the constraints on questionnaire length in MIDUS. The 3 items were chosen in consultation with the author of the original scale (Tellegen) and using factor analysis and item response theory to analyze the original items from other datasets. Cronbach’s alpha for this scale was .74 (compared to .84 in the original, longer version). Validity is indicated by virtue of the short version correlating significantly with other measures of negative affect in MIDUS, such as neuroticism (r=.69, p<.001) and negative affect from the Positive and Negative Affect Scales (r=.56, p<.001). This is comparable to validity data on the original scale, in which the stress reactivity scale correlated .76 with neuroticism, and .33-.63 with negative emotion measures related to anxiety, anger, and distress (55, 56).
To create a total shift score, responses to the two questionnaires were first standardized (because they have different ranges), and then summed. Thus higher scores indicated greater use of shift strategies.
Measure of persist
A measure of future orientation was included as an indicator of persistence at time 2 (57). The “Live for Today” subscale of the Planning and Making Sense of the Past questionnaire in MIDUS consisted of four items, each rated on a 4 point scale (from “not at all” to “a lot”). These items tapped the extent to which individuals think about their future (e.g., “I have too many things to think about today to think about tomorrow”). Items were reverse coded such that higher scores indicated higher persistence with thinking about the future. Cronbach’s alpha for the scale in MIDUS I was .73. Ten year test-retest reliability (from MIDUS I to MIDUS II) was .55, comparable to the stability of personality traits over this type of interval (52). Validity is indicated by virtue of the Live for Today scale correlating significantly with other measures from MIDUS that one would expect to co-occur with a future orientation, such as greater mastery (r=.27, p<.001), optimism (r=.21, p<.001), greater life satisfaction (r=.12, p<.001), and greater conscientiousness (r=.13, p<.001), similar to the pattern of correlations documented for a related measure of future planning (57).
Allostatic load
Allostatic load was calculated as a risk score based on measures taken of seven different physiological systems at time 2. Measures of cardiovascular functioning included resting systolic and diastolic blood pressure (SBP, DBP; average of the two most similar of three successive measurements with a 30 sec rest period in between) and resting pulse (pulse count taken for 15 sec and multiplied by 4 to obtain resting beats per min). Indicators of sympathetic nervous system (SNS) activity included overnight urinary measures of epinephrine and norepinephrine (12-hour overnight urine collections via high-pressure liquid chromatography (HPLC), and adjusted by urine creatine levels to control for hormone output as a function of body size). Measures of parasympathetic nervous system (PNS) activity included the following heart rate variability parameters (assessed with electrocardiographic (ECG) electrodes placed on the left and right shoulders and in the left quadrant, and with ECG activity monitored during an 11 min seated baseline period): low and high frequency spectral power, the standard deviation of R-R (heartbeat to heartbeat) intervals (SDRR), and the root mean square of successive differences (RMSSD). Indicators of hypothalamic pituitary adrenal (HPA) axis activity included an overnight urinary measure of the hormone cortisol (measured via HPLC) and a serum measure of the hormone dehydroepiandrosterone sulfate (DHEA-S, assessed with a Roche Modular Analytics E170 analyzer with an Elecsys kit). Measures of inflammation included plasma C-reactive protein (CRP, assayed with a particle-enhanced immunonepholometric assay using a BNII nephelometer), fibrinogen (BNII nephelometer), and serum measures of interleukin-6 (IL-6, assayed with high-sensitivity enzyme-linked immunosorbent assay, ELISA) and the soluble adhesion molecules e-selectin and intracelleular adhesion molecule-1 (ELISAs). Indicators of lipid and general metabolic activity included high density lipoprotein (HDL) cholesterol, low density lipoprotein (LDL) cholesterol, triglycerides, body mass index (BMI), and waist-hip ratio (WHR). Cholesterol panels were obtained using a Cobas Integra analyzer. LDL cholesterol levels were estimated using the Friedewald equation based on measures of total cholesterol, triglycerides, and HDL cholesterol. WHR is the ratio of waist (assessed at the narrowest point between ribs and the iliac crest) to hip circumference (measured at the maximum diameter of the buttocks), and BMI was calculated based on height and weight. Measures of glucose metabolism included levels of glycosylated hemoglobin (measured using a Cobas Integra analyzer), fasting glucose (measured with an enzymatic assay on an automated analyzer, Roche Modular Analytics P), and the homeostasis model of insulin resistance (HOMA-IR, calculated based on glucose and insulin, which was measured with a two-site sandwich immunoassay using direct chemiluminescent technology).
To calculate allostatic load, a multi-system physiological risk score was computed as the sum of the seven separate physiological system (SNS, PNS, HPA, cardiovascular, glucose metabolism, lipid metabolism, and inflammation) risk indices. System risk indices were computed as the proportion of individual biomarker indicators for each system (ranging from 2 to 6 biomarkers) for which participant values fell into high-risk quartile ranges; scores were only computed for individuals with values on at least half of the biomarkers within a system. System risk scores could range from 0 to 1 (indicating 0 - 100% of system biomarkers in high-risk range for a given participant). As the number of biomarker indicators varied across physiological systems, this average risk scoring method produced a similar ‘scaling’ of risk scores across the different systems. A multi-system physiological risk index was computed as the sum of the seven physiological system scores (possible range from 0 to 7). Multi-system risk scores were only computed for participants with information on at least 6 of the 7 systems. Similar approaches to conceptualizing and calculating allostatic load have been used in numerous previous studies (45-47).
Covariate
Covariates included in statistical analyses included the demographic covariates of age, gender, and race (White or other). Medical covariates included a history of diseases that could affect the above systems, including diabetes (yes/no) and cardiovascular disease (yes/no). Health behavior covariates included current smoking status (yes/no). To address the possibility that childhood SES merely served as a proxy for current SES, we also included current education as a covariate.
Statistical analyses
Our first analysis involved a test of the 3-way interaction between childhood SES, shift strategies, and persist strategies in predicting allostatic load. This allowed us to test our overarching hypothesis that the combination (interaction) of shift and persist strategies would have different effects across SES groups. Hierarchical multiple regression analyses were conducted in which allostatic load was predicted from (1) covariates; (2) main effects of childhood SES (low, high), shift, and persist variables; and (3) the interaction of childhood SES, shift, and persist variables. Significant 3-way interactions were then followed-up by testing 2-way interactions (shift × persist) within each childhood SES group (low, high). The 2-way interactions allowed us to ask the question of how the combination of shift-and-persist strategies related to allostatic load within each SES group. Within SES groups, hierarchical multiple regression analyses were conducted in which allostatic load was predicted from (1) covariates; (2) main effects of shift and main effects of persist variables; and (3) the interaction of shift and persist variables. Including this interaction allowed us to test the hypothesis that it would be specifically the combination of high shift and high persist strategies (rather than either on its own) that would be protective for low childhood SES individuals. Tests of interactions were conducted according to the recommendations of Aiken and West (58), whereby variables are first centered, and then the interaction is calculated as the product of centered variables.
RESULTS
Table 1 presents descriptive information on the study sample. Adults from low childhood SES backgrounds were less likely to use persist strategies than adults from high childhood SES backgrounds (t=5.13, p<.001), although they were equally likely to use shift strategies (t=0.11, p=.90). Adults from low childhood SES backgrounds had higher allostatic load scores than adults from high childhood SES backgrounds (t=5.26, p<.001).
Do the Effects of Shift-and-Persist Differ by Childhood SES?
We first tested the 3-way interaction of childhood SES, shift, and persist variables predicting allostatic load. After controlling for demographic variables, medical variables, smoking, and current SES, the 3-way interaction of childhood SES, shift, and persist variables significantly predicted allostatic load (β=-.07, p=.02), indicating that the combination of shift and persist strategies had different associations with allostatic load, depending on childhood SES. To clarify the nature of this 3-way interaction, we next investigated how the 2-way interactions between shift and persist strategies predicted allostatic load for low versus higher childhood SES participants. This analytic approach allowed us to test whether the combination of shift and persist strategies was important specifically for buffering low SES adults from elevations in allostatic load.
Associations of Shift-and-Persist Strategies with Allostatic Load in Low Childhood SES Participants
Table 2 presents results for shift-and-persist analyses in low childhood SES participants. In Model 1, the demographic variables of age, gender, and ethnicity were controlled. Neither the main effects of shift strategies (p=.73) or persist (p=.53) strategies predicted allostatic load. However, the interaction between shift and persist significantly predicted allostatic load (p=.02), such that high shift in combination with high persist resulted in the lowest levels of allostatic load among adults who came from disadvantaged childhood SES environments.
Table 2.
Multiple regression analyses of shift-and-persist strategies predicting allostatic load in MIDUS adults from low childhood SES backgrounds (N=300)
| Predictor | Controlling demographics | Controlling demographics + med history | Controlling demographics + med history + smoking | Controlling demographics + med history + smoking + current SES |
|---|---|---|---|---|
| Age | .23** | .20** | .24** | .23** |
| Gender | .05 | .05 | .07 | .04 |
| Ethnicity | -.05 | -.02 | .01 | .00 |
| CVD diagnosis | .05 | .06 | .04 | |
| Diabetes | .20** | .20** | .20** | |
| Smoking | .17** | .14* | ||
| Current SES | -.10 | |||
| Shift | -.02 | .00 | .00 | .01 |
| Persist | -.04 | -.02 | .00 | .01 |
| Shift × Persist | -.16* | -.18* | -.16* | -.15* |
Note: Coefficients presented are beta weights.
p<.05.
p<.01.
SES=socioeconomic status. CVD=cardiovascular disease. Gender (1=female, 0=male). Ethnicity (1=white, 0=nonwhite). CVD diagnosis=ever diagnosed with CVD (yes=1, no=0). Diabetes=ever diagnosed with diabetes (yes=1, no=0). Smoking=current smoker (yes=1, no=0). Current SES=educational attainment of participant.
In Model 2, medical history variables were added as covariates. In Model 3, current smoking was added as a covariate. And in model 4, current SES was included as a covariate. Patterns remained identical in all models, such that even in the fully adjusted model, after controlling for demographic variables, medical variables, smoking, and current SES, the interaction between shift and persist strategies remained significant (p=.04). As with Model 1, high shift in combination with high persist resulted in the lowest levels of allostatic load among adults who came from disadvantaged childhood SES environments.1 See Figure 1.
Figure 1.

Interaction of shift-and-persist strategies in predicting allostatic load among low childhood SES MIDUS participants. Estimated allostatic load scores are plotted at ±1 SD of the shift and persist variables.
Influence of Twin Status
To determine whether the presence of siblings in our sample affected our results, we redid analyses using Generalized Estimating Equations, specifying an exchangeable covariance matrix. This specification relaxes the assumption of independent and identically distributed observations. Patterns remained the same, in that over and above covariates, the interaction of shift-and-persist strategies (p=.03), but not the main effects of either shift (p=.92) or persist (p=.72) strategies predicted allostatic load.
Effects in Higher Childhood SES Participants
To test the specificity of these findings to low childhood SES participants, analyses were repeated for the 907 MIDUS participants who were higher in SES in childhood. The interaction between shift-and-persist strategies did not predict the allostatic load composite in this sample (β=.01, p=.36). See Figure 2. In addition, there was no main effect of shift strategies (β=.02, p=.40), and a marginal effect of persist strategies (β=-.07, p=.06), predicting allostatic load. This suggests that the combination of shift-and-persist strategies is beneficial only among those adults who came from low SES circumstances early in life.
Figure 2.
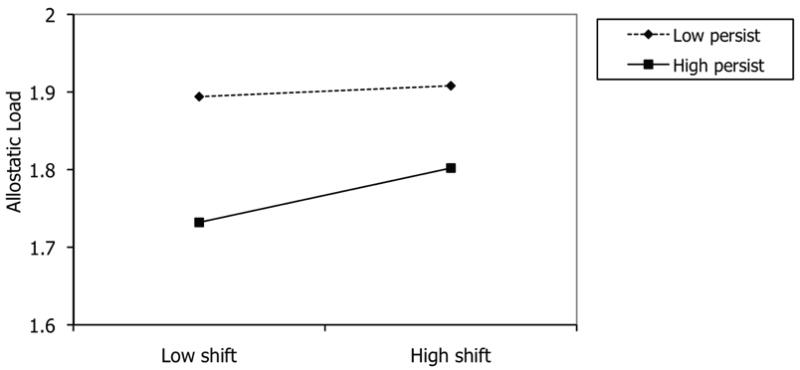
No significant interaction of shift-and-persist strategies predicting allostatic load among high childhood SES MIDUS participants. Estimated allostatic load scores are plotted at ±1 SD of the shift and persist variables.
DISCUSSION
These findings revealed that among adults who grew up in low SES households, engaging in shift-and-persist strategies was associated with reduced allostatic load. This shift-and-persist strategy entailed an approach to life that aims to positively reappraise stress and regulate negative emotions (shifting) in combination with maintaining a focus on the future (persisting). The association of shift-and-persist with lower allostatic load scores among adults who grew up in low SES households persisted after controls were implemented for a number of demographic, medical, and behavioral covariates. In addition, neither shifting nor persisting alone was associated with allostatic load in low SES individuals, suggesting that the combination of tendencies is what is important for reducing cumulative physiological risk in adults from low childhood SES backgrounds.
In this study, allostatic load was defined by a comprehensive battery of 24 measures taken from seven different physiological systems. The notion behind allostatic load is that risk may be better captured through a multisystem perspective that acknowledges the non-independence of physiological systems (45). The fact that shift-and-persist strategies were associated with this cumulative index of allostatic load suggests that shift-and-persist strategies may have implications over the long term for a number of chronic diseases of aging.
We note that shift-and-persist strategies were hypothesized to be beneficial specifically to those who live, on average, under circumstances of frequent stressors with few resources (i.e., low SES). In contrast, for those who live, on average, with fewer stressors and more abundant resources (high SES), an approach to life that in general emphasizes proactive efforts to change stressors themselves may be most effective for mitigating physiological responses to stress. In line with this theory, we found that shift-and-persist strategies were beneficial to allostatic load only in adults from low childhood SES backgrounds, but not in the much larger group of higher childhood SES adults.
Taken together, these findings suggest that the psychological qualities that are beneficial to low SES individuals’ health may be different from those that are beneficial to high SES individuals. That is, for low SES individuals who, on average, face more uncontrollable stressors in daily life (22), an approach that in general emphasizes shifting oneself by accepting and adapting the self to stressors may be most effective for slowing down the pathogenic processes that contribute to chronic diseases. In combination, persistence in terms of maintaining a focus on one’s future may provide meaning in life that promotes beneficial cardiovascular, neuroendocrine, and immune profiles. Over a lifetime, an accumulation of reduced psychosocial and physiological responses to acute stressors may slow the progression of longer-term pathogenic processes for chronic diseases of aging that low childhood SES normally sets into motion.
Our findings are consistent with the few studies that have examined positive psychological traits that buffer the effects of low current SES on current physiological or physical health outcomes. For example, adults who are low in SES but high in perceived control have profiles of self-reported health, acute health symptoms, and functional limitations that are similar to high SES individuals, and better than those of low SES individuals with low perceived control (59). Although the distinction between primary and secondary control coping was not made in this earlier study, the patterns are consistent with our notion that certain types of control strategies will be beneficial to low SES individuals. Similarly, another study found that adults who are low in SES but have high purpose in life showed lower levels of the CVD risk marker IL-6, and their levels were similar to those of high SES individuals (37). These patterns are consistent with the notion that persisting in terms of finding meaning is beneficial to low SES individuals’ health. Finally, these results are consistent with another recent paper published by our group in which we documented that among low SES children with asthma, high shift-and-persist scores predicted lower levels of asthma inflammation and reduced clinical impairment over time. In contrast, high SES children with asthma showed no benefit from shift-and-persist strategies (60). Our work extends these findings by focusing on the long-term health consequences of low early-life SES, and by identifying a unique constellation of traits that is protective among adults who grew up in low, but not high, SES families.
Our work is also consistent with previous literature that has focused on the broader social environment and that has identified protective effects of social support from others. This research has demonstrated that support and warmth from others is associated with reduced allostatic load in low SES children (61), and with beneficial cardiovascular and immune profiles among currently low SES adults (62). It is also consistent with research that has documented that positive relationships can buffer the effects of low childhood SES on adult physiological risk and gene expression profiles (63, 64). It may be the case that experiencing positive relationships with others over time helps to facilitate the development of a shift-in-persist approach within individuals. In the present study, rather than focusing on external social environments, we focused on low SES individuals themselves, and the characteristics that they can acquire to protect themselves from adverse health outcomes.
The fact that findings emerged with respect to the childhood economic circumstances from which study participants came is consistent with previous epidemiological literature documenting that childhood may represent a sensitive, or critical, period with long-lasting effects on health outcomes (6, 8, 65). One reason why this may be is that adversity that happens during certain sensitive periods of children’s development may calibrate how certain bodily systems operate going forward, establishing more permanent proclivities, such as the tendency for immune cells to exhibit stable pro-inflammatory tendencies throughout life, via mechanisms such as epigenetics, post-translational modifications, and tissue remodeling (66).
Strengths of the present study include the large sample of participants from low SES childhoods, and the extensive battery of allostatic load measures. Limitations include the cross-sectional design that makes the directionality of findings difficult to discern. Ideally, we would have had a lifecourse design so that we could assess shift-and-persist strategies during childhood, and its ability to prospectively predict trajectories of physiological profiles over adulthood, and so that we could assess an array of childhood SES measures (in addition to parent education) and potential confounding variables, such as child health, early in life. In the absence of this type of longitudinal data, it is certainly possible that shift-and-persist strategies as measured in adulthood could reflect a consequence of certain childhood factors, such as being healthier or having experienced less stressful events earlier in life. However, we do note that the measures chosen for this study have long-term stability (high test-retest reliability over 10 years), suggesting that they represent characteristics that developed earlier in life. Second, we note that our findings are consistent with another study in which we also took approximations of the shift-and-persist construct from an existing dataset and demonstrated that shift-and-persist strategies protected low SES children from asthma inflammation and impairment (60). Taken together, these two studies suggest that there are benefits to shifting-and-persisting in childhood, and that their effects are also evident into adulthood, as suggested by the present study.
Second, this study was not designed explicitly to test the shift-and-persist hypothesis. As with many new theories, the first tests often come from existing datasets in which one utilizes reasonable approximations of a construct. Hence we relied on measures within the MIDUS study that tapped constructs related to shift-and-persist. We are currently in the process of developing and validating a measure of shift-and-persist, and plan to test ability of this measure to predict physiological and health-related outcomes in a low SES sample in a future study.
Finally, we did not have the ability to verify the controllability of stressful situations when probing shift strategies, although we know that low SES individuals, on average, report more perceived constraints in life (59).
In sum, adults who came from low SES backgrounds as children and who engaged in a combination of shift-and-persist strategies (dealing with stressors by positively reappraising them, engaging in emotion regulation, and at the same time, persisting with a future orientation) showed lower allostatic load scores. Understanding the specific psychosocial qualities that naturally contribute to physiological resilience among low SES individuals represents one important approach toward developing future interventions aimed at reducing health disparities. That is, if we can identify characteristics that some low SES individuals already possess that promote positive physiological profiles, these may be ones that would be effective to alter through intervention in other low SES individuals, allowing our society to help bolster individual coping resources among those who grow up with socioeconomic disadvantage. There are of course a variety of approaches that society could take toward reducing health disparities. The ones related to shift-and-persist suggested by this paper focus around efforts to help individuals deal with adversity themselves. Alternative approaches of great importance in the public health realm center around providing low SES individuals with basic resources to improve health. No one approach is likely to eliminate the health disparities that are so prominent in our society, and hence efforts to promote shift-and-persist would ideally be utilized in conjunction with societal efforts to provide basic economic resources and health services, which together could be marshaled to begin to make a difference in improving the health of those confronting disadvantaged circumstances early in life.
Acknowledgments
This study was supported by NIH grant PO1 AG20166 (Lachman, Seeman).
Abbreviations
- SES
socioeconomic status
- CVD
cardiovascular disease
- MIDUS
Midlife in the United States
- SDRR
standard deviation of the RR interval
- RMSSD
root mean squared successive difference
- LFHRV
low frequency heart rate variability
- HFLRV
high frequency heart rate variability
- DHEA-S
dehydroepiandrosterone sulfate
- CRP
C reactive protein
- IL-6
interleukin 6
- sICAM-1
soluble intercellular adhesion molecule 1
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- HbA1c%
hemoglobin A1c %
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- BMI
body mass index
- WHR
waist hip ratio
- SNS
sympathetic nervous system
- PNS
parasympathetic nervous system
- HPA
hypothalamic pituitary adrenal axis
- ECG
electrocardiogram
- HPLC
high pressure liquid chromatography
- DHEA-S
dehydroepiandrosterone sulfate
- ELISA
enzyme-linked immunosorbent assay
- HOMA-IR
homeostasis model of insulin resistance
Footnotes
These effects were specific to the allostatic load composite, as interactions were not significant for the individual measures that comprised allostatic load.
References
- 1.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL. Socioeconomic status and health: The challenge of the gradient. American Psychologist. 1994;49:15–24. doi: 10.1037//0003-066x.49.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Marmot MG, Shipley MJ, Rose G. Inequalities in death- specific explanations of a general pattern? Lancet. 1984;i:1003–1006. doi: 10.1016/s0140-6736(84)92337-7. [DOI] [PubMed] [Google Scholar]
- 3.Adler NE, Rehkopf DH. U.S. disparities in health: descriptions, causes, and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- 4.Adler NE, Boyce WT, Chesney MA, Folkman S, Syme SL. Socioeconomic inequalities in health: No easy solution. Journal of the American Medical Association. 1993;269:3140–3145. [PubMed] [Google Scholar]
- 5.Galobardes B, Lynch JW, Smith GD. Childhood socioeconomic circumstances and cause-specific mortality in adulthood: Systematic review and interpretation. Epidemiol Rev. 2004;26:7–21. doi: 10.1093/epirev/mxh008. [DOI] [PubMed] [Google Scholar]
- 6.Galobardes B, Smith GD, Lynch JW. Systematic review of the influence of childhood socioeconomic circumstances on risk for cardiovascular disease in adulthood. Annals of Epidemiology. 2006;16:91–104. doi: 10.1016/j.annepidem.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 7.Cohen S, Doyle WJ, Turner RB, Alper CM, Skoner DP. Childhood socioeconomic status and host resistance to infectious illness in adulthood. Psychosom Med. 2004;66:553–558. doi: 10.1097/01.psy.0000126200.05189.d3. [DOI] [PubMed] [Google Scholar]
- 8.Kittleson MM, Meoni LA, Wang NY, Chu AY, Ford DE, Klag MJ. Association of childhood socioeconomic status with subsequent coronary heart disease in physicians. Archives of Internal Medicine. 2006;166:2356–2361. doi: 10.1001/archinte.166.21.2356. [DOI] [PubMed] [Google Scholar]
- 9.Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- 10.Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviors and psychosocial characteristics by stages of the socioeconomic lifecourse. Social Science and Medicine. 1997;44:809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- 11.Evans GW. The environment of childhood poverty. American Psychologist. 2004;59:77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- 12.Chen E, Matthews KA, Boyce WT. Socioeconomic differences in children’s health: How and why do these relationships change with age? Psychological Bulletin. 2002;128:295–329. doi: 10.1037/0033-2909.128.2.295. [DOI] [PubMed] [Google Scholar]
- 13.Cohen S, Janicki-Deverts D, Chen E, Matthews KA. Childhood socioeconomic status and adult health. Ann N Y Acad Sci. 2010;1186:37–55. doi: 10.1111/j.1749-6632.2009.05334.x. [DOI] [PubMed] [Google Scholar]
- 14.Masten AS, Garmezy N, Tellegen A, Pellegrini DS, Larkin K, Larsen A. Competence and stress in school children: The moderating effects of individual and family qualities. Journal of Child Psychiatry. 1988;29:745–764. doi: 10.1111/j.1469-7610.1988.tb00751.x. [DOI] [PubMed] [Google Scholar]
- 15.Masten AS. Ordinary magic: Resilience processes in development. American Psychologist. 2001;56:227–238. doi: 10.1037//0003-066x.56.3.227. [DOI] [PubMed] [Google Scholar]
- 16.Garmezy N. In: Stress-resistant children: The search for protective factors. Stevenson JE, editor. Oxford; Pergamon: 1985. pp. 213–233. [Google Scholar]
- 17.Rutter M. Psychosocial resilience and protective mechanisms. Am J Orthopsychiatry. 1987;57:316–331. doi: 10.1111/j.1939-0025.1987.tb03541.x. [DOI] [PubMed] [Google Scholar]
- 18.Werner EE. Resilience in development. Current Directions in Psychological Science. 1995;4:81–85. [Google Scholar]
- 19.Chen E, Miller GE. “Shift-and-persist” strategies: Why being low in socioeconomic status isn’t always bad for your health. doi: 10.1177/1745691612436694. manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annu Rev Psychol. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 21.Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: A review and methodological critique. Psychological Bulletin. 1984;96:435–464. [PubMed] [Google Scholar]
- 22.Brady SS, Matthews KA. The effect of socioeconomic status and ethnicity on adolescents’ exposure to stressful life events. Journal of Pediatric Psychology. 2002;27:575–583. doi: 10.1093/jpepsy/27.7.575. [DOI] [PubMed] [Google Scholar]
- 23.Attar BK, Guerra NG, Tolan PH. Neighborhood disadvantage, stressful life events, and adjustment in urban elementary-school children. Journal of Clinical Child Psychology. 1994;23:391–400. [Google Scholar]
- 24.Chen E. Why socioeconomic status affects the health of children: A psychosocial perspective. Current Directions in Psychological Science. 2004;13:112–115. [Google Scholar]
- 25.Heckhausen J, Schulz R. A life-span theory of control. Psychol Rev. 1995;102:284–304. doi: 10.1037/0033-295x.102.2.284. [DOI] [PubMed] [Google Scholar]
- 26.Heckhausen J, Wrosch C, Schulz R. A motivational theory of life-span development. Psychol Rev. 2010;117:32–60. doi: 10.1037/a0017668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens NM, Hamedani MG, Markus HR, Bergsieker HB, Eloul L. Why did they “choose” to stay? Perspectives of Hurricane Katrina observers and survivors. Psychol Sci. 2009;20:878–886. doi: 10.1111/j.1467-9280.2009.02386.x. [DOI] [PubMed] [Google Scholar]
- 28.Markus HR, Ryff CD, Curhan KB, Palmersheim K. In: In their own words: Well-being at midlife among high school and college educated adults. Brim OG, Ryff CD, Kessler RC, editors. Chicago, IL: University of Chicago Press; 2004. pp. 273–319. [Google Scholar]
- 29.James SA, Hartnett SA, Kalsbeek WD. John Henryism and blood pressure differences among black men. J Behav Med. 1983;6:259–278. doi: 10.1007/BF01315113. [DOI] [PubMed] [Google Scholar]
- 30.James SA, Strogatz DS, Wing SB, Ramsey DL. Socioeconomic status, John Henryism, and hypertension in blacks and whites. Am J Epidemiol. 1987;126:664–673. doi: 10.1093/oxfordjournals.aje.a114706. [DOI] [PubMed] [Google Scholar]
- 31.James SA, Keenan NL, Strogatz DS, Browning SR, Garrett JM. Socioeconomic status, John Henryism, and blood pressure in black adults. The Pitt County Study. Am J Epidemiol. 1992;135:59–67. doi: 10.1093/oxfordjournals.aje.a116202. [DOI] [PubMed] [Google Scholar]
- 32.Wright LB, Treiber FA, Davis H, Strong WB. Relationship of John Henryism to cardiovascular functioning at rest and during stress in youth. Annals of Behavioral Medicine. 1996;18:146–150. doi: 10.1007/BF02883390. [DOI] [PubMed] [Google Scholar]
- 33.Chen E. Impact of socioeconomic status on physiological health in adolescents: An experimental manipulation of psychosocial factors. Psychosom Med. 2007;69:348–355. doi: 10.1097/PSY.0b013e3180592b20. [DOI] [PubMed] [Google Scholar]
- 34.Taylor SE. Adjustment to threatening events: A theory of cognitive adaptation. American Psychologist. 1983;38:1161–1173. [Google Scholar]
- 35.Updegraff JA, Silver RC, Holman EA. Searching for and finding meaning in collective trauma: results from a national longitudinal study of the 9/11 terrorist attacks. J Pers Soc Psychol. 2008;95:709–722. doi: 10.1037/0022-3514.95.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonanno GA, Wortman CB, Nesse RM. Prospective patterns of resilience and maladjustment during widowhood. Psychol Aging. 2004;19:260–271. doi: 10.1037/0882-7974.19.2.260. [DOI] [PubMed] [Google Scholar]
- 37.Morozink JA, Friedman EM, Coe CL, Ryff CD. Socioeconomic and psychosocial predictors of interleukin-6 in the MIDUS national sample. Health Psychol. 2010;29:626–635. doi: 10.1037/a0021360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hobfoll SE. Conservation of resources: A new attempt at conceptualizing stress. American Psychologist. 1989;44:513–524. doi: 10.1037//0003-066x.44.3.513. [DOI] [PubMed] [Google Scholar]
- 39.Hobfoll SE. The influence of culture, community, and the nested-self in the stress process: Advancing Conservation of Resources Theory. Applied Psychology: An International Review. 2001;50:337–370. [Google Scholar]
- 40.Gallo LC, Matthews KA. Understanding the association between socioeconomic status and physical health: Do negative emotions play a role? Psychological Bulletin. 2003;129:10–51. doi: 10.1037/0033-2909.129.1.10. [DOI] [PubMed] [Google Scholar]
- 41.Aspinwall LG, Taylor SE. A stitch in time: self-regulation and proactive coping. Psychol Bull. 1997;121:417–436. doi: 10.1037/0033-2909.121.3.417. [DOI] [PubMed] [Google Scholar]
- 42.Maier SF, Seligman MEP. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105:3–46. [Google Scholar]
- 43.Evers AW, Kraaimaat FW, van Lankveld W, Jongen PJ, Jacobs JW, Bijlsma JW. Beyond unfavorable thinking: the illness cognition questionnaire for chronic diseases. J Consult Clin Psychol. 2001;69:1026–1036. [PubMed] [Google Scholar]
- 44.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 45.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: cumulative allostatic load. Ann N Y Acad Sci. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- 46.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98:4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosom Med. 2006;68:500–507. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- 48.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaption - Allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med. 1997;157:2268. [PubMed] [Google Scholar]
- 49.Dienberg Love G, Seeman TE, Weinstein M, Ryff CD. Bioindicators in the MIDUS National Study: Protocol, Measures, Sample, and Comparative Context. J Aging Health. 2010;22:1059–1080. doi: 10.1177/0898264310374355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, Posner S. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 51.Wrosch C, Heckhausen J, Lachman ME. Primary and secondary control strategies for managing health and financial stress across adulthood. Psychol Aging. 2000;15:387–399. doi: 10.1037//0882-7974.15.3.387. [DOI] [PubMed] [Google Scholar]
- 52.Roberts BW, DelVecchio WF. The rank-order consistency of personality traits from childhood to old age: a quantitative review of longitudinal studies. Psychol Bull. 2000;126:3–25. doi: 10.1037/0033-2909.126.1.3. [DOI] [PubMed] [Google Scholar]
- 53.Wrosch C, Heckhausen J. Control processes before and after passing a developmental deadline: Activation and deactivation of intimate relationship goals. Journal of Personality and Social Psychology. 1999;77:415–427. [Google Scholar]
- 54.Heckhausen J, Wrosch C, Fleeson W. Developmental regulation before and after a developmental deadline: the sample case of “biological clock” for childbearing. Psychol Aging. 2001;16:400–413. doi: 10.1037//0882-7974.16.3.400. [DOI] [PubMed] [Google Scholar]
- 55.Patrick CJ, Curtin JJ, Tellegen A. Development and validation of a brief form of the Multidimensional Personality Questionnaire. Psychol Assess. 2002;14:150–163. doi: 10.1037//1040-3590.14.2.150. [DOI] [PubMed] [Google Scholar]
- 56.Church AT. Relating the Tellegen and five-factor models of personality structure. J Pers Soc Psychol. 1994;67:898–909. doi: 10.1037//0022-3514.67.5.898. [DOI] [PubMed] [Google Scholar]
- 57.Prenda KM, Lachman ME. Planning for the future: a life management strategy for increasing control and life satisfaction in adulthood. Psychol Aging. 2001;16:206–216. [PubMed] [Google Scholar]
- 58.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. London: Sage Publications; 1991. [Google Scholar]
- 59.Lachman ME, Weaver SL. The sense of control as a moderator of social class differences in health and well-being. Journal of Personality and Social Psychology. 1998;74:763–773. doi: 10.1037//0022-3514.74.3.763. [DOI] [PubMed] [Google Scholar]
- 60.Chen E, Strunk RC, Trethewey A, Schreier HMC, Maharaj N, Miller GE. Resilience in low socioeconomic status children with asthma: Adaptations to stress. Journal of Allergy and Clinical Immunology. doi: 10.1016/j.jaci.2011.06.040. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans GW, Kim P, Ting AH, Tesher HB, Shannis D. Cumulative risk, maternal responsiveness, and allostatic load among young adolescents. Developmental Psychology. 2007;43:341–351. doi: 10.1037/0012-1649.43.2.341. [DOI] [PubMed] [Google Scholar]
- 62.Vitaliano PP, Scanlan JM, Zhang JP, Savage MV, Brummett B, Barefoot J, Siegler IC. Are the salutogenic effects of social supports modified by income? A test of an “added value hypothesis”. Health Psychology. 2001;20:155–165. [PubMed] [Google Scholar]
- 63.Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Mol Psychiatry. 2011;16:729–737. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singer B, Ryff CD. Hierarchies of life histories and associated health risks. Ann N Y Acad Sci. 1999;896:96–115. doi: 10.1111/j.1749-6632.1999.tb08108.x. [DOI] [PubMed] [Google Scholar]
- 65.Galobardes B, Lynch JW, Smith GD. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62:387–390. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 66.Miller GE, Chen E, Parker KJ. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving towards a model of behavioral and biological mechanisms. Psychological Bulletin. doi: 10.1037/a0024768. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


